Abstract
Pancreatic cancer is lethal, as it is often detected late. Thus, novel biomarkers of precursor lesions are needed to devise timely therapies. Pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN) are major precursors of pancreatic cancer. In normal gastric mucosa, gastric gland mucin‐specific O‐glycans are unique in having α1,4‐linked N‐acetylglucosamine (αGlcNAc) residues attached to MUC6. Recently we reported that αGlcNAc functions as a tumor suppressor for differentiated‐type gastric adenocarcinoma (Karasawa et al., J Clin Invest 122, 923, 2012). MUC6 is also expressed in pancreatic neoplasms, including PanIN and IPMN, but the role of αGlcNAc expression in pancreatic neoplasms remains unknown. Here, we analyze expression patterns of αGlcNAc, MUC6 and MUC5AC in pancreatic neoplasms and compare them with progression from PanIN to invasive ductal adenocarcinoma (IDAC) (the PanIN‐IDAC sequence; 20 cases) and from IPMN to IPMN with associated invasive carcinoma (IPMNAIC) (the IPMN‐IPMNAIC sequence; 20 cases). At both sequences, the frequency of MUC6‐positive and αGlcNAc‐positive lesions decreased with tumor progression. We then compared expression levels of αGlcNAc and MUC6 at each step of the progression. At the PanIN‐IDAC sequence, αGlcNAc expression significantly decreased relative to MUC6 in low‐grade PanIN (P = 0.021), high‐grade PanIN/intraductal spread of IDAC (P = 0.031) and IDAC (P = 0.013). At the IPMN‐IPMNAIC sequence, decreased αGlcNAc expression was also observed in low‐grade IPMN exhibiting gastric‐type morphology (P = 0.020). These results suggest that decreased expression of αGlcNAc relative to MUC6 occurs early and marks the initiation of tumor progression to pancreatic cancer.
Keywords: αGlcNAc, intraductal papillary mucinous neoplasms, MUC6, pancreatic cancer, pancreatic intraepithelial neoplasia
Pancreatic cancer is highly lethal due to difficulty of early diagnosis: most cases of pancreatic cancer are diagnosed at advanced stage, greatly decreasing the chance for a cure. Thus, novel biomarkers of precursor lesions of pancreatic cancer are required. An international consensus meeting held at the Johns Hopkins Hospital, Baltimore, MD, USA in 2003 assessed and reported the current definition and classification of three major precursor lesions to invasive ductal adenocarcinoma (IDAC) of the pancreas; they include pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasm (MCN).1 In 2014, a new international consensus meeting held at the Johns Hopkins Hospital revised the earlier guidelines.2 Specifically, the revised guideline recommends a two‐tiered system (i.e. low‐grade versus high‐grade), instead of a three‐tiered system used for former classification of the precursor lesions including PanIN, IPMN and MCN.
Changes in the mucin phenotype of the pancreatic epithelium, particularly acquisition of gastric mucin properties, are crucial events in early stages of pancreatic tumor progression.3, 4, 5, 6, 7 Gastric mucins are classified as surface and gland mucins that contain MUC5AC and MUC6, respectively.8 Gland mucin characteristically contains O‐linked oligosaccharides (O‐glycans) with terminal α1,4‐linked N‐acetylglucosamine residues (αGlcNAc) attached largely to a MUC6 scaffold.9, 10 In normal gastric mucosa, αGlcNAc and MUC6 are co‐expressed in gland mucous cells, such as pyloric gland and mucous neck cells.10, 11 Previously, we used expression cloning to isolate cDNA encoding α1,4‐N‐acetylglucosaminytransferase (α4GnT), which catalyzes αGlcNAc biosynthesis.12 We then reported that Α4gnt‐deficient mice, which show αGlcNAc loss in gland mucin, spontaneously develop gastric adenocarcinoma.13 These findings suggest that αGlcNAc serves as a tumor suppressor.14 In support of this idea, we observed that αGlcNAc expression is frequently lost in human gastric differentiated‐type adenocarcinoma expressing MUC6.15 We also showed that reduced αGlcNAc expression relative to MUC6 is associated with malignant potential in pyloric gland adenoma of the human stomach, a precursor of gastric adenocarcinoma.16 These studies suggest overall that αGlcNAc could serve as a critical biomarker of malignant potential in early stages of gastric epithelial neoplasias. In normal human pancreas, MUC6 and αGlcNAc are co‐expressed in periductal mucous gland cells of the main pancreatic duct.10 In addition, we and others reported that αGlcNAc is expressed in PanIN.17, 18, 19 However, the relationship between αGlcNAc expression and pancreatic tumor progression remains unknown.
Here, we used immunohistochemistry to examine expression patterns of gastric mucin markers, including MUC5AC, MUC6 and αGlcNAc, in precursor lesions of pancreatic cancer, including PanIN and IPMN, as well as invasive carcinoma. We then compared relative αGlcNAc and MUC6 expression in each lesion.
Materials and Methods
Patient samples
The present study evaluated pancreatic tissue specimens from 48 surgically resected cases of pancreatic tumors at Shinshu University Hospital, Matsumoto, Japan. Specifically, tissue specimens of IDAC (20 cases) and IPMN (28 cases), which were diagnosed based on World Health Organization classification criteria (2010),20 were retrieved from the pathology files of the Department of Laboratory Medicine of the same hospital. All specimens were fixed in 10% buffered formalin and embedded in paraffin wax. Tissue sections were stained with H&E for histopathological analysis. In 20 cases of IDAC that did not contain IPMN components, we selected lesions exhibiting low‐grade PanIN, high‐grade PanIN and IDAC classified on a recent consensus.2 Hereafter, we used high‐grade PanIN/intraducal spread of IDAC (high‐grade PanIN/IDS) for high‐grade PanIN, because it is morphologically difficult to distinguish high‐grade PanIN from intraductal spreading of IDAC when IDAC exists.2 We eventually selected 17 low‐grade PanIN lesions, 12 high‐grade PanIN/IDS lesions and 20 IDAC lesions (Table S1). For IPMN, we first excluded 8 cases of intestinal‐type IPMN, which is characterized by its MUC2 expression, from 28 cases of IPMN retrieved from the pathology file, because this particular type of IPMN does not express MUC6.17 In fact, all of the excluded cases were negative for MUC6 (Fig. S1). Thus, we classified IPMN lesions into low‐grade IPMN, high‐grade IPMN, and IPMN with associated invasive carcinoma (IPMNAIC) lesions based on a recent consensus for histological grade.2 Consequently, 19 lesions of low‐grade IPMN, 10 lesions of high‐grade IPMN and 8 lesions of IPMNAIC were selected (Table S2). Furthermore, both low‐grade IPMN and high‐grade IPMN lesions were morphologically subclassified into gastric type, pancreatobiliary type and oncocytic type based on World Health Organization classification criteria (2010).20 Because cases with oncocytic‐type IPMN were not included in the pathology file, we eventually selected 21 gastric‐type IPMN lesions, including 19 lesions of low‐grade IPMN and 2 lesions of high‐grade IPMN, and 8 lesions of pancreatobiliary‐type IPMN, which were high‐grade IPMN (Table S3). This study was approved by the Ethics Committee of the Shinshu University School of Medicine, Matsumoto, Japan (nos. 1338 and 3626) and was in accordance with the Declaration of Helsinki. The Ethics Committee also granted a waiver of informed consent to use formalin‐fixed, paraffin‐embedded tissue specimens, because diagnostic use of samples was completed before the study and there was no risk to patients involved. Samples were also coded to protect patient anonymity.
Immunohistochemistry
Primary antibodies used in this study were: anti‐MUC5AC (clone 45M1, mouse IgG; Novocastra, Newcastle, UK) diluted 1:100, anti‐MUC6 (clone CLH5, mouse IgG; Novocastra) diluted 1:200, and anti‐αGlcNAc (clone HIK1083, mouse IgM; Kantokagaku, Tokyo, Japan) diluted 1:20. Conventional immunohistochemistry for all primary antibodies was carried out using the EnVision system (DakoCytomation, Carpinteria, CA, USA). Tissue sections of 3‐μm thickness were deparaffinized in xylene and rehydrated in ethanol. Except for αGlcNAc, antigens were retrieved by boiling sections in 10‐mM Tris/HCl buffer (pH 8.0) containing 1 mM EDTA for 25 min in a microwave oven. Endogenous peroxidase activity was quenched by soaking sections in absolute methanol containing 0.3% hydrogen peroxide for 30 min. After blocking with 1% BSA (Sigma‐Aldrich, St. Louis, MO, USA) in TBS (pH 7.6) for 15 min, sections were incubated with each primary antibody at 4°C overnight followed by incubation with HRP‐conjugated anti‐mouse immunoglobulins for 60 min. The color reaction was developed with 3,3′‐diaminobenzidine (Dojindo, Kumamoto, Japan). Negative controls were established by omitting primary antibodies from the procedure, and no specific staining was seen. Immunohistochemical evaluation was undertaken in two ways. First, lesions in which >5% of the total number of tumor cells of each lesion were positively‐stained were judged positive, as described previously.15 Second, expression levels of MUC6 and αGlcNAc were further scored semi‐quantitatively from 0 to 3: 0 (≤5% positive cells), 1 (6%–33% positive cells), 2 (34%–66% positive cells) or 3 (≥67% positive cells), as described previously.16
Statistical analysis
Correlations between each grade for PanIN or IPMN and the number of positive lesions were statistically analyzed by Fisher's exact probability test. Differences between semi‐quantitative immunoreactivity scores in MUC6‐stained and αGlcNAc‐stained sections were statistically analyzed using the Wilcoxon matched pairs test. All analyses were carried out with Microsoft Office Excel 2010 (Microsoft, Redmond, WA, USA). P‐values <0.05 were considered statistically significant.
Results
Expression of mucin core proteins MUC5AC and MUC6 as well as αGlcNAc in pancreatic lesions exhibiting the PanIN‐IDAC sequence
MUC5AC was expressed in 45 (91.8%) of 49 lesions associated with the PanIN‐IDAC sequence, irrespective of histological grade (Table 1 and Fig. 1a). By contrast, MUC6 was expressed in all 17 low‐grade PanIN, 11 (91.7%) of 12 high‐grade PanIN/IDS, and 14 (70%) of 20 IDAC lesions. The number of MUC6‐positive lesions representing low‐grade PanIN was significantly higher than that seen in IDAC (P < 0.05). However, low‐grade PanIN and high‐grade PanIN/IDS did not show a significant difference (P = 0.41). In contrast, αGlcNAc expression was observed in all 17 low‐grade PanIN lesions (100%), 6 (50%) of 12 high‐grade PanIN/IDS, and 8 (40%) of 20 IDAC. The frequency of αGlcNAc‐positive lesions in both high‐grade PanIN/IDS and IDAC was significantly decreased relative to that seen in low‐grade PanIN (P < 0.01).
Table 1.
Frequency of lesions positive for MUC proteins or αGlcNAc associated with the PanIN‐IDAC sequence of pancreatic tumor progression
| Number of lesions | MUC5AC (%) | MUC6 (%) | αGlcNAc (%) | |
|---|---|---|---|---|
| PanIN‐IDAC | ||||
| Low‐grade PanIN | 17 | 16 (94.1) | 17 (100)a | 17 (100)b |
| High‐grade PanIN/IDS | 12 | 11 (91.7) | 11 (91.7) | 6 (50)b |
| IDAC | 20 | 18 (90.0) | 14 (70.0)a | 8 (40.0)b |
| Total | 49 | 45 (91.8) | 42 (85.7) | 31 (63.3) |
Significant difference in MUC6 positivity between low‐grade PanIN and IDAC (P < 0.05).
Significant difference in αGlcNAc positivity between low‐grade and high‐grade PanIN/IDS (P < 0.01) and between low‐grade PanIN and IDAC (P < 0.01).
Figure 1.
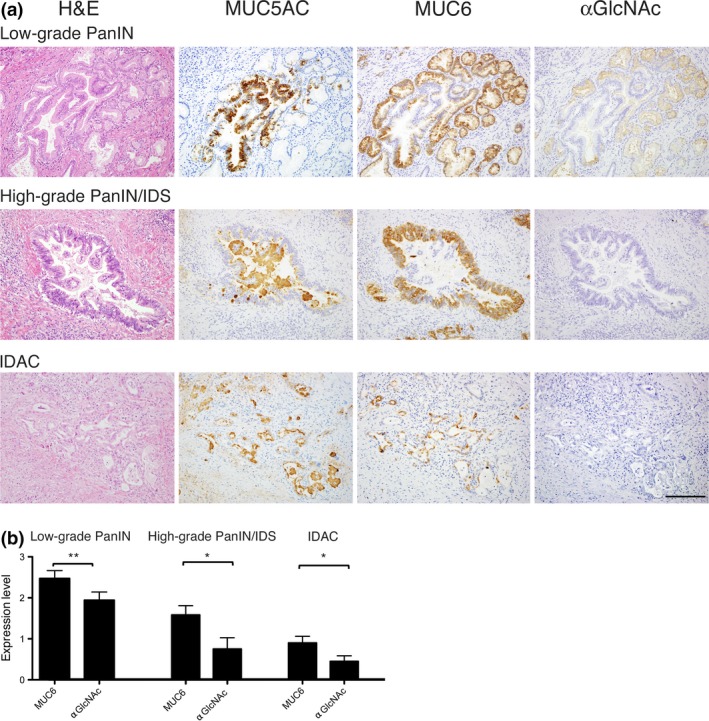
Immunohistochemical analysis of MUC5AC, MUC6 and αGlcNAc expression in PanIN and IDAC. (a) MUC5AC is expressed in tumor cells, irrespective of tumor grade. MUC6 is expressed in tumor cells showing pyloric gland phenotypes in low‐grade PanIN and high‐grade PanIN/IDS. αGlcNAc expression coincides with that of MUC6 in low‐grade PanIN. By contrast, in both high‐grade PanIN/IDS and IDAC, αGlcNAc is not expressed in MUC6‐positive tumor cells. Bar = 100 μm. (b) Semi‐quantitation of MUC6 and αGlcNAc expression in low‐grade PanIN, high‐grade PanIN/IDS, and IDAC. Data are represented as the mean ± SEM. *P < 0.05 and **P < 0.01 by Wilcoxon matched‐pair test.
Because αGlcNAc is largely attached to MUC6, and the relatively decreased αGlcNAc expression in MUC6‐positive lesions is associated with gastric cancer progression,10, 15 we compared αGlcNAc and MUC6 immunoreactivity semi‐quantitatively in low‐grade PanIN, high‐grade PanIN/IDS, and IDAC (Table S1). At any histological grade, αGlcNAc expression levels were significantly reduced relative to those of MUC6 (P < 0.01 for low‐grade PanIN, P < 0.05 for high‐grade PanIN/IDS, and P < 0.05 for IDAC) (Fig. 1b).
Expression of MUC5AC and MUC6 as well as αGlcNAc in pancreatic lesions representing the IPMN‐IPMNAIC sequence
We next examined expression of MUC5AC, MUC6 and αGlcNAc in lesions exhibiting the IPMN‐IPMNAIC sequence. MUC5AC was expressed in all 37 IPMN lesions, irrespective of histological grade (Table 2 and Fig. 2a). MUC6 was expressed in 18 (94.7%) of 19 low‐grade IPMN, 7 (70%) of 10 high‐grade IPMN, and 3 (37.5%) of 8 IPMNAIC lesions. Statistical analysis revealed that the number of MUC6‐positive lesions in low‐grade IPMN was significantly greater than that seen in IPMNAIC (P < 0.01). However, the difference in the number of MUC6‐positive lesions between low‐grade and high‐grade IPMN was not significant (P = 0.10). In contrast, αGlcNAc was expressed in 18 (94.7%) of 19 low‐grade IPMN and 5 (50%) of 10 high‐grade IPMN lesions. However, αGlcNAc was not detected in any of 8 IPMNAIC lesions. When we compared the number of αGlcNAc‐positive lesions between high‐grade IPMN and IPMNAIC or between low‐grade IPMN and high‐grade IPMN, the frequency of αGlcNAc‐positive lesions was significantly decreased in more advanced histological grades (P < 0.05 for high‐grade IPMN versus IPMNAIC and P < 0.01 for low‐grade IPMN versus high‐grade IPMN).
Table 2.
Frequency of lesions positive for MUC proteins or αGlcNAc associated with the IPMN‐IPMNAIC sequence of pancreatic tumor progression
| Number of lesions | MUC5AC (%) | MUC6 (%) | αGlcNAc (%) | |
|---|---|---|---|---|
| IPMN‐IPMNAIC | ||||
| Low‐grade IPMN | 19 | 19 (100) | 18 (94.7)a | 18 (94.7)b |
| High‐grade IPMN | 10 | 10 (100) | 7 (70.0) | 5 (50.0)b |
| IPMNAIC | 8 | 8 (100) | 3 (37.5)a | 0 (0)b |
| Total | 37 | 37 (100) | 28 (75.7) | 23 (62.2) |
Significant difference in MUC6 positivity between low‐grade IPMN and IPMNAIC (P < 0.01).
Significant difference in αGlcNAc positivity between low‐grade IPMN and high‐grade IPMN (P < 0.01), between high‐grade IPMN and IPMNAIC (P < 0.05), and between low‐grade IPMN and IPMNAIC (P < 0.01).
Figure 2.
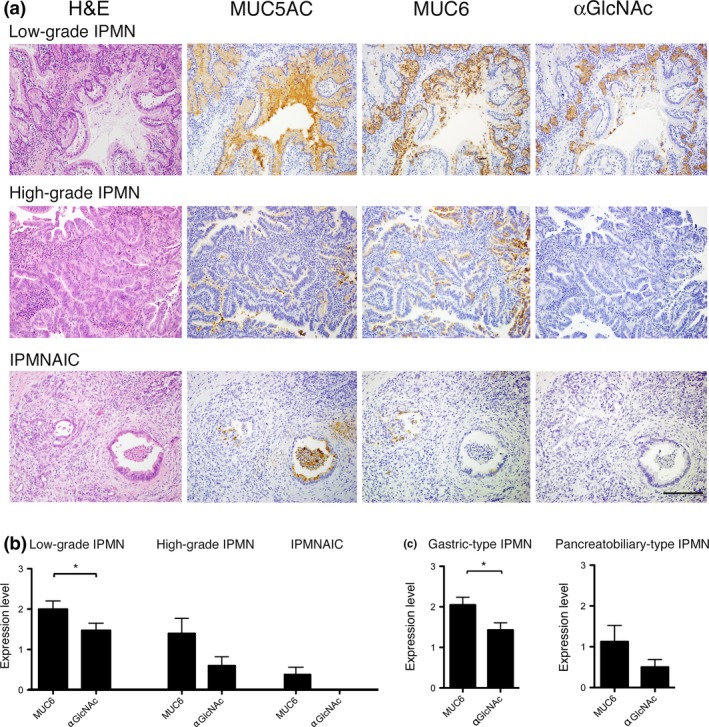
Immunohistochemical analysis of MUC5AC, MUC6 and αGlcNAc in IPMN and IPMNAIC. (a) MUC5AC is expressed in tumor cells, irrespective of histological grade. MUC6 is highly expressed in tumor cells showing a pyloric gland phenotype characteristic of low‐grade IPMN. However, MUC6 expression decreases in high‐grade IPMN and IPMNAIC. αGlcNAc expression in low‐grade IPMN coincides with that of MUC6. By contrast, in high‐grade and IPMNAIC, αGlcNAc is not expressed in MUC6‐positve tumor cells. Bar = 100 μm. (b) Semi‐quantitation of MUC6 and αGlcNAc expression in low‐grade IPMN, high‐grade IPMN and IPMNAIC. Data are represented as the mean ± SEM. *P < 0.05 by Wilcoxon matched‐pair test. (c) Semi‐quantitation of MUC6 and αGlcNAc expression in gastric‐type IPMN and pancreatobiliary‐type IPMN. Data are represented as the mean ± SEM. *P < 0.05 by Wilcoxon matched‐pair test.
Next, we assessed αGlcNAc and MUC6 immunoreactivity semi‐quantitatively in low‐grade IPMN, high‐grade IPMN, and IPMNAIC (Table S2). In low‐grade IPMN, αGlcNAc immunoreactivity was significantly decreased relative to that of MUC6 (P < 0.05) (Fig. 2b). Nonetheless, we did not observe significant differences in αGlcNAc and MUC6 immunoreactivity in either high‐grade IPMN or IPMNAIC (P = 0.071 for high‐grade IPMN and P = 0.083 for IPMNAIC) (Fig. 2b).
Finally, we semi‐quantitatively assessed αGlcNAc and MUC6 immunoreactivity from a standpoint of morphological classifications, gastric‐type and pancreatobiliary‐type IPMN (Table S3). We compared the expression level of αGlcNAc and MUC6 in all 21 gastric‐type IPMN lesions, including both 19 lesions of low‐grade IPMN and 2 lesions of high‐grade IPMN. We found that αGlcNAc immunoreactivity in gastric‐type IPMN was significantly decreased compared to MUC6 (P < 0.05) (Fig. 2c). On the other hand, the expression level of αGlcNAc in 8 lesions of pancreatobiliary‐type IPMN, all of which belonged to high‐grade IPMN, was lower than that of MUC6. However, significant differences were not obtained between them (P = 0.13) (Fig. 2c).
Discussion
The present study revealed that expression levels of αGlcNAc relative to MUC6 begin to decrease early in pancreatic tumor progression in both the PanIN‐IDAC and IPMN‐IPMNAIC sequences: specifically, lesions positive for αGlcNAc or MUC6 were most frequently detected in low‐grade PanIN and low‐grade IPMN (Tables 1 and 2). However, semi‐quantitative analysis of αGlcNAc and MUC6 immunoreactivities indicated that αGlcNAc expression relative to that of MUC6 had already decreased not only in low‐grade PanIN but also in low‐grade IPMN (Figs. 1b and 2b). In both high‐grade PanIN/IDS and IDAC, the number of αGlcNAc‐positive lesions and expression levels of αGlcNAc significantly decreased relative to MUC6 levels. Although we did not observe a significant difference between high‐grade IPMN and IPMNAIC, αGlcNAc expression in both lesions was lower than that of MUC6. These results combined together indicate that a decrease in αGlcNAc expression precedes a decrease in MUC6, even in early phases of pancreatic tumor progression. We previously demonstrated that αGlcNAc and MUC6 are largely co‐expressed in periductal accessory glands of the pancreatic duct.10 In the present study, we reveal that at the early phase of PanIN‐IDAC and of IPMN‐IPMNAIC sequence, MUC6 expression significantly predominates over αGlcNAc expression, and as histological grade progresses to pancreatic cancer, expression levels of both decrease. We recently demonstrated that αGlcNAc expression is significantly reduced in pyloric gland adenoma with high‐grade dysplasia that is a precancerous lesion of gastric adenocarcinoma.16 These results overall suggest that reduced αGlcNAc expression relative to MUC6 occurs at early stages of pancreatic tumor progression. However, molecular mechanism explaining why decreased αGlcNAc expression marks the initiation of tumor progression has yet to be elucidated. Because αGlcNAc functions as a tumor suppressor for differentiated‐type gastric adenocarcinoma,13 decrement of αGlcNAc might trigger the initiation of tumor progression. Future studies are needed to address this problem.
Morphological subclassification of IPMN into gastric, intestinal, pancreatobiliary and oncocytic type is of significance to predict the malignant potential of tumors and the prognosis of patients; that is, gastric‐type IPMN is strongly associated with low histological grade, and other IPMN types are negatively associated with low histological grade.22 In fact, all 19 lesions of low‐grade IPMN examined in the present study were classified as gastric‐type IPMN, whereas 8 of 10 lesions of high‐grade IPMN were categorized as pancreatobiliary‐type IPMN (Table S3). Significant reduction of αGlcNAc relative to MUC6 in gastric‐type IPMN shown here supported that αGlcNAc expression already decreased in the early phase of IPMN‐IPMNAIC sequence.
Recent studies show that IDAC derived from PanIN frequently exhibits K‐RAS mutations but not GNAS mutations, although IPMN typically harbors GNAS mutations.21, 23 These findings suggest that PanIN‐IDAC and IPMN‐IPMNAIC sequences employ different molecular machinery. Here, however, we observed decreased expression of αGlcNAc accompanied by progression of pancreatic neoplasia at both sequences in the tumor progression pathway, suggesting that αGlcNAc expression levels could predict malignant potentials of both PanIN and IPMN. Future studies are needed to define molecular mechanisms underlying regulation of expression of Α4GNT gene, which encodes α4GnT.
We also show that MUC5AC and MUC6 are expressed not only in both low‐grade PanIN and high‐grade PanIN/IDS but also in low‐grade and high‐grade IPMN, all precursors of pancreatic cancer (Tables 1 and 2). However, MUC6 expression in pancreatic cancer, including IDAC and IPMNAIC, was lower relative to MUC5AC expression. Kim et al. demonstrated that MUC6 expression in PanIN is an early event seen in 74% of PanIN1A lesions, 67% of PanIN1B lesions, 66% of PanIN2 lesions and 56% of PanIN3 lesions, whereas MUC6 is expressed only in 35% of IDAC lesions.5 Our results are consistent with these studies. In terms of other cancer types, Chang et al.24 demonstrate that MUC6 is expressed in metaplastic pseudopuloric glands in the gallbladder and its expression decreases in dysplasia and carcinoma. Matsukita et al.25 also showed a correlation between MUC6 expression and mucinous carcinoma of the breast, suggesting that high MUC6 expression in that context may act as a barrier to cancerous growth and antagonize tumor cell invasivity. All of these studies strongly suggest that MUC6 may play an important role as a tumor suppressor in pancreatic and other tumors, such as the gallbladder and breast.
In conclusion, the present study indicates that decreased expression of αGlcNAc relative to MUC6 is an initial event marking the early phase of pancreatic tumor progression. Further studies are needed to determine molecular mechanisms that regulate αGlcNAc expression to better understand pancreatic tumor progression.
Disclosure Statement
The authors have no conflicts of interest to declare.
Supporting information
Fig. S1. Immunohistochemical expression of MUC5AC, MUC2 and MUC6 in intestinal‐type IPMN. MUC5AC and MUC2 are expressed in tumor cells of intestinal‐type IPMN, irrespective of histological grade. By contrast, MUC6 is not detected in the tumor cells. Primary antibody used for MUC2 immunohistochemistry was anti‐MUC2 antibody (clone Ccp58, mouse IgG, Novocastra). Information about anti‐MUC5AC and anti‐MUC6 antibodies was provided in the main text. Bar = 100 μm.
Table S1. Immunohistochemical scores reflecting MUC6 and αGlcNAc staining in each lesion of 20 cases associated with the PanIN‐IDAC sequence.
Table S2. Immunohistochemical scores reflecting MUC6 and αGlcNAc staining in each lesion of 20 cases associated with the IPMN‐IPMNAIC sequence.
Table S3. Morphological subclassification of low‐grade IPMN lesions and high‐grade IPMN lesions in 20 cases in IPMN‐IPMNAIC sequence.
Acknowledgments
The authors thank Dr Elise Lamar for editing the manuscript. This work was supported by Grants‐in‐Aid for Scientific Research 15H04712 and 17K15640 from the Japan Society for the Promotion of Science.
Cancer Sci 108 (2017) 1897–1902
Funding information
Japan Society for the Promotion of Science (15H04712 and 17K15640)
References
- 1. Hruban RH, Takaori K, Klimstra DS et al An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2004; 28: 977–87. [DOI] [PubMed] [Google Scholar]
- 2. Basturk O, Hong SM, Wood LD et al A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursors lesions in the pancreas. Am J Surg Pathol 2015; 39: 1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prasad NB, Biankin AV, Fukushima N et al Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res 2005; 65: 1619–26. [DOI] [PubMed] [Google Scholar]
- 4. Ban S, Naitoh Y, Mino‐Kenudson M et al Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol 2006; 30: 1561–9. [DOI] [PubMed] [Google Scholar]
- 5. Kim GE, Bae HI, Park HU et al Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 2002; 123: 1052–60. [DOI] [PubMed] [Google Scholar]
- 6. Nagata K, Horinouchi M, Saitou M et al Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg 2007; 14: 243–54. [DOI] [PubMed] [Google Scholar]
- 7. Basturk O, Khayyata S, Klimstra DS et al Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol 2010; 34: 364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ota H, Katsuyama T, Ishii K et al A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J 1991; 23: 22–8. [DOI] [PubMed] [Google Scholar]
- 9. Ishihara K, Kurihara M, Goso Y et al Peripheral α‐linked N‐acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 1996; 318(Pt 2): 409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang MX, Nakayama J, Hidaka E et al Immunohistochemical demonstration of α1,4‐N‐acetylglucosaminyltransferase that forms GlcNAcα1,4Galβ residues in human gastrointestinal mucosa. J Histochem Cytochem 2001; 49: 587–96. [DOI] [PubMed] [Google Scholar]
- 11. Yamada S, Okamura T, Kobayashi S, Tanaka E, Nakayama J. Reduced gland mucin‐specific O‐glycan in gastric atrophy: a possible risk factor for differentiated‐type adenocarcinoma of the stomach. J Gastroenterol Hepatol 2015; 30: 1478–84. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama J, Yeh JC, Misra AK et al Expression cloning of a human α1,4‐N‐acetylglucosaminyltransferase that forms GlcNAcα1→4Galβ→R, a glycan specifically expressed in the gastric gland mucous cell‐type mucin. Proc Natl Acad Sci USA 1999; 96: 8991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karasawa F, Shiota A, Goso Y et al Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest 2012; 122: 923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakayama J. Dual roles of gastric gland mucin‐specific O‐glycans in prevention of gastric cancer. Acta Histochem Cytochem 2014; 47: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiratsu K, Higuchi K, Nakayama J. Loss of gastric gland mucin‐specific O‐glycan is associated with progression of differentiated‐type adenocarcinoma of the stomach. Cancer Sci 2014; 105: 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamanoi K, Sekine S, Higuchi K, Kushima R, Nakayama J. Decreased expression of gastric gland mucin‐specific glycan α1,4‐linked N‐acetylglucosamine on its scaffold mucin 6 is associated with malignant potential of pyloric gland adenoma of the stomach. Histopathology 2015; 67: 898–904. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi M, Fujinaga Y, Ota H. Reappraisal of the immunophenotype of pancreatic intraductal papillary mucinous neoplasms (IPMNs) ‐gastric pyloric and small intestinal immunophenotype expression in gastric and intestinal type IPMNs‐. Acta Histochem Cytochem 2014; 47: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuzawa K, Akamatsu T, Katsuyama T. Mucin histochemistry of pancreatic duct cell carcinoma, with special reference to organoid differentiation simulating gastric pyloric mucosa. Hum Pathol 1992; 23: 925–33. [DOI] [PubMed] [Google Scholar]
- 19. Ishizone S, Yamauchi K, Kawa S et al Clinical utility of quantitative RT‐PCR targeted to α1,4‐N‐acetylglucosaminyltransferase mRNA for detection of pancreatic cancer. Cancer Sci 2006; 97: 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adsay NV, Fukushima N, Furukawa T et al Intraductal neoplasms of the pancreas In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, 4th edn Lyon: IARC Press, 2010; 304–13. [Google Scholar]
- 21. Furukawa T, Kuboki Y, Tanji E et al Whole‐exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011; 1: 161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furukawa T, Hatori T, Fujita I et al Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011; 60: 509–16. [DOI] [PubMed] [Google Scholar]
- 23. Matthaei H, Wu J, Dal Molin M et al GNAS sequencing identifies IPMN‐specific mutations in a subgroup of diminutive pancreas cysts referred to as “incipient IPMNs”. Am J Surg Pathol 2014; 38: 360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang HJ, Kim SW, Lee BL, Hong EK, Kim WH. Phenotypic alterations of mucins and cytokeratins during gallbladder carcinogenesis. Pathol Int 2004; 54: 576–84. [DOI] [PubMed] [Google Scholar]
- 25. Matsukita S, Nomoto M, Kitajima S et al Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology 2003; 42: 26–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunohistochemical expression of MUC5AC, MUC2 and MUC6 in intestinal‐type IPMN. MUC5AC and MUC2 are expressed in tumor cells of intestinal‐type IPMN, irrespective of histological grade. By contrast, MUC6 is not detected in the tumor cells. Primary antibody used for MUC2 immunohistochemistry was anti‐MUC2 antibody (clone Ccp58, mouse IgG, Novocastra). Information about anti‐MUC5AC and anti‐MUC6 antibodies was provided in the main text. Bar = 100 μm.
Table S1. Immunohistochemical scores reflecting MUC6 and αGlcNAc staining in each lesion of 20 cases associated with the PanIN‐IDAC sequence.
Table S2. Immunohistochemical scores reflecting MUC6 and αGlcNAc staining in each lesion of 20 cases associated with the IPMN‐IPMNAIC sequence.
Table S3. Morphological subclassification of low‐grade IPMN lesions and high‐grade IPMN lesions in 20 cases in IPMN‐IPMNAIC sequence.


