Abstract
Background
Sleep difficulties are common in schizophrenia, however these complaints are often overshadowed by more prominent clinical concerns. The point prevalence of insomnia in this population is not well documented. Poor sleep is associated with lower quality of life, impaired cognition, and weight gain.
Objectives
The objectives of this study are to evaluate the prevalence of insomnia in schizophrenia and to explore the relationship of sleep to cognition, quality of life, and clinical variables.
Method
175 outpatients with schizophrenia or schizoaffective disorder were assessed for insomnia. Participants were evaluated for sleep difficulties, sleep patterns, body mass index, and psychiatric symptoms. Participants were also administered a brief cognitive assessment of processing speed.
Results
44% of the sample currently met the criteria for clinical insomnia. An additional 4% were successfully treated with medications. Insomnia was associated with depression and was an independent predictor of lower quality of life. Insomnia was also associated with high rates of night eating and patients with severe insomnia were significantly more obese. The type of antipsychotic did not account for the difference in body mass index. No difference between group means in cognition was detected, although those with severe insomnia did perform least well.
Conclusion
Clinical insomnia in outpatients with schizophrenia is highly prevalent and has a negative impact on quality of life and psychiatric symptoms. This study offers additional support to the association between poor sleep and higher weight, as well as indicating a potential link to night eating in this population. Assessment for sleep difficulties should be a routine part of clinical care.
Keywords: Schizophrenia, Sleep, Insomnia, Obesity, Night eating
1. Introduction
Sleep difficulties are among the most common complaints among patients with schizophrenia. Insomnia is not well studied in schizophrenia, perhaps because it is not one of the core features of this condition. The point prevalence of insomnia in patients with schizophrenia is not well known, though one prior study reported poor sleep quality at a rate of 52% (Royuela et al., 2002). The specific sleep abnormalities detected in patients with schizophrenia include shortened rapid eye movement (REM), sleep latency, decreased delta waves, and reduced slow wave sleep (Benson, 2006). In addition to these, individuals with schizophrenia report nightmares, difficulty initiating sleep, and difficulty staying asleep (Benson, 2006).
The existing studies have reported that sleep disturbances have significant impact on quality of life in patients with schizophrenia (Benca et al., 1992; Haffmans et al., 1994; Chouinard et al., 2004; Ritsner et al., 2004; Hofstetter and Mayeda, 2008). Additionally, sleep disturbances associated with schizophrenia have been linked to worsening of symptoms of psychosis and poorer overall outcomes (Goldman et al., 1996; Chemerinski et al., 2002). Insomnia has also been associated with increased positive symptoms (Poulin et al., 2003), while improved sleep has been shown to decrease the negative symptoms of schizophrenia (Kato et al., 1999).
In the general population, reduced sleep has been linked to reduced quality of physical health, including excess body weight and obesity. This includes studies indicating a negative association between sleep duration and weight (Taheri et al., 2004; Ganwisch et al., 2005; Vorona et al., 2005). This potential consequence of poor sleep is particularly concerning with respect to those with schizophrenia given the high rates of obesity in individuals taking antipsychotic medications, the associated cardiovascular risk factors, and increased mortality reported in this population (Allison et al., 1999; Goff et al., 2005; Parks et al., 2006).
Disturbances of sleep have also been associated with impairments in cognition. Sleep appears to play an important role in the consolidation of memory (Walker and Stickgold, 2006). Associations between quality of sleep, visuospatial memory, and procedural learning have been reported (Göder et al., 2004; Manoach et al., 2004). Given that deficits in cognition are part of the symptomotology of schizophrenia, exacerbation of these deficits, secondary to poor sleep, would have important negative consequences.
Despite the links of sleep disturbance to physical health and cognition in the general population and the importance of these issues for those with schizophrenia, minimal research has been carried out on the relationships of sleep and quality of life, physical health, and cognitive functioning in patients with schizophrenia. In this study we aimed to better evaluate whether such relationships exist in those with schizophrenia and explore the associations among sleep and a variety of quality of life and clinical variables in stable patients with schizophrenia. We hypothesized that insomnia would negatively impact quality of life and cognition, and would be associated with obesity.
2. Methods
2.1. Participants
175 clinically stable outpatients with documented diagnosis of schizophrenia or schizoaffective disorder were assessed for insomnia. Individuals were recruited from an urban community mental health center. Participants were interviewed regarding their current sleep quality and behaviors, quality of life, processing speed as a proxy for overall cognition, and current psychiatric symptoms. All participants provided written informed consent and study protocols were approved by the Yale University School of Medicine Human Investigation Committee. One-hundred (57.1%) of the participants were male and 75 (42.9%) were female. Fifty-nine (33.7%) identified their ethnicity as Caucasian, 99 (56.6%) as African American, and 15 (8.6%) as Hispanic. Two (1.1%) identified their ethnicity as “other”. Average length of illness for the sample was 20.3±10.4 years (Table 1). One-hundred eighteen (67%) were currently taking a second-generation antipsychotic, 32 (18%) were taking a first-generation, and 15(9%) were taking both a first- and second-generation antipsychotic. Nine (5%) patients were not currently taking an antipsychotic and one did not know the name of his antipsychotic.
Table 1.
Demographic and clinical information for sample by insomnia category.
| No insomnia | Subthreshold | Moderate | Severe | F | p value | |
|---|---|---|---|---|---|---|
| Age | 44.9±8 | 43.6±10.8 | 43.9±10.9 | 42.1±7.5 | 0.39 | 0.76 |
| Sex | χ2=9.05 | 0.03* | ||||
| Male | 18(50%) | 41(66%) | 34(62%) | 7(32%) | ||
| Female | 18(50%) | 21(34%) | 21(38%) | 15(68%) | ||
| Years of Education | 11.7±2 | 12.1±3.3 | 12±2.3 | 12.1±2.1 | 0.23 | 0.88 |
| Length of illness (in years) | 21.5±8.8 | 20.1±10.6 | 19.5±10.3 | 21.3±12.6 | 0.33 | 0.80 |
| Calgary Depression Scalea | 3±2.9 | 5.1±4 | 6.7±4.2 | 10±5.5 | 14.51 | <0.001* |
| CGIb symptom domains | ||||||
| Positive | 3.4±1.1 | 3.8±1.2 | 3.6±1.2 | 4.2±0.9 | 1.82 | 0.15 |
| Negative | 3.3±1 | 3.4±1 | 3.4±1.1 | 3.3±0.9 | 0.09 | 0.97 |
| Cognitive | 2.5±0.9 | 3±1.2 | 3.2±1.1 | 3.3±1.4 | 2.11 | 0.10 |
| Depressive | 2±1.2 | 2.4±1.3 | 2.9±1.4 | 3.4±1.6 | 3.99 | 0.01* |
| Global | 3.3±0.8 | 3.8±0.8 | 3.8±0.9 | 4.1±0.5 | 3.71 | 0.01* |
Statistically significant.
Calgary Depression Scale total score, minus the 1 item about sleep.
Clinical Global Impression Scale-Schizophrenia Version.
2.2. Instruments
Participants completed the following questionnaires under the supervision of research staff. When necessary, research staff administered questionnaires in an interview style.
2.2.1. Insomnia Severity Index (ISI)
This is a brief sleep questionnaire used to screen for insomnia (Bastien et al., 2001). Total score on the first 7 items results in the following classifications: 0–7=no clinically significant insomnia, 8–14=subthreshold insomnia, 15–21=clinical insomnia (moderate severity), 22–28=clinical insomnia (severe).
2.2.2. Pittsburgh Sleep Quality Index (PSQI)
This instrument measures subjective sleep quality and generates 7 component scores: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction (Buysse et al., 1989). This measure has been previously used in research on schizophrenia (Royuela et al., 2002; Ritsner et al., 2004; Hofstetter et al., 2005).
2.2.3. Quality of Life Enjoyment and Satisfaction Questionnaire-Abbreviated Version for Schizophrenia (QLES-Q-18)
This is a quality of life measure that has been validated for patients with schizophrenia spectrum illnesses (Ritsner et al., 2005). Scores may range from 18 to 90.
2.2.4. Calgary Depression Scale (CDS)
This is a standard depression scale used in the assessment of depression in schizophrenia spectrum patients (Addington et al., 1992). Range of scores is 0–3 for each of the 9 items.
2.2.5. Night Eating Questionnaire (NEQ)
This instrument measures night eating behavior and contains 14 self-report items, though only 13 items are included in the total score (Allison et al., 2008). Each item is scored from 0 to 4.
2.2.6. Sleep Hygiene Index (SHI)
This is a measure of how frequently individuals engage in specific sleep hygiene-related behaviors (Mastin et al., 2006). Scores on each of the 13 items range from 1(never) to 5(always).
2.2.7. Clinical Global Impression Scale-Schizophrenia Version (CGI)
This is a brief semi-structured form in which the rater indicates their global impression of severity of several domains of schizophrenia (Haro et al., 2003). A clinician’s global severity rating from 1 to 7 is assigned to the following symptom domains: positive, negative, depressive, cognitive, and overall symptoms.
2.2.8. Digit Symbol Substitution Test (DSST)
This is a subtest of the Wechsler Adult Intelligence Scale-III that measures processing speed (Wechsler, 1997). This was utilized as a brief measure of overall cognitive functioning, as it has been shown to be the strongest predictor of global cognitive impairment in schizophrenia across studies (Dickinson et al., 2007).
2.2.9. Body Mass Index (BMI)
Calculated with standard equation: (weight in kilograms/height in meters2).
2.2.10. Statistical analyses
One way between subjects ANOVAs were calculated in order to compare participants in terms of their level of reported sleep disturbance. A p<0.05 significance level was utilized for all analyses. In addition, post hoc analyses included Bonferroni correction. A Pearson’s r correlation coefficient was calculated for several variables. Regression analysis was employed where appropriate.
3. Results
3.1. Insomnia severity
Overall, 77 (44%) subjects met criteria for clinical insomnia, with an additional seven (4%) subjects in treatment with sleep aids, which is presumed to indicate that they met criteria for a diagnosis of insomnia, bringing the total insomnia diagnosis frequency to 48%. The mean ISI score across all subjects for the first seven items was 13.3± 6.6, with scores ranging from 0 to 28. Fifty-five (35%) subjects met ISI criteria for moderate insomnia and 22 (13%) for severe insomnia. Sixty-two (35%) subjects reported sleep difficulties that were categorized as “subthreshold”. Thirty-six (21%) reported no significant sleep difficulties, and thus, were categorized into the “no insomnia” group. These groups did not differ in terms of demographic information, length of illness, generation of antipsychotic used, previous diagnosis of sleep apnea, or caffeine, alcohol, and nicotine use. PSQI total score was correlated with ISI 7-item score as well as global score (both r=0.7, p<0.001) providing construct validity of these sleep instruments for use in schizophrenia populations. All PSQI component scores with the exception of the taking sleep medication component were significantly different between ISI generated insomnia sub-groups. These scores give an idea about the nature of sleep problems in patients with schizophrenia and are presented in Table 2.
Table 2.
Pittsburgh Sleep Quality Index scores by insomnia category.
| No insomnia | Subthreshold | Moderate | Severe | F | |
|---|---|---|---|---|---|
| PSQI-total score | 5.58±3.68 | 9.82±3.91 | 11.89±3.54 | 15.32±2.53 | 38.81* |
| Components: | |||||
| Sleep quality | 0.69±0.79 | 1.36±0.68 | 1.85±0.80 | 2.86±0.35 | 46.53* |
| Sleep latency | 1.17±0.91 | 1.85±0.98 | 2.39±0.83 | 2.05±1.09 | 12.47* |
| Sleep duration | 0.42±0.73 | 1.21±1.08 | 1.75±1.21 | 2.36±1 | 19.14* |
| Habitual sleep efficiency | 0.56±1.03 | 1.37±1.28 | 1.63±1.19 | 2.23±1.15 | 10.38* |
| Sleep disturbances | 1.14±0.49 | 1.79±0.34 | 1.78±0.53 | 2.23±0.61 | 18.56* |
| Use of sleep medication | 0.81±1.21 | 1.20±1.38 | 1.33±1.39 | 1.18±1.37 | 1.13 |
| Daytime dysfunction | 0.81±0.75 | 1.34±1.49 | 1.24±0.96 | 2.27±0.88 | 7.72* |
Significant at p<0.001.
3.2. Sleep hygiene
Differences between groups on Sleep Hygiene Index total score approached significance [F (3, 170)=2.58, p=0.06]. Comparison of the 13 individual behaviors assessed by this measure detected differences with respect to 5 of the items. Item #3 (I get out of bed at different times from day to day) revealed a significant difference between the no insomnia group (2.39±0.96) and those with moderate (3.15±1.25) and severe insomnia (3.23±1.45), [F (3, 171)=4.24, p<0.01], with those in the former obtaining a lower mean score than those in the latter, indicating a higher reported frequency of this behavior. Groups also differed with respect to item # 8 (I go to bed feeling stressed, angry, upset, or nervous), [F (3, 170)=7.42, p<0.001]. Those in the “no insomnia” group obtained a significantly lower mean score (1.92±0.94) than both the moderate (2.82±1.16) and severe (3.18±1.37) insomnia groups. Item # 13 (I think, plan, or worry when I am in bed), indicated that both the moderate (3.25±1.08) and severe (3.59±1.3) insomnia groups obtained higher mean scores as compared to the no insomnia group (2.33±1.01), [F(3, 170)=7.39, p<0.001]. For item # 11 (I sleep in an uncomfortable bedroom), the only significant difference was between the no insomnia and subthreshold groups, (1.81±1.17 vs. 2.48±1.22), [F (3, 170)=2.92, p=0.04]. For item #7 (I do something that wakes me up right before bedtime), there was also a difference between the no insomnia and subthreshold group (1.61±0.87 vs. 2.18±1.04), [F (3, 170)=2.94, p=0.04].
3.3. Depression and overall symptoms
The groups differed in depression scores on the CDS, [F (3, 170)= 14.51, p<0.001]. Specifically, post hoc analyses detected that those categorized in the severe insomnia group reported significantly higher mean score (9.95±5.45) than all other groups (3±2.92, 5.05±4.02, 6.67±4.2). This difference remained significant even after removal of the one CDS item which asks about sleep, [F (3, 170)=9.03, p<0.001]. On the CGI measure of depression, distinctions between groups were significant, [F (3, 109)=3.99, p=0.01], with additional analysis revealing the sole difference to be between the no insomnia (2±1.2) and severe (3.4±1.55) insomnia category. No significant differences were detected for the CGI measures of positive, negative, and cognitive symptoms, [F (3, 109)=1.82, ns., F (3, 109)=0.09, ns., F (3, 109)=2.11, ns, respectively]. See Table 1.
3.4. Quality of life
A significant effect of ISI category on total quality of life score was found, [F (3, 169)=8.52, p<0.001]. Post hoc comparisons revealed that the mean QLES score of those in the clinically-severe insomnia category (48.77±11.45) was significantly lower than that of the three other categories (65.33±12.95, 60.25±12.35, 58.60±11.94, respectively), which did not differ significantly from each other. Lower quality of life was independently predicted by both insomnia severity and level of depression, which held true after controlling for age and gender, [F=44.9, p<0.001].
3.5. Body Mass Index
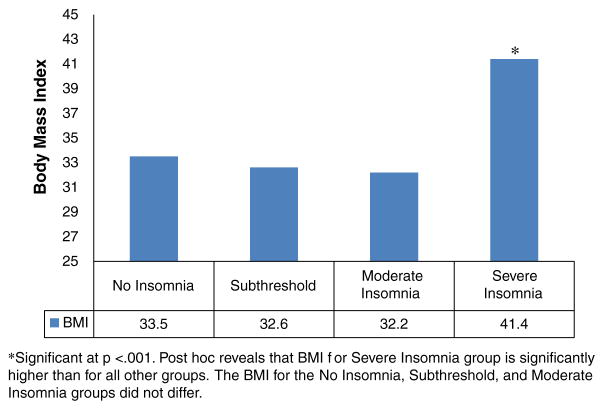
Analyses comparing the four ISI categories on the measure of BMI detected a significant effect, [F (3, 168)=7.97, p<0.001]. This effect remained significant after age, sex, type of antipsychotic medication, and CDS score was controlled for [F (3, 160)=5.36, p=0.002]. The type of antipsychotic did not account for the difference in BMI [F (3, 161)=1.48, ns]. Post hoc comparisons revealed that the mean BMI of those in the clinically-severe insomnia category (41.4±8 kg/m2) was significantly higher than that of the three other categories (mean BMI=33.5±7.1, 32.6±7.5, and 32.2±8.7 kg/m2, respectively), which did not differ significantly from each other (Fig. 1).
Fig. 1.
Mean BMI for insomnia categories.
3.6. Night eating
An ANOVA revealed a significant effect for ISI group on NEQ score, [F (3, 169)=11.53, p<0.001]. Those grouped into the “severe insomnia” category obtained a mean NEQ score (25.55±8.27) significantly higher than both the no-insomnia (15.39±5.92) and subthreshold (19.70± 7.54) insomnia groups. The moderate and severe groups did not differ in their reports of night eating (23.06±7.73 vs. 25.55±8.27). Night eating score was correlated with both ISI and PSQI global score (r=0.49 and 0.43 respectively, p<0.001). NEQ was positively correlated with depression (r=0.34, p=0.01), but not correlated with BMI. NEQ scores were not different between groups of patients who were on first-generation or second-generation antipsychotics, or combination of both.
3.7. Cognition
No differences between group means on the DSST were found [F (3, 168)=1.45, ns.] although the severe insomnia group had a lower score. Mean scores for this measure were as follows: no insomnia, 13.17±2.19; subthreshold, 12.8±1.65; moderate, 13.19±1.79; severe, 8.42±1.8. DSST score was negatively correlated with age and CGI cognitive score, (r=−0.27, 0.28, p<0.01), as expected. On the other hand, while CGI cognitive score was positively correlated with global sleep measures (ISI, r=0.28, p=0.003; PSQI, r=0.24, p=0.01), DSST was not.
4. Discussion
Results of analyses show that 44% of individuals with schizophrenia or schizoaffective disorder in our sample have significant symptoms of insomnia. Twenty-nine percent of these individuals report sleep difficulties which are categorized as severe. An additional 4% of the sample are currently receiving pharmacologic treatment for insomnia, and report no significant sleep problems at this time. This offers further evidence that individuals with schizophrenia do indeed suffer from clinically significant sleep problems. Comparatively, only 15% of the general population of Americans met the criteria for insomnia in a recent analysis of NHANES data (Ngyuen et al., 2009). Levels of insomnia in this schizophrenia sample are associated with additional clinically important factors. In concordance with previous studies, results show that individuals with schizophrenia and severe insomnia report significantly lower quality of life than those with lesser or no sleep difficulties. While depression in this population was also moderately associated with insomnia severity, the connection between insomnia and lowered quality of life persisted after controlling for depression.
In addition to its impact on quality of life, severe insomnia was also associated with significantly higher BMIs. The mean BMI (41.4) for this group was nearly 10 points higher than the average of the other three insomnia categories (Fig. 1). According to the NIH Clinical Guidelines (NIH publication No. 98–4083, 1998), a BMI of 40 and above is categorized as Class III (extreme) obesity and has a disease risk classified as extremely high. The significant elevation in BMI for patients with severe insomnia was not explained by the type of antipsychotic used but may in part be due to an increased frequency of night eating, as measured by the NEQ, though BMI and NEQ were not found to be correlated in this population. This may in part be due to the homogeneity of the sample: 88% of this population was overweight (BMI>25). Lundgren et al. (2010), too, found that overweight individuals with severe mental illness were at high risk for night eating syndrome and that BMI was not related to night eating. Perhaps in first episode samples who tend not to be overweight (Phutane et al., 2011), night eating may be a factor in initial weight gain. Those in the severe clinical insomnia group had an average NEQ score of 25.5 which was both statistically and clinically distinguishable from scores obtained by the 2 non-clinical insomnia groups. In a previous study which assessed for prevalence of night eating syndrome in psychiatric population, a cutoff score of 25 on the NEQ yielded a positive predictive value of 62% for night eating syndrome (Lundgren et al., 2006; Allison et al., 2008). Though a causal relationship cannot be drawn, this finding is alarming in an already at-risk patient population.
In terms of sleep hygiene, individuals who report no sleep difficulties seem to report a more consistent sleep schedule (specifically with respect to when they get out of bed in the morning). This is noteworthy in that an important component of proper sleep hygiene is keeping a consistent sleep cycle. Additionally, those with no insomnia do not seem to engage in worrying and/or planning while in bed, which likely results in a lower level of anxiety. Indeed, those without insomnia report experiencing negative emotional arousal less frequently while in bed. Given the success in improving sleep through sleep hygiene education in various populations (Morin et al., 2006) it might benefit patients with schizophrenia as well. Supplemental to sleep hygiene education, cognitive techniques would likely be effective in helping to address the worrying and emotional arousal reported by these patients.
As expected, subjective sleep quality in this sample was increasingly poor with increased insomnia severity. Reported sleep disturbances, daytime dysfunction, and delayed sleep latency were all reported to a higher extent by individuals with any level of insomnia than in those experiencing no insomnia. Subjective duration of sleep as well as habitual sleep efficiency (the ratio of actual sleep time to time spent in bed) clearly differed amongst those with sleep difficulties; those with moderate and severe insomnia reporting on average lower sleep duration and lower efficiency. Interestingly, despite reported sleep difficulties, the groups did not differ with respect to reported utility of sleep medication, perhaps offering more support for the notion that insomnia is inadequately addressed by providers.
One limitation to this study is the probability that some of our sample may have undiagnosed sleep apnea, which may be contributing to reported sleep difficulties. Another limitation of the study is lack of assessment of extrapyramidal side effects. Movement disorders such as restless leg syndrome may be related to nocturnal eating (Howell et al., 2010). Also, while we show an association between global sleep measures and global impression of level of cognitive dysfunction, our chosen cognitive test, DSST was not able to capture this despite previous data pointing to predictive value of DSST for overall cognition (Dickinson et al., 2007). Perhaps the relationship between insomnia and cognitive impairment is subtle or compartmentalized to a cognitive function which cannot be fully assessed by processing speed, thus should be further studied with more comprehensive neuropsychological testing. In fact, one study comparing DSST scores in primary insomnia and controls did not detect a difference (Hauri, 1997). Additionally, although we did not exclude anyone from participating in the screening, it is likely that those who volunteered for such a study did have some level of sleep problem which may have overestimated the prevalence of insomnia in schizophrenia. Although insomnia is associated with night eating and obesity, one possibility that must be considered is that some individuals sleep more poorly because they are obese. This could be due to sleep apnea or other obesity related pathologies.
The findings of this study are limited to chronic schizophrenia. Further research is needed with first episode patients. Finally, as with other self-report sleep studies, subjective reports of insomnia may be best validated by more objective measures (i.e. actigraphy) in future research.
5. Conclusion
Insomnia is not a well recognized symptom in routine care of schizophrenia, however, it is a substantial and frequent clinical problem which should be addressed given its negative impact on quality of life and psychiatric symptoms. Our finding that 44% of our sample of outpatients at an urban community health center met clinical criteria for moderate or severe insomnia supports the high prevalence of this problem and the need to address it in clinical care.
We found an association between insomnia and higher weight as well as increased night eating in this study. Further studies are needed to investigate the nature of this relationship, and to determine if improving sleep would have any beneficial effects on high rates of obesity seen in this population. Adequately addressing the problem of poor sleep and related night eating in schizophrenia should start with simple assessment during routine clinical appointments as interventions for insomnia are readily available and safe for clinical use in schizophrenia populations.
Acknowledgments
Role of funding source
This research was partly supported by a NARSAD young investigator grant and an investigator initiated grant from Sepracor/Sunovion, Inc. to Cenk Tek, M.D. The sponsor of the study had no role in the collection, analysis and interpretation of data; in the writing of this report; and in the decision to submit the paper for publication.
We thank Erin Reutenauer for her assistance in data collection, as well as the grammatical and textual check of the first draft of our manuscript.
Footnotes
Contributors
All authors have made significant scientific contributions to this manuscript.
Conflict of interest
The authors report no conflicts of interest.
References
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6(3):201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60:215–220. doi: 10.4088/jcp.v60n0402. [DOI] [PubMed] [Google Scholar]
- Allison KC, Lundgren JD, O’Reardon JP, Martino NS, Sarwer DB, Adden TA, et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. J Clin Psychiatry. 2008;60(4):215–220. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Benson KL. Sleep in schizophrenia: impairments, correlates, and treatment. Psychiatr Clin North Am. 2006;29:1033–1045. doi: 10.1016/j.psc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chemerinski E, Ho BC, Flaum M, Arndt S, Fleming F, Andreasen N. Insomnia as a predictor for symptom worsening following antipsychotic withdrawal in schizophrenia. Compr Psychiatry. 2002;43(5):393–396. doi: 10.1053/comp.2002.34627. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30:957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Ganwisch JE, Malaspina D, Boden-Albala B, Heynsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- Göder R, Boigs M, Braun S, Friege L, Fritzer G, Aldenhoff JB, et al. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res. 2004;38:591–599. doi: 10.1016/j.jpsychires.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PP, et al. Medical morbidity in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–194. doi: 10.4088/jcp.v66n0205. [DOI] [PubMed] [Google Scholar]
- Goldman M, Tandon R, DeQuardo JR, Taylor SF, Goodson J, McGrath M. Biological predictors of 1-year outcome in schizophrenia in males and females. Schizophr Res. 1996;21:65–73. doi: 10.1016/0920-9964(96)00021-7. [DOI] [PubMed] [Google Scholar]
- Haffmans PM, Hoencamp E, Knegtering HJ, van Heycop ten Ham BF. Sleep disturbance in schizophrenia. Br J Psychiatry. 1994;165:697–698. doi: 10.1192/bjp.165.5.697b. [DOI] [PubMed] [Google Scholar]
- Haro JM, Kamath SA, Ochoa S, Novivk D, Rele K, Fargas A, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand. 2003;416:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- Hauri PJ. Cognitive deficits in insomnia patients. Acta Neurol Belg. 1997;97(2):113–117. [PubMed] [Google Scholar]
- Hofstetter JR, Mayeda A. Sleep and quality of life in schizophrenia. In: Verster JC, Pandi-Perumal SR, Streiner D, editors. Sleep and Quality of Life in Clinical Medicine. Humana Press; New Jersey: 2008. pp. 299–311. [Google Scholar]
- Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13. doi: 10.1186/1471-244X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MJ, Schenck CH, Larson S, Pusalavidyasagar S. Nocturnal eating and sleep-related eating disorder (SRED) are common among patients with restless leg syndrome (RLS) Sleep. 2010;33:A227. [Google Scholar]
- Kato M, Kajimura N, Okuma T, Sekimoto M, Watanabe T, Yamadera H, et al. Association between delta waves during sleep and negative symptoms in schizophrenia. Pharmaco-eeg studies by using structurally difference hypnotics. Neuropsychobiology. 1999;39(3):165–172. doi: 10.1159/000026577. [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Allison KC, Crow S, O’Reardon JP, Berg KC, Galbraith J, et al. Prevalence of the night eating syndrome in a psychiatric population. Am J Psychiatry. 2006;163:156–158. doi: 10.1176/appi.ajp.163.1.156. [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Rempfer MV, Brown CE, Goetz J, Hamera E. The prevalence of night eating syndrome and binge eating disorder among overweight and obese individuals with serious mental illness. Psychiatry Res. 2010;175:233–236. doi: 10.1016/j.psychres.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 2006;29(3):223–227. doi: 10.1007/s10865-006-9047-6. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Ngyuen TA, Ramos G, Riolo S. Insomnia in the United States: prevalence and associated factors; results from National Health and Nutrition Examination Survey (NHANES 2004–2005). American Psychiatric Association 162nd Annual Meeting; 2009. Abstract NR3-024. http://www.psych.org/Departments/EDU/Library/APAOfficialDocumentsandRelated/Conference-Publications/Annual-Meeting-New-Research-Abstracts/2009.aspx?FT=.pdf (Abstract retrieved from) [Google Scholar]
- NIH Clinical Guidelines for the National Institutes of Health National Heart, Lung and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. 1998 (NIH publication No. 98–4083) [PubMed] [Google Scholar]
- Parks J, Svendsen D, Singer P, Foti ME, editors. Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors; Alexandria, Virginia: 2006. (Technical Report 13) [Google Scholar]
- Phutane VH, Tek C, Chwastiak L, Ratliff JC, Ozyuksel B, Woods SW, et al. Cardiovascular risk in a first-episode psychosis sample: a ‘critical period’ for prevention? Schizophr Res. 2011;127(1–3):257–261. doi: 10.1016/j.schres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naïve patients with schizophrenia. Schizophr Res. 2003;62:147–153. doi: 10.1016/s0920-9964(02)00346-8. [DOI] [PubMed] [Google Scholar]
- Ritsner M, Kurs R, Ponizovsky A, Hadjez J. Perceived quality of life in schizophrenia: relationships to sleep quality. Qual Life Res. 2004;13:783–791. doi: 10.1023/B:QURE.0000021687.18783.d6. [DOI] [PubMed] [Google Scholar]
- Ritsner M, Kurs R, Gibel A, Ratner Y, Endicott J. Validity of an abbreviated quality of life enjoyment and satisfaction questionnaire (Q-LES-Q-18) for schizophrenia, schizoaffective, and mood disorder patients. Qual Life Res. 2005;14(7):1693–1703. doi: 10.1007/s11136-005-2816-9. [DOI] [PubMed] [Google Scholar]
- Royuela A, Macias JA, Gil-Verona JA, Pastou JF, Maniega MA, Alonso J, et al. Sleep in schizophrenia: a preliminary study using the Pittsburgh Sleep Quality Index. Neurobiol Sleep-Wakefulness Cycle. 2002;2:37–39. [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):210–217. e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorona RD, Winnn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. The Psychological Corporation; San Antonio, Texas: 1997. [Google Scholar]