Abstract
Importance
Outcomes of treating high-grade squamous intraepithelial lesions (HSIL), a precursor to anal cancer, remain uncertain. Emerging evidence shows that post HSIL treatment adjuvant quadrivalent human papillomavirus (qHPV) vaccination improves the effectiveness of treatment. However, no recommendations exist regarding the use of qHPV vaccine as an adjuvant form of therapy. Our objective was to determine whether post-treatment adjuvant vaccination should be adopted in HIV-infected MSM (individuals at highest risk for anal cancer) on the basis of cost-effectiveness determined using existing evidence or whether future research is needed.
Methods
We developed a Markov (state-transition) cohort model to assess the cost-effectiveness of post-treatment adjuvant HPV vaccination of 27 years or older HIV-infected MSM. We first estimated cost-effectiveness and then performed value-of-information (VOI) analysis to determine whether future research is required by estimating the expected value of perfect information (EVPI). We also estimated expected value of partial perfect information (EVPPI) to determine what new evidences should have highest priority.
Results
With the incremental cost-effectiveness ratio (ICER) of $71,937/QALY, “treatment plus vaccination” was the most cost-effective HSIL management strategy using the willingness-to-pay threshold of 100,000/QALY. We found that population-level EVPI for conducting future clinical research evaluating HSIL management approaches was US$12 million (range $6–$20 million). The EVPPI associated with adjuvant qHPV vaccination efficacy estimated in terms of hazards of decreasing HSIL recurrence was $0 implying that additional data from a future study evaluating efficacy of adjuvant qHPV vaccination will not change our policy conclusion that “treatment plus vaccination” was cost-effective. Both the ICER and EVPI were sensitive to HSIL treatment compliance.
Conclusion
Post-treatment adjuvant qHPV vaccination in HIV-infected MSM aged 27 or above is likely to be cost-effective. Use of adjuvant qHPV vaccination could be considered as a potential strategy to reduce rising anal cancer burden among these high-risk individuals.
Keywords: Cost-effectiveness analysis, Value of information analysis, Quadrivalent human papillomavirus vaccine, Secondary/adjunct prevention, High-grade squamous intraepithelial lesions
INTRODUCTION
The incidence of anal cancer is 80 times higher in HIV-infected men who have sex with men (MSM) than the general population in the United States [1, 2]. Infection with human papillomavirus (HPV), particularly types 16 or 18, is considered the most significant risk factor for anal cancer [3]. Persistent HPV infection may lead to anal high-grade squamous intraepithelial lesions (HSIL), which might ultimately progress to invasive anal cancer [4].
Effective management of HSIL might prevent or slow down progression to invasive anal cancer; however, how best to manage HSIL is controversial as the benefits of treatment are not demonstrated by clinical trials. For this reason, there is no established standard of care. Treatment using ablation is the most commonly accepted strategy. However, HSIL recurs in about 60–70% of patients within 1 year following ablation and despite treatment for recurrent lesions [5], patients are at risk of progressing to invasive anal cancer [5–8]. Tong et al and data from the Study of Prevention of Anal Cancer (SPANC) demonstrated that HSIL might naturally regress [9]. Furthermore, given that treatment is associated with morbidity [5, 10], some suggest delaying HSIL treatment and actively monitoring patients until occurrence of early invasive cancer when treatment is known to be efficacious where five-year cancer-specific survival of 95% is achievable [11–13].
Recent data show that the effectiveness of anal HSIL treatment could be improved by the addition of post-treatment adjuvant quadrivalent human papillomavirus (qHPV) vaccination because vaccination decreases the risk of recurrence and has the potential to decrease subsequent progression to invasive anal cancer [14, 15]. Similarly, studies have shown benefits of adjuvant qHPV vaccination for prevention of recurrent cervical intraepithelial neoplasia (CIN), oral papillomas, anal condyloma, and respiratory and laryngeal papillomatosis (other HPV-associated diseases) [16]. Data from the SPERANZA randomized clinical trial (RCT) showed that the qHPV vaccine is efficacious after loop electrosurgical excision procedure (LEEP) treatment for CIN—over the median follow-up of 27 months, 11 out of 162 patients in control group developed a cervical recurrence (6%) while 2 out of 162 vaccinated women recurred (1%) (p=0.0199) [17]. Although clinical trials found no evidence on efficacy of HPV vaccination for prevention of anal persistent HPV infection [18], no empirical trial data exist regarding the effectiveness of adjuvant vaccination for prevention of recurrent anal HSIL or anal cancer in HIV-infected individuals. Considering this uncertainty, a modeling based analysis showed that “treatment plus vaccination” could be a cost-effective HSIL management strategy even under the assumption of worst-case efficacy of reducing HSIL recurrence for only up to 2 years [19]. Therefore, the questions then arise: Should the guidelines consider implementing post-treatment adjuvant qHPV vaccination despite the lack of direct evidence from empirical studies? Or should further data be collected from additional studies before making a decision?
In this study, we investigated above tradeoffs by conducting a value of information (VOI) analysis, which provides a unique perspective on the need for conducting further research, including randomized clinical trials, before making a policy decision, and an indication of the amount that society should be willing to spend to gain additional information and reduce uncertainty through further research about unknown factors (e.g., adjuvant qHPV vaccine efficacy, disease natural history, etc.). VOI analysis has been successfully used in risk analysis [20] and has recently been recommended to evaluate healthcare technologies by the Second Panel on Cost-Effectiveness in Health and Medicine, as well as by multiple agencies including the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI) in the United States [21–27]. Therefore, our objective was to determine if additional clinical studies were necessary or if the currently available data, even with uncertainty, were sufficient to inform post-treatment adjuvant HPV vaccination recommendations.
METHODS
Model overview and comparators
To conduct VOI analysis, we used a previously developed and validated state-transition model that simulated the natural history of anal carcinoma in HIV-infected MSM and followed patients after treatment for initial HSIL for any risk of subsequent recurrence or potential cancer [19, 28] (Appendix S1.1). The model compared four strategies: do nothing, active monitoring (using annual digital anorectal examination (DARE) and high resolution anoscopy (HRA)), treatment for HSIL (using ablation), and treatment plus adjuvant qHPV vaccination [16] (Appendix S1.2). The schematic model structure of the natural history of anal carcinoma and potential disease progression after HSIL treatment is presented in Appendix Figure F1.1.
Baseline cohort and disease transition
The hypothetical baseline cohort was comprised of 27 year or older HIV-infected MSM who have been diagnosed with HSIL in the U.S. We defined the Markov health states capturing anal carcinogenesis as: normal, low-grade squamous intraepithelial lesions (LSIL), HSIL, invasive anal cancer, HSIL recurrence, and death. In addition to these, the model included health states for HIV progression based on the patient’s CD4 count (i.e., CD4 >500 cells/mm3, CD4 between 200–500 cells/mm3, and CD4 <200 cells/mm3). Patient CD4 count, rates of HSIL progression or regression, and natural history post initial regression were obtained from the literature or calibrated using a separate natural history model for anal carcinogenesis (Appendix S1.3; Appendix Table T1.1 and T1.2) [2, 9, 19, 29–31]. Our model also considered the possibility of receiving antiretroviral therapy (ART) and the impact of change in CD4 count on anal carcinogenesis.
Effectiveness parameters
We defined treatment effectiveness as probability of HSIL recurrence post-treatment for initial HSIL. (Appendix Table T1.2) We defined vaccine effectiveness as the hazards (probability that an individual at a given time has an event) of developing recurrent HSIL that ultimately transitions to anal cancer (Appendix Table T1.3) [14, 28, 32]. Patients not receiving any care (do nothing) followed natural history of the disease where stage-specific anal cancer was diagnosed symptomatically while active monitoring led to early detection of early invasive anal cancer (Appendix Table T1.2).
Cost and utilities
Cost data were estimated using the Surveillance, Epidemiology, and End Results (SEER) database and Medicare claims (Appendix Table T1.4) as well as obtained from published studies [28, 29, 33]. Health-state utilities (preference-based valuation of quality of life) were identified from the literature (Appendix Table T1.4) [29].
Value of information analysis
The first step to conduct a value of information analysis was to conduct a probabilistic sensitivity analysis (PSA). We used 10,000 Monte Carlo runs to sample the probability distributions for all model parameters. We then estimated the cost-effectiveness of each HSIL management strategy using the average of the PSA runs. The decision uncertainty was presented using the cost-effectiveness acceptability frontier that provides a graphical illustration of the probability that the alternative with the highest net benefit will be cost-effective [34].
Next, we estimated the expected value of perfect information (EVPI) [35], which provided the maximum amount the healthcare system would be willing to pay to achieve perfect information about the clinical and economic value of the HSIL management approach. We first estimated patient-level EVPI, which is the difference between the payoff (expected net benefit) with perfect information and current information [36, 37]. Given that observational studies have shown that individuals who have had HSIL treatment were less likely to progress to anal cancer for at least 10 years [7, 8], We further assumed that the optimal strategy remains a viable alternative for prevention of anal cancer in MSM between 10 to 30 years (where the base case time horizon was assumed to be 20 years).
We then estimated the population-level EVPI by multiplying the individual EVPI by the number of MSM who would be potentially benefited over the next 20 years, discounted at an annual rate of 3%. To determine the population of men who will potentially benefit, we used population projection data from the US Census Bureau [38], proportion of MSM in the US from Urban Health data [39], prevalence of HIV in the US among MSM [40], and current and projected incidence of anal cancer in MSM [41, 42] (Appendix 2). Finally, we estimated EVPI by assuming the time-frame to be either 10 years or 30 years.
Similarly, we estimated the patient-level and then population-level expected value of partial perfect information (EVPPI) to find how much the healthcare system would be willing to pay to resolve uncertainty around specific parameters. This indicates what new evidence (studies) should have the highest priority when undertaking additional studies (i.e., parameters with highest informational value). This was estimated using a two-level simulation process [26], where outer simulation loop values were drawn for parameter of interest and inner loop values were drawn for all other parameters [26]. The EVPPI was estimated as the difference between the expected value with perfect and current information about the parameter(s). Particularly, we estimated the EVPPI for the group of parameters that characterize HSIL progression or regression and LSIL progression or regression in patients not treated for HSIL, and adjuvant HPV vaccination efficacy and HSIL treatment efficacy in patients receiving treatment for HSIL.
Sensitivity analysis
HSIL recurrence rate remains high and patients are likely to be lost to follow-up; therefore, we first determined cost-effectiveness and value of future research by varying treatment compliance. Since efficacy of adjuvant qHPV vaccination was a major assumption for this study, we performed sensitivity analysis to evaluated EVPI and EVPPI under the worst case scenario (i.e., lowest possible efficacy) of adjuvant qHPV vaccination efficacy. For this analysis, we assumed that that the efficacy of adjuvant qHPV vaccination was only 2% (range 0%–78%) for the time duration of first two years post treatment for initial HSIL. Then after, we assumed that there was no difference in recurrence among individuals who received adjuvant vaccine versus individuals who did not receive the vaccine post treatment.
RESULTS
Model validation
We validated our model by comparing natural history model-predicted 1-year, 2-year, and 3-year cumulative risks of HSIL to the cumulative risks reported by Burgos et al [43]. Model accuracy was established as the cumulative risks predicted by the model were within the 95% confidence interval of the cumulative risks reported in the literature. Detailed model validation is presented in Supplementary Appendix 3 and elsewhere [19, 28].
Cost-effectiveness
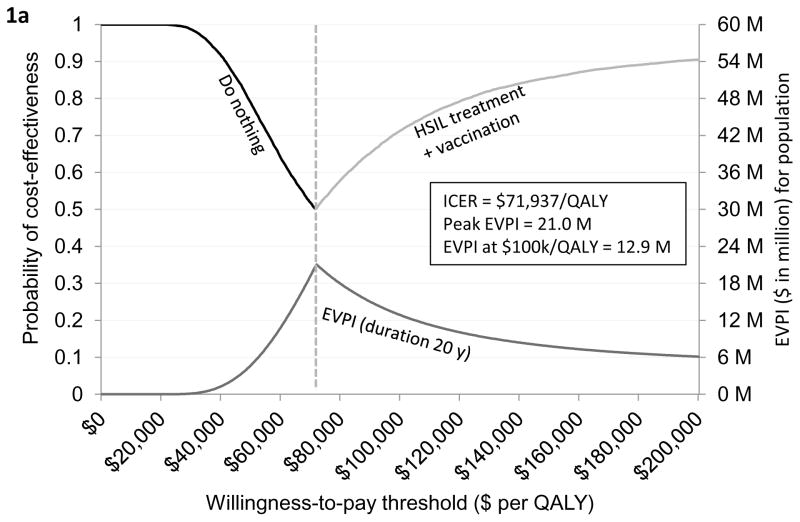
Based on the current evidence, we found that “do nothing” was likely to be a cost-effective strategy if the willingness-to-pay (WTP) threshold is below $71,937 per QALY. If the WTP threshold is greater than or equal to $71,937 per QALY, then “HSIL treatment plus vaccination” was optimal. At the WTP threshold of $100,000/QALY, “HSIL treatment plus vaccination” was likely to be most optimal with 73% probability. Detailed clinical and economic outcomes by strategies are listed in Table 1. The uncertainty associated with these strategies is represented using the cost-effectiveness acceptability frontier (Figure 1a). We also present cost-effectiveness acceptability curves (Appendix Figures F4.3 and F4.4).
Table 1.
Cost-effectiveness analysis
| Strategy | Lifetime Cost, $ | QALYs | ICER, $ per QALY | Cumulative anal cancer incidence, % | Cumulative anal cancer mortality, % |
|---|---|---|---|---|---|
| Do nothing | 367,911 | 12.93 | – | 8% | 5% |
| Active monitoring/watchful waiting | 372,268 | 12.95 | Weakly Dominated† | 8% | 4% |
| Treat HSIL | 379,056 | 13.04 | Weakly Dominated† | 3% | 2% |
| Treat HSIL+ adjuvant qHPV vaccination | 379,458 | 13.09 | 71,937 | 2% | 1% |
Abbreviations: QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; HSIL, high-grade squamous intraepithelial lesions; qHPV, quadrivalent human papillomavirus vaccine
Figure 1. Cost-effectiveness acceptability frontier and expected value of perfect and partial perfect information.
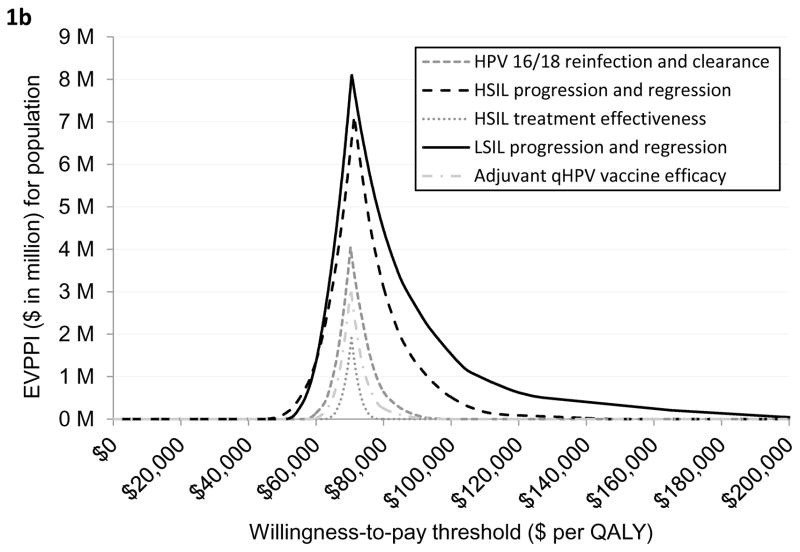
Figure 1a provides a graphical illustration of the uncertainty associated with the optimal strategy over a range of values for the willingness-to-pay thresholds. As seen in the figure, do nothing was the optimal strategy if the willingness-to-pay (WTP) threshold was below $71,937 per QALY. If the WTP threshold was greater than $71,937 per QALY then HSIL treatment plus adjuvant vaccination was the optimal strategy. The secondary vertical axis of the figure illustrates the EVPI associated with HSIL management in HIV-infected MSM. As seen in the figure, the value of further investigation was $12 million at the WTP threshold of $100,000 per QALY. Figure 1b shows EVPPI associated among HIV-infected MSM. Solid black, dashed black, dashed gray, dashed and dotted gray, and dotted gray lines represent the parameters LSIL progression and regression, HSIL progression and regression, HPV 16/18 reinfection and clearance, adjuvant qHPV vaccination efficacy, and HSIL treatment effectiveness, respectively. As seen in the figure, at the WTP threshold of $100,000 per QALY, the EVPPIs for LSIL progression and regression and HSIL progression and regression were $1.5 million and $0.5 million, respectively.
Abbreviations: EVPI, expected value of perfect information; EVPPI, expected value of partial perfect information; ICER, incremental cost-effectiveness ratio; HSIL, high-grade squamous intraepithelial lesions; QALY, quality-adjusted life-year.
Value of perfect information
Figure 1a presents the EVPI for HIV-infected MSM over WTP thresholds ranging from $0 per QALY to $200,000 per QALY. Assuming that the HSIL management approaches would remain viable for prevention of anal cancer over 20 years, the EVPI at the WTP of $100,000/QALY was $12 million (peak EVPI was $21 million). This implies that future clinical research evaluating HSIL management approaches could be potentially worthwhile as long as the cost of research is below $12 million.
When the time-frame for the analysis was decreased to 10 years, the potential value of further information dropped to $6 million, when the time–frame for the analysis was increased to 30 years, the potential value of further information increased to $20 million (Appendix Figure F4.1.).
Value of partial perfect information
The EVPPI of adjuvant qHPV vaccination efficacy was $0 at the WTP threshold of $100,000/QALY (Figure 1b). This implies that additional evidence on efficacy of adjuvant qHPV vaccination is not needed prior to implementation of post-treatment adjuvant HPV vaccination policy. In other words, despite uncertainty regarding the efficacy of adjuvant qHPV vaccination, treatment plus vaccination will remain the most cost-effective strategy.
Similarly, the EVPPI for HPV 16/18 reinfection, HSIL treatment effectiveness, and adjuvant qHPV vaccination efficacy were $0 at the WTP threshold of $100,000/QALY implying that additional future research in these individual variables will not change the cost-effectiveness results.
We found that the EVPPI was highest for LSIL progression or regression (peak EVPPI at $8.1 million), followed by HSIL progression or regression (peak EVPPI at $7.1 million). At the WTP threshold of $100,000/QALY, these values were $1.5 million and $0.5 million, respectively. High EVPPI associated with these parameters indicate that a more precise estimate of the LSIL and HSIL natural history may be required; this in turn would require an experimental design.
Sensitivity analysis
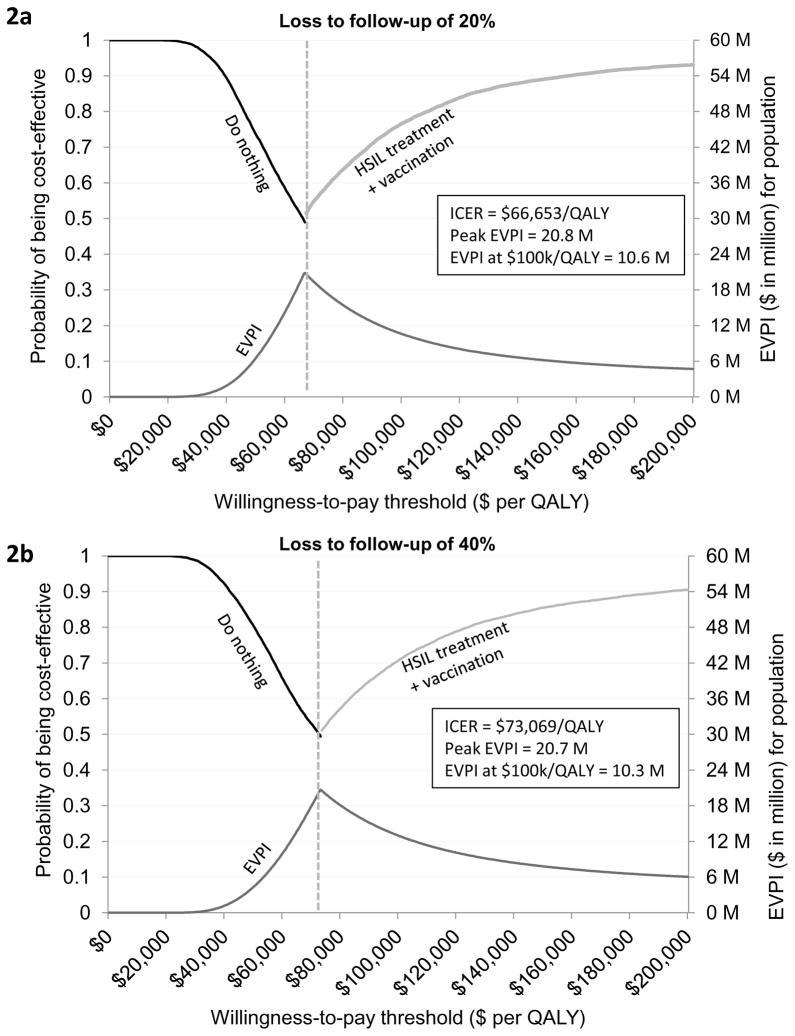
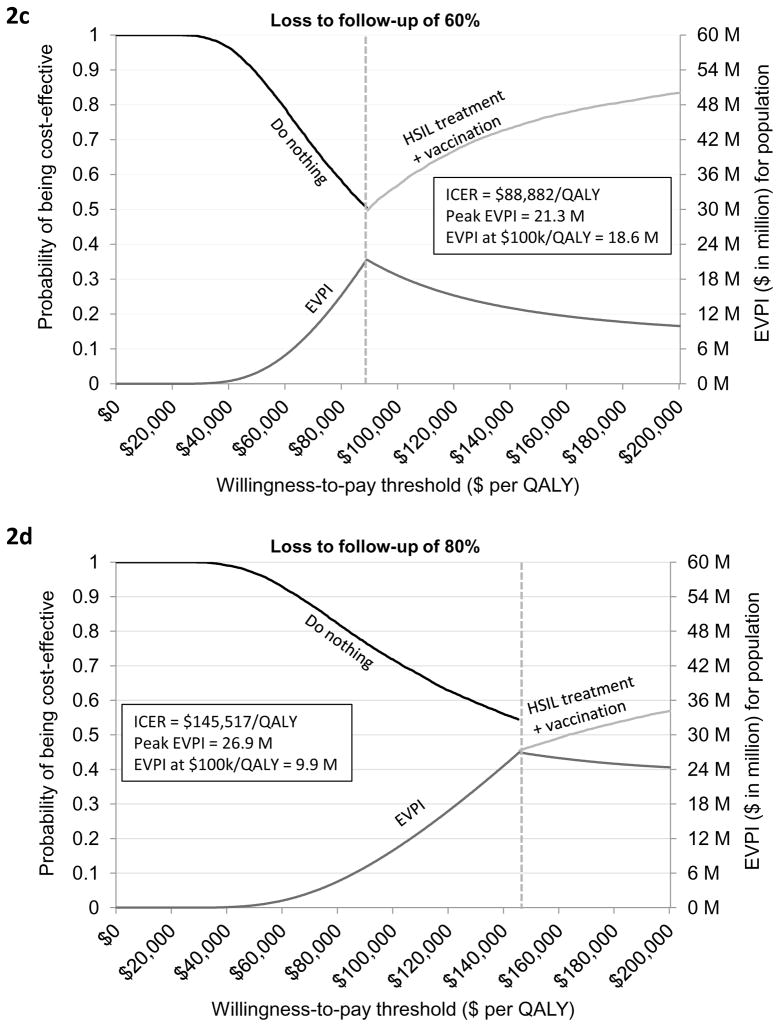
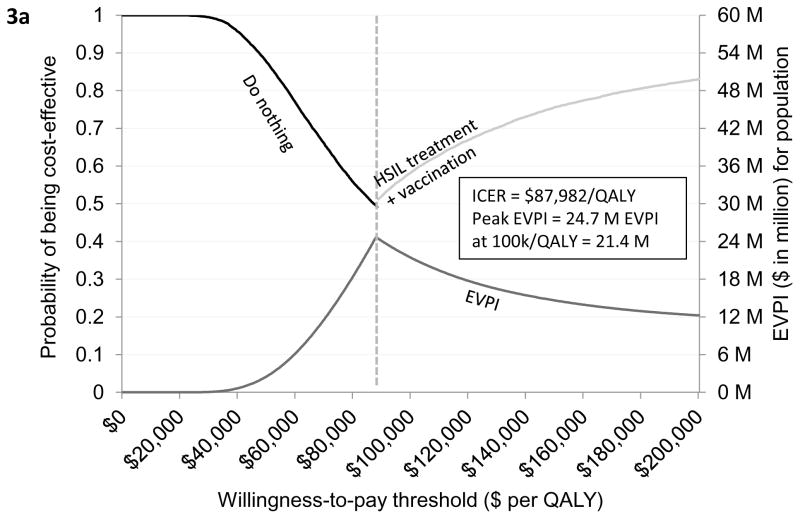
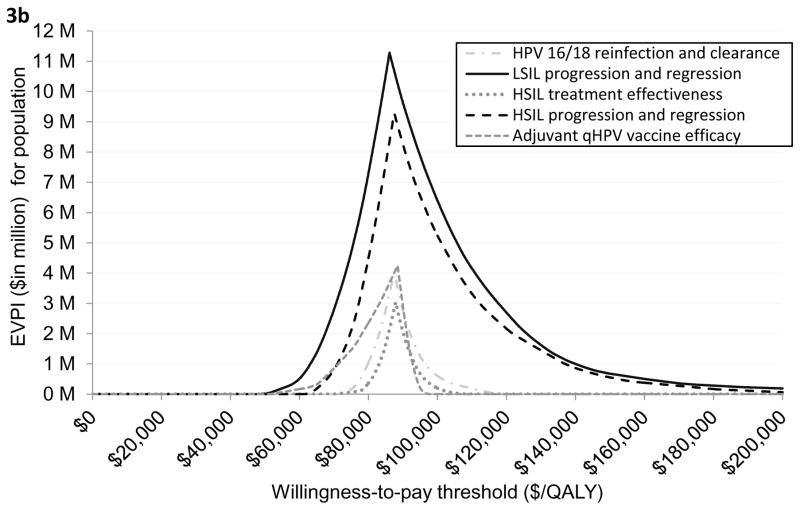
Both the ICER and EVPI were sensitivity to HSIL treatment compliance. When loss to follow-up was varied in the range of 20% to 80%, the ICER varied from $66,653/QALY to $145,517/QALY and peak EVPI varied from $20.8 million to $26.9 million (Figures 2a–2d). When borderline adjuvant vaccine efficacy was assumed, the ICER of treatment plus adjuvant qHPV vaccination compared with do nothing was increased to $87,982/QALY. (Figure 3a; Appendix Table T4.1). The EVPI under this scenario of borderline vaccine efficacy at the WTP threshold of $100,000/QALY increased to $21 million (range $10 million to $34 million) (Figure 3a and Appendix Figure 4.2). The peak EVPI was $24 million (range $12 million to $38 million). The EVPPI at $100,000/QALY when borderline efficacy was assumed was $6 million (peak EVPPI at $11 million) for LSIL progression or regression followed by $5 million (peak at $9 million) for HSIL progression or regression (Figure 3b).
Figure 2. Cost-effectiveness acceptability frontier and expected value of perfect information by treatment compliance (loss to follow-up of 20% to 80%).
Figure 2 depicts cost-effectiveness acceptability frontier and expected value of perfect information when loss to follow-up was varied from (A) 20% to (B) 40%, (C) 60%, and (D) 80%.
Figure 3. Cost-effectiveness acceptability frontier and expected value of perfect information assuming worst-case adjuvant qHPV vaccination efficacy.
Figure 3a provides a graphical illustration of the uncertainty associated with the optimal strategy over a range of values for the willingness-to-pay thresholds under the assumption of worst-case adjuvant qHPV vaccination efficacy. As seen in the figure, do nothing was the optimal strategy if the willingness-to-pay (WTP) threshold was below $87,982 per QALY. If the WTP threshold was greater than $87,982 per QALY then HSIL treatment plus adjuvant vaccination was the optimal strategy. The secondary vertical axis of the figure illustrates the EVPI associated with HSIL management in HIV-infected MSM when worst-case adjuvant qHPV vaccination efficacy was assumed. As seen in the figure, the value of further investigation was $21 million at the WTP threshold of $100,000 per QALY. Figure 3b shows EVPPI associated among HIV-infected MSM. Solid black, dashed gray, dashed brown, dashed and dotted blue, and dotted green lines represent the parameters LSIL progression and regression, HSIL progression and regression, adjuvant qHPV vaccination efficacy, HPV 16/18 reinfection and clearance, and HSIL treatment effectiveness, respectively. As seen in the figure, at the WTP threshold of $100,000 per QALY, the EVPPIs for LSIL progression and regression and HSIL progression and regression were $6 million and $5 million, respectively.
Abbreviations: EVPI, expected value of perfect information; EVPPI, expected value of partial perfect information; ICER, incremental cost-effectiveness ratio; HSIL, high-grade squamous intraepithelial lesions; QALY, quality-adjusted life-year
DISCUSSION
With increasing health care expenditures, value-based care has received significant attention in recent years [44–46]. There is an increasing demand for value-based cancer research with emphasis on improvement in clinical trial efficiency and cancer outcomes in order to increase the body of knowledge derived from scarce research funding without sacrificing scientific rigor [44]. Likewise, there is an urgent need to determine research priorities that will facilitate precision medicine. Our analysis provides a unique perspective on the value of future adjuvant qHPV vaccination research that by evaluating the impact of uncertainty regarding vaccine efficacy and other study variables on decision making, systematically determines future research needs with the objective to achieve the maximum return on limited healthcare resources and at the same time, maximize population health.
Previous studies evaluated the effectiveness or cost-effectiveness of adjuvant HPV vaccination compared to HSIL treatment alone (i.e., adding HPV vaccination to HSIL treatment regimen) [14, 15, 47] or determined the cost-effectiveness of HSIL management [19] or surveillance after HSIL treatment [48]. No guidelines exist for anal HSIL management and given the uncertainty associated with the efficacy of HSIL treatment and adjuvant HPV vaccination, in the present study, we first comprehensively compared the outcomes of all potential HSIL management options and then conducted value-of-information analysis to determine the value of future research needed to resolve all possible uncertainty among HIV-infected MSM. Finally, we determined future high priority research by identifying parameters with highest informational value.
We found that post-treatment adjuvant qHPV vaccination is likely to be a cost-effective strategy for management of HSIL and implementation of post-treatment adjuvant HPV vaccination policy sooner rather than later should be of high priority because the future data is unlikely to change the conclusion that treatment plus adjuvant qHPV vaccination would be most cost-effective. Our conclusion is based on a detailed cost-effectiveness and value of information analysis, which evaluated a tradeoff between making a decision with current, uncertain, information versus waiting to reduce uncertainty with additional research. The VOI analysis suggested that potential HSIL management research in HIV-infected MSM who are 27 years or older could potentially be worthwhile if the total cost of research is below $12 million (range $6 million – $20 million). Under an alternative assumption of borderline adjuvant qHPV vaccination efficacy, where adjuvant vaccine efficacy was at its extreme lower-bound, the maximum value of potential research increased to $21 million (range $10 million to $34 million).
To our knowledge, no previous study has evaluated the value of conducting future research for prevention of anal cancer. Our findings will help prioritize future research that will ultimately help reduce uncertainty about unobserved study variables. The reduction in uncertainty in some parameters will not affect results of future cost-effectiveness analysis. For example, future studies evaluating adjuvant qHPV vaccination efficacy, HSIL treatment effectiveness, and HPV reinfection and clearance where EVPPI was $0. The significance of this finding is that despite the uncertainty about these parameters, particularly about the efficacy of adjuvant qHPV vaccination, treatment plus adjuvant vaccination remains the most cost-effective strategy. Any future research in these parameters is unlikely to change the policy conclusion that HSIL treatment plus adjuvant HPV vaccination is cost-effective in HIV-infected MSM.
We also identified areas of future research that should have a high priority. First, future studies evaluating the natural history (progression or regression) of LSIL was likely to be of highest priority, based on its highest EVPPI. Substantial uncertainty exists about the natural history of LSIL. For instance, three studies reported LSIL progression or regression [9, 43, 49]. Two of these followed a cohort of patients [43, 49] and one retrospectively performed clinical audit.[9] All reported progression in the range of 4.7% to 11.6% per year [9, 43, 49]. In addition, all reported that the progression rate increases with age and decreases with reduced CD4 count. No study reported spontaneous LSIL regression. Such a study along with additional research to resolve uncertainty related to evaluation of age-specific progression or regression could potentially be worthwhile as long as the total cost of future research remains below $1.5 million.
The second highest priority future research should focus on measuring HSIL progression or regression in patients not receiving treatment for HSIL. In a recent meta-analysis, Machalek et al (2012) [2] theoretically calculated the progression rate of HSIL to cancer to be 1 in 377 HIV-infected MSM per year, and previous modeling-based studies have shown that progression is likely to increase with age [28, 50]; however, none of these estimates were based on empirical studies. Tong et al (2013) evaluated regression of HSIL and found that regression rate decreases with age [9]. Further research to evaluate HSIL progression and regression could potentially be worthwhile if it cost less than $0.5 million. The low EVPPI implies that the precise estimates for this variable might be obtained from less costly methods, such as observational studies.
Our study’s inherent limitation is that it only evaluates the uncertainties captured by the economic evaluation model. The analysis does not take into account parameters that may apply to particular individuals—for example, vaccination side effects like allergic reactions, pain, and fainting. We did not consider these side effects because they are extremely rare. In most instances, when they occur, these side effects have little to no impact on the overall cost-effectiveness of the vaccine. Second, we estimated the outcomes assuming a time frame of 20 years over which post-treatment adjuvant HPV vaccination might remain efficacious for prevention of anal cancer. As there is uncertainty over long term duration and degree of protection of treatment and adjuvant vaccination, we performed sensitivity analysis varying this time-frame in the range of 10 to 30 years.
In conclusion, treatment plus vaccination is potentially the most cost-effective approach for HSIL management. If implemented sooner rather than later, post-treatment adjuvant qHPV vaccination has the potential to decrease the incidence of preventable invasive anal cancer and save a large sum of healthcare dollars that would otherwise be spent on treatment for recurrent HSIL and invasive cancer. Therefore, adjuvant qHPV vaccination of HIV-infected MSM aged 27 years or older and who have had treatment for HSIL should be considered.
Supplementary Material
Research highlights.
We examined value of adoption of adjuvant qHPV vaccine for anal cancer prevention.
We performed cost-effectiveness and value of information analysis.
HPV vaccination is cost-effective for managing HIV-positive MSM with HSIL.
Future clinical research data will not change this policy conclusion.
Implementing adjuvant HPV vaccination soon will provide excellent value for money.
Acknowledgments
The authors wish to thank Chin Hur from Massachusetts General Hospital for his constructive comments and Ryan Suk from University of Florida for analytical support. We also acknowledge Amy Ninetto and Diane Hackett from The University of Texas MD Anderson Cancer Center for editorial contributions that enhanced the quality of the manuscript.
Funding/Support: This work was supported by US National Cancer Institute [R01 CA163103], the Janice Davis Gordon Memorial Postdoctoral Fellowship in Colorectal Cancer Prevention, and the National Institutes of Health through The University of Texas MD Anderson Cancer Center Support Grant [CA016672].
Footnotes
Declaration of Interest: Dr. Goldstone received personal fees from Merck & Co. Inc., grants and personal fees from Medtronic Inc., outside the submitted work. Dr. Wilkin received grant support (paid to Weill Cornell Medicine) from Bristol-Myers Squibb, Gilead Sciences, and Glaxo Smith Kline/ViiV Healthcare. Dr. Chhatwal received personal fees from Merck & Co. Inc., grants and personal fees from Gilead Sciences, outside the submitted work. There are no conflicts of interest to report for Drs. Deshmukh, Fenwick, Chiao, Cantor, Stier, and Nyitray.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–34. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 4.Gervaz P, Hahnloser D, Wolff BG, Anderson SA, Cunningham J, Beart RW, Jr, et al. Molecular biology of squamous cell carcinoma of the anus: a comparison of HIV-positive and HIV-negative patients. J Gastrointest Surg. 2004;8:1024–30. doi: 10.1016/j.gassur.2004.08.013. discussion 31. [DOI] [PubMed] [Google Scholar]
- 5.Richel O, de Vries HJ, van Noesel CJ, Dijkgraaf MG, Prins JM. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol. 2013;14:346–53. doi: 10.1016/S1470-2045(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 6.Pineda CE, Welton ML. Management of anal squamous intraepithelial lesions. Clin Colon Rectal Surg. 2009;22:94–101. doi: 10.1055/s-0029-1223840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Dis Colon Rectum. 2008;51:829–35. doi: 10.1007/s10350-008-9233-4. discussion 35–7. [DOI] [PubMed] [Google Scholar]
- 8.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: recurrence and incidence of cancer. Dis Colon Rectum. 2014;57:316–23. doi: 10.1097/DCR.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 9.Tong WW, Jin F, McHugh LC, Maher T, Sinclair B, Grulich AE, et al. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS. 2013;27:2233–43. doi: 10.1097/QAD.0b013e3283633111. [DOI] [PubMed] [Google Scholar]
- 10.Grulich A. Natural history of anal HPV infection and HSIL and implications for screening and treatment: Data from SPANC study. International Anal Neoplasia Society; San Francisco, California: 2017. 2016. [Google Scholar]
- 11.Pineda CE, Welton ML. Controversies in the management of anal high-grade squamous intraepithelial lesions. Minerva Chir. 2008;63:389–99. [PubMed] [Google Scholar]
- 12.Weis SE. Current treatment options for management of anal intraepithelial neoplasia. Onco Targets Ther. 2013;6:651–65. doi: 10.2147/OTT.S38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orchard M, Roman A, Parvaiz AC. Anal intraepithelial neoplasia--is treatment better than observation? Int J Surg. 2013;11:438–41. doi: 10.1016/j.ijsu.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891–8. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh AA, Chhatwal J, Chiao EY, Nyitray AG, Das P, Cantor SB. Long-Term Outcomes of Adding HPV Vaccine to the Anal Intraepithelial Neoplasia Treatment Regimen in HIV-Positive Men Who Have Sex With Men. Clin Infect Dis. 2015;61:1527–35. doi: 10.1093/cid/civ628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dion GR, Teng S, Boyd LR, Northam A, Mason-Apps C, Vieira D, et al. Adjuvant human papillomavirus vaccination for secondary prevention: a systematic review. JAMA Otolaryngol Head Neck Surg. 2017;143:614–22. doi: 10.1001/jamaoto.2016.4736. [DOI] [PubMed] [Google Scholar]
- 17.Ghelardi A, Bay P, Tonetti A, Ragusa AF. Speranza Study: Preliminary results of HPV vaccination after loop electrosurgical excision procedure for cervical intraepithelial neoplasia. EUROGIN. 2016 [Google Scholar]
- 18.Wilkin TJ, Chen H, Cespedes M, Pawel P, Godfrey C, Chiao E, et al. ACTG A5298: A phase 3 trial of the quadrivalent HPV vaccine in older HIV+ adults. The Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2016. [Google Scholar]
- 19.Deshmukh AA, Chiao EY, Cantor SB, Stier EA, Goldstone SE, Nyitray AG, et al. Management of precancerous anal intraepithelial lesions in HIV-positive MSM: clinical and cost effectiveness. Cancer. 2017 doi: 10.1002/cncr.31035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard RA. Information value theory. IEEE Transactions on Systems Science and Cybernetics. 1966;3:54–60. [Google Scholar]
- 21.Myers E, Sanders GD, Ravi D, Matchar D, Havrilesky L, Samsa G, et al. Evaluating the potential use of modeling and value-of-information analysis for future research prioritization within the evidence-based practice center program. 2011 [PubMed] [Google Scholar]
- 22.Myers E, McBroom A, Shen L, Posey R, Gray M. Value of information analysis for patient-centered outcomes research. Prioritization. 2012 [Google Scholar]
- 23.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 24.Fleurence RL, Meltzer DO. Toward a science of research prioritization? The use of value of information by multidisciplinary stakeholder groups. Med Decis Making. 2013;33:460–2. doi: 10.1177/0272989X13486979. [DOI] [PubMed] [Google Scholar]
- 25.Eckermann S, Karnon J, Willan AR. The value of value of information best informing research design and prioritization using current methods. Pharmacoeconomics. 2010;28:699–709. doi: 10.2165/11537370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 27.Myers E, Sanders GD, Dhurjati R, Matchar D, Havrilesky L, Samsa G, et al. Evaluating the potential use of modeling and value-of-information analysis for future research prioritization within the evidence-based practice center program. 2011 [PubMed] [Google Scholar]
- 28.Deshmukh AA, Chhatwal J, Chiao EY, Nyitray AG, Das P, Cantor SB. Long-term outcomes of adding HPV vaccine to the anal intraepithelial neoplasia treatment regimen in HIV-positive men who have sex with men. Clin Infect Dis. 2015 doi: 10.1093/cid/civ628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnon J, Jones R, Czoski-Murray C, Smith KJ. Cost-utility analysis of screening high-risk groups for anal cancer. J Public Health. 2008;30:293–304. doi: 10.1093/pubmed/fdn045. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lifson AR, Krantz EM, Eberly LE, Dolan MJ, Marconi VC, Weintrob AC, et al. Long-term CD4+ lymphocyte response following HAART initiation in a U.S. Military prospective cohort. AIDS Res Ther. 2011;8:2. doi: 10.1186/1742-6405-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32:6941–7. doi: 10.1016/j.vaccine.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozzette SA, Joyce G, McCaffrey DF, Leibowitz AA, Morton SC, Berry SH, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344:817–23. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 34.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–87. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 35.Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE) Lancet. 2002;360:711–5. doi: 10.1016/S0140-6736(02)09832-X. [DOI] [PubMed] [Google Scholar]
- 36.Sculpher M, Claxton K. Establishing the cost-effectiveness of new pharmaceuticals under conditions of uncertainty--when is there sufficient evidence? Value Health. 2005;8:433–46. doi: 10.1111/j.1524-4733.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- 37.Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making. 2004;24:207–27. doi: 10.1177/0272989X04263162. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Census Bureau. 2014 National Population Projections. Washington, DC: US Census Bureau; 2014. [Google Scholar]
- 39.Lieb S, Thompson DR, Misra S, Gates GJ, Duffus WA, Fallon SJ, et al. Estimating populations of men who have sex with men in the southern United States. J Urban Health. 2009;86:887–901. doi: 10.1007/s11524-009-9401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell DW, Johnson CH, Lansky A, Prejean J, Stein R, Denning P, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–9. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piketty C, Selinger-Leneman H, Bouvier AM, Belot A, Mary-Krause M, Duvivier C, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the french hospital database on HIV. J Clin Oncol. 2012;30:4360–6. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 43.Burgos J, Curran A, Tallada N, Guelar A, Navarro J, Landolfi S, et al. Risk of progression to high-grade anal intraepithelial neoplasia in HIV-infected MSM. AIDS. 2015;29:695–702. doi: 10.1097/QAD.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 44.Gross CP, Emanuel EJ. A call for value in cancer research. JAMA Oncol. 2016;2:11–2. doi: 10.1001/jamaoncol.2015.3706. [DOI] [PubMed] [Google Scholar]
- 45.Young RC. Value-based cancer care. N Engl J Med. 2015;373:2593–5. doi: 10.1056/NEJMp1508387. [DOI] [PubMed] [Google Scholar]
- 46.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 47.Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32:6941–7. doi: 10.1016/j.vaccine.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assoumou SA, Mayer KH, Panther L, Linas BP, Kim J. Cost-effectiveness of surveillance strategies after treatment for high-grade anal dysplasia in high-risk patients. Sex Transm Dis. 2013;40:298–303. doi: 10.1097/OLQ.0b013e31827f4fe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Pokomandy A, Rouleau D, Ghattas G, Trottier H, Vezina S, Cote P, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis. 2011;52:1174–81. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 50.Czoski-Murray C, Karnon J, Jones R, Smith K, Kinghorn G. Cost-effectiveness of screening high-risk HIV-positive men who have sex with men (MSM) and HIV-positive women for anal cancer. Health Technol Assess. 2010;14:iii–iv. ix–x, 1–101. doi: 10.3310/hta14530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.