Abstract
Background & Aims
The goal of treatment for Barrett’s esophagus (BE) with dysplasia is complete eradication of intestinal metaplasia (CEIM). The long-term durability of CEIM has not been well characterized, so the frequency and duration of surveillance are unclear. We report results from a 5-year follow-up analysis of patients with BE and dysplasia treated by radiofrequency ablation (RFA) in the randomized controlled Ablation of Intestinal Metaplasia Containing Dysplasia (AIM) trial.
Methods
Participants for the AIM dysplasia trial (18–80 years old) were recruited from 19 sites in the United States and had endoscopic evidence of non-nodular dysplastic BE ≤8 cm in length. Subjects (n=127) were randomly assigned (2:1 ratio) to receive either RFA (entire BE segment ablated circumferentially) or a sham endoscopic procedure; patients in the sham group were offered RFA treatment 1 year later, and all patients were followed for 5 years. We collected data on BE recurrence (defined as intestinal metaplasia in the tubular esophagus) and dysplastic BE recurrence among patients who achieved CEIM. We constructed Kaplan-Meier estimates and applied parametric survival analysis to examine proportions of patients without any recurrence and without dysplastic recurrence.
Results
Of 127 patients in the AIM Dysplasia trial, 119 received RFA and met inclusion criteria. Of those 119, 110 (92%) achieved CEIM. Over 401 person-years of follow-up (mean 3.6 years per patient, range 0.2 – 5.8 years), 35 of 110 (32%) patients had recurrence of BE or dysplasia, and 19 (17%) had dysplasia recurrence. The incidence rate of BE recurrence was 10.8 per 100 person-years overall (95% CI, 7.8 – 15.0); 8.3 per 100 person-years among patients with baseline low-grade dysplasia (95% CI, 4.9 – 14.0), and 13.5 per 100 person-years among patients with baseline high-grade dysplasia (95% CI 8.8 – 20.7). The incidence rate of dysplasia recurrence was 5.2 per 100 person-years overall (95% CI 3.3 – 8.2); 3.3 per 100 person-years among patients with baseline low-grade dysplasia (95% CI 1.5 – 7.2), and 7.3 per 100 personyears among patients with baseline high-grade dysplasia (95% CI 4.2 – 12.5). Neither BE nor dysplasia recurred at a constant rate. There was a greater probability of recurrence in the first year following CEIM than in the following 4 years combined.
Conclusions
In this analysis of prospective cohort data from the AIM dysplasia trial, we found BE to recur after CEIM by RFA in almost one-third of patients with baseline dysplastic disease; most recurrences occurred during the first year after CEIM. However, patients who achieved CEIM and remained BE free at 1 year after RFA had a low risk of BE recurrence. Studies are needed to determine when surveillance can be decreased or discontinued; our study did not identify any BE or dysplasia recurrence after 4 years of surveillance.
Keywords: LGD, HGD, prognostic factor, long-term outcome
Introduction
Barrett’s esophagus (BE) is a precancerous state defined by the replacement of normal esophageal squamous mucosa by intestinal metaplasia (IM). This metaplastic change predisposes to the development of esophageal adenocarcinoma (EAC). BE is thought to progress to EAC via a series of steps beginning with non-dysplastic BE (NDBE), progressing to low-grade dysplasia (LGD), and on to high-grade dysplasia (HGD). LGD and HGD carry an increasing risk of progression and malignant transformation compared to NDBE.1–3 Eradication of dysplastic BE with endoscopic ablative techniques such as radiofrequency ablation (RFA) is commonly performed to reduce the risk of malignant progression.4,5 Dysplastic BE can usually be treated to complete eradication of intestinal metaplasia (CEIM).6
After CEIM, patients undergo surveillance where serial endoscopy is performed to assess for recurrence of BE.7,8 Per recent guidelines, patients with HGD who have been treated to CEIM enter a surveillance program with 3 month intervals for the first year, 6 month intervals in the second year, and then annually thereafter. For LGD, the first year interval is 6 months and then annually thereafter. Research to date has consistently demonstrated low rates of recurrence after CEIM.9–12 However, discontinuing surveillance remains a contentious area, in large part because follow up of BE patients in most prior studies is only one to two years,10–12 leaving the long-term risk of recurrence and progression of BE in doubt.
Here we present the final report of the AIM Dysplasia Trial, representing five years of follow-up after initial treatment in a randomized controlled trial of RFA of dysplastic BE. The aim of this study was to assess the rate of recurrence of BE in prospectively followed patients who had presented with dysplasia and then achieved CEIM.
Methods
The AIM Dysplasia Trial
Participants for the AIM Dysplasia trial were recruited at 19 U.S. sites. Eligibility criteria included age (18 to 80 years) and endoscopic evidence of non-nodular dysplastic BE ≤8 cm in length. A central pathology laboratory confirmed histologic grade. Previous endoscopic mucosal resection was permissible at least 8 weeks prior to entry, provided that subsequent endoscopy demonstrated non-nodular dysplasia. Exclusion criteria included pregnancy, active esophagitis or stricture, history of esophageal malignancy, varices, uncontrolled coagulopathy, or life expectancy of <2 years as judged by the investigator.
Subjects were randomly assigned in a 2:1 ratio to receive either RFA or a sham endoscopic procedure. Randomization was stratified by grade of dysplasia (LGD vs HGD) at study entry and endoscopic length of BE (<4 cm vs 4–8 cm). All underwent upper endoscopy, esophageal intubation with a study catheter, measurement of the esophageal inner diameter, and in-room assignment of treatment group (RFA vs sham) using a computer-generated block randomization sequence. In subjects assigned to RFA, the entire BE segment was ablated circumferentially. In the sham arm, the study catheter was removed and the procedure terminated.
RFA subjects could receive up to four RFA sessions in the first year, performed at 0, 2, 4, and 9 months based on interval biopsy results, and one RFA session in the second year (15 months). An initial treatment was performed with a HALO360 device, the circumferential balloon catheter, with settings of 12 joules/cm2 and 300 watts (BARRX Medical, Inc., Sunnyvale, CA, USA, now Medtronic, Sunnyvale, CA, USA). Any necessary follow-up treatments of residual BE were performed with a HALO90 focal ablation device. After achieving CEIM, subjects underwent endoscopy with biopsy at 6 and 12 months (LGD arm), or 3, 6, 9 and 12 months (HGD arm). Endoscopic biopsies were performed with maximum-capacity or jumbo forceps in 4 quadrants every 1 cm throughout the original length of BE, with the most distal biopsies 3–5 mm above the gastric folds, plus directed biopsies of any visible abnormalities.
After completion of the 12 month assessment, subjects in the sham arm were offered cross-over to active (RFA) treatment. Because subjects initially assigned to sham spent 12 months without ablative therapy, the earliest CEIM endoscopy in the sham group occurred after the patient had been in the study for at least 14 months, two months after their first RFA.
Subjects demonstrating CEIM at 2 years after initial treatment were eligible for participation in a 3 year study extension. The purpose of this extension was to assess the long-term durability of changes induced by RFA, and outcomes associated with treatment. Subjects who did not achieve CEIM at 2 years were offered a single salvage RFA treatment at that time with repeat biopsy 2 months later. Those with CEIM at repeat biopsy were then eligible for the study extension. The study extension provided for three additional years of follow-up for these subjects (to a total of 5 years), with annual surveillance endoscopies with biopsies identical in methodology to the primary protocol. All subjects in the trial extension were maintained on esomeprazole 40 mg twice daily. All institutions’ ethics committees approved the protocols for both the parent trial and the extension. The trial was performed in accordance with the Declaration of Helsinki. Further details of patient selection and have been published previously.10,13
Statistical Methods
We examined rates of any recurrence and dysplastic recurrence among AIM Dysplasia patients who achieved CEIM. Any recurrence is defined as the first recurrence with intestinal metaplasia, dysplasia, or adenocarcinoma. Dysplastic recurrence is defined as the first recurrence with dysplasia or adenocarcinoma. For the primary analysis, CEIM was defined as a single endoscopy visit without endoscopic evidence of BE and with biopsies negative for intestinal metaplasia or dysplasia. Sensitivity analysis was performed with a definition of CEIM requiring two such endoscopy visits. Descriptive statistics were reported as mean and standard deviation for continuous variables and as number and percentage for categorical variables. We calculated the proportion of patients who achieved CEIM, who experienced recurrence, who experienced progression, and who achieved second CEIM.
We constructed right-censored survival curves for any recurrence and for dysplastic recurrence after CEIM and compared the incidence of recurrence between patients with baseline LGD and HGD using the Wilcoxon Rank-Sum test. We used the Kaplan-Meier estimate of the survival function to examine proportions and 95% confidence intervals (CI) of patients without recurrence in the overall population and stratified by baseline histologic grade continuously and at annual intervals of one through five years. We examined the Epnechnikov Kernel-Smoothed estimate of the hazard function with optimal and arbitrary bandwidth settings to examine changes in recurrence rate over time.14 We calculated incidence rates and 95% CI of recurrence and dysplastic recurrence using a Poisson model.
We also fit parametric failure time models with an exponential distribution, Weibull distribution, and spline functions to examine changes in the rate of recurrence over time.15 We also calculated the proportion and 95% bootstrap CI of patients with CEIM at each annual visit allowing interim retreatments.15 Bootstrap confidence intervals were performed with 10,000 replicates using the Mersenne-Twister random number generator.
While only patients who acheived CEIM were included for Kaplan-Meier analyses of recurrence, analysis of the proportion of patients with CEIM at selected time intervals included all treated patients. The durability analysis was performed in 2 ways, one which considered any recurrence a permanent failure, demonstrated in Kaplan-Meier analyses, and a second which allowed for patients to be re-treated and re-gain CEIM, represented in tabular form. All statistical analyses were performed using SAS software (SAS version 9.4, SAS Institute Inc., Cary, NC).
Results
Inclusion and Censoring of Patients in the Surveillance Cohort
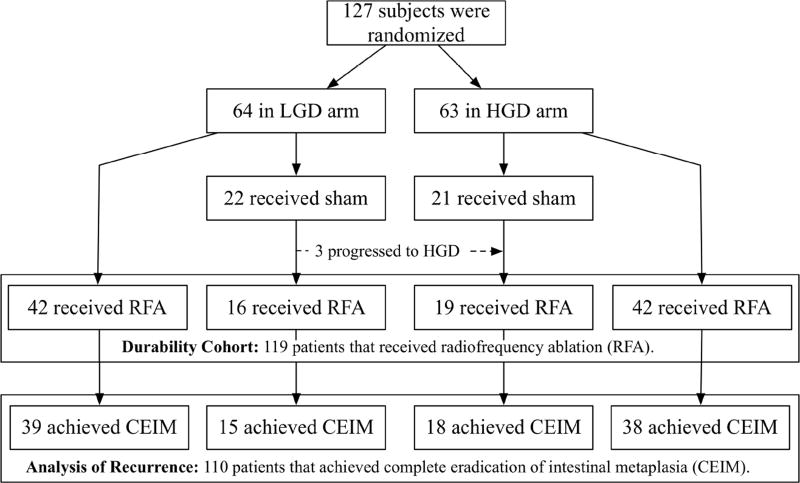
Of 127 patients initially randomized in the AIM Dysplasia trial, 64 had LGD and 63 had HGD. After crossover, 119 received RFA and were included for analysis of the proportion with CEIM. Of these 119, 110 (92%) achieved CEIM and were included for analysis of recurrence (Figure 1). These 110 patients had an average age of 66 years and were predominantly male (87%) and Caucasian (95%) (Table 1). Patients were observed for 401.1 person-years (mean 3.6 years per patient, range 0.2 – 5.8 years) after they achieved CEIM.
Figure 1.
Inclusion of 119 Patients in the Durability Cohort Allowing Interim “Touch-up” Treatments and 110 Patients in the Analysis of Recurrence not Allowing “Touch-up” Treatments.
Table 1.
Baseline Characteristics of Patients in the Analysis of Recurrence (N = 110)
| Age, years (mean ± SD) | 66.2 ± 8.5 |
| Male sex, n (%) | 96 (87.3) |
| Race - Caucasian, n (%) | 105 (95.5) |
| BMI (mean ± SD) | 29.2 ± 5.3 |
| Years with BE Prior to Enrollment (mean ± SD) | 5.5 ± 5.0 |
| Years with Dysplasia Prior to Enrollment (mean ± SD) | 2.5 ± 2.9 |
| Prague C Length, cm (mean ± SD) | 2.4 ± 2.5 |
| Prague M Length, cm (mean ± SD) | 3.7 ± 2.7 |
| Baseline Characteristics of Patients in the Durability Cohort (N = 119) | |
| Age, years (mean ± SD) | 66.0 ± 8.9 |
| Male sex, n (%) | 102 (85.7_ |
| Race - Caucasian, n (%) | 113 (95.0) |
| BMI (mean ± SD) | 29.2 ± 5.3 |
| Years with BE Prior to Enrollment (mean ± SD) | 5.4 ± 4.9 |
| Years with Dysplasia Prior to Enrollment (mean ± SD) | 2.4 ± 2.8 |
| Prague C Length, cm (mean ± SD) | 2.4 ± 2.5 |
| Prague M Length, cm (mean ± SD) | 3.7 ± 2.7 |
Of 110 patients entering surveillance, 5 were lost to follow-up during the first two years and 6 were ineligible due to not being in CEIM at the two-year visit. The 5 losses to follow-up consisted of two subjects who had progression of disease during treatment, one due to diagnosis of amyotrophic lateral sclerosis, and two who were unreachable. Of the remaining 99 patients eligible for the study extension at the end of year 2, two dropped out because their site did not participate in extension, four died of unrelated causes, two could not be reached, and 16 subjects declined to participate in the extension. The remaining 75 enrolled in the study extension and returned for the year 3 visit.
Any Recurrence of Intestinal Metaplasia, Dysplasia, or Progression to Adenocarcinoma
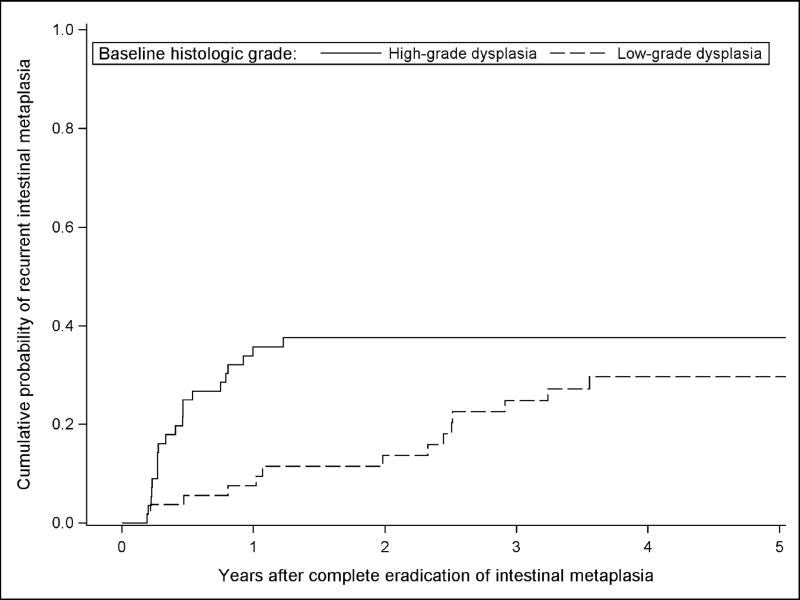
Over 324.5 person-years of follow-up (mean 2.9 years per patient, range 0.2 – 5.5 years), 35 of 110 (32%) patients experienced any recurrence. Among patients with baseline LGD, 14 (26%) had recurrence, while 21 (38%) with baseline HGD had recurrence. The incidence rate of any recurrence was 10.8 (95% CI 7.8 – 15.0) per 100 person-years overall, 8.3 (95% CI 4.9 – 14.0) per 100 person-years among patients with LGD, and 13.5 (95% CI 8.8 – 20.7) per 100 person-years among patients with HGD. Patients with baseline HGD had a numerically higher rate of any recurrence than patients with baseline LGD, but were not at a statistically significant increased risk of any recurrence with a rate ratio of 1.63 (95% CI 0.83 – 3.20) compared to patients with baseline LGD (Figure 2). Of the 35 recurrences, 10 (29%) had subsquamous intestinal metaplasia on one or more fragments from the biopsy level with recurrence. Of these 10 recurrences showing subsquamous metaplasia, 4 had one or more biopsies from the same endoscopy showing recurrence with no subsquamous metaplasia. Therefore, 6 out of the 35 recurrences were solely subsquamous (4 non-dysplastic BE, 1 indefinite for dysplasia, and 1 with LGD). No recurrence with subsquamous intestinal metaplasia was higher than low-grade dysplasia. Six (17%) of the recurrences were on targeted biopsies and these consisted of two patients with adenocarcinoma, one with high-grade dysplasia, one with low-grade dysplasia, and two with intestinal metaplasia recurrence.
Figure 2.
Estimated Proportion of Subjects with Any Recurrence Stratified by Baseline Histologic Grade After Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Recurrence with Dysplasia or Progression to Adenocarcinoma
Over 363.1 person-years of follow-up (mean 3.3 years per patient, range 0.2 – 5.7 years) 19 (17%) experienced dysplastic recurrence The incidence rate of dysplastic recurrence was 5.2 (95% CI 3.3 – 8.2) per 100 person-years overall, 3.3 (95% CI 1.5 – 7.2) per 100 person-years among patients with LGD, and 7.3 (95% CI 4.2 – 12.5) per 100 person-years among patients with HGD. Patients with baseline HGD had a numerically higher rate of dysplastic recurrence than patients with baseline LGD, but were not at a statistically significant increased risk of dysplastic recurrence with a rate ratio of 2.24 (95% CI 0.85 – 5.89) compared to patients with baseline LGD. Of the 35 patients with recurrence, 24 (69%) went on to acheive second CEIM after further endoscopic therapy. Of the 19 patients with dysplastic recurrence, 14 (74%) achieved second CEIM. During surveillance, four (7%) patients with LGD and two (4%) with HGD had progression of dysplasia to a condition worse than their baseline histologic grade.
Change in the Rate of Recurrence over Time
There was a greater probability of any recurrence or dysplastic recurrence in the first year following CEIM than in the following four years combined (Table 2). Fitting time to any recurrence and dysplastic recurrence to an exponential distribution, which assumes a constant rate of recurrence, produced a poor fit, indicating that recurrence did not occur at a constant rate. A Weibull distribution significantly improved fit, yielding an estimated shape parameter k of 0.62 (95% CI: [0.55, 0.71]) for any recurrence and 0.53 (95% CI: [0.45, 0.65]) for dysplastic recurrence. The shape parameter was significantly less than 1, demonstrating decreasing rates of recurrence and dysplastic recurrence over time. The rates of recurrence with high-grade dysplasia and mucosal adenocarcinoma recurrence also decreased with time after CEIM, but this did not reach statistical significance.
Table 2.
Kaplan-Meier Estimates of the Cumulative Incidence and 95% Confidence Limits (CL) of Recurrence of Barrett’s Associated Neoplasia by Year following Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
| High-Grade Dysplasia at Baseline |
Low-Grade Dysplasia at Baseline |
Combined Surveillance Cohort | ||||
|---|---|---|---|---|---|---|
| Any Recurrence | ||||||
| Year | Events | Cumulative Incidence % (95% CL) |
Events | Cumulative Incidence % (95% CL) |
Events | Cumulative Incidence % (95% CL) |
| First | 20 | 35.8 (23.2, 48.3) | 4 | 7.5 (0.4, 14.6) | 24 | 22.0 (14.2, 29.8) |
| Second | 1 | 37.7 (24.9, 50.4) | 3 | 13.7 (4.2, 23.1) | 4 | 26.0 (17.7, 34.3) |
| Third | 0 | 37.7 (24.9, 50.4) | 5 | 24.8 (12.5, 37.1) | 5 | 31.4 (22.5, 40.4) |
| Fourth | 0 | 37.7 (24.9, 50.4) | 2 | 29.7 (16.5, 43.0) | 2 | 33.8 (24.5, 43.0) |
| Fifth | 0 | 37.7 (24.9, 50.4) | 0 | 29.7 (16.5, 43.0) | 0 | 33.8 (24.5, 43.0) |
| Recurrence with Low-grade Dysplasia or Higher | ||||||
| First | 10 | 17.9 (7.8, 27.9) | 2 | 3.8 (0.0, 8.7) | 12 | 10.9 (5.1, 16.7) |
| Second | 2 | 21.7 (10.8, 32.6) | 1 | 5.7 (0.0, 11.9) | 3 | 13.8 (7.3, 20.3) |
| Third | 0 | 21.7 (10.8, 32.6) | 2 | 10.1 (1.7, 18.6) | 2 | 16.1 (9.0, 23.1) |
| Fourth | 1 | 24.1 (12.6, 35.6) | 1 | 12.7 (3.1, 22.4) | 2 | 18.6 (10.9, 26.2) |
| Fifth | 0 | 24.1 (12.6, 35.6) | 0 | 12.7 (3.1, 22.4) | 0 | 18.6 (10.9, 26.2) |
| Recurrence with High-grade Dysplasia or Higher | ||||||
| First | 7 | 12.5 (3.8, 21.2) | 1 | 1.9 (0.0, 5.4) | 8 | 7.3 (2.4, 12.2) |
| Second | 0 | 12.5 (3.8, 21.2) | 1 | 3.8 (0.0, 9.0) | 1 | 8.3 (3.1, 13.4) |
| Third | 0 | 12.5 (3.8, 21.2) | 2 | 8.2 (0.5, 16.0) | 2 | 10.5 (4.6, 16.4) |
| Fourth | 2 | 17.1 (6.8, 7.4) | 1 | 10.9 (1.8, 20.0) | 3 | 14.2 (7.2, 21.1) |
| Fifth | 0 | 17.1 (6.8, 7.4) | 0 | 10.9 (1.8, 20.0) | 0 | 14.2 (7.2, 21.1) |
| Recurrence with Intramucosal Adenocarcinoma | ||||||
| First | 2 | 3.6 (0.0, 8.4) | 0 | 0.0* | 2 | 1.8 (0.0, 4.3) |
| Second | 0 | 3.6 (0.0, 8.4) | 1 | 2.0 (0.0, 5.9) | 1 | 2.8 (0.0, 5.9) |
| Third | 0 | 3.6 (0.0, 8.4) | 0 | 2.0 (0.0, 5.9) | 0 | 2.8 (0.0, 5.9) |
| Fourth | 0 | 3.6 (0.0, 8.4) | 0 | 2.0 (0.0, 5.9) | 0 | 2.8 (0.0, 5.9) |
| Fifth | 0 | 3.6 (0.0, 8.4) | 0 | 2.0 (0.0, 5.9) | 0 | 2.8 (0.0, 5.9) |
Confidence limits are omitted when no events occurred,
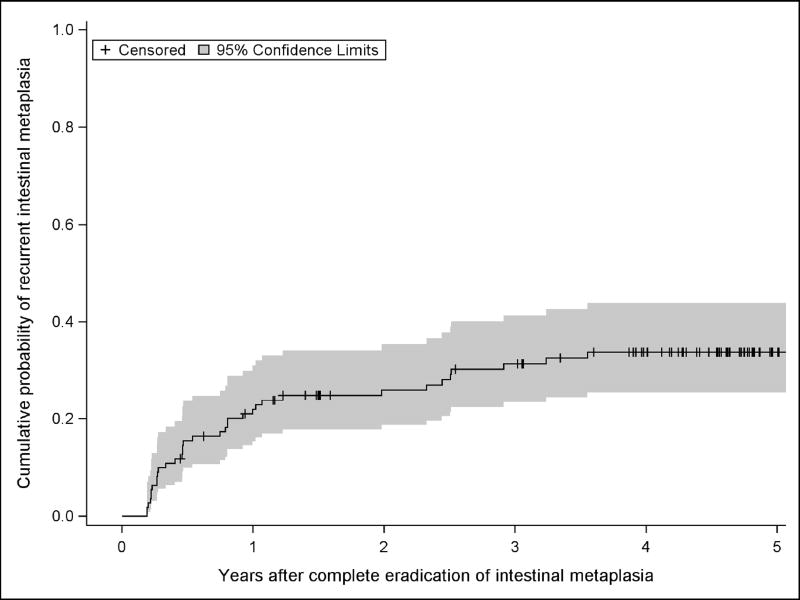
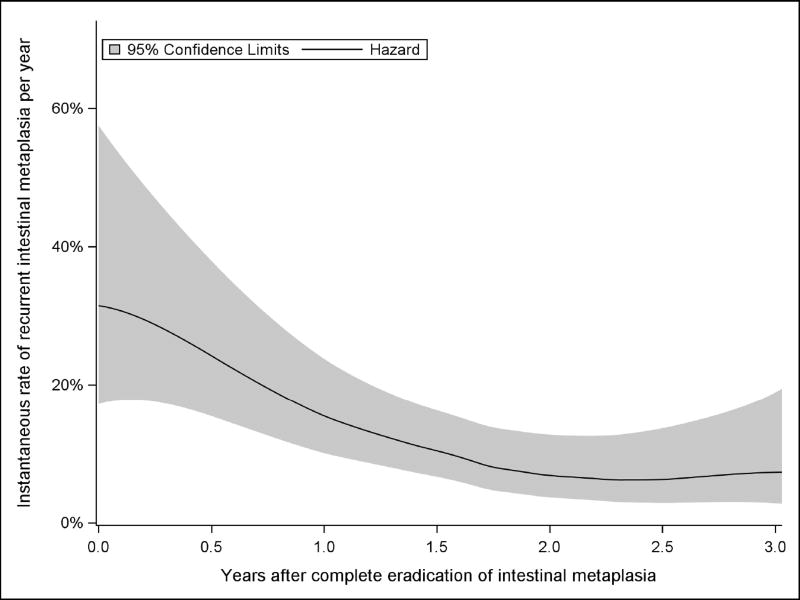
Examination of Kaplan-Meier estimates (Figures 2 and 3) and the smoothed hazard function (Figure 4) show an increased rate of recurrence at the three- and six-month visits immediately after CEIM was achieved. Thereafter, the predicted rate of recurrence for each subsequent year decreased to a plateau. Whether rates decreased to a constant plateau or continued to decrease in years four and five was sensitive to the model chosen for parametric survival analysis or the smoother bandwidth in the case of the smoothed hazard function.
Figure 3.
Estimated Proportion of Subjects with Any Recurrence After Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Figure 4.
Estimated Hazard Function, or Instantaneous Rate, for Recurrence of Intestinal Metaplasia Following Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Proportion of Patients with Current Remission of Intestinal Metaplasia
Patients in this trial received a prescribed surveillance endoscopy protocol, with further endoscopic therapy for recurrences. An additional important outcome in describing the durability after CEIM is the proportion of patients with CEIM or complete eradication of dysplasia (CE-D) allowing for patients to receive retreatment. Tabular estimates by year show that among 73 patients completing five years of surveillance, 72 (99%) had complete eradication of dysplasia (CE-D) and 66 (90%) had CEIM, when allowing for intervening treatment of recurrences (Table 3).
Table 3.
Proportion of 119 Patients Treated with RFA with Complete Eradication of Intestinal Metaplasia and Complete Eradication of Dysplasia by Year Following First Treatment allowing Interim “Touchup” Treatments.
| All enrolled patients | ||||
| Complete Eradication of Dysplasia | Complete Eradication of Intestinal Metaplasia | |||
| Year | Number / N* | % (95% CL) | Number / N | % (95% CL) |
| First | 103 / 115 | 89.6 (84.0, 95.2) | 90 / 115 | 78.3 (70.7, 85.8) |
| Second | 101 / 106 | 95.3 (91.2, 99.3) | 99 / 106 | 93.4 (88.7, 98.1) |
| Third | 74 / 75 | 98.7 (96.1, 100.0) | 71 / 75 | 94.7 (89.6, 86.9) |
| Fourth | 73 / 77 | 94.8 (89.8, 99.8) | 67 / 77 | 87.0 (79.5, 94.5) |
| Fifth | 72 / 73 | 96.8 (96.0, 100.0) | 66 / 73 | 90.4 (83.7, 97.2) |
| Patients with baseline low-grade dysplasia | ||||
| Complete Eradication of Dysplasia | Complete Eradication of Intestinal Metaplasia | |||
| Year | Number / N | % (95% CL) | Number / N | % (95% CL) |
| First | 53 / 55 | 96.4 (91.4, 100.0) | 45 / 55 | 81.8 (71.6, 92.0) |
| Second | 51 / 52 | 98.1 (94.3, 100.0) | 51 / 52 | 98.1 (94.3, 100.0) |
| Third | 39 / 39 | 100.0 (91.0, 100.0) | 36 / 39 | 92.3 (83.9, 100.0) |
| Fourth | 38 / 40 | 95.0 (88.2, 100.0) | 32 / 40 | 80.0 (67.6, 92.4) |
| Fifth | 32 / 38 | 84.2 (72.6, 95.8) | 32 / 38 | 84.2 (72.6, 95.8) |
| Patients with baseline high-grade dysplasia | ||||
| Complete Eradication of Dysplasia | Complete Eradication of Intestinal Metaplasia | |||
| Year | Number / N | % (95% CL) | Number / N | % (95% CL) |
| First | 50 / 56 | 89.3 (81.2, 97.4) | 45 / 56 | 80.4 (70.0, 90.8) |
| Second | 50 / 54 | 92.6 (85.6, 99.6) | 48 / 54 | 88.9 (80.5, 97.3) |
| Third | 35 / 36 | 97.2 (91.9, 100.0) | 35 / 36 | 97.2 (91.9, 100.0) |
| Fourth | 35 / 37 | 94.6 (87.3, 100.0) | 35 / 37 | 94.6 (87.3, 100.0) |
| Fifth | 34 / 35 | 97.1 (91.6, 100.0) | 34 / 35 | 97.1 (91.6, 100.0) |
N is the number of patients remaining at risk at the respective time points.
Sensitivity Analysis
Our sensitivity analysis defining CEIM as two clean endoscopies did not significantly alter these findings. Even after requiring 2 negative endoscopies for CEIM, subjects still had a higher rate of recurrence in year one of follow-up than the subsequent 4 years combined (Table S1, Figures S1 – S3).
Discussion
In this prospective, multicenter study assessing the 5-year risk of recurrence of BE among patients with dysplastic BE at baseline, the vast majority were successfully treated to CEIM. However, recurrence after CEIM was common, occurring in 32% of the cohort. Of 35 recurrence events, 24 occurred in the first year and none during the fifth year. While some portion of this decline is attributable to the fact that not all patients completed five years of surveillance, parametric survival analysis suggests two distinct phases of recurrence risk, rather than a continuum: an acute phase of elevated risk during the first year which is followed by a longer term trend toward decreased rates of recurrence over time. While some early recurrence may be due to sampling error, attributing these findings solely to sampling error (and premature declaration of CEIM) is not supported by our sensitivity analysis, where requiring two clean endoscopies prior to CEIM did not meaningfully change our findings. Even with this more rigorous definition, over half of recurrence events occurred in the first year of follow-up. Rather, our results suggest a true propensity to early versus late recurrence, lending credence to current recommendations based on expert opinion for aggressive screening during the first year after CEIM followed by a broader spacing of surveillance timing. The absence of BE recurrence after the fourth year of surveillance suggests that current open ended surveillance programs may need to be re-evaluated, and that cessation of surveillance for a subgroup demonstrating no recurrence by that point may be possible. However, the relatively small numbers of subjects in this study demand further research to solidify such a recommendation.
In our previous report of AIM-D trial results, we demonstrated that 95% of patients achieving CEIM had CE-D at 2 years, and 93% had CEIM (our previously published data for year 3 is superseded by the data published here as additional patients completed 3 years of surveillance since the last report).10 In the current data, 99% still had CE-D and 90% of patients had persistent CEIM, allowing for interim “touch up” treatments. These results indicate that surveillance will protect the vast majority of patients with dysplastic BE from recurrence of high risk disease.
Our BE recurrence frequency is comparable to prior results exploring the rate of recurrence in patients with dysplastic Barrett’s.11,16–18 However, the shape of the recurrence curve has varied, with some studies suggesting a relatively constant rate of recurrence,18 while the present data suggest a front-loaded risk of recurrence in year one. The long follow up period of dysplastic BE in the current study is unique in the literature, as is the statistical approach which tests (and rejects) the hypothesis that the rate of recurrence after ablation is constant.
This study has several limitations. Though use of bootstrap confidence intervals allows a robust estimate of the shape parameter k, which indicates decreasing rates of recurrence over time after CEIM, censored data are inherently at risk of bias due to nonrandom censoring. This may result in continued inclusion of patients who, for reasons of genetics, disease, or unknown factors, have easier-to-treat Barrett’s. This concern is mitigated by the durability analysis presented here which allows for retreatment, thus ensuring that patients with more aggressive disease are also well sampled. These data describe treatment and surveillance in expert centers, and their generalizability to community centers is not certain. Finally, biopsy samples included in this study did not sample directly at the top of gastric folds but rather sampled 0.5 cm proximal to this landmark, which may reduce the sensitivity of sampling for recurrence.
These data demonstrate that, while recurrence of BE after CEIM is common, re-treatment to CEIM is usually achievable, and rates of both CE-D and CEIM out to 5 years are high. Importantly, nearly all recurrence risk occurs in the first year after CEIM. Patients who achieve CEIM after an initial diagnosis of dysplasia can be assured that their risk of malignancy is small. However, it would be premature to recommend stopping surveillance endoscopy at a fixed number of years after CEIM based on this study. Further research will be needed to clarify the long term risk of BE recurrence and the necessary surveillance interval and duration. The timing of discontinuing surveillance for BE recurrence remains unclear. While our data suggest that four years may be enough, further work with larger numbers of patients treated in real world settings will be necessary to provide guidance.
Supplementary Material
Table 1-S. Kaplan-Meier Estimates of the Cumulative Incidence and 95% Confidence Limits (CL) of Recurrence of Barrett’s Associated Neoplasia by Year following Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Figure 1-S. Estimated Proportion of Subjects with Incidence of Any Recurrence Stratified by Baseline Histologic Grade After Complete Eradication of Intestinal Metaplasia Defined as Two Clean Endoscopies not Allowing Interim “Touch-up” Treatments.
Figure 2-S. Estimated Proportion of Subjects with Incidence of Any Recurrence After Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Figure 3-S. Estimated Hazard Function, or Instantaneous Rate, for Recurrence of Intestinal Metaplasia Following Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Acknowledgments
Dr. Shaheen receives research funding from Barrx/Covidien/Medtronic, CSA Medical, C2 Therapeutics, Boston Scientific, Interpace Diagnostics, and CDx Medical. The other authors received research funding from Barrx/Covidien/Medtronic for this study.
This research was funded by T32 DK07634 and K24DK100548 from the National Institutes of Health and Barrx/Covidien/Medtronic.
The AIM Dysplasia Trial Group consists of Richard E. Sampliner, David E. Fleischer, Virender K. Sharma, Glenn M. Eisen, M. Brian Fennerty, John G. Hunter, Mary P. Bronner, John R. Goldblum, Ana E. Bennett, Hiroshi Mashimo, Richard I. Rothstein, Stuart R. Gordon, Steven A. Edmundowicz, V. Raman Muthusamy, Kenneth J. Chang, Michael B. Kimmey, Stuart J. Spechler, Ali A. Siddiqui, Rhonda F. Souza, Anthony Infantolino, John A. Dumot, Gary W. Falk, Blair A. Jobe, Robert H. Hawes, Brenda J. Hoffman, Prateek Sharma, and Amitabh Chak.
Abbreviations
- BE
Barrett’s esophagus
- CE-D
complete eradication of dysplasia
- CEIM
complete eradication of intestinal metaplasia
- CI
confidence interval
- EAC
esophageal adenocarcinoma
- HGD
high grade dysplasia
- LGD
low grade dysplasia
- NDBE
non-dysplastic Barrett’s esophagus
- RFA
radiofrequency ablation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No author has other financial, personal, or professional conflicts to declare.
Author Contributions:
CCC: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis
WAW: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis
BFO: study concept and design, critical revision of the manuscript for important intellectual content
NL: analysis and interpretation of data, statistical analysis
CJL: study concept and design, critical revision of the manuscript for important intellectual content
HCW: study concept and design, critical revision of the manuscript for important intellectual content
DK: drafting of the manuscript
SP: critical revision of the manuscript for important intellectual content
KKW: study concept and design, critical revision of the manuscript for important intellectual content
NJS: study concept and design, analysis and interpretation of data, drafting of the manuscript, study supervision
References
- 1.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, Sharma P. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–398. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Weston AP, Sharma P, Topalovski M, Richards R, Cherian R, Dixon A. Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–1893. doi: 10.1111/j.1572-0241.2000.02234.x. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 5.Sharma VK, Jae Kim H, Das A, Wells CD, Nguyen CC, Fleischer DE. Circumferential and focal ablation of Barrett's esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310–317. doi: 10.1038/ajg.2008.142. [DOI] [PubMed] [Google Scholar]
- 6.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clinical Gastroenterology and Hepatology. 2014;12:1840–1847. e1. doi: 10.1016/j.cgh.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Falk GW, Iyer PG, Gerson L. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2015;111:30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 9.Wolf WA, Pasricha S, Cotton C, et al. Incidence of Esophageal Adenocarcinoma and Causes of Mortality After Radiofrequency Ablation of Barrett’s Esophagus. Gastroenterology. 2015;149:1752–1761. e1. doi: 10.1053/j.gastro.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011;141:460–468. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orman ES, Kim HP, Bulsiewicz WJ, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett's esophagus with radiofrequency ablation. Am J Gastroenterol. 2013;108:187–95. doi: 10.1038/ajg.2012.413. quiz 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett's esophagus: results from a US Multicenter Consortium. Gastroenterology. 2013;145:79–86. e1. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 14.Muller H, Wang J. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994:61–76. [PubMed] [Google Scholar]
- 15.Crowther MJ, Lambert PC. A general framework for parametric survival analysis. Stat Med. 2014;33:5280–5297. doi: 10.1002/sim.6300. [DOI] [PubMed] [Google Scholar]
- 16.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamoorthi R, Singh S, Ragunathan K, Katzka DA, Wang KK, Iyer PG. Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointest Endosc. 2016;83:1090–1106. e3. doi: 10.1016/j.gie.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthikonda A, Cotton CC, Madanick RD, et al. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett’s Esophagus With Radiofrequency Ablation. Am J Gastroenterol. 2017;112(1):87–94. doi: 10.1038/ajg.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1-S. Kaplan-Meier Estimates of the Cumulative Incidence and 95% Confidence Limits (CL) of Recurrence of Barrett’s Associated Neoplasia by Year following Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Figure 1-S. Estimated Proportion of Subjects with Incidence of Any Recurrence Stratified by Baseline Histologic Grade After Complete Eradication of Intestinal Metaplasia Defined as Two Clean Endoscopies not Allowing Interim “Touch-up” Treatments.
Figure 2-S. Estimated Proportion of Subjects with Incidence of Any Recurrence After Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.
Figure 3-S. Estimated Hazard Function, or Instantaneous Rate, for Recurrence of Intestinal Metaplasia Following Complete Eradication of Intestinal Metaplasia not Allowing Interim “Touch-up” Treatments.