Abstract
Membrane voltages are ubiquitous throughout cell biology. Voltage is most commonly associated with excitable cells such as neurons and cardiomyocytes, although many other cell types and organelles also support electrical signaling. Voltage imaging in vivo would offer unique capabilities in reporting the spatial pattern and temporal dynamics of electrical signaling at the cellular and circuit levels. Voltage is not directly visible, and so a longstanding challenge has been to develop genetically encoded fluorescent voltage indicator proteins. Recent advances have led to a profusion of new voltage indicators, based on different scaffolds and with different tradeoffs between voltage sensitivity, speed, brightness, and spectrum. In this review, we describe recent advances in design and applications of genetically-encoded voltage indicators (GEVIs). We also highlight the protein engineering strategies employed to improve the dynamic range and kinetics of GEVIs and opportunities for future advances.
Graphical abstract
Introduction
Membrane voltage is an important signal that affects many fundamental aspects of cellular physiology. The transmembrane electric field perturbs the energy landscape of biomolecules embedded in the lipid membrane, including ion channels, G protein-coupled receptors and membrane-associated enzymes [1,2], and is affected in turn by the dynamics of voltage- and ligand-gated ion channels, as well as electrogenic transporters and pumps. While bioelectrical signaling is most commonly associated with neurons and cardiomyocytes, membrane voltage also forms the basis for signaling in other cell types including bacteria [3,4], fungi [5] and plants [6]. Compared with electrode-based techniques such as patch clamp electrophysiology, optical measurements offer spatial resolution, non-invasiveness, ease of operation, and high measurement throughput. Much effort has gone toward development of fluorescent genetically encoded voltage indicators (GEVIs), though current-generation GEVIs are still far from optimal. Here we review recent advances in GEVI designs and their applications in voltage imaging of bioelectric phenomena. We hope that this article will help the reader select the right GEVI for his or her application, and will inspire novel ideas for better GEVIs.
The family of GEVIs
Fluorescent protein-based GEVIs
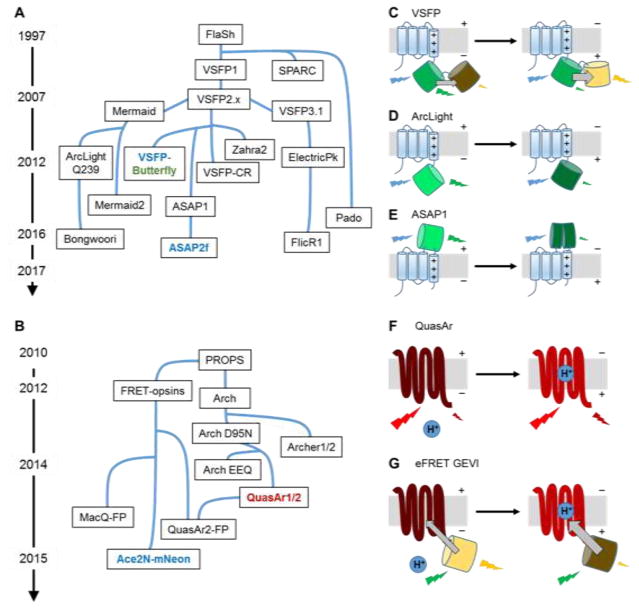
The history of GEVI development is marked by occasional introduction of qualitatively new scaffold designs, followed by periods of iterative optimization and refinement (Fig. 1). The earliest GEVI designs comprised naturally occurring ion channel voltage sensor domains fused to fluorescent proteins (FPs) (FlaSh [7], SPARC [8]) or F rster resonance energy transfer (FRET) FP pairs (VSFP1 [9]). GEVIs based on ion channel scaffolds typically exhibited modest voltage sensitivity (<5% ΔF/F per 100 mV) and slow kinetics (typically 10–200 ms), and many suffered from poor membrane trafficking [10].
Figure 1. The family of GEVIs.
A) and B) Approximate lineages of the major classes of GEVIs. The GEVIs highlighted in bold have shown the greatest sensitivity for use in vivo and are colored with their approximate excitation wavelengths. A) GEVIs based on voltage-sensor domains. B) GEVIs based on microbial rhodopsins. C)–G) Voltage sensing mechanisms in the major classes of GEVIs.
The transmembrane voltage-sensing domain (VSD) of the ascidian Ciona intestinalis voltage-sensing phosphatase (Ci-VSP) [11], turned out to be a good transducer for many GEVIs (VSFP2.x [12,13], Mermaid [14,15], Butterfly [16], VSFP-CR [17], ArcLight [18], ASAP [19,20], Bongwoori [21], FlicR1 [22]). GEVIs based on Ci-VSP had much improved membrane trafficking, presumably due to the monomeric nature of the VSD. Ci-VSP VSDs fused with FRET pairs showed good voltage sensitivity (ΔR/R approaching 48% per 100 mV for Mermaid2 [14,15]) and have been applied to report large-area membrane voltage fluctuations in vivo (optical EEG with Butterfly 1.2 [16]). While the ratiometric nature of FRET signals is useful for canceling motion and blood flow artifacts, the broad spectrum of FRET GEVIs often precludes multiplex imaging with other sensors. Surprisingly, a VSD fused with a single FP also reported membrane voltage (VSFP3.1), suggesting that a mechanism other than FRET was responsible for some of the observed fluorescence change [23]. One drawback of these monochromic GEVIs was their limited sensitivity.
A breakthrough came in 2012, when Jin et al. discovered that an unintended mutation (A227D) in the FP boosted voltage sensitivity 14-fold, and further engineering through mutagenesis and linker optimization resulted in ArcLight Q239 with voltage sensitivity 35% ΔF/F per 100 mV [18]. However, the slow temporal response of ArcLight (10 ms for its fast component) hindered action potential detection (3.2% ΔF/F in cultured neurons). Replacing the Ci-VSP VSD with homologues from chicken and zebrafish VSPs resulted in faster kinetics, but at the cost of reduced sensitivity [24]. Sequence alignment analysis and a cassette mutagenesis screen led to Bongwoori, a triple mutant of ArcLight with truncated linker, which resolved neuronal action potentials at 60 Hz [21]. Recently, the Ci-VSP VSD was fused to NanoLuc luciferase and Venus to create a bioluminescent GEVI called LOTUS-V. This GEVI had ΔR/R of 21% in response to 100 mV membrane voltage change, but its response was dominated by a ~200 ms time constant and the GEVI required an exogenous substrate to luminesce [25].
Another way to capitalize on the conformational change in the VSD is to insert a circularly permuted FP (cpFP) on the C-terminus (ElectricPk) [26,27]. Recently, Abdelfattah et al. applied this design to a screen of VSD-cpmApple mutants. Through a combination of directed protein evolution and rational design they created a red-shifted GEVI, FlicR1, which faithfully detected action potentials, albeit with modest signal amplitude (~3% ΔF/F) [22]. In 2014, St-Pierre et al. reported a new design, called ASAP1, where a cpGFP was inserted into the extracellular loop connecting the third and fourth transmembrane helices of the Ci-VSP VSD. The combination of high speed (~2 ms) and high sensitivity (18–29% ΔF/F per 100 mV) enabled detection of action potential waveforms up to 200 Hz [19]. Further mutagenesis of the linker connecting the VSD and cpGFP led to ASAP2f, which produced larger fluorescence changes in vivo than ASAP1 and was applied in two-photon voltage imaging of Drosophila visual system [20].
Microbial rhodopsin-based GEVIs
Microbial rhodopsin proteins were initially used for optogenetic control of membrane potential, but in 2011 these proteins were shown to function as fluorescent voltage reporters too [3]. The endogenous retinal chromophore shows weak near infrared fluorescence, but only when a proton resides on the Schiff base linking the retinal to the protein core. Changes in membrane potential shift the local electrochemical potential of protons, and thereby tune fluorescence-determining acid-base equilibrium. A green-absorbing proteorhodopsin mutant (PROPS) revealed that bacteria generate spontaneous electrical spikes [3]. However, PROPS did not traffic to the plasma membrane in eukaryotic cells, and thus was not a useful mammalian voltage sensor.
Archaerhodopsin 3 (Arch) from Halorubrum sodomense expressed and trafficked well in mammalian cells [28]. This protein functioned as a near-infrared GEVI that resolved individual action potentials in mammalian neurons in vitro [29]. Arch has sub-millisecond response kinetics and exhibits large voltage sensitivity (~35% ΔF/F per 100 mV). Directed evolution identified mutations that improved brightness, kinetics, and voltage sensitivity, while eliminating the proton-pumping photocurrent (Archers [30,31] and QuasArs [32]). Surprisingly, some of these mutations are far away from the retinal chromophore, suggesting subtle influences on the rhodopsin conformation and photocycle dynamics [32]. Despite their broad utility, Arch-derived GEVIs suffer from weak fluorescence, typically 30–80-fold dimmer than GFP. Voltage imaging with microbial rhodopsin endogenous fluorescence requires intense red laser illumination, typically 200–1000 W/cm2 [29,32]. Efforts to evolve Arch variants that accept unnatural chromophores have increased the quantum yield, but have not yet produced usable voltage sensors [33].
Electrochromic FRET (eFRET) provides a means to improve the brightness of microbial rhodopsin-derived GEVIs while maintaining their fast kinetics. In eFRET, voltage-induced changes in the rhodopsin absorption spectrum alter the degree of non-radiative quenching of an appended FP [34,35]. The first generation eFRET GEVIs used either QuasAr2 or a proton-pumping rhodopsin derived from Leptosphaeria maculans (Mac). Microbial rhodopsin absorption spectra are very broad, so FPs with different colors can be used as eFRET donors, leading to a palette of GEVIs spanning much of the visible spectrum [34,35]. While these sensors are sufficiently bright to detect action potentials in cultured neurons their sensitivity is lower than the parent rhodopsin (e.g. 13%/100 mV for QuasAr2-Citrine eFRET vs. 90%/100 mV for QuasAr2) [34]. A recently developed eFRET GEVI fused a proton-pumping rhodopsin mutant from a green algae Acetabularia acetabulum (Ace) to a bright and photostable FP, mNeonGreen [35,36]. This GEVI showed remarkably bright and fast signals, enabling voltage imaging with single-cell and single-spike resolution in mouse and fly brains in vivo.
Near infrared GEVIs open combinatorial possibilities
Pairing of voltage imaging with patterned optogenetic stimulation greatly facilitates exploration of neural circuits [32,37–40]. Optogenetic stimulation allows one to explore neural behavior over a wider range of parameters, and in a more systematic fashion, than one can achieve through passive observation or sensory stimulation alone. Knowledge of input-output properties under defined conditions can then shed insight into observations of native function.
To combine stimulation and measurement, the actuator and reporter must be spectrally orthogonal. While there exist red-shifted channelrhodopsin variants [41–44], none is truly spectrally orthogonal to GFP or its derivatives. All retain 20–30% activation at wavelengths used for GFP excitation [45]. Thus it has not been feasible to pair red-shifted optogenetic actuation with blue-excited GEVIs. Examination of optical action spectra shows that pairing of a blue-shifted channelrhodopsin with a red-excited near infrared (NIR) emitting GEVI provides better protection from optical crosstalk.
Hochbaum et al. paired a NIR GEVI, QuasAr2, with a blue-shifted channelrhodopsin, CheRiff, to create an all-optical method for simultaneous optogenetic stimulation and voltage imaging [32]. This ‘Optopatch’ technique has been used to probe neuronal excitability in cultured neurons, organotypic [32] and acute [46] brain slices and mouse somatosensory ganglia in vivo [46]. The high-throughput nature of Optopatch measurements has also been applied to screening for pharmacological modulators of heterologously expressed voltage-gated sodium channels [47,48].
NIR GEVIs can also be paired with GFP-based fluorescent reporters, to study the interrelation of voltage and soluble analytes. For example, fusion of QuasArs and GCaMP-series calcium indicators (CaViar) has enabled simultaneous measurement of calcium and voltage signal in zebrafish heart in vivo [47,49] and in human iPSC-derived cardiomyocytes [47,49]. NIR GEVIs could in principle be paired with a great many other GFP-based fluorescent reporters, e.g. for vesicle release, neurotransmitters, cAMP, or kinase activity.
There are also technical merits to working with NIR GEVIs. In brain tissue, autofluorescence is 136-fold lower with excitation at 640 nm than at 488 nm [46]. Thus, despite the fact that Arch-based GEVIs are ~30–80-fold dimmer than mCitrine, the signal-to-background ratio of QuasAr is similar to that of mCitrine when the two proteins are expressed as a 1:1 fusion and imaged with 1-photon excitation in vivo. Photochemical toxicity in cultured cells is > 100-fold lower at 640 nm than at 488 nm [50]. Optical scattering lengths are ~50% longer at 640 nm than at 488 nm, enabling correspondingly deeper imaging [51]. The greatest concern for imaging NIR GEVIs in tissue is photothermal heating. At 640 nm, optical absorption of brain tissue, and hence laser-induced heating, is ~20-fold lower than at 488 nm, and 3–5-fold lower than in the 800–1000 nm band used for conventional 2P imaging [51]. Optical powers up to 280 mW (λ = 925 nm) [52] have been safely used in 2P imaging in vivo, suggesting that similar optical powers are acceptable for imaging NIR GEVIs. Thus development of brighter NIR GEVIs would be an enabling technology for neuroscience.
Biological applications of GEVIs
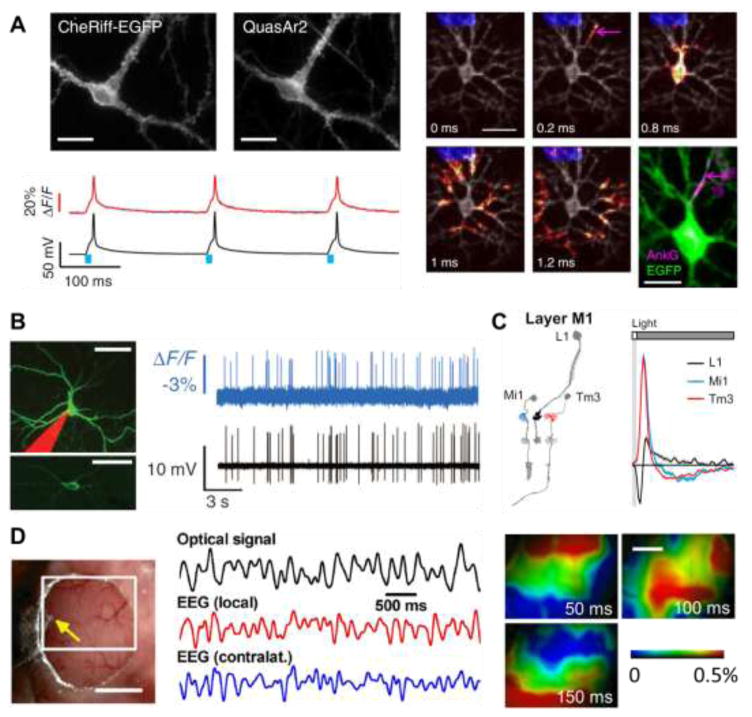
GEVIs have been applied to study electrical dynamics across spatial scales, from single dendritic branches to macroscopic brain regions (Fig. 2). On the smallest scale, Optopatch-based measurements in cultured neurons probed the microsecond-timescale dynamics of action potential initiation and propagation [32]. On an intermediate scale, two-photon imaging of the ASAP2f GEVI in Drosophila revealed that the ON and OFF selectivity to visual stimuli arose in the intracellular transformation of voltage to Ca2+ responses [20]. Imaging of single neurons expressing Ace2N-mNeon in mouse visual cortex probed propagation delays associated with dendritic activation [36]. On the largest scale, wide-field imaging of VSFP Butterfly 1.2 in mouse cortex showed changes in large-scale dynamics associated with waking from anesthesia [53].
Figure 2. Applications of GEVI imaging.
A) All-optical electrophysiology (‘Optopatch’) in neurons co-expressing a blue-shifted channelrhodopsin, CheRiff, and a red-shifted NIR GEVI, QuasAr2. Left: Optical stimuli evoke electrical spikes and closely corresponding fluorescence transients. Right: Patterned optogenetic stimulation on a dendritic region evokes action potentials whose sub-cellular propagation initiates at the axon initial segment, marked by an Ankyrin G immunostain. Figures from Ref. [32]. B) Ace2N-mNeon reports neuronal spikes in mouse visual cortex in vivo. Figure from Ref. [36]. C) Two-photon imaging of ASAP2f in Drosophila visual neurons reports sub-cellular details of stimulus-evoked electrical responses. Figure from Ref. [20]. D) Wide-field optical voltage mapping in mouse cortex in vivo. The optical signal correlates with the EEG from the ipsilateral but not the contralateral hemisphere. Figure from Ref. [16].
Despite these early results, biological application of GEVIs remains in its infancy. Improvements in GEVI sensitivity could enable a host of new applications (for an in-depth discussion see [54]). Voltage imaging could enable (1) to measure sub-threshold post-synaptic potentials as a direct probe of synaptic strength. In combination with targeted optogenetic stimulation, such a technique could be used for functional circuit mapping; (2) to resolve subcellular details of voltage propagation in intact tissue. These measurements could probe the ways in which excitatory inputs, inhibitory inputs, and action potentials propagate through the dendritic tree; and how these signals interact with each other when they overlap in space and time; and (3) to make precise measurements of spike timing and action potential waveform in genetically specified cells to probe circuit mechanisms that are masked by the lower temporal resolution of Ca2+ imaging. For instance, in fast-spiking interneurons, Ca2+ levels do not follow the individual spike dynamics [55], so spike timing is best determined by measuring voltage.
The future of GEVIs: brighter, more sensitive, redder
To achieve these goals will require dramatic improvements in GEVI performance. An ideal GEVI should show large voltage-induced changes in fluorescence over a physiological range, have sub-millisecond response kinetics, traffic efficiently to the neuronal plasma membrane, be bright and photostable, and fluoresce in the near infrared part of the spectrum. No such GEVI exists. In engineering new GEVIs, it is important to understand how tradeoffs among these different parameters affect overall GEVI performance.
Ultimately, the ability to record neural activity is governed by the signal-to-noise ratio (SNR) of fluorescent voltage measurements. The shot noise-limited SNR of any fluorescent reporter is:
| [Eq. 1] |
where ΔF is the change in fluorescence associated with the electrical event of interest, F is the basal fluorescence of the indicator, and B is the background fluorescence due to improperly trafficked GEVI molecules, tissue autofluorescence, and out-of-focus fluorescence. All fluorescence values are measured in photon counts. Eq. 1 provides guidance on how tradeoffs in brightness (affects F and ΔF proportionally), trafficking (affects B), speed (affects ΔF), and voltage sensitivity (affects ΔF) impact GEVI performance.
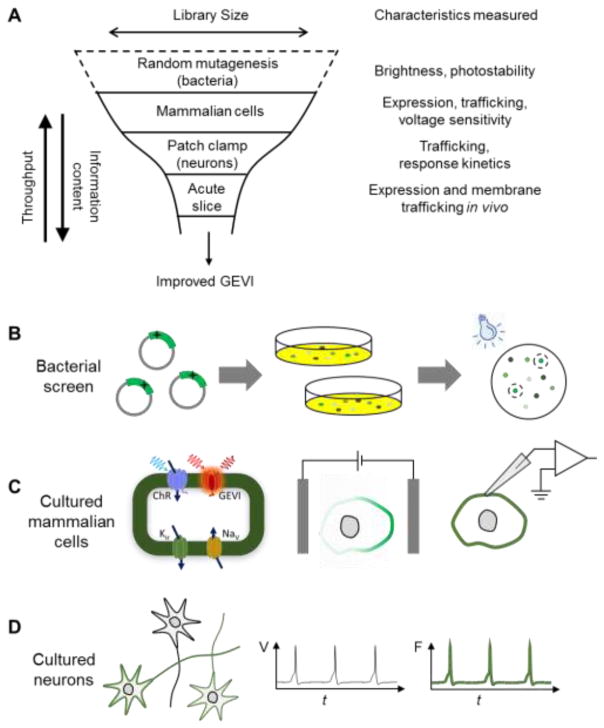
Iterative improvement in GEVIs has historically been slow and labor intensive, due to the multiple parameters governing GEVI performance. In recent years a hierarchical approach has been employed to screen mutant libraries first in bacteria and then in mammalian cells (Fig. 3; Archers [30,31] and QuasArs [32]). While bacteria offer large library size and can be efficiently used to optimize F, trafficking in bacteria is not predictive of trafficking in mammalian cells, nor can one readily perturb membrane voltage in bacteria.
Figure 3. GEVI screening pipeline.
A) A hierarchical approach screens large libraries for the most easily measured parameters (brightness), and then characterizes hits for voltage sensitivity and speed. Trafficking must ultimately be tested by GEVI expression in vivo and imaging in acute slice. Figure modified from Ref. [56]. B) Bacterial screens for brightness can be performed in a pooled library assay where highly fluorescent colonies are manually selected for propagation. To distinguish brightness from colony size, it is important to have a spectrally distinct reference fluorophore expressed at a constant level. C) Tests for voltage sensitivity and kinetics can be performed in cultured mammalian cells, using either (left) spiking HEK cells, (middle) induced transmembrane voltage, or (right) manual patch-clamp electrophysiology. D) Tests in cultured neurons probe trafficking and high-speed kinetics. Neural activity can be induced either optogenetically or via field-stimulation electrodes.
The hardest part of GEVI screening is to measure voltage response kinetics and sensitivity, which together determine ΔF. Mammalian HEK cell lines have been engineered which can produce either spontaneous [56] or optogenetically triggered [48,57] electrical spikes, analogous to cardiac action potentials. These spiking HEK cells provide a robust platform for evaluating trafficking, brightness, voltage sensitivity, and speed in a high-throughput format. An alternate technique is to apply bulk electric fields which induce oppositely-directed transmembrane potentials on opposite sides of each cell. This technique of induced transient voltage (ITV) does not require cell lines expressing exogenous ion channels, but is less precise and lower throughput than measurements in spiking HEK cells [58]. Whole-cell voltage clamp provides the highest precision and accuracy, but is slow and laborious. Membrane trafficking can differ dramatically between stable cell lines, primary cultured neurons, and neurons in vivo. Thus it is essential to validate every promising GEVI in cultured neurons and ultimately in acute brain slice.
New forms of molecular logic
A parallel thread is to engineer GEVIs to implement new forms of molecular logic which may enable alternate approaches to studying neural activity. In every GEVI, optical excitation transfers the molecule to a short-lived (ps–ns) electronic excited state. In microbial rhodopsin-based GEVIs, illumination can further drive transitions among distinct retinal isomerization states and protein conformations in a complex multi-state photocycle [59]. One can use the interaction of light- and voltage-driven dynamics to implement several novel forms of voltage sensing.
The numerical values, in millivolt, of the resting potential and action potential height are important biological parameters, but most GEVIs only report relative changes, not the numerical value, of the voltage. Even with FRET-based ratiometric sensors it is challenging to determine absolute voltage because the two FPs may have different rates of folding, maturation, and photobleaching; and it is challenging to maintain accurately calibrated illumination intensities and detection sensitivities at multiple wavelengths.
In some GEVIs, changes in membrane voltage modulate the electronic excited state lifetime, which can be measured by illuminating the GEVI with a pulsed source and performing time-correlated single-photon counting for detection. Time can be measured with absolute accuracy, so lifetime imaging reports absolute voltage. This strategy has been employed with ASAP1 and an eFRET-based GEVI called CAESR [60]. Voltage also affects the kinetics of transitions within the Arch photocycle, and mutants of Arch have been engineered in which optical measurements of these kinetics report absolute voltage [61].
The short duration of neuronal action potentials poses a severe challenge for GEVI imaging: real-time measurements require a frame rate of ~1 kHz. It has not yet been technically feasible to attain this frame rate over a large field of view in vivo. The complex photocycles of Arch-based GEVIs provide a potential solution to this challenge too[62]. Some Arch mutants undergo a photochemical conversion into a metastable fluorescent state, only in the simultaneous presence of illumination and a depolarizing voltage. By flood-illuminating a neural circuit during a user-selected ‘write’ interval, one can form a photochemical imprint within each cell of the amount of electrical activity during the write interval. Cells that fire more during this interval produce more fluorescent product. This fluorescence can then be probed at a later time using a slow imaging technique. A key merit of this ‘Flash Memory’ approach is that the light used for the write interval does not need to follow a straight-line path through the tissue. The light can propagate diffusively, enabling penetration deep into the tissue. The Flash Memory scheme has only been demonstrated in cultured neurons [63]. Improvements in sensitivity are needed before this can be applied in tissue or in vivo.
Conclusions: better GEVIs are only part of the solution
The ideal GEVI has not yet been found. Three protein scaffolds have been explored in depth, with distinct voltage-sensing mechanisms: ion channels and VSPs that undergo voltage-induced conformational changes, and microbial rhodopsins that have voltage-dependent chromophore protonation. Engineering of these scaffolds has led to improvements, but progress is often incremental. Many potential novel scaffolds remain to be tested. Of the many thousands of microbial rhodopsins identified via metagenomic sequence analysis [64], only a handful have been tested as putative voltage sensors. Mitochondria and chloroplasts have molecular machinery for transmembrane electron transfer. These proteins have endogenous cytochrome chromophores, which might show voltage-dependent spectral properties. Recently developed electrically spiking HEK cell lines should greatly facilitate testing of new GEVI concepts. There remains great scope for enterprising researchers to explore new GEVI scaffolds.
Even with best GEVI imaginable, voltage imaging in the brain will remain technically challenging, far harder than Ca2+ imaging. Action potentials are 100–1000-fold faster than Ca2+ transients, demanding correspondingly faster imaging speeds. Even if brightness and sensitivity were matched between voltage and Ca2+ indicators, the higher imaging speed for voltage would require proportionally higher illumination intensity to maintain comparable SNR, leading to higher rates of photobleaching and phototoxicity. Furthermore, voltage signals in cells come exclusively from the 2-D plasma membrane, while Ca2+ reporters reside in the bulk cytoplasm. Thus voltage signals sources are sparsely and irregularly distributed in intact tissue. Light-gated voltage integrators and related concepts open the possibility to relax the stringent requirements on imaging speed, possibly allowing new types of microscopy to be applied to GEVI imaging.
Another difference between voltage and Ca2+ imaging comes from the differing surface-to-volume ratios in soma and neuropil. In CNS neurons, the ratio of soma volume to axo-dendritic volume is ~3:2, so most Ca2+ signals come from the somata. The ratio of soma surface area to axo-dendritic surface area is ~1:20 [65,66], so most voltage signals come from optically unresolvable neuropil. Signals from soma membranes can be overwhelmed by surrounding neuropil, unless the GEVI is either expressed in a sparse subset of neurons or is constrained to reside in the soma membrane only.
Despite these technical challenges, recent progress in GEVIs has brought us closer to the dream of sensitively mapping electric fluctuations in the brain with high spatial and temporal resolution. Successful application of GEVIs in vivo will require advances not just in GEVI scaffolds, but also in techniques for gene delivery and sub-cellular control of protein trafficking; in microscopes for high-speed imaging in scattering tissues; and in software for analyzing the torrents of data that emerge. This progress will require close collaboration of protein engineers, microscope developers and neuroscientists.
Table 1.
Recently developed GEVIs appropriate for in vivo voltage imaging
| GEVI name | Voltage-sensing structure | Fluorescence reporter (Emission wavelength) | ΔF/F or ΔR/R (%) | τon (ms) | τoff (ms) | |||
|---|---|---|---|---|---|---|---|---|
| 100 mV | AP** | fast | slow | fast | slow | |||
| Mermaid2 [15] | Ci-VSP | mUKG (499 nm)/mKOκ (563 nm) | 48.5 | 2.6 | 0.92 | 13 | 10.3 | - |
| VSFP-Butterfly 1.2 [16] | Ci-VSP | mCitrine (529)/mKate2 (633 nm) | ~6 | 0.3 | 2 | 10 | ~80 | - |
| ArcLight Q239 [18] | Ci-VSP | s.e. pHluorin A227D* (509 nm) | ~−39 | −3.2 | 9 | 48 | 17 | 60 |
| Bongwoori [21] | Ci-VSP/Kv chimera | s.e. pHluorin A227D* (509 nm) | ~−16 | −1.5 | 8 | NA | 7 | - |
| ASAP2f [20] | chicken VSP | cpGFP (509 nm) | ~−25 | ~−8 | 2.8 | 135 | 2.4 | 155 |
| QuasAr1 [32] | Archaerhodopsin 3 | retinal cofactor (715 nm) | 33 | 21 | 0.05 | 3.2 | 0.07 | 1.9 |
| QuasAr2 [32] | Archaerhodopsin 3 | retinal cofactor (715 nm) | 90 | 48 | 1.2 | 11.8 | 1 | 15.9 |
| Ace2N-mNeon [36] | Acetabularia rhodopsin | mNeongreen (517 nm) | −18 | −12 | 0.36 | 4.2 | 0.42 | 5.2 |
Super ecliptic pHluorin A227D
The ΔF/F amplitude associated with an action potential (AP) values reported here are for cultured neurons. This value varies depending upon the efficiency of membrane trafficking and the method of fluorescence extraction (peripheral membrane only vs. whole cell) and the method of background subtraction.
The numbers given should be considered approximate.
Highlights.
Recent advances in genetically encoded voltage indicators (GEVIs) have brought neural voltage imaging in vivo within reach
Recently introduced tools such as electrically spiking HEK cells facilitate rapid testing of new GEVIs
Light-gated voltage integrators and reporters of absolute voltage reporters implement new forms of molecular logic
Many potentially useful GEVI scaffolds and designs remain to be tested
Improvements in microscopy and software will be needed to attain full benefit from the newest GEVIs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AE, Venkatachalam V. Bringing bioelectricity to light. Annu Rev Biophys. 2014;43:211–232. doi: 10.1146/annurev-biophys-051013-022717. [DOI] [PubMed] [Google Scholar]
- **3.Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333:345–348. doi: 10.1126/science.1204763. The discovery of microbial rhodopsins as a novel GEVI scaffold. This probe revealed surprising spontaneous voltage fluctuations in bacteria. [DOI] [PubMed] [Google Scholar]
- 4.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slayman CL, Slayman CW. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974;71:1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–426. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- 7.Siegel MS, Isacoff EY. A genetically encoded optical probe of membrane voltage. Neuron. 1997;19:735–741. doi: 10.1016/s0896-6273(00)80955-1. [DOI] [PubMed] [Google Scholar]
- 8.Ataka K, Pieribone VA. A genetically targetable fluorescent probe of channel gating with rapid kinetics. Biophys J. 2002;82:509–516. doi: 10.1016/S0006-3495(02)75415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai R, Repunte-Canonigo V, Raj CD, Knopfel T. Design and characterization of a DNA-encoded, voltage-sensitive fluorescent protein. Eur J Neurosci. 2001;13:2314–2318. doi: 10.1046/j.0953-816x.2001.01617.x. [DOI] [PubMed] [Google Scholar]
- 10.Baker BJ, Lee H, Pieribone VA, Cohen LB, Isacoff EY, Knopfel T, Kosmidis EK. Three fluorescent protein voltage sensors exhibit low plasma membrane expression in mammalian cells. J Neurosci Methods. 2007;161:32–38. doi: 10.1016/j.jneumeth.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 12.Dimitrov D, He Y, Mutoh H, Baker BJ, Cohen L, Akemann W, Knopfel T. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS One. 2007;2:e440. doi: 10.1371/journal.pone.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutoh H, Perron A, Dimitrov D, Iwamoto Y, Akemann W, Chudakov DM, Knopfel T. Spectrally-resolved response properties of the three most advanced FRET based fluorescent protein voltage probes. PLoS One. 2009;4:e4555. doi: 10.1371/journal.pone.0004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui H, Jinno Y, Tomita A, Niino Y, Yamada Y, Mikoshiba K, Miyawaki A, Okamura Y. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J Physiol. 2013;591:4427–4437. doi: 10.1113/jphysiol.2013.257048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knopfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol. 2012;108:2323–2337. doi: 10.1152/jn.00452.2012. [DOI] [PubMed] [Google Scholar]
- 17.Lam AJ, St-Pierre F, Gong Y, Marshall JD, Cranfill PJ, Baird MA, McKeown MR, Wiedenmann J, Davidson MW, Schnitzer MJ, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9:1005–1012. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.St-Pierre F, Marshall JD, Yang Y, Gong Y, Schnitzer MJ, Lin MZ. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci. 2014;17:884–889. doi: 10.1038/nn.3709. The first report of GEVI with a circularly permuted GFP inserted between the S3 and S4 helices of a voltage-sensing domain. Screening of VSDs from different species and linker optimization led to the discovery of ASAP1 with fast and sensitive voltage response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Yang HH, St-Pierre F, Sun X, Ding X, Lin MZ, Clandinin TR. Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo. Cell. 2016;166:245–257. doi: 10.1016/j.cell.2016.05.031. The development and application of ASAP2f enabled subcellular voltage imaging in Drosophila visual cortex. This study revealed that the ON and OFF selectivity to visual stimuli arose in the intracellular transformation of voltage to Ca2+ responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piao HH, Rajakumar D, Kang BE, Kim EH, Baker BJ. Combinatorial mutagenesis of the voltage-sensing domain enables the optical resolution of action potentials firing at 60 Hz by a genetically encoded fluorescent sensor of membrane potential. J Neurosci. 2015;35:372–385. doi: 10.1523/JNEUROSCI.3008-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelfattah AS, Farhi SL, Zhao Y, Brinks D, Zou P, Ruangkittisakul A, Platisa J, Pieribone VA, Ballanyi K, Cohen AE, et al. A Bright and Fast Red Fluorescent Protein Voltage Indicator That Reports Neuronal Activity in Organotypic Brain Slices. J Neurosci. 2016;36:2458–2472. doi: 10.1523/JNEUROSCI.3484-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundby A, Mutoh H, Dimitrov D, Akemann W, Knopfel T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One. 2008;3:e2514. doi: 10.1371/journal.pone.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Han Z, Jin L, Platisa J, Cohen LB, Baker BJ, Pieribone VA. Fluorescent protein voltage probes derived from ArcLight that respond to membrane voltage changes with fast kinetics. PLoS One. 2013;8:e81295. doi: 10.1371/journal.pone.0081295. The discovery of chicken and zebrafish-based VSDs that offer faster response kinetics than Ciona intestinalis-based VSD. This forms the basis for subsequent protein engineering efforts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki S, Tsutsui H, Suzuki K, Agetsuma M, Arai Y, Jinno Y, Bai G, Daniels MJ, Okamura Y, Matsuda T, et al. Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci Rep. 2017;7:42398. doi: 10.1038/srep42398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautam SG, Perron A, Mutoh H, Knopfel T. Exploration of fluorescent protein voltage probes based on circularly permuted fluorescent proteins. Front Neuroeng. 2009;2:14. doi: 10.3389/neuro.16.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnett L, Platisa J, Popovic M, Pieribone VA, Hughes T. A fluorescent, genetically-encoded voltage probe capable of resolving action potentials. PLoS One. 2012;7:e43454. doi: 10.1371/journal.pone.0043454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 2011;9:90–95. doi: 10.1038/nmeth.1782. The first demonstration of voltage imaging with a microbial rhodopsin-derived GEVI in neurons. Arch reported action potential spikes with high fidelity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flytzanis NC, Bedbrook CN, Chiu H, Engqvist MK, Xiao C, Chan KY, Sternberg PW, Arnold FH, Gradinaru V. Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons. Nat Commun. 2014;5:4894. doi: 10.1038/ncomms5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIsaac RS, Engqvist MK, Wannier T, Rosenthal AZ, Herwig L, Flytzanis NC, Imasheva ES, Lanyi JK, Balashov SP, Gradinaru V, et al. Directed evolution of a far-red fluorescent rhodopsin. Proc Natl Acad Sci U S A. 2014;111:13034–13039. doi: 10.1073/pnas.1413987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. Hierarchical screening in bacteria and mammalian cells led to the discovery of archaerhodopsin-based GEVIs (QuasAr1 and QuasAr2) with improved brightness, dynamic range and kinetics compared with the parent GEVI, Arch. These NIR GEVIs were paired with blue-shifted optogenetic actuator, CheRiff, to enabel all-optical interrogation of electrophysiology in cultured neurons and in brain slices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herwig L, Rice AJ, Bedbrook CN, Zhang RK, Lignell A, Cahn JK, Renata H, Dodani SC, Cho I, Cai L, et al. Directed Evolution of a Bright Near-Infrared Fluorescent Rhodopsin Using a Synthetic Chromophore. Cell Chem Biol. 2017;24:415–425. doi: 10.1016/j.chembiol.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou P, Zhao Y, Douglass AD, Hochbaum DR, Brinks D, Werley CA, Harrison DJ, Campbell RE, Cohen AE. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat Commun. 2014;5:4625. doi: 10.1038/ncomms5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Y, Wagner MJ, Zhong Li J, Schnitzer MJ. Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors. Nat Commun. 2014;5:3674. doi: 10.1038/ncomms4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Gong Y, Huang C, Li JZ, Grewe BF, Zhang Y, Eismann S, Schnitzer MJ. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 2015;350:1361–1366. doi: 10.1126/science.aab0810. This study employed electrochromic FRET (also known as FRET-opsin configuration) to design a new GEVI that combined the fast voltage response of a rhodopsin mutant (Ace) with the brightness of a recently engineered FP (mNeonGreen). Ace-mNeon has sub-millisecond response time constant and faithfully reports neuronal action potentials in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emiliani V, Cohen AE, Deisseroth K, Hausser M. All-Optical Interrogation of Neural Circuits. J Neurosci. 2015;35:13917–13926. doi: 10.1523/JNEUROSCI.2916-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Packer AM, Russell LE, Dalgleish HW, Hausser M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat Methods. 2015;12:140–146. doi: 10.1038/nmeth.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rickgauer JP, Deisseroth K, Tank DW. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat Neurosci. 2014;17:1816–1824. doi: 10.1038/nn.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrillo-Reid L, Yang W, Bando Y, Peterka DS, Yuste R. Imprinting and recalling cortical ensembles. Science. 2016;353:691–694. doi: 10.1126/science.aaf7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erbguth K, Prigge M, Schneider F, Hegemann P, Gottschalk A. Bimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegans. PLoS One. 2012;7:e46827. doi: 10.1371/journal.pone.0046827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatachalam V, Cohen AE. Imaging GFP-based reporters in neurons with multiwavelength optogenetic control. Biophys J. 2014;107:1554–1563. doi: 10.1016/j.bpj.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Lou S, Adam Y, Weinstein EN, Williams E, Williams K, Parot V, Kavokine N, Liberles S, Madisen L, Zeng H, et al. Genetically Targeted All-Optical Electrophysiology with a Transgenic Cre-Dependent Optopatch Mouse. J Neurosci. 2016;36:11059–11073. doi: 10.1523/JNEUROSCI.1582-16.2016. The first demonstration of in vivo voltage imaging with QuasAr2 in a Cre-dependent transgenic Optopatch2 mouse line (Floxopatch) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dempsey GT, Chaudhary KW, Atwater N, Nguyen C, Brown BS, McNeish JD, Cohen AE, Kralj JM. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J Pharmacol Toxicol Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- *48.Zhang H, Reichert E, Cohen AE. Optical electrophysiology for probing function and pharmacology of voltage-gated ion channels. Elife. 2016;5 doi: 10.7554/eLife.15202. Application of Optopatch to study the activity-dependent modulation of Nav1.7 ion channels. All-optical electrophysiology allows high-throughput screening of over 300 compounds for their binding kinetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou JH, Kralj JM, Douglass AD, Engert F, Cohen AE. Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front Physiol. 2014;5:344. doi: 10.3389/fphys.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldchen S, Lehmann J, Klein T, van de Linde S, Sauer M. Light-induced cell damage in live-cell super-resolution microscopy. Sci Rep. 2015;5:15348. doi: 10.1038/srep15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:R37–61. doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 52.Mittmann W, Wallace DJ, Czubayko U, Herb JT, Schaefer AT, Looger LL, Denk W, Kerr JN. Two-photon calcium imaging of evoked activity from L5 somatosensory neurons in vivo. Nat Neurosci. 2011;14:1089–1093. doi: 10.1038/nn.2879. [DOI] [PubMed] [Google Scholar]
- *53.Scott G, Fagerholm ED, Mutoh H, Leech R, Sharp DJ, Shew WL, Knopfel T. Voltage imaging of waking mouse cortex reveals emergence of critical neuronal dynamics. J Neurosci. 2014;34:16611–16620. doi: 10.1523/JNEUROSCI.3474-14.2014. This study applied GEVI imaging of cortex-wide electrical activity in awake mice. It revealed changes in large-scale dynamics associated with waking from anesthesia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Antic SD, Empson RM, Knopfel T. Voltage imaging to understand connections and functions of neuronal circuits. J Neurophysiol. 2016;116:135–152. doi: 10.1152/jn.00226.2016. A comprehensive review of the history, design principles, and performance of voltage indicators. It discusses applications of GEVIs at cellular, circuit and system-levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camire O, Topolnik L. Dendritic calcium nonlinearities switch the direction of synaptic plasticity in fast-spiking interneurons. J Neurosci. 2014;34:3864–3877. doi: 10.1523/JNEUROSCI.2253-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J, Werley CA, Venkatachalam V, Kralj JM, Dib-Hajj SD, Waxman SG, Cohen AE. Screening fluorescent voltage indicators with spontaneously spiking HEK cells. PLoS One. 2013;8:e85221. doi: 10.1371/journal.pone.0085221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNamara HM, Zhang H, Werley CA, Cohen AE. Optically Controlled Oscillators in an Engineered Bioelectric Tissue. In. Phys Rev X. 2016;6:031001. [Google Scholar]
- 58.Pucihar G, Kotnik T, Miklavcic D. Measuring the induced membrane voltage with Di-8-ANEPPS. J Vis Exp. 2009 doi: 10.3791/1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *59.Maclaurin D, Venkatachalam V, Lee H, Cohen AE. Mechanism of voltage-sensitive fluorescence in a microbial rhodopsin. Proc Natl Acad Sci U S A. 2013;110:5939–5944. doi: 10.1073/pnas.1215595110. This paper studies Arch voltage-sensing photocycle with fluorescence spectroscopy. It discoveres that the fluorescence of Arch arises through a sequential three-photon process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brinks D, Klein AJ, Cohen AE. Two-Photon Lifetime Imaging of Voltage Indicating Proteins as a Probe of Absolute Membrane Voltage. Biophys J. 2015;109:914–921. doi: 10.1016/j.bpj.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou JH, Venkatachalam V, Cohen AE. Temporal dynamics of microbial rhodopsin fluorescence reports absolute membrane voltage. Biophys J. 2014;106:639–648. doi: 10.1016/j.bpj.2013.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinks D, Adam Y, Kheifets S, Cohen AE. Painting with Rainbows: Patterning Light in Space, Time, and Wavelength for Multiphoton Optogenetic Sensing and Control. Acc Chem Res. 2016;49:2518–2526. doi: 10.1021/acs.accounts.6b00415. [DOI] [PubMed] [Google Scholar]
- 63.Venkatachalam V, Brinks D, Maclaurin D, Hochbaum D, Kralj J, Cohen AE. Flash memory: photochemical imprinting of neuronal action potentials onto a microbial rhodopsin. J Am Chem Soc. 2014;136:2529–2537. doi: 10.1021/ja411338t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinhassi J, DeLong EF, Beja O, Gonzalez JM, Pedros-Alio C. Marine Bacterial and Archaeal Ion-Pumping Rhodopsins: Genetic Diversity, Physiology, and Ecology. Microbiol Mol Biol Rev. 2016;80:929–954. doi: 10.1128/MMBR.00003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubota Y, Karube F, Nomura M, Gulledge AT, Mochizuki A, Schertel A, Kawaguchi Y. Conserved properties of dendritic trees in four cortical interneuron subtypes. Sci Rep. 2011;1:89. doi: 10.1038/srep00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birinyi A, Antal M, Wolf E, Szekely G. The Extent of the Dendritic Tree and the Number of Synapses in the Frog Motoneuron. Eur J Neurosci. 1992;4:1003–1012. doi: 10.1111/j.1460-9568.1992.tb00127.x. [DOI] [PubMed] [Google Scholar]