Abstract
More Americans are consuming diets higher in saturated fats and refined sugars than ever before, and based on increasing obesity rates, this is a growing trend among older adults as well. While high saturated fat diet (HFD) consumption has been shown to sensitize the inflammatory response to a subsequent immune challenge in young adult rats, the inflammatory effect of HFD in the already-vulnerable aging brain has not yet been assessed. Here, we explored whether short-term (3 days) consumption of HFD would serve as a neuroinflammatory trigger in aging animals, leading to cognitive deficits. HFD impaired long-term contextual (hippocampal-dependent) and auditory-cued fear (amygdalar-dependent) memory in aged, but not young adult rats. Short-term memory performance for both tasks was intact, suggesting that HFD impairs memory consolidation processes. Microglial markers of activation Iba1 and cd11b were only increased in the aged rats, while MHCII was further amplified by HFD. Furthermore, these HFD-induced long-term memory impairments were accompanied by IL-1β protein increases in both hippocampus and amygdala in aged rats. Central administration of IL-1RA in aged rats following conditioning mitigated both contextual and auditory-cued fear memory impairments caused by HFD, strongly suggesting that IL-1β plays a critical role in these effects. Voluntary wheel running, known to have anti-inflammatory effects in the hippocampus, rescued hippocampal-dependent but not amygdalar-dependent memory impairments caused by HFD. Together, these data suggest that short-term consumption of HFD can lead to memory deficits and significant brain inflammation in the aged animal, and strongly suggest that appropriate diet is crucial for cognitive health.
Introduction
In the last 100 years, diet in developed countries has seen astounding increases in the amounts of fats and sugar consumed (Guyenet and Carlson, 2015, Putnam and Allshouse, 1999). Not surprisingly, these increases are correlated with rising obesity rates. Approximately 40% of the adult U.S. population is currently obese, a dramatic increase from the 13% incidence of 1960 (Fryar, et al., 2016, Ogden, et al., 2015). Importantly, the prevalence among older individuals (aged 60–74) has nearly doubled since just 1980 (Fakhouri, et al., 2012), and with the population of Americans over the age of 65 expected to reach 25% by the year 2030 (Alzheimer’sAssociation, 2016, Wimo, et al., 2013), any health risks associated with aging and unhealthy diets are likely to become even greater in the future.
Aging is a major risk factor for inflammation-induced mild cognitive impairments, as demonstrated in both the clinical and preclinical literature (Alzheimer’sAssociation, 2016, Barrientos, et al., 2015, Corona, et al., 2012, Holmes, et al., 2003, Moller, et al., 1998, Murray, et al., 2010). Importantly, mild cognitive impairments among older individuals increase the probability for developing Alzheimer’s disease later in life (Alzheimer’sAssociation, 2016, Miller and Spencer, 2014). As many have shown, microglia, the brain’s resident immune cells, become sensitized with age, lowering their threshold for activation (Frank, et al., 2006, Frank, et al., 2010b, Perry, et al., 1993, Rogers, et al., 1988, Rozovsky, et al., 1998). When either a peripheral or central inflammatory challenge is experienced, these sensitized microglia become hyper-activated, and produce pathological levels of pro-inflammatory cytokines, thereby interfering with synaptic plasticity processes, potentially resulting in precipitous memory declines (Chapman, et al., 2012, Chapman, et al., 2010, Combrinck, et al., 2002, Cunningham, et al., 2009). Prior research has demonstrated that this sequence occurs in aged rodents in response to bacterial and viral infections, and surgical insults (Abraham, et al., 2008, Barrientos, et al., 2012, Barrientos, et al., 2006, Chen, et al., 2008, Rosczyk, et al., 2008). We hypothesize that acute high fat diet (HFD), such as occurs with binge-eating and fast-food meals, may be an important pathological trigger, similar to infection or surgery, to induce overt brain inflammation and memory loss in the already-sensitized aging brain.
While HFD in young adults produces low-grade inflammation in the circulation and peripheral tissues (Cano, et al., 2009, Coppack, 2001, Xu, et al., 2002), its ability to directly induce inflammation within the brain is limited to the hypothalamus (De Luca, et al., 2016, Maric, et al., 2014, Milanski, et al., 2009), except after extremely long-term HFD during which diabetes-like symptoms are starting to occur (Jeon, et al., 2012). In the hippocampus, HFD in the young adult is not directly inflammatory and only sensitizes cells to over-respond to future inflammatory stimuli (Sobesky, et al., 2014, Sobesky, et al., 2016). In the absence of such stimuli no inflammatory response is detected, nor memory impairments observed. Interestingly, even as little as 3 days HFD is sufficient to sensitize the hippocampus in this way (Sobesky, et al., 2014, Sobesky, et al., 2016). Despite these pronounced effects of short-term HFD on extra-hypothalamic regions of the brain in the young adult, the inflammatory effect of HFD in the already-vulnerable aging brain has not yet been assessed. Here, we hypothesized that consumption of a high saturated fat diet would serve as a neuroinflammatory trigger in aging animals, leading to cognitive deficits, without having an overt pro-inflammatory or cognitive effect in young adults.
Materials and Methods
Subjects
Subjects were male F344xBN F1 rats obtained from the National Institute on Aging Rodent Colony maintained by Envigo (Indianapolis, IN). Upon arrival at our facility, aged rats were 24 mos. old and weighed approximately 500 g. Young adult rats were 3 mos. old and weighed approximately 300 g. Following arrival, animals were allowed to acclimate to the facility for at least 7 days prior to diet modifications. With the exception of the exercise experiments, where they were individually housed, rats were pair-housed in standard cages (46cm × 26cm × 21cm; L × W × H) with food and water available ad libitum. The colony room was maintained at 22° C on a 12-h light/dark cycle (lights on at 07:00h). All experiments were performed during the light phase. All experiments were conducted in accordance with protocols approved by the University of Colorado Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Diet
Animals were assigned to either continue consuming their regular chow (Teklad Diets, TD. 8640, energy density of 3.0 kcal/g; 29% calories from protein, 54% from carbohydrates (no sweetener added), and 17% from fat [0.9% saturated, 1.2% monounsaturated, 2.7% polyunsaturated]), or the HFD, which is an adjusted calorie HFD (TD.06414, Envigo, energy density of 5.1 kcal/g; 18.4% calories from protein, 21.3% from carbohydrates (90g/kg sucrose, 160g/kg maltodextrin), and 60.3% from fat [37% saturated, 47% monounsaturated, 16% polyunsaturated]). Body mass, and food and water consumption was weighed (in g) daily at the same time of day (9:00 – 9:30 am). Energy intake was calculated by multiplying the kcal/g by the total grams consumed per cage, divided by the starting average body mass per cage.
Contextual Fear Conditioning
Three days after consuming their respective diets, rats were taken two at a time from their home cage and each was placed in a conditioning chamber (26 L × 21 W × 24 H, cm) made of clear plastic and topped with a wire mesh top. Each chamber was housed inside an ice chest (54 L × 30 W × 27 H, cm). A speaker, a fan, and two 24 V DC lightbulbs (one white, one red) were mounted on the ceiling of each ice chest. Rats were allowed to explore the chamber for 2 min before the onset of a 15 s tone (76 dB), followed immediately by a 2 s footshock (1.5 mA) delivered through a removable floor of stainless steel rods. Each rod was wired to a shock generator and scrambler (Coulbourn Instruments, Allentown, PA). To assess obvious signs of lethargy or sickness, locomotion was scored during conditioning. Immediately after the termination of the shock, rats were removed from the chamber and returned to their home cage. At this point, HFD-fed rats were switched back to chow. Four days later, all rats were tested for fear of the conditioning context, a hippocampal-dependent task (Matus-Amat, et al., 2004, Rudy, et al., 2002), and then for fear of the tone, an amygdala-dependent task (Kim and Fanselow, 1992, Phillips and LeDoux, 1992). Chambers were cleaned with water and a mild detergent before each animal was conditioned or tested. For the context fear test, rats were placed in the exact context in which they were conditioned and were observed for 6 min and scored for freezing behavior. For the auditory-cued fear test, rats were placed in an altered context (e.g., different shaped and sized chamber, red light, no grid floor) and scored for freezing behavior for 3 min. Following the 3 min, the tone was activated and freezing behavior was scored for an additional 3 min. All scoring was done in real time by researchers who were blind to the experimental conditions. Freezing is the rat’s dominant defensive fear response and it is a common measure of conditioned fear (Kim and Fanselow, 1992). Freezing was defined as the absence of all visible movement, except for respiration. Using a time-sampling procedure, every 10 s each rat was judged as either freezing or active at the instant the sample was taken. Inter-rater reliability exceeded 97% for all experiments. For the long-term memory experiments, memory was assessed 4 days post-conditioning; for short-term memory experiments, memory was assessed 1–2 hr post-conditioning. Separate cohorts of rats were used for the long-term and the short-term memory tests. An additional experiment was conducted whereby rats were maintained on the HFD throughout the acquisition and retention phases of the experiment (8 days), to rule out the possibility that any impairments observed with the 3-day protocol was due to stress related to switching diets mid-way through the behavioral paradigm. Conditioning coincided with day 3 on the respective diets, as with the other studies presented here. Memory testing occurred on day 7.
Morris Water Maze
Spatial learning and memory was assessed using the Morris water maze. Rats received acquisition training on day 4 of the diet regimen and were tested for long-term spatial memory 4 days later. Diet conditions were maintained through day 8. The water maze consisted of a circular galvanized steel pool approximately 110 cm in diameter and 58 cm deep. A movable escape platform constructed of a Plexiglass base column having a height of 43 cm and topped by a round platform 15 cm in diameter, was placed in one quadrant of the pool and was maintained there throughout acquisition of the task. The water was filled to a height of 47 cm and maintained at 26° ± 1° C, and rendered opaque with non-toxic, water-soluble white paint. Water level was maintained at 2 cm above the platform’s surface. There were many prominent visual cues that remained constantly positioned around the testing room throughout the study, to serve as distal spatial cues to the location of the platform. No local cues were present within the pool. Latencies to reach the platform during acquisition trials were measured with a stopwatch. Probe trials were recorded with a video camera that was mounted on the ceiling.
All animals received four trials per session and four sessions throughout a single day, for a total of 16 acquisition trials, with a 2.5 hr inter-session interval, as we have shown that this protocol produces stable and robust spatial memory in young and aged rats (Barrientos, et al., 2016). Each training trial started by placing the rat into the water at any of four randomly chosen quadrants with its snout facing the wall of the pool. The rat was allowed to search for the escape platform for up to 60s and when located allowed to remain on the platform for 15s. If the rat failed to find the platform within 60s it was gently guided to the platform. The rat was returned to a holding cage until the next trial. Rats were run in squads of 12 at a time. The inter-trial interval was ~3–8 min. At the end of each session, rats were dried with a towel, placed back into their home cage and returned to their colony until the beginning of the next session. A retention probe trial was given 4 days after the last acquisition trial, during which the platform was removed from the pool and the rat was allowed to swim freely for 30s. Spatial memory was assessed by examining dwell times in each of the pool’s quadrants, latency to swim to the platform’s original location and total number of platform crosses. Swim speed was also calculated.
Tissue Collection
In all experiments, separate cohorts of rats were used for behavior testing and physiological measurements. Rats were injected (i.p.) with a lethal dose of sodium pentobarbital until unresponsive to toe-pinch. Blood was collected through a cardiac puncture. Then rats were transcardially perfused with ice cold 0.9% saline for 3 min. Following saline perfusion, brains were extracted from the skull and placed on a clean glass dish on ice wherein hippocampus and amygdala were dissected according to landmarks identified by Paxinos and Watson (Paxinos and Watson, 1986). Dissected tissues from each hemisphere of both brain regions were placed into separate pre-labeled Eppendorf tubes and flash frozen in liquid nitrogen. All samples were stored at −80° C until further processed.
Glucose and Insulin Measurement
Morning blood glucose (after 2 hour fasting) was measured in whole blood using a commercial (Bayer Contour) glucometer. Plasma insulin was measured using a commercial ELISA kit for rat insulin from Abnova (Taiwan) with a detection range of 0μLU/mL – 140 μLU/mL, and a sensitivity of < 5 μLU/mL.
Rt-PCR
RNA Isolation from whole tissue samples
RNA was isolated from whole tissue utilizing a standard method of phenol:chloroform extraction (Chomczynski and Sacchi, 1987). Briefly, tissue samples were rapidly homogenized in 1 mL Trizol reagent (Invitrogen, Carlsbad, CA). Samples were homogenized using a Tissue Tearor homogenizer. After incubation at room temperature for 5 min, chloroform was added to supernatant, the sample vortexed for 2 min and centrifuged (4°C, 12,000 g, 15 min) to achieve phase separation of nucleic acid. Isopropyl alcohol (0.5 volume of Trizol volume) was added to precipitate nucleic acid. Samples were briefly vortexed and incubated at room temperature for 10 min followed by centrifugation (4°C, 12,000 g) for 10 min. Nucleic acid precipitate was washed in 75% ethanol (1 mL) and centrifuged (4°C, 7500 g, 5 min). The ethanol was gently poured out, the RNA pellet allowed to dry, followed by resuspension with 40 μL of nuclease-free water (Ambian).
cDNA Synthesis of Whole-Tissue Derived RNA
Total RNA was reverse transcribed into cDNA using the SuperScript II First Strand Synthesis System for Rt-PCR (Invitrogen). A standard amount of sample was added to nucleic acid-free water to equate to 11 μL. This RNA was incubated for 5 min at 65°C in a total reaction volume of 13 μL containing random hexamer primers (5 ng/μL) and dNTPs (1 mM). Samples were chilled on ice for at least 1 min. A cDNA synthesis buffer (6 μL) was added to the reaction and the sample incubated at 20°C for 2 min. Reverse transcriptase (1 μL; 200 units SuperScript II) was added to the reaction and incubated at 25°C for 10 min followed by 42°C for 50 min. Reaction was terminated by heating to 70°C for 15 min.
Primer Specifications
cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using the Eurofins MWG Operon Oligo Analysis & Plotting Tool (http://www.operon.com/technical/toolkit.aspx) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul, et al., 1997). Primers were obtained from Invitrogen and primer specificity was verified by melt curve analysis. Gene function and primer sequences of the genes of interest are presented in Table 1.
Table 1.
PCR Primer Description and Sequences
| Gene | Primer Sequence: 5′ -> 3′ | Function |
|---|---|---|
| β-Actin | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (housekeeping gene) |
| CD11b | F: CTGGGAGATGTGAATGGAG R: ACTGATGCTGGCTACTGATG |
Macrophage/Microglial antigen marker |
| Iba-1 | F: GGCAATGGAGATATCGATAT R: AGAATCATTCTCAAGATGGC |
Macrophage/Microglial antigen marker |
| MHCII | F: AGAFACCATCTGGAGACTTG R: CATCTGGGGTGTTGTTGGA |
Macrophage/Microglial antigen marker |
| NLRP3 | F: AGAAGCTGGGGTTGGTGAATT R: GTTGTCTAACTCCAGCATCTG |
Rate limiting protein in NLRP3 inflammasome formation |
| HMGB1 | F: GAGGTGGAAGACCATGTCTG R: AAGAAGAAGGCCGAAGGAGG |
Endogenous danger signal |
Abbreviations: CD11b: cluster of differentiation 11b; Iba-1: ionized calcium-binding adaptor molecule-1; MHCII: Major Histocompatibility Complex II; NLRP3: nod-like receptor protein 3; HMGB1: high mobility group box 1
Quantitative Real Time Rt-PCR
Rt-PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Quiagen, Valencia, CA). cDNA (1 μL) was added to a reaction master mix (25 μL) containing 2.5 mM MgCl2, HotStar Taq DNA polymerase, SYBR Green I, dNTPs, fluorescein (10 nM) and gene-specific primers (500 nM of each of forward and reverse primer). For each experimental sample, triplicate reactions were conducted in 96-well plates (BioRad, Hercules, CA). PCR cycling conditions consisted of a hot-start activation of HotStart Taq DNA polymerase (94°C, 15 min) and 40 cycles of denaturation (95°C, 15 s), annealing (55–58°C, 30 s), and extension (72°C, 30 s). A melt curve analysis was conducted to assess uniformity of product formation, primer dimer formation, and amplification of non-specific products. PCR product was denatured (95°C, 1 min) and annealed (55°C, 1 min) prior to melt curve analysis, which consisted of incrementally increasing the reaction temperature (0.5°C/10 s) from 55 to 95°C.
Real-Time Detection and Relative Quantification of PCR Product
Formation of the PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad). Fluorescence of SYBR Green I was captured at 72°C. The threshold for detection of PCR product above background was set at 10x the standard deviation of mean background fluorescence for all reactions. Background fluorescence was determined from 1 to 5 cycles prior to exponential amplification of product and subtracted from raw fluorescence of each reaction/cycle. The threshold for detection of PCR product fell within the exponential phase of amplification for each reaction. Threshold cycle (CT; number of cycles to reach threshold of detection) was determined for each reaction.
Relative Quantitation of Gene Expression
Relative gene expression was determined using the 2−ΔΔCT method (Livak and Schmittgen, 2001). Mean CT of triplicate measures was computed for each sample. Sample mean CT of the internal control (β actin) was subtracted from the sample mean CT of the respective gene of interest (ΔCT). For each gene of interest, the sample with the absolute highest mean ΔCT was selected as a calibrator and the mean ΔCT of each experimental sample (ΔΔCT) was subtracted from this value. 2ΔΔCT yielded fold change in gene expression of the gene of interest normalized to the internal control gene expression and relative to the calibrator sample. Relative gene expression for each sample was calculated.
Tissue Processing for ELISA
In preparation for assays, tissue samples were sonicated in 0.3 mL sonication buffer (Invitrogen). Tissues were then mechanically homogenized using an ultrasonic cell disrupter (Thermo Fisher Scientific). Sonication consisted of 20 s of cell disruption at 52% amplitude. Sonicated samples were centrifuged (4°C, 10,000 g, 10 min) and supernatants were removed and stored at 4°C until ELISA assays were performed. Bradford protein assays determined total protein concentrations of sonicated tissue.
ELISA
Levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)α protein (R&D Systems; Cat#: RLB00, RTA00, R6000B, respectively) were determined using commercially available rat-specific enzyme linked immunosorbant assays (ELISA). The assays were performed according to the manufacturer instructions. Detection limits for each assay is as follows: IL-1β: <5 pg/mL, IL-6: 21 pg/mL, and TNF: <5 pg/mL. All values were determined and normalized to total protein.
Intra-cisterna magna (icm) administration of hIL-1RA
A subset of the aged rats received a non-invasive central injection of human IL-1 receptor antagonist, (hIL-1RA; 112 μg/3μL/rat; Kineret) or an equal volume of the vehicle, sterile saline, as previously described (Barrientos, et al., 2012). Immediately following conditioning, rats were anesthetized with halothane, the dorsal aspect of the skull was shaved and swabbed with 70% EtOH. A 27-gauge needle attached via PE50 tubing to a 25 μL Hamilton syringe was inserted by hand into the cisterna magna. To verify entry into the cisterna magna for each rat, ~2 μL of clear cerebral spinal fluid was drawn and gently pushed back in and 3 μL total volume of hIL-1RA or vehicle was administered. The whole procedure lasted less than 5 min.
Voluntary wheel running
Aged rats were single-housed with in-cage running wheels, whereas sedentary control rats were single-housed with a locked wheel. Voluntary, unrestricted access to running or locked wheels was maintained for a total of 6 weeks prior to experimentation and through until the end of the study. Wheel running was recorded digitally using Vital View software (Mini Mitter), and distance traveled was calculated by multiplying the number of wheel revolutions by the wheel circumference (1.08 m). After 6 weeks under these conditions, HFD was introduced to all animals for 3 days. On the third day of HFD, one cohort of rats was conditioned as described above, with memory tests occurring 4 days later. A separate cohort of rats was euthanized 2 hr after conditioning to harvest hippocampus and amygdala to measure IL-1β protein levels at the time of memory consolidation, as described above.
Data Analysis
All data are presented as means ± SEM. Statistical analyses were computed using Graphpad Prism version 7. All experiments had 6–10 rats per group; exact numbers for each experiment are described in the results section with each experiment. Unequal sample sizes both within and across experiments are typically related to attrition of aged rats due to naturally occurring ailments, such as tumors. In addition, due to animal ordering limitations by the National Institute on Aging, the desired number of animals are not always available. A three-way repeated measures ANOVA was conducted to analyze % body weight change between the two age groups and the two diets over the three days. two-way ANOVAs were run for the experiments that had a 2×2 factorial design. In the case of significant main effects and interactions, Tukey’s multiple comparisons post hoc tests were run. For experiments with only two groups, Student’s unpaired t-tests were conducted. Threshold for significance was set at α= 0.05.
Results
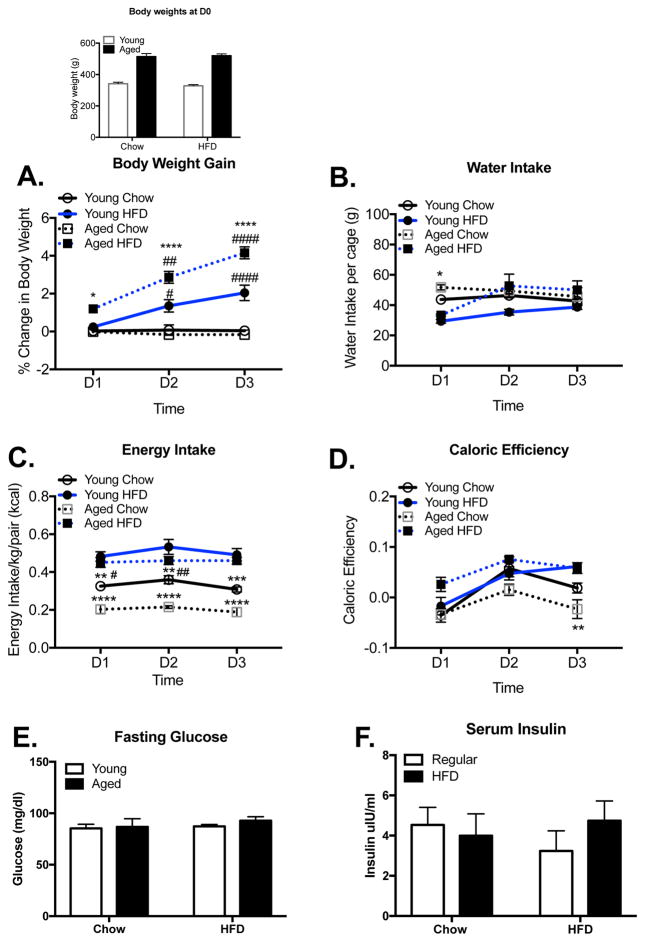
3 days HFD increases body mass, particularly in aged rats
Young and aged rats were weighed on the day of their introduction to the HFD (day 0) and every day for 3 days. As expected, body weight averages for aged rats at the start of the experiment were significantly greater than those of young rats (F(1,36)= 198.6, p < 0.0001 Fig. 1, inset; n=10 for each group). With regard to percent change in body weight over the 3 days, a significant interaction effect of age x diet (F(1, 2) = 32.98, p < 0.0001) and day x diet (F(2, 2) = 23.41, p < 0.0001) followed by post hoc tests revealed that young and aged rats that were maintained on regular chow did not differ from each other (p > 0.05) and did not exhibit significant changes from their D0 body weights across the three days (p > 0.999). In contrast, HFD-fed young and aged rats exhibited substantial weight increases across the three days that significantly differed from their chow-fed counterparts (young: D1 (p > 0.05); D2 (p < 0.05); D3(p < 0.0001); aged: D1 (p < 0.05); D2 (p < 0.0001); D3(p < 0.0001). In addition, HFD-fed aged rats exhibited even greater gains than their younger counterparts (D2 (p < 0.05); D3 (p < 0.0001); Fig. 1A).
Figure 1.
Metabolic measures. Body weights on day 0 of rats assigned to the two diet groups (Inset). Daily % change in body mass (A), water intake (B), energy intake (C), and caloric efficiency (D) over the 3 days during which young and aged rats had free access to the regular chow or HFD. Glucose (E) and insulin (F) levels after 3 days on their respective diets. Data are means ± SEM. * indicates significant difference between age-matched groups. # indicates significant difference between diet-matched groups. */# p<0.05; **/##p<0.01; ***/###p<0.001; ****/####p<0.0001.
3 days HFD on water, energy intake, and caloric efficiency
A three-way repeated measures ANOVA of water consumption data indicated a significant time x diet interaction (F(2,2) = 5.75, p = 0.009; Fig. 1B). Post hoc tests revealed a significant difference on day 1 between young HFD-fed rats and aged chow-fed rats (p = 0.008); and between aged HFD-fed and aged chow-fed rats (p = 0.049). On day 2, the difference between the aged groups was eliminated, as the aged HFD-fed rats drank significantly more than on day 1 (p = 0.03). No other differences were found between the four groups on days 2 and 3. Water intake among young and aged chow-fed rats did not differ on any day. Analysis of energy intake indicated a significant age x diet interaction (F(1,2) = 9.48, p = 0.005; Fig. 1C). Post-hoc tests indicated that young and aged HFD-fed rats did not differ in their energy intake across all days. However, both HFD-fed groups had greater intake than their age-matched chow-fed counterparts on all days (p < 0.01). Young chow-fed rats had greater intake than aged chow-fed rats on days 1 and 2 (p < 0.05). Analysis of caloric efficiency indicated a significant age x diet interaction (F(1,2) = 11.63, p = 0.002; Fig. 1D). There were no significant differences between the four groups on days 1 and 2, but on day 3 both young and aged HFD-fed rats had greater caloric efficiency than aged chow-fed rats (p < 0.01). Young chow-fed rats showed a rise in caloric efficiency from day 1 to day 2 (p < 0.01). Young HFD-fed rats showed a rise in caloric efficiency from day 1 to day 2 (p < 0.05) and day 3 (p < 0.01).
3 days HFD does not affect glucose or insulin
A two-way ANOVA (age x diet) indicated that glucose (F(1,20)= 0.17, p = 0.69) and insulin (F(1,20)= 1.06, p = 0.32; n=6 in each group) levels were not different between the four groups (Fig 1E & F).
Experiment 1: HFD impairs long-term, but not short-term contextual and auditory-cued fear memory, and partially impairs spatial memory in aged rats
Long-term contextual memory
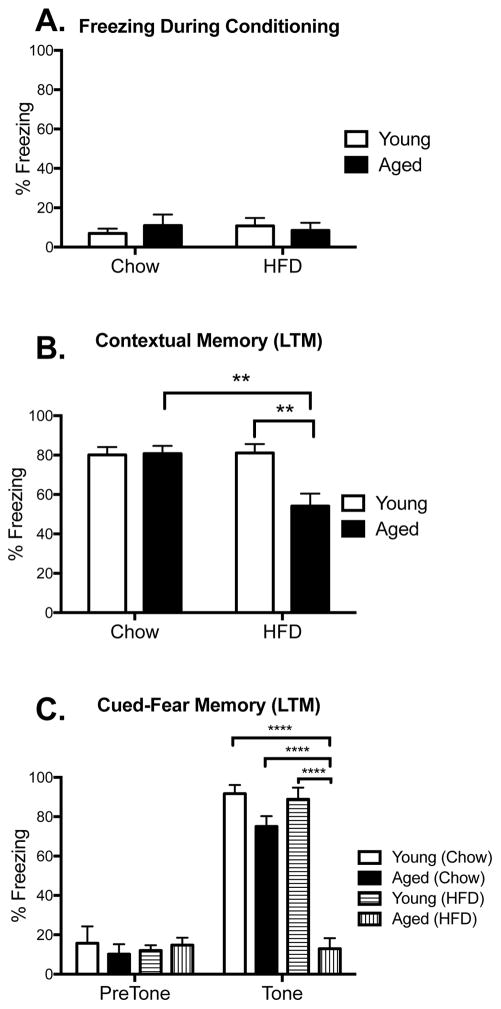
To investigate the influence of HFD and aging on hippocampus- and amygdala-dependent memory function, young and aged rats were given either chow or HFD for 3 days prior to conditioning. During conditioning, all groups explored the novel context with equal interest, freezing less than 15% of the time spent in the conditioning chamber, suggesting that none of the rats were lethargic, ill, or anxious/stressed during conditioning (p > 0.05; Fig. 2A). Long-term memory was tested 4 days after conditioning. For the contextual memory test, a two-way ANOVA revealed a significant (age x diet) interaction (F(1,33) = 7.58, p < 0.01; n=10 in each group, except aged chow n=7). Post-hoc tests confirmed that HFD-fed aged rats froze significantly less in the context compared to chow-fed aged rats (p < 0.01) and HFD-fed young rats (p < 0.01), indicating a substantial contextual memory deficit in these rats. All other groups performed well and did not differ from one another (Fig. 2B). For the amygdala-dependent auditory-cued test, separate two-way ANOVAs were conducted for the pre- and post-tone freezing data. Analyses on the pre-tone freezing data confirmed no significant differences between the two factors (age x diet; p > 0.05; n=6 in each group), indicating that the novel context alone did not induce any generalized fear or anxiety in any of the groups (Fig. 2C, pre-tone). Analyses of the tone-induced freezing data produced a significant age x diet interaction (F(1, 20) = 31.51, p < 0.0001). Post hoc tests revealed an astounding impairment in HFD-fed aged rats compared to every other group (p < 0.0001; Fig. 2C, tone), with a complete absence of tone-induced freezing. No other groups differed from each other.
Figure 2.
Long-term contextual memory. Percentage of time spent freezing during conditioning (A), or during test 4 days later in the conditioning context (B), or in novel context in the presence or absence of the tone (C) among young and aged rats that were fed chow or HFD. Data are means ± SEM. **p<0.01; ****p<0.0001.
Maintaining the rats on the HFD through the acquisition and retention phases of the behavioral experiment (a total of 8 days) did not affect the outcome. As with the 3 day protocol, analyses of the contextual memory test revealed a significant (age x diet) interaction (F(1,20) = 5.37, p < 0.05; n=6 in each group). Post-hoc tests confirmed that HFD-fed aged rats froze significantly less in the context compared to chow-fed aged rats (p < 0.01) and HFD-fed young rats (p < 0.05; data not shown). Analyses of the auditory-cued test also replicated the findings of the 3 day protocol. Pre-tone freezing was not significantly different between the two factors (age x diet; p > 0.05), but freezing to the tone was significantly different (F(1, 20) = 11.03, p < 0.01; n=6 in each group), with HFD-fed aged rats freezing significantly less than every other group (p < 0.01; data not shown).
Short-term contextual memory
To determine whether the phenomena reported above were specific to long-term memory, short-term contextual and auditory-cued memory were measured in a separate cohort of rats. Since young rats displayed no impairments on either test, only aged rats were included here (n=6 in each group). As before, both diet groups explored the conditioning context equally (p > 0.05; Fig. 3A). Unlike the long-term memory tests, short-term memory for the context and for the tone were not impaired; percentage freezing for the diet groups did not differ (p > 0.05; Fig. 3B and C).
Figure 3.
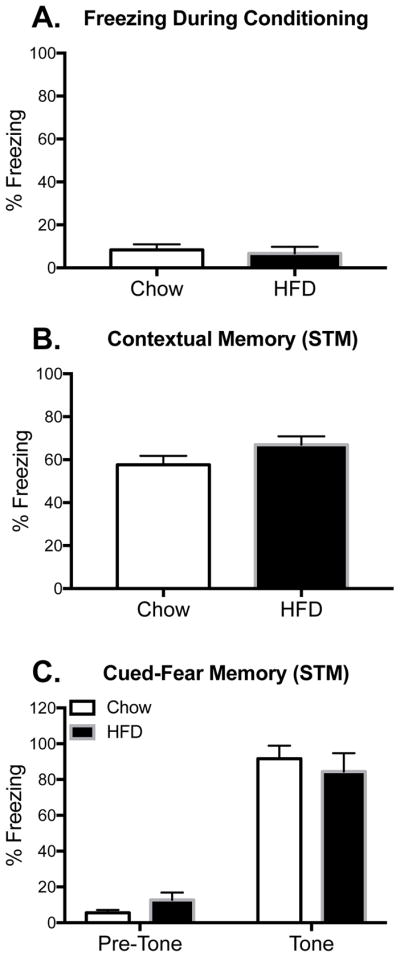
Short-term contextual memory. Percentage of time spent freezing during conditioning (A), or during test 2 hr later in the conditioning context (B), or in novel context in the presence or absence of the tone (C) among aged rats that were fed chow or HFD. Data are means ± SEM.
Long-term Spatial memory
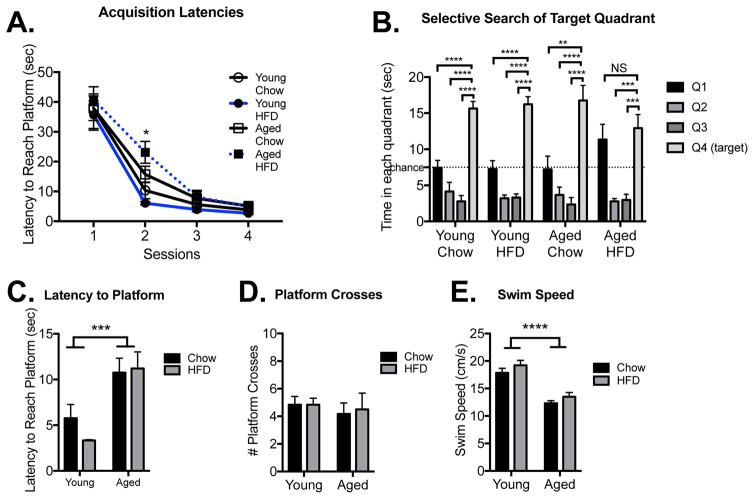
In the Morris water maze, acquisition latencies produced significant time and age main effects (Fig. 4A) with latencies to reach the platform being significantly reduced over the four sessions (F(3,80)=109.08, p < 0.0001; n=6 in each group) and aged rats being slower to reach the platform than young rats (F(1,80)=9.98, p < 0.01), consistent with their slower swim speed (F(1,20)=55.11, p < 0.0001; Fig 4E). Post hoc tests revealed that on session 2 HFD-fed aged rats were significantly slower than young HFD-fed rats (p < 0.05).
Figure 4.
Long-term spatial memory in the Morris water maze. Latency to reach the platform during acquisition training among young and aged rats that were fed chow or HFD (A). Dwell times in each quadrant during the retention probe trial (B). Latency to reach the platform location (C), number of platform crosses (D), and swim speed (E) during the retention probe trial. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Retention performance is presented in Fig. 4B, C, and D. One-way ANOVAs of dwell times in each of the pool’s quadrants were run for each condition. Each group demonstrated significant main effect differences across the quadrant dwell times (Young Chow: F(3,20)=31.09, p < 0.0001; Young HFD: F(3,20)=51.05, p < 0.0001; Aged Chow: F(3,20)=17.48, p < 0.0001; Aged HFD: F(3,20)=13.03, p < 0.0001). Post hoc tests revealed that all groups except the HFD-fed aged group spent significantly more time in the target quadrant than every other quadrant (p < 0.0001), indicating strong long-term spatial memory for the target quadrant. The HFD-fed aged rats exhibited greater dwell times in the target quadrant compared to two of the other quadrants, but were unable to discriminate between the target quadrant and one of the adjacent quadrants (p > 0.05; Fig. 4B). An analysis of dwell times in the target quadrant across the four groups indicated no significant differences (F(3,20)=1.17, p = 0.35). Aged rats exhibited longer latencies to first reach the platform location during the probe trial compared to young rats (F(1,20)=20.54, p < 0.001; Fig. 4C), but there was no effect of diet (p > 0.05). Finally, there were no significant differences in the number of platform crosses made during the probe trial (p > 0.05; Fig. 4D). Together, these data suggest only a mild spatial memory impairment in HFD-fed aged rats.
Experiment 2: HFD induces markers of neuroinflammation in hippocampus and amygdala of aged rats
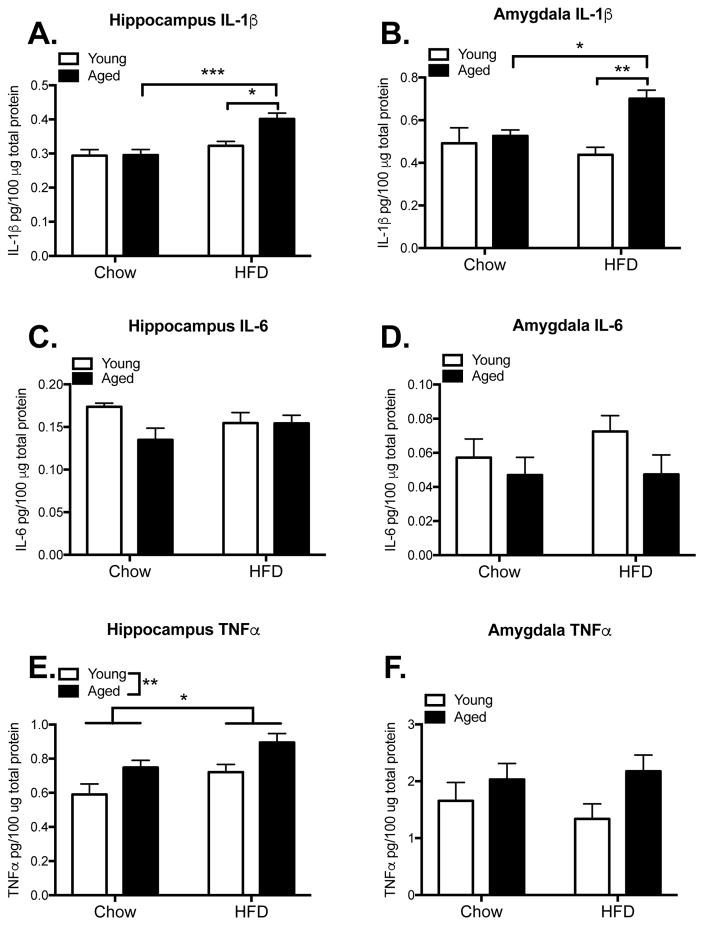
To examine whether the combination of HFD and aging would produce a neuroinflammatory response in the hippocampus and amygdala (the two regions of the brain that mediate contextual fear and auditory-cued fear memories, respectively), these tissues were collected from a separate cohort of rats 2 hr following conditioning, a time point at which activity-induced changes in cytokine expression were predicted based on prior studies (Barrientos, et al., 2011, Barrientos, et al., 2009, Sobesky, et al., 2014), and protein levels of IL-1β, IL-6, and TNFα were measured (n=8 for aged groups, n=6 for young groups). A two-way ANOVA analysis on the hippocampal IL-1β data showed a significant interaction between HFD and age (F(1,24) = 5.07, p < 0.05; Fig. 5A). Post-hoc tests indicated that HFD-fed aged rats had significantly more IL-1β protein than HFD-fed young rats (p < 0.05) and chow-fed aged rats (p < 0.001). All other groups did not differ from one another. Similar findings were observed in amygdala. Thus, there was a significant HFD x age interaction in the amygdala (F(1,24) = 6.35, p < 0.05; Fig. 5B), with HFD-fed aged rats exhibiting a significant increase in IL-1β protein compared to HFD-fed young rats (p < 0.01) and to chow-fed aged rats (p < 0.05). IL-6 levels did not differ between the groups in the hippocampus (F(1,24) = 2.70, p > 0.05; Fig. 5C) or amygdala (F(1,24) = 0.47, p > 0.05; Fig. 5D). TNFα levels in the hippocampus were increased by advanced age (F(1,24) = 10.34, p < 0.01) and by HFD (F(1,24) = 7.14, p < 0.05), but their interaction was not significant (F(1,24) = 0.02, p > 0.05; Fig. 5E). TNFα levels in the amygdala were not significantly different between any of the groups (F(1,24) = 0.61, p > 0.05; Fig. 5F).
Figure 5.
Neuroinflammation. Protein levels of the proinflammatory cytokines IL-1β, IL6, and TNFα in hippocampus (A, C, E) and amygdala (B, D, F) among young and aged rats that were fed chow or HFD. Data are means ± SEM. *p<0.05; **p<0.01; ***p<0.001.
In light of the potentiated IL-1β protein levels in aged HFD-fed rats, we assessed gene expression for microglial activation markers ionized calcium binding adaptor molecule 1 (Iba1), cluster of differentiation molecule 11b (cd11b), and major histocompatibility complex (MHC)II in hippocampus and amygdala (n=6 in each group). Expression of the housekeeping gene, βactin, was not different between the groups in either the hippocampus (F(1,20) = 0.18, p > 0.05) or the amygdala (F(1,20) = 0.74, p > 0.05; data not shown). In the hippocampus, there was a main effect of age for both Iba1 and cd11b (Iba1: F(1,20) = 10.52, p < 0.01); cd11b: (F(1,20) = 6.69, p < 0.05; Fig. 6A & C), with aged rats expressing significantly more of both than young rats. MHCII expression showed a significant interaction of age and diet (F(1,20) = 4.45, p < 0.05; Fig. 6E). In the amygdala, only main effects of age were observed for all three genes (Iba1: F(1,20) = 4.94, p < 0.05); cd11b: (F(1,20) = 8.01, p < 0.05); MHCII: (F(1,20) = 31.24, p < 0.0001) Fig. 6B, D, & F). In most cases, aged HFD-fed rats exhibited greater gene expression than age-matched control rats, but this increase was not statistically significant. Expression of the inflammasome component nucleotide-binding domain, leucine-rich repeat, pyrin domain containing 3 (NLRP3) and the danger signal high mobility group box 1 (HMGB1) were also measured. Analyses of NLRP3 expression indicated a main effect of age in both the hippocampus (F(1,20) = 11.52, p < 0.01; Fig. 6G) and amygdala (F(1,20) = 5.51, p < 0.05; Fig. 6H), with aged rats expressing higher values than young rats. HMGB1 was not significantly different between the groups in the hippocampus (p > 0.05; Fig. 6I), but in the amygdala there was a significant interaction between the factors (F(1,20) = 5.69, p < 0.05; Fig. 6J), with chow-fed aged rats expressing greater HMGB1 than chow-fed young rats (p < 0.05), but in HFD-fed aged rats HMGB1 was not elevated.
Figure 6.
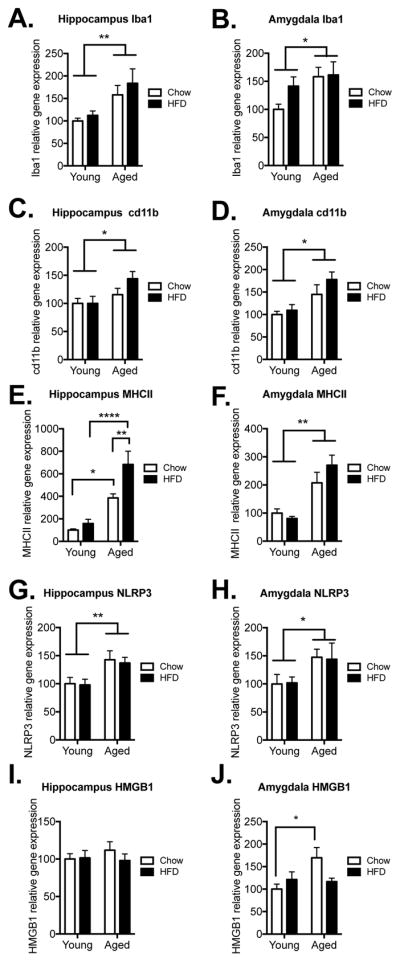
Microglial activation markers, inflammasome, and danger signal. Gene expression of Iba1, cd11b, MHCII, NLRP3, and HMGB1 in hippocampus (A, C, E, G, I) and amygdala (B, D, F, H, J) among young and aged rats that were fed chow or HFD. Data are means ± SEM. *p<0.05; **p<0.01, ****p<0.0001.
Experiment 3: IL-1β plays a critical role in HFD potentiated aging memory impairments
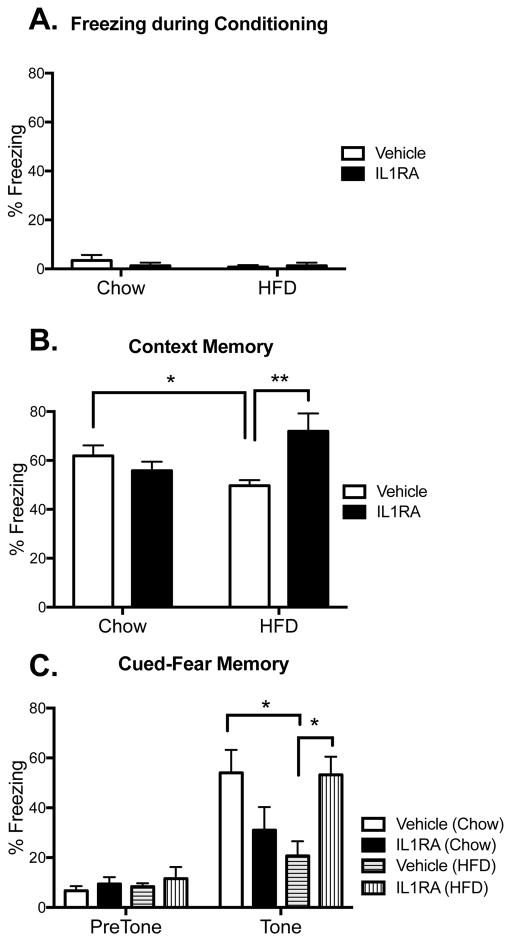
To investigate whether HFD-induced increases in IL-1β detected in hippocampus and amygdala play a causal role in HFD-induced hippocampal- and amygdala-dependent memory disruptions, the IL-1 receptor antagonist hIL-1RA (or vehicle) was administered centrally to a separate cohort of aged rats immediately following conditioning and then long-term memory was assessed (n=9 in vehicle groups, n=6 in IL-1RA groups). It should be noted that overall freezing values were lower in all groups in this experiment compared to those in experiment 1, and this may have been due to handling stress during the icm injection procedure. Nonetheless, a significant two-way ANOVA interaction between diet and drug (F(1,25) = 10.60, p < 0.01) followed by post-hoc tests confirmed that HFD impaired hippocampal-dependent contextual memory compared to that of chow-fed rats (p < 0.05). Importantly, IL-1RA treatment robustly rescued this impairment (p < 0.01; Fig. 7B). IL-1RA was equally effective at rescuing the amygdala-dependent auditory-cued memory (Fig. 7C). Post hoc tests run after a significant diet x drug interaction effect (F(1,26) = 11.36, p < 0.01) also revealed that HFD-fed rats exhibited significantly reduced freezing to the tone compared to chow-fed rats (p < 0.05), and IL-1RA rescued this impairment (p < 0.05). Though not statistically significant, there was a notable decline in freezing in chow-fed rats that received IL-1RA (p = 0.22).
Figure 7.
Long-term memory. Percentage of time spent freezing during conditioning (A), or during test 4 days later in the conditioning context (B), or in novel context in the presence or absence of the tone (C) in aged rats that were fed chow or HFD and treated with IL-1RA or vehicle. Data are means ± SEM. *p<0.05; **p<0.01.
Experiment 4: Voluntary wheel running rescues hippocampal-dependent but not amygdala-dependent memory impairments caused by HFD, a role for IL-1β
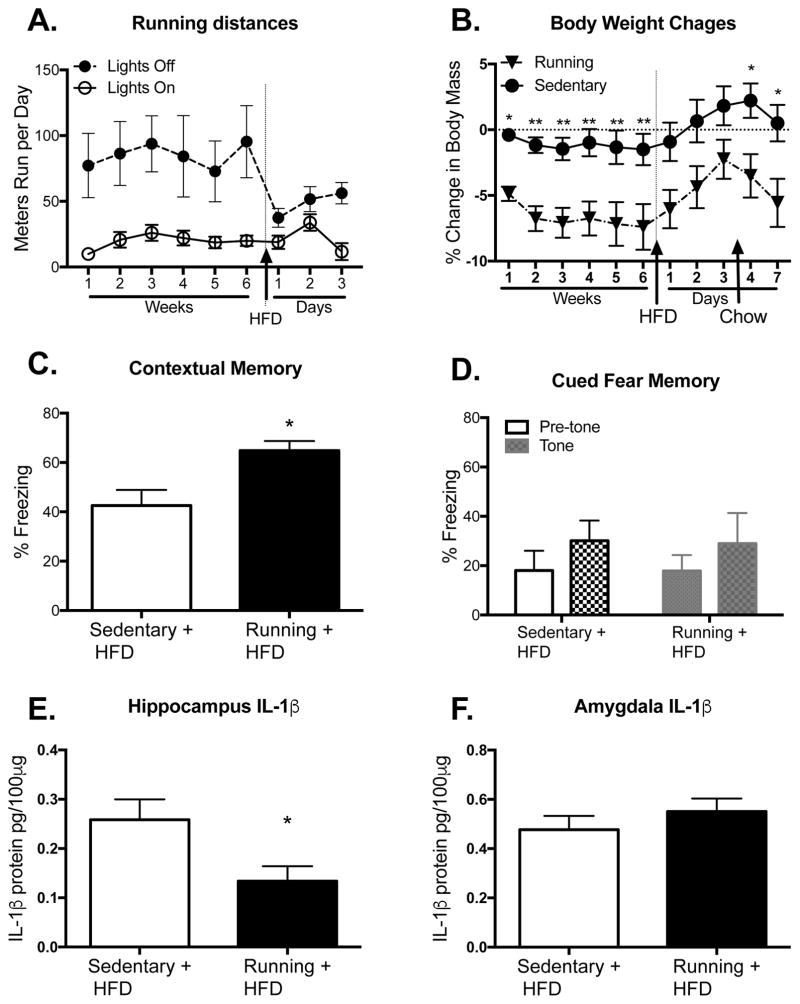
Based on prior data demonstrating that voluntary wheel running attenuates the inflammatory response to bacterial infection, stress, and surgery (Barrientos, et al., 2011, Grace, et al., 2016, Speaker, et al., 2014), here we assessed whether voluntary exercise in aged rats would prevent hippocampal- and amygdala-dependent memory impairments caused by HFD, and if it would do this by altering levels of IL-1β (n=6 in each group). Rats ran an average of ~100 meters per day over 6 weeks, as we have reported previously (Barrientos, et al., 2011). As expected, they ran a significant portion during the lights-off period compared to the lights-on period (F(1,10) = 9.25, p < 0.05; Fig. 8A). Interestingly, lights-off running was dramatically reduced on the first day of eating the HFD (p < 0.01) compared to the running averages from week 6, but running was no longer significantly different on days 2 and 3 (p > 0.05). Despite the small amounts of exercise exhibited, the rats with access to a running wheel showed marked weight loss compared to rats housed with a locked wheel (F(5,45) = 3.81, p < 0.01; Fig. 8B). This significant weight loss was apparent by the end of the first week (p < 0.05), and was maintained for the duration of the 6 weeks prior to HFD (p < 0.01). During the 3 days on the HFD, all rats gained weight and there were no longer significant differences between the groups (p > 0.05). Interestingly, once the rats were switched back to regular chow, weight differences were once again significantly different between the groups (p < 0.05). Crucially, unpaired t-tests revealed that voluntary wheel running rescued the hippocampal-dependent contextual memory impairment caused by HFD (t10 = 2.99, p < 0.05; Fig. 8C), but it was not effective at rescuing the amygdala-dependent auditory-cued memory impairment (t10 = 0.07, p > 0.05; Fig. 8D). In addition, t-tests revealed that voluntary wheel running significantly reduced HFD-induced IL-1β levels in the hippocampus (t10 = 2.34, p < 0.05; Fig. 8E), but did not change IL-1β levels in the amygdala (t10 = 0.095, p > 0.05; Fig. 8F).
Figure 8.
Effects of voluntary wheel running. Running distances during the light and dark phases across the 6 weeks prior to HFD and the 3 days during HFD consumption (A). Percent body weight changes throughout the experiment among sedentary or running aged rats (B). Percentage of time spent freezing in the conditioning context (C) and in the novel context in the absence or presence of the tone (D) among sedentary or running aged rats that were fed HFD. Protein levels of the proinflammatory cytokine IL-1β in hippocampus (E) and amygdala (F) of sedentary or running aged rats that were fed HFD. Data are means ± SEM. *p<0.05; **p<0.01.
Discussion
Together, these findings suggest that aged rats are particularly vulnerable to contextual and cued fear memory disruptions following short-term consumption of a HFD. That is, HFD appears to affect both hippocampal and amygdalar functioning. A previous study demonstrated long-term HFD-induced impairments of hippocampal spatial memory in aged rats. In this study, 14 month old F344 rats that were fed a 36% fat diet supplemented with 10% coconut oil and 2% cholesterol for 6 months were impaired on a water radial arm maze working memory task (Ledreux, et al., 2016). Other aging studies, however, have failed to detect hippocampal-dependent memory impairments caused by HFD consumption (Kesby, et al., 2015, Pancani, et al., 2013). In one of these studies, 14 month-old C57BL/6N mice were fed a 60% fat diet for 3 months, and did not exhibit impaired object or place recognition memory above and beyond that exhibited by chow-fed aging mice (Kesby, et al., 2015). In the other study, 13 month-old F344 rats were fed a 42% fat diet for 4.5 months and did not exhibit spatial memory impairments in the Morris water maze (Pancani, et al., 2013). Although it is difficult to pinpoint the exact reasons for the discrepancy between these and our findings, it is likely due to differences in the composition of the diets, the consumption duration, the strain and species of animals used, the exact behavioral parameters used (e.g. retention interval), and the various combinations of these factors.
In contrast to the robust impairments in contextual and cued-fear memory, HFD-fed aged rats exhibited only mild impairments in spatial reference memory. That is, during the retention probe of the Morris water maze task, these rats could not clearly discriminate between the target quadrant and the adjacent one. However, on number of platform crosses and latency to reach the platform location, they performed comparably to their chow-fed aged counterparts. One possible explanation for this discrepancy is that HFD may have altered autonomic or stress responses, and since contextual fear conditioning involves an acute stressor (foot shock) while Morris water maze does not, fear memories may be more vulnerable to impairment. Although we currently do not know if autonomic activation or the stress axis were altered by fear conditioning in HFD-fed rats, we can point to the equal freezing levels of all groups during the conditioning phase, the equal freezing levels of all groups during the pre-tone phase of the auditory cued-fear test, and the fact that short-term memory was not affected at all. The finding that all rats froze very little and equally during the pre-tone test also indicates that the stress response to the conditioning task was not altered with HFD. However, these areas represent avenues for future research. An alternative explanation for this modest spatial memory impairment is that the 1-day training protocol used here, which requires quite a bit of swimming throughout the day, may have induced increases in brain-derived neurotrophic factor (BDNF), a growth factor known to support hippocampal-dependent learning and memory through growth and differentiation of neurons and synapses (Huang and Reichardt, 2001). Thus, this training may have buffered against the negative effects of HFD. Although purely speculative, this idea is plausible, as even a single 30-minute session of mild exercise has been shown to significantly increase BDNF protein levels in the hippocampus of adult rats and it remains elevated for at least 6 hours (Soya, et al., 2007).
Prior work has demonstrated that inflammatory challenges in aged rats, such as infection (Abraham, et al., 2008, Barrientos, et al., 2006, Chen, et al., 2008) and surgery (Barrientos, et al., 2012, Rosczyk, et al., 2008), produce robust hippocampal-dependent memory impairments, but leave amygdala-dependent memory intact. Thus, HFD is unique among the challenges so far tested in affecting both hippocampal- and amygdala-dependent tasks, suggesting that additional or different circuitry is being activated or disrupted with HFD compared to other inflammatory challenges. Importantly, long-term contextual memory was impaired, but short-term memory was not, in aged rats after 3 days HFD, suggesting that synaptic plasticity processes that are involved in facilitating long-term memory consolidation, including long-term potentiation, protein synthesis, and BDNF upregulation, may be particularly affected in these key regions.
To begin to explore whether microglia may be an important cell type to target in the hippocampus and/or amygdala in this model of neuroinflammation, gene expression of the microglial activation markers, Iba1, cd11b, and MHCII were measured. Consistent with aging’s known effects on microglial priming, both Iba1 and cd11b were significantly elevated in hippocampus and amygdala in both aging groups compared with young adult rats, but were not further increased with the addition of HFD. MHCII gene expression however was not only significantly increased in the aged hippocampus, it was also potently increased by the addition of HFD. In the amygdala, MHCII was also elevated in aged rats, but was not further increased by HFD. Microglial priming is an increase in pro-inflammatory mRNA and protein as well as a microglial profile consistent with hyper-activation in the absence of overt inflammation. Together, these data suggest a potential role for microglia in the aging-related hypersensitivity to the “second hit” of acute HFD we report here, as has been suggested by others (Nadjar, et al., 2016, Valdearcos, et al., 2014). Increased microglial activation markers in the aging hippocampus have been reported previously (Frank, et al., 2006, Frank, et al., 2010b, Henry, et al., 2009), and here we replicate those findings. In addition, we report for the first time evidence that expression of microglia markers in the aged amygdala are also increased. Although these markers trended toward greater expression in the HFD-fed aged rats compared to chow-fed controls, this was not statistically significant, perhaps due to a ceiling effect. NLRP3, a structural component of one of the inflammasomes, which regulates cleavage and release of IL-1 through activation of caspase 1 (Khare, et al., 2010), was significantly elevated by aging in both hippocampus and amygdala, but was not further elevated with HFD. Similarly, HMGB1, the endogenous danger signal that can stimulate inflammation through activation of pattern recognition receptors (Yanai, et al., 2012) was elevated by aging in the amygdala, but was not elevated with HFD. These results are consistent with previous findings that have shown aging-induced increases, and independently, HFD-induced increases in NLRP3 and HMGB1 gene expression (Fonken, et al., 2016, Robblee, et al., 2016, Sobesky, et al., 2016). We did not find potentiated expression of these two genes when aging and HFD were combined, consistent with previous findings that also failed to show amplified expression of NLRP3 or HMGB1 when HFD was combined with LPS (Sobesky, et al., 2016).
Consistent with our hypothesis that HFD stimulates a neuroinflammatory microenvironment when encountered on the background of aging-associated microglial priming, IL-1β protein was significantly elevated at the time of memory consolidation in both the hippocampus and the amygdala in HFD-fed aged rats compared to HFD-fed young rats and chow-fed controls. IL6 and TNFα did not play prominent roles here. Notably, IL-1β increases in the aged hippocampus are known to impair long-term potentiation (Chapman, et al., 2010), blunt learning-induced BDNF upregulation (Barrientos, et al., 2011, Barrientos, et al., 2003, Barrientos, et al., 2004, Chapman, et al., 2012), and inhibit other proteins critical for memory consolidation, such as Arc (Czerniawski and Guzowski, 2014, Frank, et al., 2010a). These IL-1β-induced effects have never been examined directly in amygdala, however, it is known that microinjections of the proinflammatory cytokine TNFα into the amygdala impair auditory-cued fear memory (Jing, et al., 2015), providing precedence for a neuroinflammatory-mediated decline of amygdala function. These mechanisms were not directly examined in the present study, but will be the focus of future studies. Previous work has shown that aging serves to sensitize key memory-sustaining regions of the brain such as the hippocampus, making it vulnerable to a secondary inflammatory challenge, which then produces sufficient neuroinflammation to cause memory impairment (Barrientos, et al., 2006, Chen, et al., 2008, Frank, et al., 2010b, Godbout, et al., 2005). The data presented here suggest that in the context of aging, HFD operates as a secondary challenge to cause both neuroinflammation and memory decline. Research examining HFD consumption in adolescent rats has reported that, like old age, adolescence renders the brain similarly primed to respond to inflammatory challenges, compared with young adulthood. As with the aging rat, the brain is sensitized during adolescence to hyper-respond to a HFD with a robust neuroinflammatory response causing significant cognitive impairments (Boitard, et al., 2014). These effects are likely to be due, in part, to an IL-1β-mediated effect.
Thus, central administration of IL-1RA to aged HFD-fed rats was able to reverse both contextual and cued-fear memory impairments, further suggesting that neuroinflammation plays a critical role in these cognitive impairments, and that IL-1β is an important component of this circuitry. Notably, adolescents fed HFD have hyper-elevated hippocampal IL-1β after a peripheral immune challenge (Boitard, et al., 2014), suggesting that the mechanisms underlying the pro-inflammatory effects of HFD on cognitive-processing regions in adolescents and aged subjects might be similar. It is worth noting that IL-1RA administered to chow-fed rats caused a modest, but not significant, decrease in freezing in both memory tests, reflecting a trend toward mild memory impairment under these conditions. Basal levels of IL-1β are necessary for memory formation processes, and therefore reducing IL-1β signaling with IL-1RA under normal conditions may cause disruptions in memory. The use of IL-1RA as a prophylactic in healthy adults is therefore not recommended at this stage (Goshen, et al., 2007).
There is an abundance of data demonstrating anti-inflammatory and pro-neurogenesis effects in the hippocampus of voluntary exercise (Barrientos, 2011, Cotman and Berchtold, 2002, Cotman, et al., 2007, Funk, et al., 2011, Nichol, et al., 2008). Voluntary exercise studies conducted in aged rats have demonstrated that despite the aged performing much less exercise than their younger counterparts, exercise is still remarkably effective at rescuing hippocampal-dependent memory dysfunction caused by various inflammatory insults (Barrientos, et al., 2011, Gibbons, et al., 2014, Kohman, et al., 2013, Kohman, et al., 2012). Less is known about whether (and if so, how) these beneficial effects also extend to the amygdala. Aged rats that were given unrestricted access to a running wheel ran approximately 50 times less than the distance the young adult rats ran in the same amount of time, yet they maintained a ~7% body weight reduction throughout the 6 weeks. Importantly, voluntary running prevented the HFD-induced impairment of the hippocampal-dependent contextual fear memory. Interestingly, however, running did not prevent the amygdala-dependent cued-fear impairment. Furthermore, running significantly reduced the HFD-induced levels of IL-1β protein in the hippocampus, but not in amygdala. These findings again support the idea that neuroinflammation is a key mediator in producing these HFD-induced memory impairments, and that mild voluntary exercise is only an effective intervention to mitigate the effects of HFD caused to the hippocampus, but not those caused to the amygdala.
Together our data suggest that acute consumption of HFD can lead to memory deficits and significant brain inflammation in the aged animal without such overt effects in the young adult. These deficits are likely to be at least partly mediated by aging-associated microglial priming that extends beyond the hypothalamus into memory processing regions including hippocampus and amygdala. This microglial priming leads to hyper-responsive IL-1β in the face of an inflammatory challenge (such as HFD) and strongly suggests that appropriate diet, particularly in the aging, is crucial for cognitive health.
Highlights.
3 days HFD impairs hippocampal- & amygdalar-dependent memory in aged rats
HFD-induced memory impairments are caused by IL-1β increases in aged rats
Central IL-1RA administration prevented HFD-induced memory impairments in aged rats
Exercise reduced hippocampal IL-1 & prevented HFD-induced hippocampal memory decline
Exercise did not reduce amygdalar IL1 & did not prevent HFD-induced amygdalar memory decline
Acknowledgments
This work was supported by grants from the National Institute on Aging R01AG028271 to L.R.W., S.F.M., & R.M.B.; and by an Australian Research Council Future Fellowship (FT110100084), an RMIT University Vice Chancellor’s Senior Research Fellowship, and a Club Melbourne Fellowship to S.J.S.
Footnotes
All authors declare no actual or potential conflicts of interests.
All authors declare no competing financial interests.
All experiments were conducted in accordance with protocols approved by the University of Colorado Animal Care and Use Committee.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents, and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29(4):614–21. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’sAssociation. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):1–80. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Barrientos RM. Voluntary exercise as an anti-neuroinflammatory therapeutic. Brain Behav Immun. 2011;25(6):1061–2. doi: 10.1016/j.bbi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31(32):11578–86. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32(42):14641–8. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–32. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, D’Angelo HM, Watkins LR, Rudy JW, Maier SF. Stable, long-term, spatial memory in young and aged rats achieved with a one day Morris water maze training protocol. Learn Mem. 2016;23(12):699–702. doi: 10.1101/lm.043489.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121(4):847–53. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155(1–2):119–26. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, Ferreira G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Cano P, Cardinali DP, Rios-Lugo MJ, Fernandez-Mateos MP, Reyes Toso CF, Esquifino AI. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity (Silver Spring) 2009;17(10):1866–71. doi: 10.1038/oby.2009.200. [DOI] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol Aging. 2012;33(4):832e1–e14. doi: 10.1016/j.neurobiolaging.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30(22):7598–603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301–11. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112(1):7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60(3):349–56. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharmacol. 2012;7(1):7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65(4):304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J Neurosci. 2014;34(37):12470–80. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca SN, Ziko I, Sominsky L, Nguyen JC, Dinan T, Miller AA, Jenkins TA, Spencer SJ. Early life overfeeding impairs spatial memory performance by reducing microglial sensitivity to learning. J Neuroinflammation. 2016;13(1):112. doi: 10.1186/s12974-016-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri TH, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS Data Brief. 2012;(106):1–8. [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF. The Alarmin HMGB1 Mediates Age-Induced Neuroinflammatory Priming. J Neurosci. 2016;36(30):7946–56. doi: 10.1523/JNEUROSCI.1161-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27(5):717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010a;24(2):254–62. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010b;226(1–2):181–4. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD, Ogden CL. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults Aged 20 and Over: United States, 1960–1962 Through 2013–2014. NCHS Data Brief. 2016:1–6. [Google Scholar]
- Funk JA, Gohlke J, Kraft AD, McPherson CA, Collins JB, Jean Harry G. Voluntary exercise protects hippocampal neurons from trimethyltin injury: Possible role of interleukin-6 to modulate tumor necrosis factor receptor-mediated neurotoxicity. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons TE, Pence BD, Petr G, Ossyra JM, Mach HC, Bhattacharya TK, Perez S, Martin SA, McCusker RH, Kelley KW, Rhodes JS, Johnson RW, Woods JA. Voluntary wheel running, but not a diet containing (−)-epigallocatechin-3-gallate and beta-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav Brain Res. 2014;272:131–40. doi: 10.1016/j.bbr.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8–10):1106–15. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–23. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet SJ, Carlson SE. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv Nutr. 2015;6(6):660–4. doi: 10.3945/an.115.009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23(3):309–17. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74(6):788–9. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61(6):1444–54. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Hao Y, Bi Q, Zhang J, Yang P. Intra-amygdala microinjection of TNF-alpha impairs the auditory fear conditioning of rats via glutamate toxicity. Neurosci Res. 2015;91:34–40. doi: 10.1016/j.neures.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, Jeste DV, Achim CL, Semenova S. Spatial Cognition in Adult and Aged Mice Exposed to High-Fat Diet. PLoS One. 2015;10(10):e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Crit Rev Immunol. 2010;30(5):463–87. doi: 10.1615/critrevimmunol.v30.i5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2012;26(5):803–10. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledreux A, Wang X, Schultzberg M, Granholm AC, Freeman LR. Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res. 2016;312:294–304. doi: 10.1016/j.bbr.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maric T, Woodside B, Luheshi GN. The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav Immun. 2014;36:35–45. doi: 10.1016/j.bbi.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, Leyrolle Q, Joffre C, Laye S. Bioactive lipids as new class of microglial modulators: When nutrition meets neuroimunology. Prog Neuropsychopharmacol Biol Psychiatry. 2016 doi: 10.1016/j.pnpbp.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- Pancani T, Anderson KL, Brewer LD, Kadish I, DeMoll C, Landfield PW, Blalock EM, Porter NM, Thibault O. Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiol Aging. 2013;34(8):1977–87. doi: 10.1016/j.neurobiolaging.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7(1):60–7. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Putnam JJ, Allshouse JE. Food Consumption, Prices, and Expenditures, 1970–97. ERS, USDA Statistical Bulletin. 1999;965:1–189. [Google Scholar]
- Robblee MM, Kim CC, Porter Abate J, Valdearcos M, Sandlund KL, Shenoy MK, Volmer R, Iwawaki T, Koliwad SK. Saturated Fatty Acids Engage an IRE1alpha-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep. 2016;14(11):2611–23. doi: 10.1016/j.celrep.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9(4):339–49. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43(9):840–6. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19(1):97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–8. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, Maier SF. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun. 2014;42:22–32. doi: 10.1016/j.bbi.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky JL, D’Angelo HM, Weber MD, Anderson ND, Frank MG, Watkins LR, Maier SF, Barrientos RM. Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1. eNeuro. 2016;3(4) doi: 10.1523/ENEURO.0113-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358(4):961–7. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Speaker KJ, Cox SS, Paton MM, Serebrakian A, Maslanik T, Greenwood BN, Fleshner M. Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behav Immun. 2014;39:87–98. doi: 10.1016/j.bbi.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9(6):2124–38. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Jonsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11. e3. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes. 2002;51(6):1876–83. doi: 10.2337/diabetes.51.6.1876. [DOI] [PubMed] [Google Scholar]
- Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol. 2012;33(12):633–40. doi: 10.1016/j.it.2012.10.005. [DOI] [PubMed] [Google Scholar]