Abstract
OBJECTIVE
To date, most comorbidity studies have analyzed either a subgroup of frequent diseases, or used summary instruments such as the Charlson score or the Cumulative Illness Rating Scale-Geriatric (CIRS-G). Yet, comorbidity is a multidimensional construct and impacts function, treatment tolerance, and survival. We assessed how heat maps can unveil specific patterns of comorbidities associated with overall survival (OS) in older cancer patients treated with chemotherapy.
MATERIAL AND METHODS
We reviewed four trials that prospectively evaluated comorbidities using CIRS-G. Eligible patients were 65 years or older and had solid tumors with 30 or more patients per tumor site. Heat maps were constructed based on CIRS-G scores and correlated with OS.
RESULTS
Among 818 patients accrued, 399 were eligible: Median follow-up was 53.4 months and median OS was 19.6 months (95% CI: 16.5–24.2). In the univariate model for OS, patients with a severe CIRS-G score in 6 organ categories (3–4 in heart, hematopoietic, respiratory, and musculoskeletal-integument and 2–4 in upper GI and liver) had statistically worse OS than those with lower scores. According to a total risk score (TRS) based on hazard ratios for OS, OS of the low risk group (N=309, TRS<2) was significantly higher (24.3 m vs. 10.8m, HR=2.05, 95% CI: 1.58–2.66). TRS was a predictor for OS independently from stage, primary site, prior chemotherapy, ECOG performance status, and IADL (HR=1.94, 95% CI: 1.47–2.57).
CONCLUSIONS
High TRS was a predictor of poor survival. Comorbidity heat maps appear promising to identify diseases most affecting the OS of older cancer patients.
Keywords: CIRS-G, comorbidity, heat map, older cancer patient, overall survival
Introduction
In 2015, in the United States, more than 50% of cancers occurred in people aged 65 and older.(1) Analyzing characteristics of these patients is very important in an aging society and increasingly older cancer patients.
Comorbidity increases with aging and cancer patients aged 70 and older have three or more comorbidities on average.(2) Comorbidity has been reported to affect the survival of cancer patients,(3–6) and treatment-related toxicities.(7–10) Furthermore, comorbidity and its potential for related complications, or frailty interfere with physicians’ treatment decisions for older cancer patients in poorly defined ways.(11) Comorbidity in the elderly should be considered as a factor when determining cancer therapy. Therefore, it is very important to define more precisely the role of comorbidity in older cancer patients.
Comorbidity is by essence a multidimensional construct, with highly variable physiopathologies and impact on function,(2) treatment tolerance,(12) cancer behavior,(13–15) and survival(3–6, 16) in cancer patients. The Cumulative Illness Rating Scale-Geriatric (CIRS-G) consists of fourteen organ categories including cardiac, vascular, respiratory disease, and so on and 5 levels of severity within each comorbid category.(17) It reflects the variety and complexity of the comorbidities. However, most comorbidity studies have analyzed either a subgroup of frequent diseases, or instruments summarizing the comorbidity burden, such as the Charlson score or CIRS-G. Although validated, these instruments usually rely on one end-point or expert consensus to build a severity rating. One can reasonably hypothesize that the subset of comorbidities that would most influence survival could be different from those that influence most physical function, or toxicity from treatment.
In order to further our understanding of comorbidity in cancer patients, we tested an analytic approach used in other multidimensional problems such as gene expression or epigenetics, namely a heat map approach. Heat maps allow a two-dimension visualization of complex variables, helping distinguish how they cluster by outcome, synchronously with a visualization of their overall frequency and level of severity (or overexpression). They may also help visualize how sets of data associate with each other (e.g. comorbidities within patients). Heat maps are a supple way of displaying associations generated by a wide range of statistical methods. Although stricto sensu the same results could be expressed by data output tables, the length and complexity of these tables prevents an intuitive grasp of those results. In this article, we apply this approach to the correlation of comorbidity and survival in a large cohort of patients.
Materials and Methods
This is a retrospective study to assess the association of comorbidity, modeled with heat maps, with overall survival in a population of older cancer patients treated with chemotherapy. We reviewed four clinical trials that prospectively evaluated comorbidities using CIRS-G in our institution (H. Lee Moffitt Cancer Center)(Table 1). Eligible patients were 65 years or older and had solid tumors for which the number of patients by primary site was 30 or more. Among 818 patients accrued from July 2003 to June 2013, 399 were eligible: 90 with breast cancer, 77 with head and neck cancer, 71 with lung cancer, 66 with pancreatobiliary cancer, 53 with prostate and bladder cancer, and 42 with colorectal cancer. The reasons for exclusions were as below: 182 patients were treated in other locations than Moffitt Cancer Center, 99 were not evaluable for the original trials’ end-points, 60 had hematologic malignancies, 48 had other solid tumors, 28 were less than 65 years old, and two had incomplete CIRS-G. All data were collected from the clinical trials databases, electronic records of Moffitt Cancer Center, and Total Cancer Care (TCC) Database.
Table 1. Trials used in the present project.
Patients retained needed to be age 65 and older, have survival data available, and have a tumor type with 30 or more patients analyzable.
| Trial | Cancer types | Treatment | Sites | #patients | characteristics | #patients retained in project |
|---|---|---|---|---|---|---|
| Extermann et al. 2012(10) | All except leukemia | chemotherapy | Moffitt + community practices | 518 | Age 70 and older | 207 |
| Extermann et al. 2012(31) | H&N | chemoradiation | Moffitt | 50 | Age 65 and older | 50 |
| Raj et al. 2013(32) | H&N | chemotherapy | Moffitt | 50 | Adults | 22 |
| Extermann et al. 2015(33) | All except leukemia | chemotherapy | Moffitt | 200 | Age 70 and older | 120 |
Comorbidity was assessed by the CIRS-G which includes 14 organ categories with 5 levels of severity of comorbidities (score 0–4).(17, 18) The five summary scores are as follows: total number of categories endorsed, total score, ratio of total score/number of endorsed categories (severity index) and number of categories at level 3 and 4 for a given patient in CIRS-G. The 14 organ categories are as follows: Heart; Vascular; Hematopoietic; Respiratory; Eyes, ears, nose, throat & larynx; Upper GI; Lower GI; Liver; Renal; Genitourinary; Musculoskeletal/Integument; Neurological; Endocrine/Metabolic and Breast; and Psychiatric illness. We reviewed the 14 organ categories and 5 CIRS-G scores of the CIRS-G of our patients.
In addition, we reviewed patients’ demographics, histology, year of diagnosis, cancer stage, prior and current cancer treatments, MAX2 index,(12, 19) and functional status (ECOG performance status (PS)) and Lawton’s 9-item Instrumental Activities of Daily Living (IADL) scale (20) at study baseline. The MAX2 index ranks the average toxicity of a chemotherapy regimen based on the most frequent reported severe toxicities. Lawton’s 9-item IADL is scored from 9 to 29 points (3 levels per item and 2 points for medications), 29 being best function.
Heat maps were created to visualize the comorbidity distributions using the following steps: 1) Each patient was attributed a line; 2) organ systems were attributed columns, and the heat color was based on each organ’s CIRS-G severity rating, from blue (0) to red (4); and 3) the comorbidity types and levels of expression were grouped according to their relationship with overall survival, according to the statistical analysis described below. This study was approved by the Institutional Review Board of the University of South Florida.
Statistics
Patients’ clinical and demographical characteristics were summarized using descriptive statistics: frequency and proportion for categorical measures and mean, standard deviation, median, and range for continuous measures. Overall survival (OS) was measured from the date of initiation of treatment to date of death or last follow-up date. The association of comorbidity with categorical and continuous outcome was evaluated by the Chi-square test and the Kruskal-Wallis test. The survival function was estimated by the Kaplan-Meier method, and the difference between the functions was assessed by the log-rank test. The Cox proportional hazards regression model was used to assess the association with OS. The impact of comorbidity on OS was evaluated, and the risk score was developed based on the hazard ratio and significance; risk score 1 was given to those who had CIRS-G categories with p-value of < 0.1 and hazard ratio of 1 to 2, and score 2 was assigned to those with p-value of < 0.1 and hazard ratio of >2 (Table 3). The total risk score (TRS) was defined as the sum of risk scores. The distribution of TRS and the impact on OS were illustrated in Table 4. Based on the result of Table 4, patients were divided into two risk groups. The high risk patients were defined as those who had a TRS of 2 or more, while the low risk patients were those who had a TRS of 0 or 1. The multivariable model was selected by the backward elimination method to assess the association between the risk group and OS, when adjusting for potential confounding variables. A variable with p-value of >0.15 was eliminated at each step. No multiple comparisons were considered. All p-values were two-sided and p-value of <0.05 was considered statistically significant. All data analysis was conducted by SAS version 9.4 and heat maps were created by MATLAB software version R2016a.
Table 3.
Univariate model for overall survival by CIRS-G comorbidity categories
| Comorbidity | CIRS-G score | p-value | HR | Risk score | ||
|---|---|---|---|---|---|---|
| Point | 95% CI | |||||
| Lower | Upper | |||||
| HEART | 0–1 | Reference | 0 | |||
| 2 | 0.358 | 1.17 | 0.84 | 1.65 | 0 | |
| 3–4 | 0.013 | 1.48 | 1.09 | 2.03 | 1 | |
| VASCULAR | 0–1 | Reference | 0 | |||
| 2 | 0.704 | 0.95 | 0.73 | 1.24 | 0 | |
| 3–4 | 0.781 | 1.04 | 0.77 | 1.42 | 0 | |
| HEMATOPOIETIC | 0–1 | Reference | 0 | |||
| 2 | 0.626 | 1.16 | 0.64 | 2.13 | 0 | |
| 3–4 | 0.007 | 2.65 | 1.30 | 5.38 | 2 | |
| RESPIRATORY | 0–1 | Reference | 0 | |||
| 2 | 0.402 | 1.14 | 0.84 | 1.56 | 0 | |
| 3–4 | 0.011 | 1.43 | 1.08 | 1.88 | 1 | |
| EENT & LARYNX | 0–1 | Reference | 0 | |||
| 2 | 0.943 | 1.02 | 0.64 | 1.62 | 0 | |
| 3–4 | 0.905 | 1.07 | 0.34 | 3.35 | 0 | |
| UPPER GI | 0–1 | Reference | 0 | |||
| 2–4 | 0.014 | 1.38 | 1.07 | 1.79 | 1 | |
| LOWER GI | 0–1 | Reference | 0 | |||
| 2 | 0.105 | 1.32 | 0.94 | 1.83 | 0 | |
| 3–4 | 0.638 | 0.81 | 0.33 | 1.97 | 0 | |
| LIVER | 0–1 | Reference | 0 | |||
| 2–4 | 0.028 | 1.57 | 1.05 | 2.36 | 1 | |
| RENAL | 0–1 | Reference | 0 | |||
| 2 | 0.308 | 1.48 | 0.70 | 3.14 | 0 | |
| 3–4 | 0.473 | 1.24 | 0.69 | 2.21 | 0 | |
| GENITOURINARY | 0–1 | Reference | 0 | |||
| 2 | 0.408 | 1.19 | 0.79 | 1.80 | 0 | |
| 3–4 | 0.561 | 1.19 | 0.67 | 2.12 | 0 | |
| MUSCULOSKELETAL INTEGUMENT | 0–1 | Reference | 0 | |||
| 2 | 0.830 | 0.96 | 0.64 | 1.43 | 0 | |
| 3–4 | 0.062 | 1.38 | 0.98 | 1.95 | 1 | |
| NEUROLOGICAL | 0–1 | Reference | 0 | |||
| 2 | 0.730 | 0.91 | 0.52 | 1.58 | 0 | |
| 3–4 | 0.223 | 1.66 | 0.74 | 3.73 | 0 | |
| ENDOCRINE METABOLIC AND BREAST | 0–1 | Reference | 0 | |||
| 2 | 0.547 | 1.10 | 0.80 | 1.51 | 0 | |
| 3–4 | 0.581 | 1.22 | 0.60 | 2.47 | 0 | |
| PSYCHIATRIC ILLNESS | 0–1 | Reference | 0 | |||
| 2–4 | 0.176 | 1.63 | 0.80 | 3.29 | 0 | |
Table 4.
Total Risk Score (TRS) distribution and mortality hazard ratios
| Total risk score | No. of patients | % | p-value | HR | ||
|---|---|---|---|---|---|---|
| Point | 95% CI | |||||
| Lower | Upper | |||||
| 0 | 160 | 40 | Reference | |||
| 1 | 149 | 37 | 0.252 | 1.17 | 0.89 | 1.54 |
| 2 | 66 | 17 | <.0001 | 2.05 | 1.48 | 2.83 |
| 3 | 19 | 5 | <.0001 | 2.72 | 1.66 | 4.44 |
| 4 | 5 | 1 | 0.029 | 3.06 | 1.13 | 8.32 |
Results
Patients’ characteristics
Patients’ characteristics are summarized in Table 2. The median age was 74 years old (range, 65–92). Fifty one percent of patients were male. Baseline ECOG PS 0 was the most common with 177 (44.4%) patients. The median score for baseline IADL was 27 (range, 12–29).
Table 2. Patients Characteristics.
Low-risk and high-risk mortality groups are defined on the basis of the comorbidity Total Risk Score we constructed from our heat map analysis (see text for details)
| Characteristics | All (N=399) |
TRS Low Risk (N=309) |
TRS High Risk (N=90) |
P value |
|---|---|---|---|---|
|
| ||||
| Age (y) median, range | 74 (65–92) | 74 (65–91) | 74 (65–92) | 0.27 |
|
| ||||
| Gender n (%) | 0.027 | |||
| Male | 203 (50.9) | 148 (47.9) | 55 (61.1) | |
| Female | 196 (49.1) | 161 (52.1) | 35 (38.9) | |
|
| ||||
| ECOG PS n (%) | 0.33 | |||
| 0 | 177 (44.4) | 143 (46.3) | 34 (37.8) | |
| 1 | 157 (39.4) | 116 (37.5) | 41 (45.6) | |
| 2 | 55 (13.8) | 41 (13.3) | 14 (15.6) | |
| 3 | 10 (2.5) | 9 (2.9) | 1 (1.1) | |
|
| ||||
| IADL median (range) | 27 (12–29) | 27 (12–29) | 27 (15–29) | 0.0503 |
|
| ||||
| Primary tumor site n (%) | <.0001 | |||
| Breast | 90 (22.6) | 81 (26.2) | 9 (10.0) | |
| Head & Neck | 77 (19.3) | 66 (21.4) | 11 (12.2) | |
| Lung | 71 (17.8) | 37 (12.0) | 34 (37.8) | |
| Pancreas, biliary | 66 (16.5) | 57 (18.5) | 9 (10.0) | |
| Prostate, bladder | 53 (13.3) | 36 (11.7) | 17 (18.9) | |
| Colon, rectum | 42 (10.5) | 32 (10.4) | 10 (11.1) | |
|
| ||||
| Stage* n (%) | 0.13 | |||
| I | 16 (4.0) | 15 (4.9) | 1 (1.1) | |
| II | 48 (12.1) | 41 (13.3) | 7 (7.8) | |
| III | 78 (19.6) | 56 (18.2) | 22 (24.4) | |
| IV | 256 (64.3) | 196 (63.6) | 60 (66.7) | |
|
| ||||
| MAX2 index, median, range | 0.13 (0.03–0.36) | 0.13 (0.03–0.36) | 0.12 (0.04–0.35) | 0.15 |
|
| ||||
| Current chemotherapy n (%) | ||||
| Cisplatin-containing | 54 (13.5) | 44 (14.2) | 10 (11.1) | 0.45 |
| Combined (≥ 3) | 54 (13.5) | 50 (16.2) | 4 (4.4) | 0.004 |
|
| ||||
| Prior therapy n (%) | ||||
| Chemotherapy | 131 (32.8) | 106 (34.3) | 25 (27.8) | 0.25 |
| Surgery | 248 (62.2) | 191 (61.8) | 57 (63.3) | 0.79 |
| Radiotherapy | 94 (23.6) | 79 (25.6) | 15 (16.7) | 0.08 |
For one Head & Neck patient stage was not available. IADL = Instrumental Activities of Daily Living
The majority of patients: (n=256, 64.3%) had stage IV cancer, with the proportion varying by tumor site:, 33.3% for breast cancer, 83.1% for head and neck cancer, 62.0% for lung cancer, 66.7% for pancreatobiliary cancer, 77.4% for prostate and bladder cancer, and 78.6% for colorectal cancer. The majority (268, 67.2%) of patients were chemotherapy-naïve. The patients received 74 chemotherapy regimens. The most frequently employed agents employed (single or combined) were platinum compounds (44%), taxanes (34%), and gemcitabine (33%). Fifty head and neck patients (12.5%) received concurrent chemotherapy and radiotherapy. The median MAX2 index of the regimens was 0.13 (range, 0.03–0.36).
Univariable models for overall survival: identification of key comorbidities by CIRS-G score
Figure 1A shows the distribution of the CIRS-G score by 14 comorbidity categories. All but one patient had one or more comorbidities (Fig. 1B). The mean value and standard deviation of the total score of all patients was 9.0 ± 4.2. The mean value and standard deviation of the total number of categories endorsed of all patients was 5.4 ± 2.2. The most common comorbidities were vascular disease, musculoskeletal/integument disease, respiratory disease, EENT and laryngeal disease, genitourinary disease, and endocrine/metabolic and breast disease.
Fig. 1A.
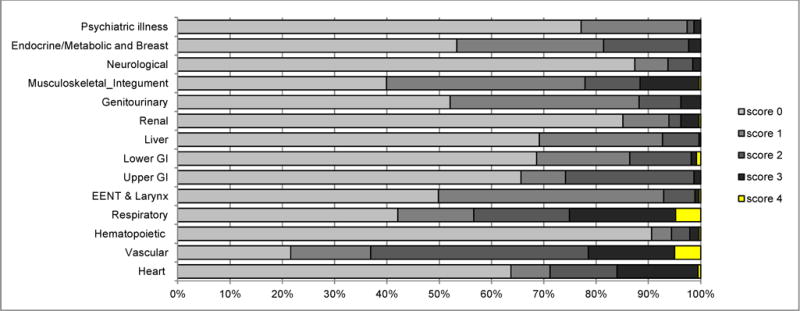
CIRS-G severity score incidence of score by category for all 399 patients
Fig. 1B.
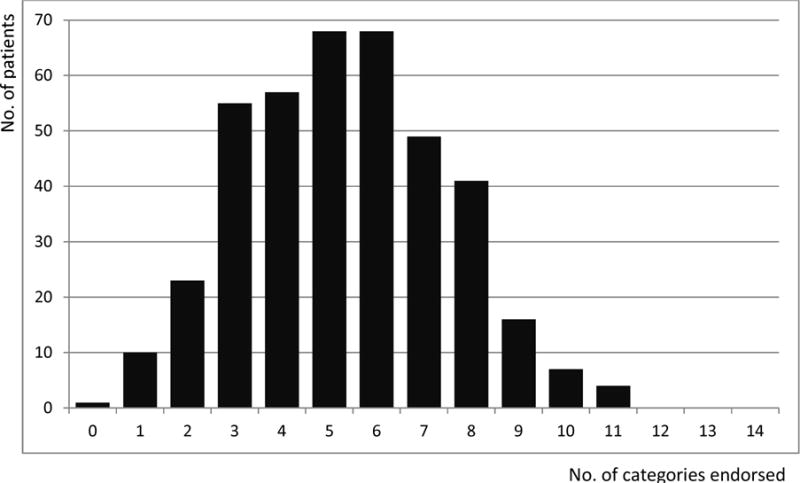
Total number of CIRS-G categories endorsed for all 399 patients
The median follow-up duration was 53.4 months (0.2–132.8 months). The median overall survival of all patients was 19.6 months (95% CI: 16.5–24.2). In univariate analyses of the 14 CIRS-G categories, the survival of patients with a severe CIRS-G score was statistically worse than those with lower scores in several categories (Table 3): CIRS-G score 3 to 4 in heart (p=0.013, HR=1.48, 95% CI: 1.09–2.03); hematopoietic (p=0.007, HR=2.65, 95% CI: 1.30–5.38); respiratory (p=0.011, HR=1.43, 95% CI: 1.08–1.88); and musculoskeletal-integument (p=0.062, HR=1.38, 95% CI: 0.98–1.95); CIRS-G score 2 to 4 in upper GI (p=0.014, HR=1.38, 95% CI: 1.07–1.79); and liver (p=0.028, HR=1.57, 95% CI: 1.05–2.36). Among them, the hazard ratio in patients with a 3 to 4 score in the hematopoietic category was the highest (p=0.007, HR=2.65, 95% CI: 1.30–5.38). Patients’ characteristics by risk groups are shown in Table 2. Ninety (23%) of all 399 patients were in the high risk group and 309 (77%) in the low risk group. Survival in the low risk group was significantly longer than that of the high risk group (median survival: 24.3 months vs. 10.8 months, p<0.0001, HR=2.05, 95% CI: 1.58–2.66) (Fig. 2).
Fig. 2.
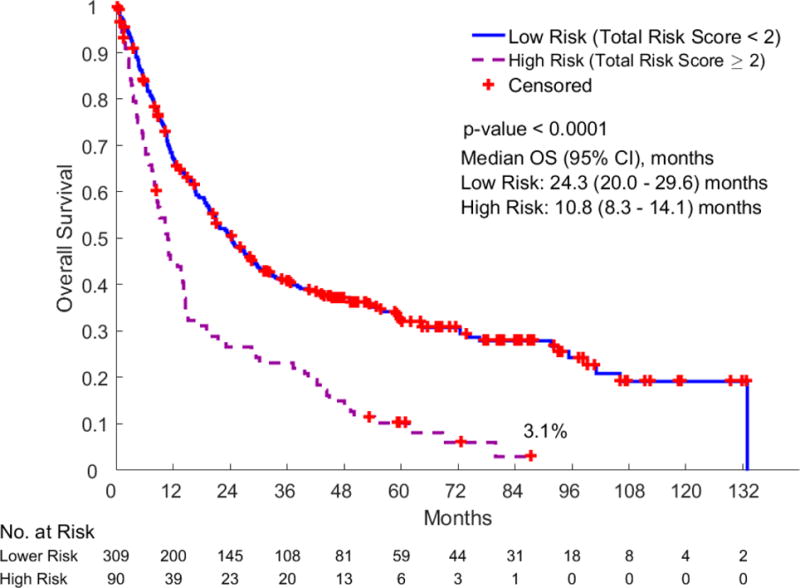
Overall survival by CIRS-G risk groups
Comorbidity heat map for overall survival
A comorbidity heat map was created to observe the association of 14 comorbidities and survival in Figure 3. The order of the CIRS-G categories was rearranged according to whether they were statistically associated or not with OS in univariate analyses. The map powerfully illustrates how diseases with very different prevalence correlate with survival. One can also visualize which level of severity is the driver of the differences: for hematologic, cardiac, and respiratory diseases, grade 3 and 4 diseases drive the associations, whereas for liver and upper GI, the association is mostly driven by grade 2 disease. The figure is more heterogeneous for locomotive/integument diseases. Patients in the high TRS risk group had an average CIRS-G score of 5.8 in the non-TRS organ categories (45.6% of them having at least one category G3 or 4) vs 4.5 in the non-TRS organ categories (26.2% of them having at least one category G3 or 4) in the low TRS risk group. The higher non-TRS CIRS-G score likely reflects some degree of associative trend with comorbidities in the high TRS group (e.g. vascular disease has some association with cardiac disease).
Fig. 3.
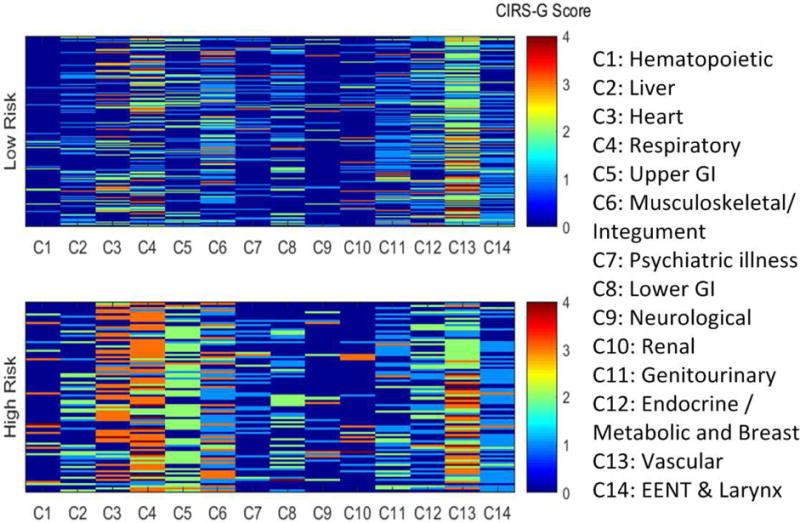
Comorbidity heat map for overall survival. Categories on the left have the highest odds ratio for OS. In addition, the heat map allows to visualize how segregated the distribution of comorbidities is between groups. It can be noted that grade 3–4 hematologic, cardiac, and respiratory disease is almost entirely clustered in the high risk group. On the other hand, the association of liver and upper GI diseases with OS is mostly due to grade 2 disease.
Multivariable model for overall survival
In order to contextualize the association of comorbidity with OS, we built a multivariable analysis model. The variables tested for univariate association were: comorbidity TRS, gender, stage, primary tumor sites, prior chemotherapy or not, ECOG PS, age, MAX2 index, and IADL scale (Table 5). The survival of patients with a high TRS; male gender; stage 3 or 4; prior chemotherapy received; ECOG PS 1 to 3; age increase; MAX2 index decrease; and IADL scale decrease; was significantly worse compared with reference patients. Among primary tumor sites, the OS of patients with head and neck cancer (median 44.4 months: 95% CI 28.4–72.3), colorectal cancer (median 19.6; 10.4–24.6), lung cancer (median 14.0; 10.5–17.3), prostate and bladder cancer (median 14; 8.1–21.4), and pancreatobiliary cancer (median 10.2 (7.8–13.4), were significantly worse than those with breast cancer (median 95.2; 55.1-not reached). The OS of patients with no prior chemotherapy was longer than that of patients with prior chemotherapy (median survival: 24.4 months vs. 12.4 months, p<0.0001). The proportion of stage 4 disease among patients with prior chemotherapy was 85%, and 53% among those without prior chemotherapy. In particular, the hazard ratios for patients with a TRS of 2 to 4 (HR=2.05, 95% CI: 1.58–2.66), stage 4 (HR=3.44, 95% CI: 2.32–5.10), colorectal cancer (HR=3.35, 95% CI: 2.13–5.26), pancreatobiliary cancer (HR=5.74, 95% CI: 3.79–8.70), lung cancer (HR=3.60, 95% CI: 2.40–5.41), prostate and bladder cancer (HR=4.05, 95% CI: 2.66–6.16,) and ECOG PS 2 to 3 (HR=3.48, 95% CI: 2.50–4.83) were significantly high (HR > 2.00).
Table 5.
Univariable and Multivariable model for overall survival
| Variables | Level | Univariable Model
|
Multivariable Model
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-value | Point | Hazard Ratio | p-value | Point | Hazard Ratio | |||||
| 95% CI | 95% CI | |||||||||
| Lower | Upper | Lower | Upper | |||||||
| Total risk | Low risk (0–1) | Reference | reference | |||||||
| score | High risk (2–4) | <.0001 | 2.05 | 1.58 | 2.66 | <.0001 | 1.94 | 1.47 | 2.57 | |
| Male | Reference | |||||||||
| Gender | ||||||||||
| Female | 0.0001 | 0.63 | 0.50 | 0.80 | ||||||
| 1 or 2 | Reference | reference | ||||||||
| Stage | 3 | 0.008 | 1.85 | 1.18 | 2.91 | 0.19 | 1.38 | 0.86 | 2.22 | |
| 4 | <.0001 | 3.44 | 2.32 | 5.10 | 0.0002 | 2.32 | 1.49 | 3.62 | ||
| Breast | Reference | reference | ||||||||
| CRC | <.0001 | 3.35 | 2.13 | 5.26 | 0.004 | 2.00 | 1.25 | 3.22 | ||
| HNC | 0.039 | 1.60 | 1.02 | 2.50 | 0.86 | 0.96 | 0.59 | 1.55 | ||
| Site | PB | <.0001 | 5.74 | 3.79 | 8.70 | <.0001 | 4.36 | 2.82 | 6.75 | |
| Lung | <.0001 | 3.60 | 2.40 | 5.41 | 0.0004 | 2.22 | 1.42 | 3.45 | ||
| Prostate/Bladder | <.0001 | 4.05 | 2.66 | 6.16 | 0.005 | 1.95 | 1.23 | 3.09 | ||
| Prior | No | Reference | Reference | |||||||
| Chemo | Yes | <.0001 | 1.76 | 1.39 | 2.24 | 0.028 | 1.40 | 1.04 | 1.88 | |
| 0 | Reference | Reference | ||||||||
| ECOG PS | 1 | <.0001 | 1.95 | 1.50 | 2.54 | 0.009 | 1.45 | 1.10 | 1.93 | |
| 2 or 3 | <.0001 | 3.48 | 2.50 | 4.83 | 0.001 | 1.98 | 1.33 | 2.95 | ||
| Age (per 5 years old increase) | 0.001 | 1.20 | 1.08 | 1.33 | ||||||
| Max2 index (per 0.05 increase) | <.0001 | 0.78 | 0.71 | 0.84 | ||||||
| Baseline IADL (per 1 increase) | <.0001 | 0.90 | 0.87 | 0.93 | 0.098 | 0.96 | 0.92 | 1.01 | ||
IADL = Instrumental Activities of Daily Living
A multivariable model for overall survival was then constructed with these variables (Table 5). The TRS was an independent predictor for OS (HR= 1.94, 95% CI: 1.47–2.57) when adjusting for stage, primary tumor sites, prior chemotherapy, ECOG PS, and Baseline IADL. In multivariate analysis, the survival of patients with a TRS of 2 or more; stage 4; colorectal, pancreatobiliary, lung, and prostate and bladder primaries; prior chemotherapy; and ECOG PS 1 to 3 was significantly worse (Table 4). The survival for patients with breast cancer was better than that of the other 5 primary tumor sites.
Discussion
Comorbidity was highly prevalent in our study population, consistent with previous findings.(2, 18, 21, 22) All patients but one had one or more comorbidities, with a median of 5 comorbidities (range 0–11) per patient. As we mentioned in the introduction, this clearly presents a challenge for studies attempting to analyze one disease at a time or simply relying on general summary scores.
Using a more detailed heat-map style method, we were able to identify a high risk group of diseases that correlated with survival. The survival for high risk patients with two or more severe comorbidities from this group was significantly worse (10.8 months vs. 24.3 months, p<0.0001). The high risk group included CIRS-G score 3 or 4 in the heart, hematopoietic, respiratory and musculoskeletal & integument categories and CIRS-G score 2 to 4 in the upper gastrointestinal and liver categories. The TRS was also an independent predictor for overall survival (HR= 1.94, 95% CI: 1.47–2.57) when adjusting for stage, primary tumor site, prior chemotherapy, and ECOG PS. According to TRS, 309 patients (77%) of low risk group had better survival although they had multiple complicated or severe comorbidities. For the low risk group, median number of comorbidity was 5 (vs. 7 for the high risk group) and the proportion of patients having at least one or more severe comorbidities out of all 14 organ categories was 57.0%. For non-TRS organ categories, the proportion of at least one or more severe comorbidity was 26.2% in low risk group (vs. 45.6% in high risk group). Therefore our approach could identify different thresholds of severity in various diseases subgroups as relevant for OS in older cancer patients. As shown in Figure 3, the difference in prevalence of the14 comorbidity categories and the diseases’ severity were not only identified but also differently recognizable and easily visualized for each risk group. Heat maps allow the reader to assess which severity of diseases is the most prevalent and the most differentially expressed between groups, and if desired track patient by patient which of the sever diseases in the lower risk categories were associated with high TRS diseases. Heat maps and the associated analytic methods offer high potential for the understanding of the correlation of comorbidity with outcomes of cancer patients. For this first approach, we relied on the CIRS-G severity rating by organ category. As we had only 14 comorbidity categories, we did not need to use false discovery reduction (FDR) algorithms to screen out features not associated with survival. Future research could assess diseases individually, without presumed weights within an organ category, and FDR algorithms which have been used in omics data analysis could allow handling a very large number of diseases and end-points. This approach could also allow differential rating of the impact of individual or groups of comorbidities on survival, treatment tolerance, or function for example. The identification of clusters of diseases associated with individual toxicities would be a setting where FDR algorithms could clearly be of use.
Studies using traditional statistical approaches have mostly focused on single diseases, such as cardiopulmonary disease or diabetes mellitus, due to the difficulty of identifying enough patients with both a specific cancer and comorbidity.(23–27) However, older cancer patients have not only been reported to have substantial number of comorbidities (the median was 5 in the present study), but also the degree of severity of these comorbidities is variable. Compared with this unidimensional approach, the present study evaluated a complex pattern of 14 categories and severity of comorbidities simultaneously in an integrated approach by identifying a cluster of diseases grouped in the TRS that allowed dividing patients into two groups with a clearly different survival duration (24.3 vs 10.8 months). To our knowledge, our study provided the most detailed identification to date of a group of comorbidities collectively associated with poor survival in cancer patients.
A multidimensional approach is more sensitive than a short instrument such as the Charlson Comorbidity Index. Tammemagi et al, for example demonstrated that fact in lung cancer patients(16, 22). Nineteen out of 56 comorbidities reviewed had a deleterious effect on lung cancer survival in multivariate analysis, explaining 6.1% of survival variation compared to 2.0% for the Charlson score (and 3.7% for age, 9.2% for treatment, and 25.4% for stage)(16). Among them, anemia, COPD, asthma, pulmonary fibrosis, liver disease, gastrointestinal bleeding, connective tissue disease, and osteoporosis are belonging to the organ categories we identified in the TRS. The present study has the advantage that not only were the data extracted from prospective studies, but they also included functional data with ECOG PS and IADL. Including functional measures in studies analyzing the association of comorbidity with prognosis is important, and in older patients the use of a couple of instruments is advisable, as ECOG PS, ADL, and IADL are only moderately correlated in older cancer patients.(2). In our study, both ECOG PS and IADL had a univariable association with prognosis, although only ECOG PS remained significant in multivariable analysis. When comorbidity is assessed with short summary instruments such as the Charlson score, its association with survival may be blurred by concomitant functional impairments. For example, in the Multicenter Italian Lung Cancer in the Elderly Study (MILES) study, a worse baseline IADL score had a significant correlation with poor survival in multivariate analysis (HR=1.31, 95% CI: 1.00–1.71, p=0.04), but not ADL or comorbidity rated by Charlson.(28) A study of advanced NSCLC treated with gemcitabine and vinorelbine reported that patients dependent in two or more IADL (RR=3.33, p=0.0025) had poor survival as well as those having an ECOG PS 2 (RR=2.92, p=0.0074) in multivariate analysis, but not comorbidity by Charlson or Kaplan-Feinstein score.(29). On the other hand, a study of the independent role of age, severe comorbidity by CIRS-G and functional impairment for survival reported that IADL was not a significant predictor for OS in multivariate analysis (HR=1.209, p=0.252) but PS was significant (HR= 1.455, p=0.021), as well as severe comorbidity (HR 1.424; 95%–CI 1.012–2.003; P=0.043).(30) These literature data support the validity of an approach analyzing the largest possible number of comorbidities and our heat-map based process could identify a core of diseases that had a robust independent association with OS. The paradoxical inverse association of the MAX2 value of chemotherapy with OS likely reflects the fact that regimens used in the palliative setting are typically less intense than those used in the curative intent setting.
Our study has several limitations. First this was an ad hoc sample of patients included in clinical trials who had an available CIRS-G score. Although this allowed for high quality prospective collection of comorbidity data, it is subject to selection bias. Furthermore the patients enrolled in these studies had heterogeneous characteristics, such as primary tumor types, stages, and prior treatment. Nevertheless the high risk group by CIRS-G was an independent predictor for worse survival across the board. Our candidate high-risk comorbidity pattern should be evaluated further in larger samples of patients with more homogeneous tumor type, stage, and treatment.
In conclusion, older cancer patients with severe comorbidities in the heart, hematopoietic, respiratory, musculoskeletal/integument, upper GI and liver categories had a worse survival as a high risk group with a total risk score of 2 to 4. Given the high prevalence of multiple comorbidities in older cancer patients, methods of analysis allowing a multidimensional evaluation of comorbidity, such as heat mapping, appear to have a high potential for improving our contextual understanding of the role of these diseases in the outcomes and management of these patients. The trend in oncology is in precision medicine, and in geriatric oncology, this applies not only to the tumor, but also to the patient.
Acknowledgments
This work was supported in part by the following grants: American Cancer Society RSG-03-151-01-CCE; National Institutes on Aging grant 1R03AG034245-01; and National Cancer Institute grant P30-CA076292 (NCI Comprehensive Cancer Core grant: Moffitt Cancer Center Biostatistics Core).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest disclosures from any authors.
References
- 1.Howlader N, N A, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: http://seercancergov/csr/1975_2012/. April 2015. [Google Scholar]
- 2.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998 Apr;16(4):1582–7. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 3.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002 Oct 1;54(2):357–64. doi: 10.1016/s0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]
- 4.Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002 Mar 15;52(4):1047–57. doi: 10.1016/s0360-3016(01)02741-9. [DOI] [PubMed] [Google Scholar]
- 5.Wieringa A, Boslooper K, Hoogendoorn M, Joosten P, Beerden T, Storm H, et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. British journal of haematology. 2014 May;165(4):489–96. doi: 10.1111/bjh.12765. [DOI] [PubMed] [Google Scholar]
- 6.Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005 Sep;55(3):231–40. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Geresi K, Megyeri A, Szabo B, Szabo Z, Aradi J, Nemeth J, et al. Myelotoxicity of carboplatin is increased in vivo in db/db mice, the animal model of obesity-associated diabetes mellitus. Cancer chemotherapy and pharmacology. 2015 Mar;75(3):609–18. doi: 10.1007/s00280-015-2679-x. [DOI] [PubMed] [Google Scholar]
- 8.Gronberg BH, Sundstrom S, Kaasa S, Bremnes RM, Flotten O, Amundsen T, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. European journal of cancer (Oxford, England: 1990) 2010 Aug;46(12):2225–34. doi: 10.1016/j.ejca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Klepin HD, Pitcher BN, Ballman KV, Kornblith AB, Hurria A, Winer EP, et al. Comorbidity, Chemotherapy Toxicity, and Outcomes Among Older Women Receiving Adjuvant Chemotherapy for Breast Cancer on a Clinical Trial: CALGB 49907 and CALGB 361004 (Alliance) J Oncol Pract. 2014 Sep;10(5):e285–92. doi: 10.1200/JOP.2014.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012 Jul 1;118(13):3377–86. doi: 10.1002/cncr.26646. Epub 2011/11/11. eng. [DOI] [PubMed] [Google Scholar]
- 11.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999 Dec 30;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 12.Extermann M, Chen H, Cantor AB, Corcoran MB, Meyer J, Grendys E, et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur J Cancer. 2002 Jul;38(11):1466–73. doi: 10.1016/s0959-8049(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 13.Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007 Jan;14(1):13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 14.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. Jama. 1995 May 24–31;273(20):1605–9. [PubMed] [Google Scholar]
- 15.Tavani A, La Vecchia C, Franceschi S, Serraino D, Carbone A. Medical history and risk of Hodgkin’s and non-Hodgkin’s lymphomas. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 2000 Feb;9(1):59–64. doi: 10.1097/00008469-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003 Mar 1;103(6):792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 17.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992 Mar;41(3):237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 18.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000 Mar;36(4):453–71. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 19.Extermann M, Meyer J, McGinnis M, Crocker TT, Corcoran MB, Yoder J, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004 Jan;49(1):69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP. Scales to measure competence in everyday activities. Psychopharmacol Bull. 1988;24(4):609–14. [PubMed] [Google Scholar]
- 21.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000 Sep;35(3):181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 22.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004 Jun;57(6):597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Semrau S, Zettl H, Hildebrandt G, Klautke G, Fietkau R. Older patients with inoperable non-small cell lung cancer: long-term survival after concurrent chemoradiotherapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2014 Nov;190(12):1125–32. doi: 10.1007/s00066-014-0710-5. [DOI] [PubMed] [Google Scholar]
- 24.Louwman WJ, Janssen-Heijnen ML, Houterman S, Voogd AC, van der Sangen MJ, Nieuwenhuijzen GA, et al. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. European journal of cancer (Oxford, England: 1990) 2005 Mar;41(5):779–85. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Datema FR, Ferrier MB, van der Schroeff MP, Baatenburg de Jong RJ. Impact of comorbidity on short-term mortality and overall survival of head and neck cancer patients. Head & neck. 2010 Jun;32(6):728–36. doi: 10.1002/hed.21245. [DOI] [PubMed] [Google Scholar]
- 26.Dy SM, Sharkey P, Herbert R, Haddad K, Wu AW. Comorbid illnesses and health care utilization among Medicare beneficiaries with lung cancer. Critical reviews in oncology/hematology. 2006 Sep;59(3):218–25. doi: 10.1016/j.critrevonc.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007 Jun 15;109(12):2410–9. doi: 10.1002/cncr.22726. [DOI] [PubMed] [Google Scholar]
- 28.Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005 Oct 1;23(28):6865–72. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 29.Maestu I, Munoz J, Gomez-Aldaravi L, Esquerdo G, Yubero A, Torregrosa MD, et al. Assessment of functional status, symptoms and comorbidity in elderly patients with advanced non-small-cell lung cancer (NSCLC) treated with gemcitabine and vinorelbine. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2007 Feb;9(2):99–105. doi: 10.1007/s12094-007-0019-2. Epub 2007/03/03. eng. [DOI] [PubMed] [Google Scholar]
- 30.Wedding U, Rohrig B, Klippstein A, Pientka L, Hoffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007 Dec;133(12):945–50. doi: 10.1007/s00432-007-0233-x. [DOI] [PubMed] [Google Scholar]
- 31.Extermann M, Fulp WJ, Lee JH, Kish JA, Sehovic M, Poon D, Gwede C. Quality of life of elderly patients undergoing concomitant chemoradiation therapy for head and neck cancers, including assessment of geriatric parameters. J Clin Oncol. 2012;30(suppl) abstr 6100. [Google Scholar]
- 32.Raj S, Kish JA, Sehovic M, Extermann M. A Study of the Impact of Palliative Chemotherapy and the Effect of Age on the Quality of Life of Patients with Recurrent or Metastatic Squamous Cell Head and Neck Cancer. J Geriatr Oncol. 2013;4(suppl):S96. [Google Scholar]
- 33.Extermann M, Reich RR, Sehovic M. Chemotoxicity recurrence in older patients: Risk factors and effectiveness of preventive strategies-a prospective study. Cancer. 2015 Sep 1;121(17):2984–92. doi: 10.1002/cncr.29423. [DOI] [PMC free article] [PubMed] [Google Scholar]


