Abstract
Previous work has debated about comparisons of hearing abilities faced with alterations in hearing thresholds and evoked potentials between groups following acoustic trauma- or age-related changes. This study compares envelope following responses (EFRs) of young and aged rats when sound levels were matched according to (1) wave I amplitudes of auditory brainstem responses (ABRs) elicited by 8 kHz tones or (2) EFR amplitudes evoked by sinusoidally amplitude modulated (SAM) tones at 100 % depth. Matched wave I amplitudes across age corresponded to approximately 20 dB sound level differences. For matched wave I, no age-related differences were observed in wave V amplitudes. However, EFRs recorded in quiet were enhanced with aging at 100 % but not 25 % depth, consistent with enhanced central gain in aging. For matched EFRs, there were no age-related differences in EFRs of AM depth and AM frequency processing. These results suggest novel, objective measures beyond threshold to compensate for differences in auditory nerve activation and to differentiate peripheral and central contributions of EFRs.
1 Introduction
Auditory temporal processing deficits can be due to age-related cochlear neuropathy [Bharadwaj et al., 2014, Sergeyenko et al., 2013], endocochlear potential reduction following age-related changes in stria vascularis and diminished prestin activity [Buckiova et al., 2007, Bielefeld et al., 2008, Chen et al., 2009] or imbalances in excitation versus inhibition in the central auditory pathway [Caspary et al., 2008]. These deficits exist in nearly all listeners and proceed gradually throughout adulthood [Fitzgibbons and Gordon-Salant, 2010, Snell and Frisina, 2000, Snell et al., 2002, Ruggles et al., 2012]. Early stages of a “normal” age-related auditory functional decline may include relatively small threshold shifts, mainly at high frequencies, as well as reductions in wave I ABR amplitudes due to cochlear synaptopathy [Sergeyenko et al., 2013]. Cochlear synaptopathy, which can be caused by aging even with no history of noise exposure, is a phenomenon marked by disappearance of synapses and cochlear-nerve terminals innervating the hair cell [Sergeyenko et al., 2013, Viana et al., 2015]. Following synaptic loss, degeneration of spiral ganglion cell bodies occurs after a delay of several months and the whole process is termed cochlear neuropathy [Makary et al., 2011]. Cochlear neuropathy results in a decrease in auditory nerve fiber (ANF) population size, but does not significantly affect pure tone thresholds [Sergeyenko et al., 2013]. Instead, precise coding of temporal fine-structure and envelope cues of supra-threshold sounds are compromised in these older listeners with clinically normal hearing thresholds (NHTs; ≤20 dB hearing level) [Ruggles et al., 2012, Moore, 2016]. As this type of auditory disorder is found in listeners with clinically normal audiogram, it has been described as "hidden hearing loss" [Schaette and McAlpine, 2011].
Temporal cues, including temporal fine structure and envelope or amplitude modulation (AM), as well as the depth of signal modulations can be used by the auditory system for speech recognition and sound perception in complex environments such as the presence of competing sounds, background noise and reverberated energy [Shannon et al., 1995, Zeng et al., 2005, Snell and Frisina, 2000, Srinivasan and Zahorik, 2014]. Due to reduced temporal salience and resolution, listeners with temporal processing deficits have perceptual difficulties in spatial sound localization, pitch coding, speech-on-speech as well as speech-in-noise masking release [Stellmack et al., 2010, Jørgensen and Dau, 2011, Christiansen et al., 2013]. Many behavioral studies reveal older listeners with NHTs have poor performance on psychoacoustic tasks that heavily rely on temporal information in supra-threshold sounds as well as speech-in-noise tests [Ruggles et al., 2012, Grose and Mamo, 2010, Strelcyk and Dau, 2009, Frisina and Frisina, 1997, Strouse et al., 1998, Dubno, 1984]. Moreover, brain responses to AM stimuli reflecting reduced auditory temporal acuity in older subjects are also demonstrated in evoked potential studies [Purcell et al., 2004, Parthasarathy and Bartlett, 2011, Parthasarathy and Bartlett, 2012, Boettcher et al., 2001]. Age-related decline in temporal processing has also been reported in the auditory mid-brain in some single-unit studies [Walton et al., 2002, Walton et al., 1998, Rabang et al., 2012].
To assess auditory temporal processing, envelope-following responses (EFRs), elicited by temporally modulated stimuli, are used because these responses reflect how faithfully subcortical regions along the central auditory pathway phase-lock to the envelope of an input signal [Cunningham et al., 2001, Aiken and Picton, 2008, Parthasarathy et al., 2010, Parthasarathy and Bartlett, 2011, Parthasarathy and Bartlett, 2012, Dolphin and Mountain, 1993]. The primary generators of EFRs have shown to be the auditory brainstem and midbrain [Herdman et al., 2002, Kuwada et al., 2002, Picton et al., 2003, Chandrasekaran and Kraus, 2010, Parthasarathy and Bartlett, 2012]. Purcell et al. (2004) measured EFRs using temporally modulated sounds in quiet and showed that EFRs are correlated with behavior in humans. Purcell’s study used the same sound level in EFRs and psychoacoustic tasks for young and aged subjects. The prevailing assumption is that larger EFR amplitudes or speech-evoked ABR responses are correlated with better behavioral perception [Parthasarathy and Bartlett, 2011, Mamo et al., 2016].
One major factor that may complicate interpretations is the sound level at which stimuli are presented. In the past, most evoked potential studies have presented sounds at equal levels [Boettcher et al., 2001, Purcell et al., 2004, Clinard et al., 2010] or similar sensation levels [Parthasarathy et al., 2010, Parthasarathy and Bartlett, 2011], meaning a similar amount above an individual’s hearing threshold. However, equal levels do not account for hearing threshold differences given the wide range of clinically normal hearing. Equal sensation levels may not account for diminished auditory nerve activation due to cochlear synaptopathy and/or neuropathy. In fact, cochlear synaptopathy and neuropathy has added challenges to studies that compare temporal coding in young and aged listeners because central effects are usually confounded and compounded by peripheral effects. A degradation in the peripheral auditory system will definitely affect subsequent auditory processing in the central auditory system. As a result, without matching peripheral neural activation, a reduction in central neural responses would imply either a true degradation in central auditory processing independent of the peripheral auditory system or a decline originating from the peripheral auditory system. To remove or reduce confounding effects from the auditory periphery, there is a need to test out some other stimulus presenting methods which may allow more appropriate comparisons across age to be performed.
In order to address this issue, we compared neural temporal coding of young and aged Fischer-344 rats using stimulus intensities that matched according to wave I amplitudes of ABRs or according to EFRs at different modulation frequencies. Sergeyenko et al. (2013) have shown that reductions in auditory brainstem response (ABR) wave I amplitude indicate reduced ANFs or decreased synaptic transmission between ANFs and inner hair cells (IHCs). Hence, we attempted to equate peripheral activation by adjusting sound levels in young animals to match to aged animals’ median tone 8 kHz ABR wave I amplitudes at 85 dB pSPL. In addition to age-related changes in the auditory periphery, gain of the central auditory system has also been reported to increase to compensate for reduced ANF activation in aging [Parthasarathy et al., 2014, Kotak and Sanes, 2003, Miko and Sanes, 2009]. Therefore, we attempted to match central population activation by matching the young EFRs to the median of the aged EFRs evoked by 100 % depth AM tones at 85 dB SPL. Matching ABR wave I amplitudes should putatively match AN activation and synchronization across age, which should enable better comparisons of central auditory temporal responses. Meanwhile, matching EFR amplitudes at 100 % AM depth may equate synchronization of central neurons, which may aid in identifying whether population synchronization is robust to reduced salience of modulation, as well as demonstrating the extent of central gain relative to their peripheral activation.
As the envelope and AM depth of a sound are crucially relevant to speech perception [Krause and Braida, 2009] and neural temporal coding, we utilized sinusoidal AM (SAM) tones at various AM frequencies (AMFs) and at various AM depths in this study.
2 Materials and Methods
2.1 Subjects
Young (3–6 months, 13 male and 5 female) and aged (21–24 months, 14 male and 9 female) Fischer-344 rats obtained from Taconic were used. The numbers of animals that were used in each recording session were mentioned in each respective figure legend in the results. All animals were housed in the animal care facility during the period of this study in a relatively quiet and standard condition. All protocols were approved by the Purdue Animal Care and Use Committee (PACUC-1111000167).
2.2 Experimental procedures
The experimental protocols used for ABR and EFR recordings were similar to previously described details in Lai and Bartlett (2015), and Parthasarathy and Bartlett (2012). All auditory evoked potential recordings were performed in a 9’x9’ double-walled anechoic chamber (Industrial Acoustic Corporation). The animals were anesthetized with isofluorane at 4 % initially. While maintaining the animals under 1.5–2 % isofluorane, subdermal needle electrodes (Ambu) were placed on the animals’ scalps in a two-channel configuration. For channel 1, a positive electrode was placed along the midline of the forehead in the Cz to Fz position. For channel 2, another positive electrode was placed horizontally along the interaural line, which is above the location of the inferior colliculus. For both channels, the negative electrode was placed under the ipsilateral ear, along the mastoid, while the ground electrode was placed in the nape of the neck. Electrode impedance was confirmed to be less than 3 kΩ as tested using a low-impedance amplifier (RA4LI, Tucker Davis Technologies or TDT). Before removing from anesthesia, the animals were injected (intramuscular) with dexmedetomidine (Dexdomitor, 0.2 mg/kg), an α-adrenergic agonist acting as a sedative and an analgesic. After a 15-minute waiting period for the effect of the anesthesia to wear off, the animals were maintained in an unanesthetized condition, where they still had a foot pinch response but were immobile and relaxed for 2.5–3 hours during recording.
All stimuli were presented free-field to the right ear of the animal at a distance of 115 cm from the speaker. The speaker was calibrated using a Bruel Kjaer microphone placing at the same distance and SigCal software (TDT). Stimuli were generated using SigGenRP (TDT) at a 100-kHz sampling rate. Stimulus presentation and response acquisition were conducted using BioSig software (TDT). Waveforms were converted to sounds and delivered through a multichannel processor (RX6, TDT) via a Bower and Wilkins DM601 speaker. Digitized response waveforms were recorded with a multichannel recording and stimulation system (Rz5, TDT). Responses were analyzed with BioSig or custom written program in MATLAB (MathWorks).
2.3 Auditory stimuli
2.3.1 Matching of peripheral activation
Tone 8 kHz ABRs were recorded using brief 8 kHz pure tones of 2 ms duration (0.5 ms cos2 rise/fall time), alternating polarity and presenting at 26.6/sec. The acquisition window was set to 30 ms and each ABR was acquired as an average of 1500 repetitions. Stimulus intensity of the pure tone was decreased from 95 dB pSPL to 15 dB pSPL in 5-dB steps. This enabled us to obtain the animal’s hearing threshold at 8 kHz as well as the magnitude of wave I at each sound level, which was used as an indicator for the amount of activated ANFs. The median of tone 8 kHz ABR wave I amplitudes at 85 dB pSPL from aged animals was calculated and used for stimulus intensity matching of peripheral activation in young animals in subsequent EFR recordings.
2.3.2 Matching of central activation
SAM stimuli with a carrier frequency of 8 kHz and 200 ms duration (5 ms cos2 rise/fall time) were used as stimuli to elicit EFRs. The carrier frequency was chosen to be near the most sensitive regions of the rat’s audiogram where fewer age-related threshold changes in that frequency’s threshold have been observed [Parthasarathy et al., 2014]. Stimuli were presented at a rate of 3.1/sec and each EFR response was acquired as an average of 200 repetitions using an acquisition window of 300 ms. SAM stimuli modulated at 100 % depth at 45, 128 and 256 Hz were presented at 85 dB SPL to all aged animals to obtain a median EFR magnitude for each AMF. In young animals, SAM stimuli at each AMF were presented from 60 to 85 dB SPL in 5-dB steps. We found that this level range was appropriate to identify sound intensities for matching EFR amplitude. Young animals’ EFR magnitudes of each AMF at each sound level were estimated and were then used to determine stimulus intensity that matched to central activation of aged animals in subsequent recordings.
2.3.3 Stimuli used for EFRs
Stimulus intensities were fixed at 85 dB SPL for all stimuli when presented to aged animals. In young animals, however, lower stimulus intensities were used. To equate peripheral activation, sound levels were selected based on tone 8 kHz ABR wave I amplitudes that were closest to aged animals’ median wave I amplitudes at 85 dB pSPL. To equate central activation, sound levels were determined based on EFR magnitudes (evoked by each AMF at 100 % depth) that were closest to aged animals’ median EFR magnitudes at 85 dB SPL.
The AM depths of the same SAM stimuli (45, 128 and 256 Hz) were varied in the following steps: 0 dB (100 %), −2.5 dB (75 %), −6 dB (50 %), −12 dB (25 %), −18 dB (12.5 %), −24 dB (6.25 %) and −30 dB (3.125 %). In addition, at 100 % or 25 % AM depth, the AMF was systematically increased from 16 to 2048 Hz in 0.5-octave steps to yield the temporal modulation transfer function (tMTF). The Q10 at an 8 kHz center frequency channel is estimated to be approximately 5–6 in young rats. This estimation was extrapolated from the Q10 measured at 1–4 kHz by Moller (1977) [Moller, 1977]. Due to widening of the auditory filter, Q10s of aged animals are generally smaller than young animals. Therefore, modulation frequencies below 667 Hz [8000 Hz/6)/2] should produce unresolved components in young animals, even at 10 dB above threshold. At 30 dB or so above threshold, where most of the sounds are played in this study, it is likely that AMFs up to 1024 Hz are unresolved in young and aged rats.
2.4 Data analysis
2.4.1 ABR wave amplitude measurements
ABR waveforms were high-pass filtered at 30 Hz and low-pass filtered at 3000 Hz. ABR wave amplitudes of wave I and V were measured using BioSig software (TDT) by placing a cursor at the average of the noise floor and another cursor at the peak of the wave. The difference between the two cursors indicated the wave amplitude.
2.4.2 EFR magnitude measurements
All collected EFRs were low-pass filtered at 3000 Hz. EFRs were also high-pass filtered at a frequency below the AMF for the slower AMFs (e.g. 30 Hz for 45 Hz AM stimuli) and at 80 Hz for AMFs faster than 90 Hz. Filtered data were then exported as text files and analyzed using custom written MATLAB programs. Fast Fourier transforms (FFTs) were performed on the time-domain waveform from 10 to 190 ms relative to stimulus onset to exclude transient ABRs at the beginning. The maximum magnitude of the evoked response at one of the three frequency bins (3 Hz/bin) around AMF was measured as the peak FFT amplitude. The noise floor was calculated as the average magnitude of five frequency bins above and below the central three bins. A peak response was taken to be significantly above noise level if the FFT amplitude was at least 6 dB above the noise floor for the slower AMFs and at least 10 dB above the noise floor for AMFs faster than 64 Hz, where the noise floor is lower.
2.5 Statistical analysis
Non-parametric rank-sum tests were used to compare ABR or EFR amplitudes between young and aged animals because the distributions of the aged amplitudes may not be normally distributed. Repeated measures ANOVAs (rmANOVAs) were performed to compare FFT amplitudes of young and aged groups as well as across different stimulus conditions using custom written scripts in SAS (Proc MIXED, SAS Institute, Cary, NC, USA). Main effects and interaction effects of each factor were analyzed based on comparisons of least squares (LS) means. Data distributions were checked for normality using normal probability plots of the residuals (proc UNIVARIATE). The differences in LS means with a confidence level of 95 % were used when reporting significant differences. LS means +/− standard error of mean are shown in the figures.
3 Results
3.1 Equivalent peripheral and central activation protocol
The average ABR thresholds for clicks were 50.1 +/− 2.1 dB SPL in the aged and 28.7 +/− 1.6 dB SPL in the young. For 8 kHz tone ABRs, the average thresholds were 39.5 +/− 1.5 dB SPL in the aged and 23 +/− 1.3 dB SPL in the young. The thresholds of click ABRs were 10.89 +/− 1.48 dB and 5.96 +/− 0.98 dB significantly higher than tone 8 kHz ABRs in aged and young animals, respectively. F344 rats have the most sensitive hearing regions at 6–16 kHz, and the current 8 kHz threshold differences are slightly larger than previously reported [Parthasarathy et al., 2014]. All the stimulus intensities used in aged animals were fixed at 85 dB SPL, which is typically near the peak EFR amplitude for aged animals [Parthasarathy et al., 2014]. Table 1 shows the means of the sound levels that were used in the young for equivalent peripheral or central activation, respectively. For central matching, the young sound levels were matched specifically for each AMF (45, 128 or 256 Hz) at 100 % modulation depth. A rmANOVA showed that sound intensities for peripheral matching were significantly lower than sound intensities for central matching at 45 (t=3.15, p < 0.05) and 256 Hz (t=3.15, p < 0.05) AMFs, respectively.
Table 1.
Sound levels used for central matching in the young were slightly higher than those used for peripheral matching according to the protocol. Mean sound levels presented to the young for matched peripheral and central activation are reported in the table. Standard errors of means (S.E.M.) are shown in parentheses.
| Matching | Mean sound level dB SP (S.E.M.) |
|---|---|
|
| |
| Peripheral activation (ABR wave I amp.) | 66.3 (1.33), N = 17 |
|
| |
| Central activation (EFR amp.) | 45 Hz: 71.0 (1.39), N = 15 |
| 128 Hz: 68.3 (1.18), N = 9 | |
| 256 Hz: 69.9 (1.34), N = 18 | |
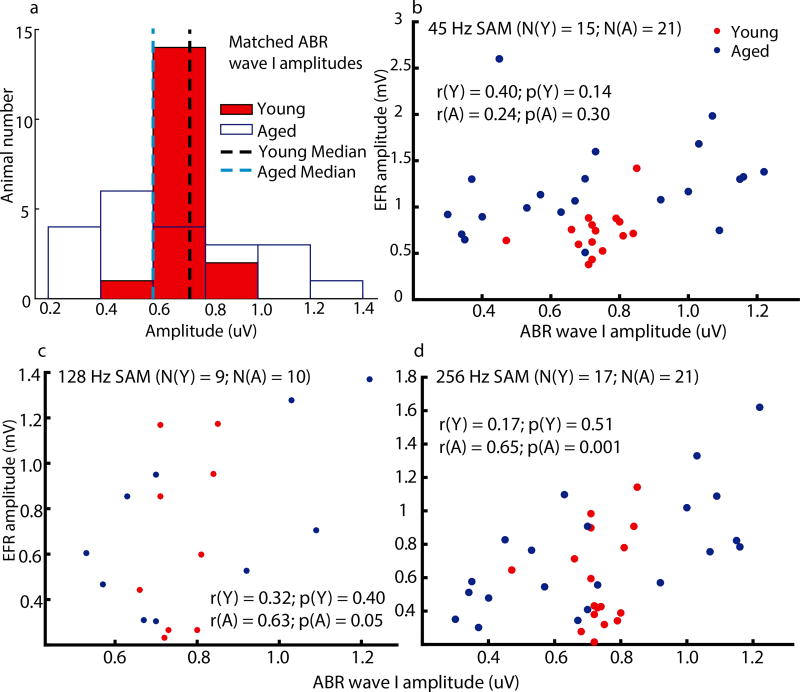
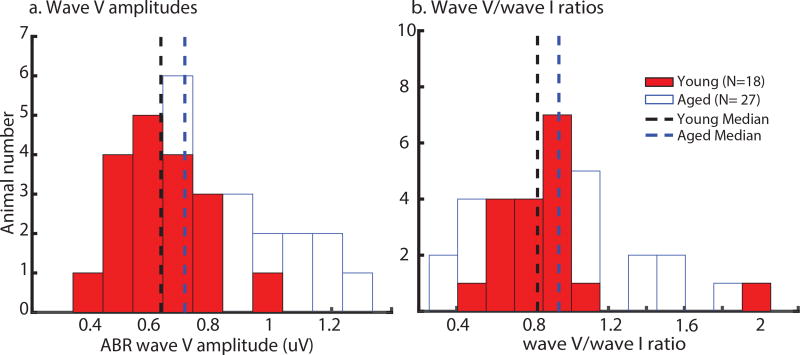
Figure 1a shows the distributions of tone 8 kHz ABR wave I amplitudes that were used for matching of peripheral activation in young and aged animals. Figures 1b–d are scatter plots showing EFR amplitudes at each AMF which were elicited following tone 8 kHz ABR wave I amplitude matching. Although the medians of young and aged animals were matched, there was a wider range in aged animals. For aged animals, correlations between ABR wave I amplitudes and EFR amplitudes were significant for 256 Hz and marginally significant for 128 Hz. For young animals, correlations between ABR wave I amplitudes and EFR amplitudes were not observed for any of the tested SAM because EFR amplitudes of the young were selected to be close (within 1 +/− SD) to the aged median. Again, EFR amplitudes of the aged were found to be varied more than the young. ABR wave V amplitudes and wave V/wave I ratios were also measured in all animals at matched peripheral activation. The histograms of wave V amplitudes or wave V/wave I ratios for the two age groups are shown in Fig. 2. The young histograms had a narrower spread than the aged histograms. However, there were no significant age-related differences in wave V amplitudes and wave V/wave I ratios according to the results of a non-parametric rank-sum test.
Figure 1. Tone 8 kHz ABR wave I amplitudes of young animals were matched to the median of aged animals’ amplitudes to equate neural excitation in the auditory periphery.
(a) Histograms of tone 8 kHz ABR wave I amplitudes of all young (red solid bars) and aged (white bars) animals show that the range of the younger amplitudes was narrower and their median was close to the aged median. Scatter plots of each individual animal’s EFR amplitude to (b) 45, (c) 128, or (d) 256 Hz at matched ABR wave I amplitude also show that variation was larger in aged animals. In the scatter plots, N denotes number of animals, Y denotes the young, A denotes the aged, r indicates correlation coefficient, and p indicates p-value.
Figure 2. Wave V amplitude and wave V/wave I ratio histograms of the young (red bars) had a narrower spread, but the aged histograms (white bars) were more right-skewed with a longer tail.
Statistical comparison showed no significant age-related differences in tone 8 kHz ABR wave V amplitudes and wave V/wave I ratios at matched peripheral activation.
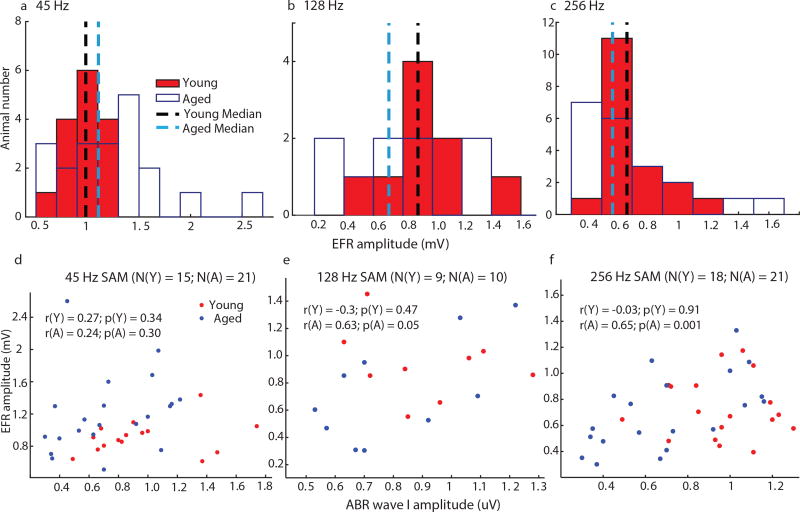
Figures 3a–c are the histograms showing EFR amplitudes, at each tested AMF, of young and aged animals that were used for matched central activation. Although the spreads of the histograms of the young and the aged were not completely overlapping, there were no significant age-related differences using non-parametric rank-sum tests. The scatter plots of EFR amplitude vs. ABR wave I amplitude at matched central activation for each AMF were plotted in Figures 3d–f. The correlations between ABR wave I amplitudes and EFR amplitudes were significant for 256 Hz and marginally significant for 128 Hz in aged animals. The variations of the young ABR wave I amplitudes at each AMF were larger because wave I amplitudes were not matched when central activation was equated.
Figure 3. In order to equate neural activation in the central auditory system, EFR amplitudes to either (a) 45, (b) 128 or (c) 256 Hz of young animals were matched to those of aged animals.
The medians of aged animals were used as references in the neural activation matching protocol.
3.2 Temporal processing of AM depth and AM frequency
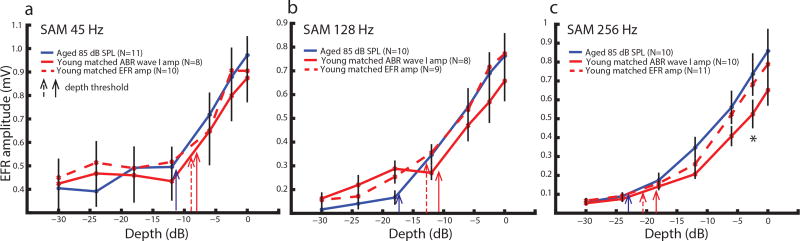
When EFR amplitudes were recorded at sound levels that evoked maximal EFRs, there were significant age-related differences at larger modulation depths for 128 and 256 Hz AMFs [Parthasarathy and Bartlett, 2012]. The sound levels used, however, could synchronize different amount of neurons peripherally or centrally. Therefore, AM depth processing was compared at matched neural activation to test the existences of age-related differences at a "fairer" condition. SAM tones at 45, 128 or 256 Hz with modulation depths from −30 dB (3.125 %) to 0 dB (100 %) were presented to young and aged animals at matched peripheral or central activation. EFR amplitudes as a function of modulation depth were plotted in Figure 4 for the modulation frequencies tested. Almost no significant age-related differences were observed at the tested SAM frequencies and modulation depths, except 256 Hz SAM tone at −2.5 dB (75 %) depth for matched peripheral activation. Statistical analysis using rmANOVA revealed no significant main effect of age or type of matching. There was only a significant main effect of AM depth, which was unsurprising. This implies that young and aged animals have similar auditory processing in quiet using sound levels that matched central or peripheral activation.
Figure 4. Minimal age-related differences in EFR amplitudes elicited by SAM tones presented in quiet at equivalent central or peripheral activation.
Similar results were observed in EFR amplitudes to SAM stimuli at (a) 45, (b) 128 and (c) 256 Hz AM as a function of modulation depth. The asterisk indicates p < 0.05 for the young matched wave I vs. the aged using least squares means comparison from rmANOVA.
Table 2 shows the mean modulation depth neurometric thresholds (in dB re:100% modulation) of the young and aged EFRs for AM depth processing. Two separate rmANOVAs were performed on the aged vs. the young at matched wave I and the aged vs. the young at matched EFRs. The AMF effect was significant for matched wave I (F = 22.1, p < 0.05) and matched EFRs (F = 26.11, p < 0.05). The age effect was only significant for matched wave I (F = 5.54, p < 0.05). Statistical analysis of differences of LS means only shows significance for thresholds of the aged vs. the young at matched wave I for 128 Hz AM (t = −2.23, p < 0.05).
Table 2.
Mean modulation depth neurometric thresholds (in dB) based on EFR amplitudes show trends of lower thresholds in the aged than the young at equivalent peripheral and central activation. Thresholds were determined at the cut-off of 6 dB (45 Hz) or 10 dB (128 and 256 Hz) above noise floor for the specific FFT peak. Standard errors of means are shown in parentheses.
| Animals | 45 Hz AM | 128 Hz AM | 256 Hz AM |
|---|---|---|---|
|
| |||
| Aged | −11.75 (1.91) | −17.47 (3.06) | −23.42 (1.93) |
| Young - Wave I matched | −8.12 (1.81) | −10.67 (1.97) | −18.67 (1.56) |
| Young - EFR matched | −8.74 (2.29) | −13.21 (1.75) | −20.46 (1.51) |
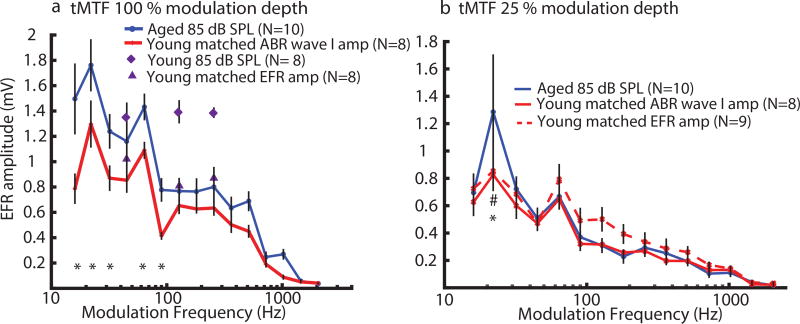
EFRs of tMTF at 100 % AM depth, for matched wave I amplitude (peripheral activation), showed a significant increase in amplitudes in aged animals especially at lower SAM frequencies (16–90 Hz; Fig. 5a). This observation agrees with our previous findings of an increase in central gain in aged animals to compensate for diminished AN activities in the auditory periphery [Parthasarathy et al., 2014]. When the young EFRs at 85 dB SPL were compared to the aged EFRs at 85 dB SPL, their EFRs at 45 Hz were close to each other but EFRs at 128 and 256 Hz were higher in the young suggesting reduced EFRs in the aged at higher AMFs at equal sound level. However, the phenomenon of increased central gain in aged animals was not observed in EFRs of tMTF at 25 % AM depth (Fig. 5b). For matched EFR amplitudes (central activation), a slight elevation of EFR amplitudes in young animals was observed at 90 to 181 Hz SAM frequencies but no significant age-related differences were observed. For tMTF at 100 % AM depth, significant main effects of Age (F = 6.02, p < 0.05) and SAM Frequency (F = 48.53, p < 0.0001) as well as Age*SAM Frequency interaction (F = 2.20, p < 0.05) effect were observed.
Figure 5. EFR amplitudes to SAM stimuli with lower AM frequencies (16–90 Hz) at 100 % depth were larger in aged animals than in young animals, even when peripheral activation was matched, but not at 25 %.
EFRs of temporal modulation transfer function (tMTF) with SAM frequencies of 16–2048 Hz at either (a)100 % or (b)25 % modulation depth. The asterisks indicate p < 0.05 for the young peripheral matching vs. the aged while the pound sign indicates p < 0.05 for the young central matching vs. the aged. Statistically significant differences were obtained using least squares means comparison from rmANOVA.
4 Discussion
4.1 Matching protocol for auditory evoked potential recordings
In this study, we investigated age-related changes of EFRs elicited by SAM tones at equivalent peripheral or central neural activation. As recent evidence has demonstrated that listeners with cochlear synaptopthy and/or neuropathy have NHTs but degraded temporal coding of supra-threshold sounds [Bharadwaj et al., 2014], it has become clear that simply compensating hearing threshold shift or ensuring similar audiograms in young and aged listeners is not sufficient. Furthermore, the 20 dB range of clinically normal hearing can permit a wide range of audibility in human studies. However, to our knowledge, no studies have attempted to compare across age based on comparable physiological responses. To account for cochlear synaptopathy and neuropathy as well as age-related changes in hearing sensitivity, we attempted to equate peripheral neural activation by matching ABR wave I amplitudes in young and aged animals. ABR wave I amplitudes reflect a combined measure of the number of activated ANFs and their synchrony [Rowe, 1981, Chen and Chen, 1991]. Previous studies in rodents and mammals also implied that ABR wave I amplitude was correlated with the number of surviving ANFs and IHC-ANF synapses [Kujawa and Liberman, 2009, Lin et al., 2011]. Speech perceptual ability in noise has been found to correlate with ABR wave I amplitude as well [Bramhall et al., 2015]. In the auditory periphery, cochlear filter shapes and frequency specificity could be different between young and aged animals [May et al., 2006, Ananthakrishnan et al., 2016, Lai and Bartlett, 2015].
The sound levels that we were using for young animals were ~ 43 dB on average above the young mean 8 kHz hearing threshold (~ 23 dB SPL). For aged animals, the sound level was ~ 46 dB above the aged mean 8 kHz hearing threshold (~ 39.5 dB SPL). The filter shapes of auditory neurons at 8 kHz for young and aged animals were estimated using an auditory nerve model [Parthasarathy et al., 2016] in order to calculate the Q-values. The estimated Q-values at the tested mean sound levels were about 1.12 and 0.61 for young and aged animals, respectively(data not shown). The low-frequency boundaries of the young and aged filters are rather similar and the difference in Q-values was due more to an upward shift in hearing threshold. Therefore, the region of basilar membrane excited in order to achieve comparable wave I amplitudes was larger with aging, which may partially compensate for the loss of IHC-AN synapses. It also suggests that at these supra-threshold levels, auditory neurons above the carrier frequency will provide a greater proportional contribution in aged animals. Therefore, the effects of high frequency hearing loss will extend to lower frequency carriers at moderate to high sound levels.
In addition, our previous work and other studies have shown that central auditory neurons may adjust their gain in aged animals to compensate for diminished AN input [Parthasarathy et al., 2014, Kotak and Sanes, 2003, Miko and Sanes, 2009]. Hence, to account for age-related differences in central gain, we attempted to equate central neural activation in a separate test by matching EFR amplitudes in young and aged animals. As EFR amplitude reflects the faithfulness of populations of neurons in the auditory brainstem and midbrain in encoding and phase-locking to the modulation envelope of complex sounds [Picton et al., 2003, Cunningham et al., 2001, Aiken and Picton, 2008, Clinard et al., 2010, Parthasarathy and Bartlett, 2011], matching EFR amplitudes should produce comparable central activation across age.
In young animals, the sound levels used for matching peripheral activation were similar to the sound levels used for matching central activation. Table 1 shows that mean sound level for peripheral matching was generally lower than mean sound levels of all the three tested AMFs for central matching. Differences in sound level were statistically significant when comparing sound levels for peripheral matching to those for central matching at 45 Hz AMF. This suggests that matching of central activation can be achieved with smaller reduction in sound intensities compared to equivalent peripheral activation. This 3–5 dB difference potentially represents the degree of enhanced gain in this population of older animals, since a slightly larger wave I amplitude and sound level was needed to evoke the same EFR response in younger animals, consistent with previous findings [Parthasarathy et al., 2014]. The enhanced gain with aging, however, does not fully restore central activation, since peak EFR amplitudes in older animals are smaller than those in younger animals.
4.2 Temporal processing of AM depth and AM frequency
Beyond reduced audibility and declined cognitive function in aging, speech intelligibility is very likely to be affected by age-related changes in temporal processing [Gordonsalant and Fitzgibbons, 1993, Snell, 1997, Strouse et al., 1998, Fullgrabe et al., 2015]. Temporal resolution has proven to be vital for precise speech understanding in quiet as well as in challenging listening conditions [Dubno et al., 2003, Drullman, 1995]. Temporal processing deficits have been associated with reduced identification of words and sentences presented in steady-state and modulated noise [Gordonsalant and Fitzgibbons, 1993, Helfer and Vargo, 2009]. Therefore, more stringent conditions, for example, compensating for shifts in hearing thresholds or ANF loss, addition of noise, etc., should be applied in aging studies of auditory processing. We believe that this may aid in teasing apart the central and the peripheral effects in age-related changes of auditory temporal processing.
Our findings in SAM depth processing (Fig. 4) and modulation frequency processing at 25 % modulation depth (Fig. 5b) showed minimal EFR amplitude differences in young and aged animals for both equivalent peripheral and central activation. For SAM depth processing, there was no age effect for the tested SAM frequencies (45, 128 and 256 Hz). This suggests that the population-level synchrony in midbrain declines with depth similarly across age. For SAM frequency processing, aged animals had higher EFR amplitudes at 100 % modulation depth than the young at matched ABR wave I amplitude at AMFs of 16–90 Hz (Fig. 5a) as well as an overall main effect of age across the tMTF. This is consistent with our previous finding which suggested age-related changes in gain mechanisms in the the relationship between ABRs and EFRs [Parthasarathy et al., 2014]. At higher AMFs (≥ 90 Hz), the aged EFRs tended to be higher than the young EFRs although the aged EFRs at 85 dB SPL were lower than the young EFRs at 85 dB SPL (Fig. 5a). This is consistent with a previous study showing enhanced speech sound-evoked magnetic fields (the middle-latency P1m wave and the long-latency N1m) in older normal-hearing human subjects, suggesting age-related changes during healthy aging due to experience-related neuroplasticity [Soros et al., 2009]. However, age-related increases in EFRs were not observed when the modulation depths of SAM stimuli reduced to 25 % (Fig. 5b). This suggests that the compensatory mechanisms are not robust to reductions in the salience of temporal cues. Overall, similar EFRs in young and aged animals for AM depth processing and AMF processing at 25 % modulation depth are analogous to other findings that older listeners have auditory processing comparable to young listeners in ideal listening conditions [Ruggles et al., 2012, Gordonsalant and Fitzgibbons, 1993, Snell and Frisina, 2000, Yonan and Sommers, 2000].
Comparison of tone 8 kHz ABR Wave V amplitudes between aged and young animals at equal peripheral activation (Fig. 2) did not produce statistically significant differences, although the aged distribution is more right-skewed and has a longer tail than the young. These results suggest that onset transients evoked by the brief 2 ms tone pips are comparable in young and aged animals, whereas gain enhancement appears to occur for sustained modulated stimuli measured by EFRs. Previous work from our lab has shown that stimuli with rapid onsets were not significantly different in aged animals [Parthasarathy and Bartlett, 2011].
On the other hand, increased EFR amplitudes at matched peripheral activation that we observed in the aged could also be due to intact low-frequency cochlear filters in aged animals but impaired high-frequency cochlear filters. Their high-frequency impairment could potentially lead to lower EFRs at high AMFs which then matches the young EFRs. However, additional empirical measurements on the cochlear filters of young and aged animals would be required to test the validity of this hypothesis.
4.2.1 Aging, synaptopathy, and central gain
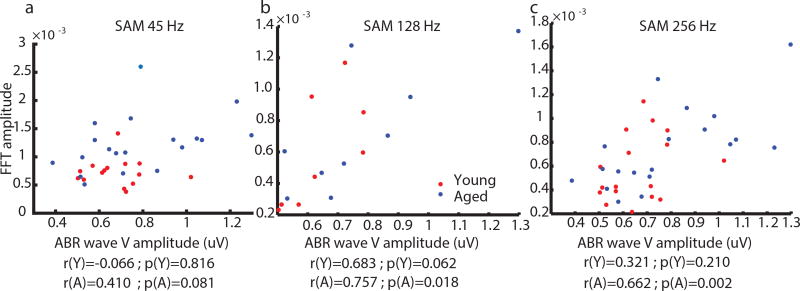
To further explore the relationship of aging, synaptopathy and central gain, correlations of tone ABR wave V amplitudes versus EFR amplitudes (at AMFs of 45, 128, or 256 Hz) were measured at matched wave I amplitudes (Fig. 6). For AMF of 45 Hz, correlations were not significant in either young or aged animals. Contributions from the auditory cortex may be involved at 45 Hz AMF as well as lower AMFs that could decorrelate the two measures [Joris et al., 2004, Coffey et al., 2016, Coffey et al., 2017]. For AMFs of 128 and 256 Hz, correlations of ABR wave V amplitudes versus EFR amplitudes were significant in the aged but not in young animals. This suggests that EFRs in aged animals may be measuring a simpler relay of brainstem inputs through the inferior colliculus, whereas young animals may transform their inputs more significantly, potentially via stronger inhibition, altered inferior colliculus neuron properties, or more complex network activities [Parthasarathy et al., 2014, Caspary et al., 2008, Burianova et al., 2009, Godfrey et al., 2017].
Figure 6. Correlations of ABR wave V and EFR amplitudes were only significant in aged animals at 128 and 256 Hz AM frequencies.
Correlations were not significant in young animals for either 45, 128 or 256 Hz AM frequencies. Both ABRs and EFRs were recorded at 85 dB SPL in aged animals whereas various sound levels were used in young animals based on wave I matching. The correlation coefficients (r) and the corresponding p-values (p) are shown below the plots. Y (red dots) denotes the young and A (blue dots) denotes the aged.
We have investigated a method that allows animals to be compared across groups by enforcing comparable measures of supra-threshold peripheral activation, enabling one to more clearly identify changes in central processing. Peripheral activation in the present study was measured using ABR wave I, which is proportional to the number of intact IHC-AN synapses [Chambers et al., 2016, Möhrle et al., 2016, Sergeyenko et al., 2013]. Comparable wave I amplitudes therefore suggest comparable aggregate numbers of IHC-AN synapses synchronously and transiently activated by the tone ABR stimuli used. Although IHC-AN synapses are not required for some degrees of sound detection and discrimination [Chambers et al., 2016, Wang et al., 1997], their function is correlated with rapid temporal processing [Chambers et al., 2016, Möhrle et al., 2016]. The present study used supra-threshold sound levels (40–50 dB SL) that would drive activation of high and low spontaneous rate auditory nerve fibers. It would be helpful in future studies to compare measures closer to threshold (10–20 dB SL) where activation should primarily be driven by high spontaneous rate AN fibers. A previous study showed that moderate noise-induced synaptopathy, which may selectively damage low spontaneous rate fibers, could still produce intact tone responses in inferior colliculus at low to moderate levels [Hesse et al., 2016] likely to be mediated by high spontaneous rate AN fibers.
Our results showed that compensation of wave I amplitudes between young and aged produces enhanced EFR responses (Fig. 5A), implying an enhancement of central gain. This is consistent with other work in aging [Sergeyenko et al., 2013, Parthasarathy et al., 2014, Möhrle et al., 2016] and noise exposure or tinnitus [Schaette and McAlpine, 2011, Möhrle et al., 2016, Hesse et al., 2016]. Decline in OHC function as measured by DPOAE will also play a role in auditory declines with aging [Lai and Bartlett, 2015, Sergeyenko et al., 2013] but does not appear to affect estimates of central gain [Verhulst et al., 2016] since all AN fibers would be affected. Although there are differences between aging and noise exposure, both can produce mixed OHC and IHC-AN damage, and the relative degree of that damage may affect individual changes in ABR and EFR measures [Bharadwaj et al., 2014, Verhulst et al., 2016, Zhong et al., 2014] and auditory nerve measures [Kale and Heinz, 2012]. As shown previously [Parthasarathy et al., 2010] and in Figs. 1 and 3, ABR and EFR measures may be poorly correlated, implying that they can provide complementary information. An enhancement of onset gain, as measured by comparison of ABR waves I and V, may be different than the enhancement of sustained modulations measured in unit recordings or EFR. This idea is supported by measurements in humans, where onset and envelope components related to perception and DPOAE measurements could be separated [Dhar et al., 2009].
5 Conclusions
This study revisited the issue of age-related changes in auditory temporal processing by testing different ways of manipulating stimulus intensities to attain comparable peripheral or central neural activation for auditory evoked potential recordings. This study aimed to provide a measurable basis for sound-level comparisons of auditory temporal processing, which can be applied not only to aging studies, but also other studies involving auditory damage, such as noise-induced hearing loss, blast-related acoustic trauma, or diseases affecting hearing [Lew et al., 2007, Seemungal et al., 2015, Alberti, 1992, Race et al., 2017]. Tone 8 kHz ABR wave I amplitude was used as an indicator for peripheral matching while EFR amplitude to 100 % SAM tone was used for central matching. We observed negligible differences in EFRs between aged and young animals at both equivalent peripheral and central activation for SAM depth processing at the modulation frequencies tested. Higher EFRs in AMF processing were obtained in aged animals for SAM tones with 100 % depth, suggestive of enhanced central gain, but not 25 % depth, when compared to the young at matched peripheral activation. Overall, these results demonstrate that compensating for peripheral or central activation can substantially aid in differentiating changes in neural representations of temporally modulated sounds.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NIDCD R01DC011580). The authors would like to thank Dr. Aravindak-shan Parthasarathy for his help in generating the auditory neuron filters using modeling, and Nicholas Race for his helpful comments on an initial draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have no conflict of interest to disclose.
References
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hearing Research. 2008;245(1–2):35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Alberti P. Noise induced hearing-loss. British Medical Journal. 1992;304(6826):522. doi: 10.1136/bmj.304.6826.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan S, Krishnan A, Bartlett E. Human Frequency Following Response: Neural Representation of Envelope and Temporal Fine Structure in Listeners with Normal Hearing and Sensorineural Hearing Loss. Ear and hearing. 2016;37(2):91–103. doi: 10.1097/AUD.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Frontiers in systems neuroscience. 2014;8:1–18. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld EC, Coling D, Chen GD, Li M, Tanaka C, Hu BH, Henderson D. Age-related hearing loss in the Fischer 344/NHsd rat substrain. Hearing Research. 2008;241(1–2):26–33. doi: 10.1016/j.heares.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Poth EA, Mills JH, Dubno JR. The amplitude-modulation following response in young and aged human subjects. Hearing Research. 2001;153(1–2):32–42. doi: 10.1016/s0378-5955(00)00255-0. [DOI] [PubMed] [Google Scholar]
- Bramhall N, Ong B, Ko J, Parker M. Speech Perception Ability in Noise is Correlated with Auditory Brainstem Response Wave I Amplitude. Journal of the American Academy of Audiology. 2015;26(5):509–17. doi: 10.3766/jaaa.14100. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Popelar J, Syka J. Aging cochleas in the F344 rat: morphological and functional changes. Exp Gerontol. 2007;42(7):629–38. doi: 10.1016/j.exger.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Experimental gerontology. 2009;44(3):161–9. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781–91. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron. 2016;89(4):867–879. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology. 2010;47(2):236–46. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Li M, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hearing Research. 2009;248(1–2):39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chen T, Chen S. Generator study of brainstem auditory evoked potentials by a radiofrequency lesion method in rats. Experimental brain research. 1991;85(3):537–42. doi: 10.1007/BF00231737. [DOI] [PubMed] [Google Scholar]
- Christiansen C, MacDonald EN, Dau T. Contribution of envelope periodicity to release from speech-on-speech masking. The Journal of the Acoustical Society of America. 2013;134(3):2197–204. doi: 10.1121/1.4816409. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings. Hearing Research. 2010;264(1–2):48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey EB, Musacchia G, Zatorre RJ. Cortical Correlates of the Auditory Frequency-Following and Onset Responses: EEG and fMRI Evidence. The Journal of Neuroscience. 2017;37(4):830–838. doi: 10.1523/JNEUROSCI.1265-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey EBJ, Herholz SC, Chepesiuk AMP, Baillet S, Zatorre RJ. Cortical contributions to the auditory frequency-following response revealed by MEG. Nature Communications. 2016;7:11070. doi: 10.1038/ncomms11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol. 2001;112(5):758–67. doi: 10.1016/s1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- Dhar S, Abel R, Hornickel J, Nicol T, Skoe E, Zhao W, Kraus N. Exploring the relationship between physiological measures of cochlear and brainstem function. Clinical Neurophysiology. 2009;120:959–966. doi: 10.1016/j.clinph.2009.02.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin WF, Mountain DC. The envelope following response (EFR) in the Mongolian gerbil to sinusoidally amplitude-modulated signals in the presence of simultaneously gated pure-tones. The Journal of the Acoustical Society of America. 1993;94(6):3215–30. doi: 10.1121/1.407227. [DOI] [PubMed] [Google Scholar]
- Drullman R. Temporal envelope and fine structure cues for speech intelligibility. The Journal of the Acoustical Society of America. 1995;97(1):585–92. doi: 10.1121/1.413112. [DOI] [PubMed] [Google Scholar]
- Dubno JR. Effects of age and mild hearing loss on speech recognition in noise. The Journal of the Acoustical Society of America. 1984;76(1):87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Recovery from prior stimulation: Masking of speech by interrupted noise for younger and older adults with normal hearing. The Journal of the Acoustical Society of America. 2003;113(4):2084–94. doi: 10.1121/1.1555611. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Behavioral studies with aging humans: Hearing sensitivity and psychoacoustics. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System, volume 34 of Springer Handbook of Auditory Research. Springer New York; New York, NY: 2010. pp. 111–34. [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hearing Research. 1997;106(1–2):95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Fullgrabe C, Moore BCJ, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Frontiers in Aging Neuroscience. 2015;6:1–25. doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DA, Chen K, O’Toole TR, Mustapha AI. Amino acid and acetylcholine chemistry in the central auditory system of young, middle-aged and old rats. Hearing Research. 2017;350:173–188. doi: 10.1016/j.heares.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Gordonsalant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. Journal of Speech and Hearing Research. 1993;36(6):1276–85. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of Temporal Fine Structure as a Function of Age. Ear and Hearing. 2010;31(6):755–60. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer KS, Vargo M. Speech Recognition and Temporal Processing in Middle-Aged Women. Journal of the American Academy of Audiology. 2009;20(4):264–71. doi: 10.3766/jaaa.20.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman AT, Lins O, Van Roon P, Stapells DR, Scherg M, Picton TW. Intracerebral sources of human auditory steady-state responses. Brain topography. 2002;15(2):69–86. doi: 10.1023/a:1021470822922. [DOI] [PubMed] [Google Scholar]
- Hesse LL, Bakay W, Ong H-C, Anderson L, Ashmore J, McAlpine D, Linden J, Schaette R. NonMonotonic Relation between Noise Exposure Severity and Neuronal Hyperactivity in the Auditory Midbrain. Frontiers in Neurology. 2016:7. doi: 10.3389/fneur.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen S, Dau T. Predicting speech intelligibility based on the signal-to-noise envelope power ratio after modulation-frequency selective processing. The Journal of the Acoustical Society of America. 2011;130(3):1475–87. doi: 10.1121/1.3621502. [DOI] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiological Reviews. 2004;84:541–77. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Temporal modulation transfer functions measured from auditory-nerve responses following sensorineural hearing loss. Hearing Research. 2012;286(1–2):64–75. doi: 10.1016/j.heares.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Gain adjustment of inhibitory synapses in the auditory system. Biological Cybernetics. 2003;89(5):363–70. doi: 10.1007/s00422-003-0441-7. [DOI] [PubMed] [Google Scholar]
- Krause JC, Braida LD. Evaluating the role of spectral and envelope characteristics in the intelligibility advantage of clear speech. The Journal of the Acoustical Society of America. 2009;125(5):3346–57. doi: 10.1121/1.3097491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. Journal of Neuroscience. 2009;29(45):14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D’Angelo WR. Sources of the scalp-recorded amplitude-modulation following response. Journal of the American Academy of Audiology. 2002;13(4):188–204. [PubMed] [Google Scholar]
- Lai J, Bartlett EL. Age-related shifts in distortion product otoacoustic emissions peak-ratios and amplitude modulation spectra. Hearing Research. 2015;327:186–98. doi: 10.1016/j.heares.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. J Rehabil Res Dev. 2007;44(7):921–8. doi: 10.1682/jrrd.2007.09.0140. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary Neural Degeneration in the Guinea Pig Cochlea After Reversible Noise-Induced Threshold Shift. Journal of the Association for Research in Otolaryngology. 2011;12(5):605–16. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. Journal of the Association for Research in Otolaryngology. 2011;12(6):711–7. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo SK, Grose JH, Buss E. Speech-evoked ABR: Effects of age and simulated neural temporal jitter. Hearing Research. 2016;333:201–9. doi: 10.1016/j.heares.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ, Kimar S, Prosen CA. Auditory filter shapes of CBA/CaJ mice: Behavioral assessments. The Journal of the Acoustical Society of America. 2006;120(1):321–30. doi: 10.1121/1.2203593. [DOI] [PubMed] [Google Scholar]
- Miko IJ, Sanes DH. Transient gain adjustment in the inferior colliculus is serotonin- and calcium-dependent. Hearing Research. 2009;251(1):39–50. doi: 10.1016/j.heares.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhrle D, Ni K, Varakina K, Bing D, Lee SC, Zimmermann U, Knipper M, Rüttiger L. Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiology of Aging. 2016;44:173–184. doi: 10.1016/j.neurobiolaging.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Moller AR. Frequency selectivity of single auditory-nerve fibers in response to broadband noise stimuli. The Journal of the Acoustical Society of America. 1977;62(1):135–42. doi: 10.1121/1.381495. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. Effects of Age and Hearing Loss on the Processing of Auditory Temporal Fine Structure. Advances in experimental medicine and biology. 2016;894:1–8. doi: 10.1007/978-3-319-25474-6_1. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett E. Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hearing Research. 2012;289(1–2):52–62. doi: 10.1016/j.heares.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192:619–30. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Frontiers in Aging Neuroscience. 2010;2:1–10. doi: 10.3389/fnagi.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JA, Hopkins C, Bartlett EL. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. Journal of the Association for Research in Otolaryngology. 2014;15(4):649–61. doi: 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Lai J, Bartlett EL. Age-Related Changes in Processing Simultaneous Amplitude Modulated Sounds Assessed Using Envelope Following Responses. Journal of the Association for Research in Otolaryngology. 2016;17(2):119–32. doi: 10.1007/s10162-016-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. International journal of audiology. 2003;42(4):177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- Purcell DW, John SM, Schneider BA, Picton TW. Human temporal auditory acuity as assessed by envelope following responses. The Journal of the Acoustical Society of America. 2004;116(6):3581–93. doi: 10.1121/1.1798354. [DOI] [PubMed] [Google Scholar]
- Rabang CF, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, Bartlett EL. A Computational Model of Inferior Colliculus Responses to Amplitude Modulated Sounds in Young and Aged Rats. Frontiers in Neural Circuits. 2012;6(77):1–25. doi: 10.3389/fncir.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race N, Lai J, Shi R, Bartlett EL. Differences in post-injury auditory system pathophysiology after mild blast and non-blast acute acoustic trauma. Journal of Neurophysiology. 2017 doi: 10.1152/jn.00710.2016. page [Eprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MJ. The brainstem auditory evoked response in neurological disease: a review. Ear and Hearing. 1981;2(1):41–51. doi: 10.1097/00003446-198101000-00008. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Why Middle-Aged Listeners Have Trouble Hearing in Everyday Settings. Current Biology. 2012;22(15):1417–22. doi: 10.1016/j.cub.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. Journal of Neuroscience. 2011;31(38):13452–7. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal B, Kaski D, Lopez-Escamez JA. Early Diagnosis and Management of Acute Vertigo from Vestibular Migraine and Ménière’s Disease. Neurologic Clinics. 2015;33(3):619–28. doi: 10.1016/j.ncl.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. Journal of Neuroscience. 2013;33(34):13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270(5234):303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. The Journal of the Acoustical Society of America. 1997;101(4):2214–20. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. The Journal of the Acoustical Society of America. 2000;107(3):1615–26. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Snell KB, Mapes FM, Hickman ED, Frisina DR. Word recognition in competing babble and the effects of age, temporal processing, and absolute sensitivity. The Journal of the Acoustical Society of America. 2002;112(2):720–7. doi: 10.1121/1.1487841. [DOI] [PubMed] [Google Scholar]
- Soros P, Teismann IK, Manemann E, Lutkenhoner B. Auditory temporal processing in healthy aging: a magnetoencephalographic study. BMC Neuroscience. 2009;10(34):1–9. doi: 10.1186/1471-2202-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan NK, Zahorik P. Enhancement of speech intelligibility in reverberant rooms: Role of amplitude envelope and temporal fine structure. The Journal of the Acoustical Society of America. 2014;135(6):EL239–EL245. doi: 10.1121/1.4874136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellmack MA, Byrne AJ, Viemeister NF. Extracting binaural information from simultaneous targets and distractors: effects of amplitude modulation and asynchronous envelopes. The Journal of the Acoustical Society of America. 2010;128(3):1235–44. doi: 10.1121/1.3466868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelcyk O, Dau T. Relations between frequency selectivity, temporal fine-structure processing, and speech reception in impaired hearing. The Journal of the Acoustical Society of America. 2009;125(5):3328–45. doi: 10.1121/1.3097469. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. The Journal of the Acoustical Society of America. 1998;104(4):2385–99. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Jagadeesh A, Mauermann M, Ernst F. Individual Differences in Auditory Brainstem Response Wave Characteristics: Relations to Different Aspects of Peripheral Hearing Loss. Trends in Hearing. 2016;20(0) doi: 10.1177/2331216516672186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18(7):2764–76. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. Journal of neurophysiology. 2002;88(2):565–78. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hearing research. 1997;107(1–2):67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Yonan CA, Sommers MS. The effects of talker familiarity on spoken word identification in younger and older listeners. Psychology and Aging. 2000;15(1):88–99. doi: 10.1037//0882-7974.15.1.88. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Stickney GS, Kong YY, Vongphoe M, Bhargave A, Wei C, Cao K. Speech recognition with amplitude and frequency modulations. Proc Natl Acad Sci U S A. 2005;102(7):2293–8. doi: 10.1073/pnas.0406460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Henry KS, Heinz MG. Sensorineural hearing loss amplifies neural coding of envelope information in the central auditory system of chinchillas. Hearing Research. 2014;309:55–62. doi: 10.1016/j.heares.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]