Abstract
The extracellular matrix (ECM) is a complex and dynamic structure made up of an estimated 300 different proteins. The ECM is also a rich source of cytokines and growth factors in addition to numerous bioactive ECM degradation products that influence cell migration, proliferation, and differentiation. The ECM is constantly being remodeled during homeostasis and in a wide range of pathological contexts. Changes in the ECM modulate immune responses, which in turn regulate repair and regeneration of tissues. Here, we review the many components of the ECM, enzymes involved in ECM remodeling, and the signals that feed into immunological pathways in the context of a dynamic ECM. We highlight studies that have taken an integrative approach to studying immune responses in the context of the ECM and studies that use novel proteomic strategies. Finally, we discuss research challenges relevant to the integration of immune and ECM networks and propose experimental and translational approaches to resolve these issues.
Immune responses to infection and injury are often tissue-specific. Migration, proliferation, and differentiation of immune cells depend on cytokines and growth factors that accumulate in the tissue microenvironment. The extracellular matrix (ECM) is a major component of any tissue and helps define its structure and function. Disruptions and alterations in the ECM feed into immunological pathways, which in turn regulate repair and regeneration of the ECM. The ultimate outcome of these regulatory circuits determines whether the tissue regains adequate function in a manner supportive of host recovery. Here we review the evidence that the ECM plays a critical role in modulating tissue-specific immune responses to infection and injury. We will primarily drawn on examples from the lung, an organ with an extensive extracellular matrix that is constantly remodeled in response to infection and other insults. We will describe the major components that make up the ECM structure, enzymes that are involved in remodeling the ECM, and cytokines and growth factors associated with the ECM that modulate host immune responses. We propose a unified theory of immunology and ECM biology in which host immune responses to infection and injury are carried out in the context of the ECM. For many diseases, persistent inflammation is associated with poor outcome. Given the important role that the ECM plays in modulating inflammation mediated by the immune system, improved understanding of the basic mechanisms underlying these interactions will inform the development of therapeutics that seek to limit immunopathology and promote restoration of tissue function.
ECM Proteins
The extracellular matrix is a complex and dynamic structure made up of an estimated 300 different proteins in mammals (1). The ECM can be generally divided into two main components: the interstitial matrix and the basement membrane. Collectively, these ECM proteins are often referred as the matrisome. In addition to providing structural support to all tissues, the ECM plays a critical role in most basic cellular functions, including differentiation, migration, proliferation, and turnover. Generally, these ECM proteins can be segregated into broad, diverse groups of collagens, proteoglycans, and other complex ECM glycoproteins. The majority of proteins that have been identified in the ECM belong to the ‘other’ category, highlighting the need for more research to better define their functional roles.
ECM genes are evolutionarily ancient, and a core set of genes that encode proteins in basement membranes likely existed in basal metazoans (1). Comparative genetic analysis of vertebrate genomes with those of closely related invertebrates, suggest that when the vertebrate lineage diverged there was a dramatic expansion in the number of ECM genes and corresponding remodeling enzymes (2,3). This expansion appears to mostly be the result of gene duplication and subsequent diversification (1,2). The evolution of the complex mammalian immune system, including both innate and adaptive branches, occurred after this expansion of ECM-related genes (4). Thus, the mammalian immune system and all of its constituents, including many innate and adaptive immune cell types, soluble mediators, and molecular effectors, has developed in the context of this dynamic and diverse extracellular matrix structure. In addition to using signals from the ECM to coordinate host responses to infection and injury, immune cells play an active role in remodeling the ECM and promoting tissue repair.
In the following section, we outline the major groups of ECM proteins and highlight important functions of specific proteins within each. For in-depth analysis of these groups of ECM proteins, we refer readers to an excellent review of the matrisome by Hynes and Naba (1)
Collagens
Collagens confer tensile strength to the ECM of tissues and are characterized by the presence of Gly-X-Y repeats, where X and Y can be any amino acid, but are frequently proline and hydroxyproline (5). Through homotypic interactions between these repeats, collagens form stable, trimeric structures. These trimeric structures also form higher order oligomers that contribute to the strength of the ECM. Impressively, 28 different types of collagen have been identified in vertebrates (6). Fibrillar collagens, including types I and III, are predominantly found in the interstitial matrix. In contrast, network forming collagens, including type IV, are found in the ECM basement membrane and provide a rigid surface for epithelial and endothelial cells (5,6).
Proteoglycans
Proteoglycans are glycoproteins that contain repeating glycosaminoglycans (GAGs). These disaccharides have attached carboxyl and sulfate groups that confer a strong negative charge to the molecule. Due to these GAGs, proteoglycans are able to bind numerous cytokines and growth factors and retain them in the ECM (7). Approximately 36 ECM proteoglycans have been identified in mammals, and these proteins have diverse functions in multiple different tissues (1,8). For example, the proteoglycan hyaluronan is abundant in the lung and plays a major role in maintaining tissue homeostasis and in responding to lung injury (9). Another proteoglycan, versican, associates with hyaluronan to form long filaments in the ECM (10). These filaments have been demonstrated to play an important role in modulating inflammatory responses to infection and tissue injury and in immune cell adhesion and migration (11). Proteoglycans are found in both interstitial and basement membrane matrices. While hyaluronan and versican are localized in the interstitial ECM, the heparin sulfate proteoglycan perlecan is found in the basement membrane and is critical for its formation across many species (12). Interestingly, hyaluronan has been demonstrated to engage innate immune sensors present on epithelial cells in the lung (13–15). A recent study showed that hyaluronan engagement of toll-like receptor-4 (TLR4) promoted renewal of alveolar progenitor cells and tissue repair, preventing lung fibrosis. In TLR-4-deficient mice, bleomycin-induced injury was exacerbated and tissue repair was compromised due to impaired renewal capacity of type 2 alveolar epithelial cells. Taken together, these studies indicate that proteoglycans in the lung ECM interact with innate immune sensors to regulate tissue-repair mechanisms highlighting the important interplay between immunity and the ECM.
Other ECM Glycoproteins
In addition to the collagens and GAG containing proteoglycans, there are numerous other complex proteins that have been identified in the ECM. Unbaised approaches to defining the matrisome have identified approximately 200 of these proteins that comprise a diverse set of molecules that mediate ECM-cell interactions, cell signaling, and binding to growth factors, among other functions. Another main function of these proteins is to serve as linkers in the ECM connecting other ECM proteins and helping to define the structure of a tissue. Laminins, present in basement membranes, and fibronectin, found in the interstitial matrix, are among the most abundant and well studied glycoproteins in the lung ECM (16). Although hundreds of ECM glycoproteins have been identified, few of them have been extensively studied, and their roles in homeostasis and in disease are largely unknown. As we gain better understanding of the functional roles of these additional ECM proteins, a more narrow and informative categorization of this diverse group of molecules will be possible.
As previously mentioned, the ECM can generally be divided into the interstitial matrix and the basement membranes. The interstitial matrix is made up primarily of fibrillar collagens and fibronectin and serves as a scaffold to the tissue. In the lung, elastin, an ECM glycoprotein, is a major component of the interstitial matrix. The basement membrane, composed primarily of type IV collagen and laminins, is a more rigid and compact ECM structure that interacts directly with epithelial and endothelial cells (17). The compact network of the basement membrane poses a potential barrier to the infiltration of immune cells surveying the tissue or responding to infection or injury. Although protease-mediated degradation of the basement membrane has been proposed as an important factor in promoting extravasation of immune cells into the interstitium, this process would involve the disruption of an extensive ECM network (18). An alternative hypothesis has been proposed in which variability in the composition of the basement membrane determines extravasation of immune cells at sites with a lower density of ECM proteins. In support of this idea, neutrophils and monocytes have been demonstrated to preferentially migrate through areas of low collagen IV expression in an in vivo mouse model (19,20).
Recently, there have been efforts to characterize the matrisome in an unbiased and comprehensive manner across different tissues using proteomic approaches. These studies have attempted to quantify the ECM in a variety of tissues ranging from the lung and heart to bone and cartilage and across different species, including humans, mice, and pigs (21). Interestingly, a study by Naba et al. comparing the ECM composition of lung and colon tissue in mice found that approximately 10 – 30% of the ECM proteins are tissue-specific (16). As previously mentioned, the ECM of a given tissue is highly dynamic both during homeostasis and in pathologic conditions. Several groups have begun to assess ECM composition temporally over the course of certain disease states, particularly in the lung. For example, Decaris et al. characterized the alterations in ECM turnover following bleomycin-induced lung fibrosis, and Talmi-Frank et al. measured changes in lung ECM protein abundance in the context of influenza infection (22,23). Proteomic analysis of samples after differential extraction and from different soluble fractions has the potential to provide improved resolution of the matrisome based on lung compartment (24).
Another disease state in which there are dramatic changes in ECM composition is cancer. Changes in ECM composition can help generate microenvironments conducive to tumor cell growth (25). For example, in a murine model of lung cancer cell metastasis, fibronectin is upregulated in future metastatic niches (26). Just as the ECM plays an important role in normal cell migration, it also influences cancer cell motility. Enzymes that remodel ECM proteins, which will be discussed below in more detail, facilitate cancer metastasis by permitting migration of these cells across extracellular matrices and into distant tissues (17). Recently, proteomics approaches have also been used to analyze the ECM in tumor microenvironments, in particular for colorectal cancer. These studies have identified ECM signatures, including increased collagen deposition and cross-linking, in both humans (27) and mice (28), that promote tumor invasion and growth.
There are numerous examples of dysregulation of the ECM and its contribution to various disease states. Going forward, proteomic approaches to defining ECM composition and changes over the course of a particular disease will be important for identifying specific ECM pathways to target with novel therapeutics. These changes in ECM composition influence immune cells that mediate both inflammatory and tissue repair processes. The tissue and regeneration process can be quite long and represents a large window for intervention. Two recent studies demonstrated that a distinct stem cell lineage, characterized by Krt5 expression, continues proliferating more than 40 days after lung injury induced by influenza infection or bleomycin treatment in mice (29,30). Another study analyzing the transcriptomic regulation of tissue repair after influenza infection found that numerous genes encoding inflammatory cytokines and chemokines, stem cell markers (including Krt5), ECM proteins, and remodeling enzymes were significantly upregulated at 35 days post-infection, indicative of persistent repair and remodeling (31).
The combination of proteomic approaches and transcriptional profiling to assess ECM composition and dynamics has the potential to identify critical pathways or networks that contribute to a disease outcome. In a recent study, Schiller et al. performed a comprehensive analysis of proteomic and transcriptomic changes in ECM following bleomycin-induced lung injury (24). Analysis of multiple time points, out to 56 days post-injury, and of multiple compartments of the lung, including soluble and insoluble fractions of total tissue and bronchoalveolar lavage fluid, provided a high-resolution data set to explore pathways involved in host response to injury. Analysis of cell-specific protein signatures also provided insight into key immune cell subsets enriched at different stages of disease. Using these methods, the authors identified and validated two novel lung ECM components, Emilin-2 and collagen-XXVIII, that are dynamically regulated during the course of lung injury and repair. Applied to different pathological contexts, tissues, or model systems, similar approaches have the potential to generate a wealth of data on ECM and immune system dynamics that will inform mechanistic studies of critical tissue repair responses.
ECM Remodeling Enzymes
Central to the interactions between the host immune system and the ECM are remodeling enzymes capable of modifying and degrading ECM proteins. Localized ECM breakdown facilitates the migration of immune cells into areas of disease-induced tissue damage. In addition, the products of ECM degradation can serve as early signals to the innate immune system that initiate responses to infection. ECM remodeling enzymes are often produced by immune cells, in particular those of the myeloid lineage, and contribute to their ability to modulate inflammation and tissue repair processes in response to injury and infection (32).
Matrix metalloproteinases (MMPs) and adamalysins are the major families of enzymes that degrade ECM proteins. MMPs are expressed as zymogens and are activated upon proteolytic cleavage by other MMPs or serine proteases (17). MMPs can be membrane-bound (MT-MMPs) or secreted as soluble factors. In humans, there are 23 MMPs that together degrade most, if not all, ECM proteins (33). Adamlysins were relatively recently discovered and include A disintegrin and metalloproteinases (ADAM) (34) and A disintegrin and metalloproteinase with thrombspondin motifs (ADAMTS) (35). ADAMs are membrane-bound while ADAMTSs are secreted due to the presence of a thrombospondin type-I repeats. Although 22 ADAM genes have been identified in humans, only a small subset has been demonstrated to have proteolytic activity for ECM proteins (17). ADAMs are also able to cleave ectodomains of various proteins in proximity to the cell membrane, including cytokines and growth factors, releasing them from the cell. Nineteen ADAMTS genes have been identified in humans. Although there are a number of ADAMTS proteins with unknown function, many ADAMTS enzymes are able to degrade ECM proteoglycans and pro-collagens (36). Within each family of MMP, ADAM, and ADAMTS genes, the enzymes are further divided into subclasses based on the presence or absence of protein domains that contribute to ECM substrate specificity. Similar to the expansion of ECM genes during the course of vertebrate evolution, the diversity of MMP and ADAMTS genes has mostly been generated by gene duplication events (36,37). Given the importance of the ECM and remodeling enzymes in development, the expansion of the genes encoding these proteins has likely facilitated the development of the impressive diversity of tissues and organisms among vertebrates.
Regulation of MMPs and ADAMTSs
Because of their ability to degrade the ECM and cause tissue damage and their role in development, matrix proteases must be tightly regulated during both homeostasis and in response to injury or disease. Indeed, excessive activity of matrix proteases has been associated with a number of different pathological conditions, including cardiac dysfunction (38), osteoarthritis (39,40), breast cancer (41), and respiratory infections (23,42), among many others (43). ECM remodeling enzymes are regulated at multiple levels from transcription to post-translation modifications (44–47). Matrix proteases are regulated at the transcription level by both cis- and trans- promoter elements, including AP-1, β-catenin/Tcf-4, and NF-κB, and promoter polymorphisms have been identified that modulate gene expression (46,47). Although there are specific MMP and ADAMTS genes that are clustered together around gene loci, they are present on various chromosomes throughout the human genome (36,37). Experiments performed in vitro have demonstrated that numerous cytokines and growth factors, including EGF, VEGF, TGF-β, TNF-α, and IL-1β, among others, can stimulate expression of MMPs and ADAMTSs (46).
These large, multi-domain enzymes also undergo post-translational modifications that regulate their activity. As previously mentioned, most MMP and ADAMTS enzymes are zymogens requiring activation by proteolytic cleavage. The pro-domains of these enzymes are often cleaved by other matrix proteases. For example, MMP-3 and MMP-10 have the ability to cleave the pro-domains of MMP-1, MMP-8, and MMP-13 (33). Another level of regulation of matrix protease activity is localization, both subcellular and outside the cell in the ECM. Matrix proteases can be localized to the migrating front of cells to aid in migration. In addition, proteinases can be sequestered in the ECM in their inactive form requiring activation by other enzymes (48).
A final level of regulation is direct inhibition by endogenous protein inhibitors. Tissue inhibitors of metalloproteinases (TIMPs) are able to directly bind to MMP, ADAM, and ADAMTS enzymes and inhibit their activity. Four TIMPs have been identified in humans, and together they can inhibit a wide range of matrix proteases (49). Although TIMPs are structurally very similar, they appear to show preferential binding to specific matrix proteases (49,50). Balance between the activity of matrix proteases and their corresponding inhibitors is critical for allowing migration of immune cells into a site of inflammation or injury and maintaining structural integrity of the tissue to avoid widespread destruction.
Multiple levels of regulation of enzymatic activity that impose tight control on the remodeling process have been demonstrated in numerous pathological situations and in homeostasis (33,41,48). The diversity of matrix protease enzymes and regulation of their activity underlies the ability to respond to and repair damage caused by the many infections and other pathological conditions that humans experience. Nonetheless, the precise mechanisms that regulate these tissue- and cell-specific responses and how their activities are coordinated by immune cell subsets remain unclear. Recent evidence suggests that MMPs expressed by macrophages, including MMP-1, 3, 8, and 12 play an important role in coordinating the infiltration of polymorphonuclear immune cells into the lung during inflammation and promote the transition to tissue repair (32,51,52). For example, MMP-12 has been demonstrated to cleave and inactivate chemokines, including CXCL-1, 2, and 8, that attract neutrophils and inflammatory monocytes (52). Interestingly, MMP-12 has also been implicated in the extracellular degradation of IFNα in the context of viral infection providing another potential anti-inflammatory role for this enzyme (53). The role that matrix proteases play in regulating both local and systemic levels of cytokines and chemokines in different disease contexts warrants further research. Given that the resolution of inflammation is often associated with improved outcomes following tissue injury, for example following severe lung damage, matrix protease activity is an attractive therapeutic target. Integration of the earliest signals resulting from ECM remodeling with downstream immune responses and corresponding repair pathways will provide a more comprehensive understanding of tissue-specific responses to disease.
ECM as a reservoir of cytokines and growth factors
The ECM is a ubiquitous structure and a major source of molecules with potential immunomodulatory activity. These molecules include cytokines and growth factors that are secreted by cells and bound in the ECM along with bioactive fragments that are produced from the activity of matrix proteases. These cytokines and growth factors influence immune cell proliferation and differentiation. There are many examples of cytokines and growth factors that are bound in the ECM, and several studies have identified interactions between specific ECM proteins and growth factors. For example, fibronectin domains have been found to bind to a number of different growth factors, including vascular endothelial growth factor (VEGF) and hepatic growth factor (HGF) (1,54–56). Also, heparin sulfate proteoglycans (HSPGs), abundant in the ECM, bind FGFs and sequester these molecules for storage (57). Proteolytic cleavage of ECM proteins releases growth factors, such as FGFs, in a spatially restricted manner and contributes to localized cell proliferation and differentiation.
Perhaps because of its pleiotropic effects, transforming growth factor beta (TGF-β) has been one of the most well studied ECM-bound molecules. The ECM helps impose tight regulation over the activation and activity of TGF-β (58), and there are multiple levels of post-translational regulation. Pro-TGF-β is initially associated with its pro-peptide, latency-associated peptide (LAP). This complex also binds to latent TGF-β-binding protein (LTBP) to form the large latent complex, which then binds to ECM proteins (58–60). MMPs and ADAMTSs help regulate TGF-β activity by cleaving ECM fibers and increasing its bioavailability (61). In addition, several matrix proteases can activate TGF-β by cleaving latency peptides (58). Interestingly, there is also evidence suggesting that the mechanical stiffness of the ECM may lower the activation threshold of TGF-β. In this model, stiff ECM provides additional resistance to cell pulling and induces a conformational change in LAP facilitating release (62).
TGF-β has many functions in diverse biological processes, including critical roles in development, tissue repair, and immune cell function. During tissue repair, TGF-β stimulates fibroblasts and myofibroblasts, signaling through the SMAD pathway, to express numerous ECM-related genes including those encoding for collagens, TIMPs, and MMPs (17,63). In this way, TGF-β contributes to the deposition of newly synthesized ECM following tissue damage and remodeling of the ECM. Although TGF-β signaling is critical for successfully repairing damaged tissues, dysregulation of this pathway can lead to tissue fibrosis. Persistent inflammation, immune activation, and fibroblast stimulation via TGF-β can lead to excess deposition of ECM proteins and the generation of fibrotic tissue. For more details on the role of immune activation and TGF-β in fibrotic disease, we refer readers to the following reviews (58,64,65).
TGF-β has also been reported to have effects on nearly all immune cell types, including cells of both the innate and adaptive immune systems (66,67). The pleiotropic effects of TGF-β and the need to tightly regulate its bioavailability and activation are evident in host immune responses to infection. TGF-β modulates nearly all stages of the immune response from early immune to later adaptive response and modulates immune cell activation, proliferation, and differentiation. TGF-β can have both pro-inflammatory and anti-inflammatory effects depending on the surrounding cytokine milieu and cell type. The effects of TGF-β on CD4+ T cell responses to infection and differentiation into distinct functional subsets have been extensively studied (67,68). For example, TGF-β is required for differentiation of pro-inflammatory IL-17 producing (TH17) cells and anti-inflammatory regulatory T cells (Tregs), and the differentiation into these two subsets depends on the concentration of TGF-β (69). In a dramatic example, targeted deletion of TGF-β in T cells resulted in lethal immunopathology in multiple organs in mice (70).
In addition to serving as a reservoir of growth factors and cytokines that modulate cell functions, the ECM interacts directly with cells and directs cell motility through integrins expressed on the surface of numerous cell types. The ECM itself provides a scaffold that cells utilize for their migration. At the same time that the ECM serves as a guide for some cell types, it can also serve as an obstacle, for example, to neutrophils that migrate to the site of infection. Integrins are expressed as αβ heterodimers on the cell surface. The extracellular domain interacts with ECM proteins while the intracellular domain interacts with the actin cytoskeleton affecting polarization and motility (71). Migration of adhesive mesenchymal cells depends on ECM proteolysis and interaction of integrins with ECM proteins. In addition, integrins are important for leukocyte interactions with endothelial cells as they move through blood vessels. Subsequently, integrin interactions with the endothelial basement membrane are criticial for transmigration of these cells as they move to sites of inflammation (72). The importance of integrin-ECM interactions in directing cell motility in the interstitial matrix remains unclear (73,74). There is evidence suggesting that lymphocyte motility and retention in certain compartments is influenced by integrin-ECM interactions, in particular in inflamed tissues with altered ECM composition and integrin expression, for example in the context of influenza infection (75,76). The contribution of integrin-ECM interactions in the interstitial matrix may depend on the tissue being studied and the extent of inflammation.
Integrins also play a role in activation of ECM-bound cytokines and growth factors. For example, the integrin α4β6 activates latent TGF-β regulating the spatial bioavailability of the growth factor (77). Integrin activation introduces another level of regulation of ECM-bound molecules. The effect of α4β6 activation of TGF-β is likely context dependent. An early study found that mice lacking α4β6 develop airway hyperresponsiveness due to infiltration of inflammatory cells in to the lungs and skin (78). A more recent study found that mice lacking α4β6 were protected against challenge with multiple respiratory pathogens, likely due to higher levels of type-I interferon produced by alveolar macrophages in the presence of lower levels of active TGF-β (79). Activation of TGF-β by α4β6 may also contribute to fibrotic lung disease following influenza infection due to increased collagen deposition (80).
The presence of cytokines and growth factors in the ECM provides a means for host cells to rapidly respond to infection or injury as these molecules are released and/or activated. In this manner, these ECM-bound molecules may be some of the earliest signals to the host immune system to promote rapid responses. In the following section, we will explore the idea that ECM proteins themselves can act as stimulation to the host immune system providing an additional source of signals that can initiate the tissue-repair response.
Bioactive ECM Fragments: Matrikines
During tissue inflammation, matrix proteases degrade ECM proteins into a heterogeneous mixture of peptide fragments. There is growing evidence that the ECM fragments generated from proteolysis are bioactive molecules that modulate responses to tissue damage. These bioactive fragments, sometimes referred to as ‘matrikines,’ can have chemoattractant properties, similar to chemokines, and can have pro-inflammatory effects, similar to some cytokines. Matrikines generated from proteolysis of elastin were among the first identified in the 1980s (81,82). Since that time matrikines generated from cleavage of many ECM proteins have been identified, and determining the functions of these bioactive fragments is an active area of research.
Elastin Fragments
Several early studies identified a six amino acid repeating sequence (VGVAPG) elastin fragment with biological activity. In subsequent studies, elastin-derived matrikines were demonstrated to be chemoattracants for fibroblasts and monocytes (83), and as inducers of matrix protease expression in fibroblasts, endothelial cells, and lung cancer cells (84–86). MMP12, also known as macrophage elastase, and neutrophil elastase, a serine protease, are capable of generating the VGVAPG elastin matrikine (87,88). Studies in mice have demonstrated that elastin fragments are capable of mediating macrophage recruitment to the lungs and contributing to the development of emphysema (89,90).
Collagen Fragments
Collagen-derived fragments are the best studied of the matrikines, perhaps because collagen, with its 28 different types, is highly abundant in both the interstitial matrix and basement membrane. In the mid 1990’s, collagen-derived peptides containing a proline-glycine-proline (PGP) sequence were demonstrated to have chemoattractant activity for immune cells, including neutrophils (91,92). Originally, these bioactive peptides were isolated from chemically degraded cornea tissue. In a subsequent study, Weathington et al. demonstrated that N-terminal acetylated PGP peptides facilitated neutrophil recruitment into the lungs after exposure to LPS (93). The authors suggested that the collagen-derived PGP peptides have structural homology to other chemokines, including IL-8, CXCL1, and CXCL2, involved in immune cell recruitment. They further demonstrated that PGP interacts with CXCR1 and CXCR2 receptors expressed on human neutrophils providing a potential mechanism for recruitment by collagen-derived matrikines. Collagen-derived PGP matrikines are thought to be generated by the sequential activity of MMP-8, MMP-9, and serine prolyl endopeptidase (94). Given the potent effect that PGPs can have on neutrophil recruitment to sites of tissue damage, these matrikines must also be tightly regulated. Snelgrove et al. have demonstrated that leukotriene A4 hydrolase (LTA4H) is capable of degrading PGPs and limiting neutrophilic inflammation in variety of pathogenic settings in the lung (95,96). Degradation of collagen-derived matrikines represents another level of regulation of ECM proteins during inflammation.
Other ECM Fragments
In addition to elastin- and collagen- derived fragments, matrikines generated from cleavage of several other ECM proteoglycans and glycoproteins have been identified. Laminin, a major component of ECM basement membranes, can be cleaved by numerous MMPs and ADAM9 to generate matrikines that influence epithelial cell migration (97–99), neutrophil infiltration (100), and alveolar regeneration by engaging EGF receptors expressed on epithelial progenitor cells (101). Proteolysis of the associated proteoglycans hyaluronan and versican also produces biologically active fragments that modulate inflammatory responses to infection and injury (102,103). A number of studies have demonstrated that hyaluronan fragments promote inflammation by signaling through toll-like receptors (TLRs) and interacting with CD44 present on epithelial cells and various immune cell subsets (13,104,105). Versican fragments appear to play a role in a wide range of biological processes. Degradation of versican by ADAMTS enzymes during development of mice induces apoptosis in the interdigital tissue and promotes proper limb formation (106). Recently, versican proteolysis and production of the matrikine versikine was found to induce production of the inflammatory cytokines IL-6 and IL-1β in the myeloma microenvironment (107). Given the large number of proteoglycans and glycoproteins that can be present in the ECM in various tissues, there are almost certainly additional matrikines that play important roles in other biological functions that have yet to be identified. The interaction of matrikines with innate immune sensors, such as TLRs, raises the possibility that these bioactive ECM degradation products modulate downstream immune responses, including the development of adaptive immune responses. The role of these molecules in all aspects of immune responses warrants further investigation.
Challenges and Future Directions
It has become clear over the last several decades that the immune system and the extracellular matrix are intimately linked. The ECM serves as a scaffold for migrating cells, a reservoir of cytokines and growth factors, and a source of bioactive peptides and damage signals that modulate immune responses (Figure 1). In this review, we have attempted to highlight studies that have taken an integrated approach to studying immune responses to infection and injury in the context of the ECM. The diversity of ECM proteins found in any given tissue, the dynamic nature of this structure, and the fact that an even greater diversity of molecules is generated following proteolysis present challenges to identifying the most important basic mechanisms contributing to a particular outcome. ‘Omics’ approaches to survey gene transcription profiles or protein profiles of extracellular matrices from different pathological contexts will be useful is assessing changes in the abundance of ECM-related proteins. Several recent studies have begun to utilize mass spectrometry to generate protein profiles in the context of viral infection, lung fibrosis, and cancer revealing major differences in ECM composition compared to healthy controls (16,21–24,27,28). Particularly promising is the approach taken by Schiller et al. in a recent study combining mass spectrometry and RNAseq to identify ECM and immunological pathways contributing to resolution of bleomycin-induced lung injury (24). Such studies provide excellent resources for identifying ECM-immunological pathways to pursue mechanistic studies that will inform novel therapeutic strategies.
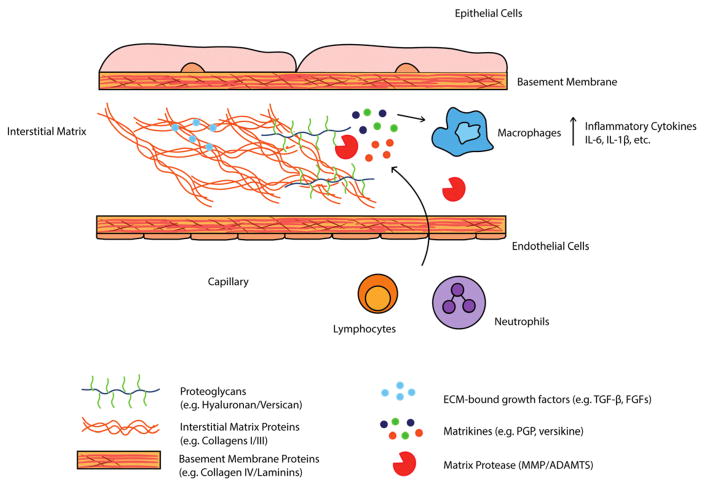
Figure 1.
The extracellular matrix as a critical mediator of immune reponses. The ECM serves as a rich source of cytokines, growth factors, and other bioactive degradation products (matrikines) that modulate immune responses to various pathological contexts. Matrix proteases, including MMP and ADAMTS enzymes, release cytokines/growth factors sequestered in the ECM and are capable of degrading ECM proteins to produce matrikines that influence immune cell migration and cytokine production. Signals provided by growth factors and matrikines to immune cells are critical for maintaining tissue homeostasis and in response to injury in a wide range of pathological contexts, including viral infection and cancer.
The diversity in composition of the ECM and related remodeling enzymes in different tissues also presents a challenge in translating findings across different model systems. There are numerous ECM proteins and remodeling enzymes with structural homology. Some groups have suggested that redundancy has evolved in order to serve as a fail-safe in the event that a particular enzyme is inhibited (37). Another possibility, however, is that enzymes with structural homology are involved in distinct ECM remodeling pathways depending on the tissue and the nature of the insult. The latter hypothesis is supported by recent data demonstrating that two matrix proteases with structural homology and similar substrate specificity, MMP-1 and MMP-13, produce distinct ECM protein profiles that mediate different ECM-cell interactions (108). As we improve our knowledge of the regulation of ECM protein and remodeling enzyme expression in different tissues and disease states, we will gain a better understanding of the level of redundancy or specificity in ECM remodeling. Identification of tissue- and disease-specific upstream signals that result from ECM remodeling and modulate immune responses will help clarify these ECM pathways. The issue of redundancy or specificity also alludes to an important caveat to in vitro studies of ECM remodeling. Many remodeling enzymes exhibit altered activity in vitro at high concentrations of the enzyme compared to that in vivo at physiologically relevant concentrations.
The ultimate goal of immunity is to prevent disease and restore tissue integrity. The host response must balance inflammation and/or clearance of a pathogen with tissue repair using the same key players. In order to mount an effective immune response, some level of tissue damage, including the ECM, is inevitable, as large numbers of cells are recruited to the affected site. The transition between inflammation and tissue repair is important for a favorable outcome, and regulation of the ECM is critical to this process. The diversity of ECM proteins and remodeling enzymes represents a large number of potential targets to manipulate inflammation and tissue repair to improve recovery from injury and infection. It is increasingly apparent that the activity and regulation of these proteins are tissue- and disease-specific adding to the complexity of these biological networks. Unbiased ‘omics’ studies are a good place to start as we begin to define these networks and narrow the field to the most promising candidates for therapeutics.
Highlights.
Reviews extracellular matrix (ECM) proteins, remodeling enzymes, and signals that feed into immunological pathways
Emphasizes recent studies that have utilized proteomic and transcriptomic approaches to assess ECM and immunological changes in different disease contexts
Proposes experimental approaches to resolve the challenges of integrating ECM and immune networks
Acknowledgments
This work was supported by HHSN272201400006C (St. Jude Center of Excellence for Influenza Research and Surveillance), R01 AI121832, and ALSAC. We apologize to any investigators whose relevant work was not included due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO, Naba A. Overview of the Matrisome--An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb Perspect Biol. 2012 Jan 1;4(1):a004903–a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley-Jones J, Robertson DL, Boot-Handford RP. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 2007 Jan;26(1):2–11. doi: 10.1016/j.matbio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Huxley-Jones J, Apte SS, Robertson DL, Boot-Handford RP. The characterisation of six ADAMTS proteases in the basal chordate Ciona intestinalis provides new insights into the vertebrate ADAMTS family. Int J Biochem Cell Biol. 2005 Sep;37(9):1838–45. doi: 10.1016/j.biocel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010 Jan;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricard-Blum S. The Collagen Family. Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a004978–a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010 Jan;339(1):247–57. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarrazin S, Lamanna WC, Esko JD. Heparan Sulfate Proteoglycans. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10(5):598–614. [PubMed] [Google Scholar]
- 9.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. AJP Lung Cell Mol Physiol. 2011 Aug 1;301(2):L137–47. doi: 10.1152/ajplung.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014 Apr;35:152–61. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012 Mar;31(2):90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iozzo RV. Perlecan: A Gem of a Proteoglycan. Matrix Biol. 1994;14:203–8. doi: 10.1016/0945-053x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 13.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005 Nov;11(11):1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 14.Taylor KR, Yamasaki K, Radek KA, Nardo AD, Goodarzi H, Golenbock D, et al. Recognition of Hyaluronan Released in Sterile Injury Involves a Unique Receptor Complex Dependent on Toll-like Receptor 4, CD44, and MD-2. J Biol Chem. 2007 Apr 16;282(25):18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016 Oct 3;22(11):1285–93. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol Cell Proteomics. 2012 Apr 1;11(4):M111.014647–M111.014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014 Nov 21;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010 Oct;10(10):712–23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Voisin M-B, Larbi KY, Dangerfield J, Scheiermann C, Tran M, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006 Jun 12;203(6):1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voisin M-B, Pröbstl D, Nourshargh S. Venular Basement Membranes Ubiquitously Express Matrix Protein Low-Expression Regions. Am J Pathol. 2010 Jan;176(1):482–95. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016 Jan;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decaris ML, Gatmaitan M, FlorCruz S, Luo F, Li K, Holmes WE, et al. Proteomic Analysis of Altered Extracellular Matrix Turnover in Bleomycin-induced Pulmonary Fibrosis. Mol Cell Proteomics. 2014 Jul 1;13(7):1741–52. doi: 10.1074/mcp.M113.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talmi-Frank D, Altboum Z, Solomonov I, Udi Y, Jaitin DA, Klepfish M, et al. Extracellular Matrix Proteolysis by MT1-MMP Contributes to Influenza-Related Tissue Damage and Mortality. Cell Host Microbe. 2016 Oct;20(4):458–70. doi: 10.1016/j.chom.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Schiller HB, Fernandez IE, Burgstaller G, Schaab C, Scheltema RA, Schwarzmayr T, et al. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol. 2015 Jul 14;11(7):819–819. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009 Apr;9(4):285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005 Dec 8;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nebuloni M, Albarello L, Andolfo A, Magagnotti C, Genovese L, Locatelli I, et al. Insight On Colorectal Carcinoma Infiltration by Studying Perilesional Extracellular Matrix. Sci Rep. 2016 Mar 4;6:22522. doi: 10.1038/srep22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016 Oct 17;213(11):2315–31. doi: 10.1084/jem.20151193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2014 Dec 24;517(7536):621–5. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo W, Zhang T, Wu DZ, Guan SP, Liew A-A, Yamamoto Y, et al. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature. 2014 Nov 12;517(7536):616–20. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pociask DA, Robinson KM, Chen K, McHugh KJ, Clay ME, Huang GT, et al. Epigenetic and Transcriptomic Regulation of Lung Repair during Recovery from Influenza Infection. Am J Pathol [Internet] 2017 doi: 10.1016/j.ajpath.2016.12.012. [cited 2017 Feb 27]; Available from: http://www.sciencedirect.com/science/article/pii/S0002944017300627. [DOI] [PMC free article] [PubMed]
- 32.Chan MF, Werb Z, Chou J. Metalloproteinases: a Functional Pathway for Myeloid Cells. Microbiol Spectr [Internet] 2016 Apr 1;4(2) doi: 10.1128/microbiolspec.MCHD-0002-2015. [cited 2016 Sep 6] Available from: http://www.asmscience.org/content/journal/microbiolspec/10.1128/microbiolspec.MCHD-0002-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu P, Takai K, Weaver VM, Werb Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a005058–a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hite LA, Shannon JD, Bjarnason JB, Fox JW. Sequence of a cDNA clone encoding the zinc metalloproteinase hemorrhagic toxin e from Crotalus atrox: evidence for signal, zymogen and disintegrin-like structures. Biochemistry (Mosc) 1992;31(27):6203–6211. doi: 10.1021/bi00142a005. [DOI] [PubMed] [Google Scholar]
- 35.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. J Biol Chem. 1997;272(1):556–62. doi: 10.1074/jbc.272.1.556. J. Biol. Chem.-1997-Kuno-556-62.pdf. [DOI] [PubMed] [Google Scholar]
- 36.Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol [Internet] 2015 Dec;16(1) doi: 10.1186/s13059-015-0676-3. [cited 2015 Nov 2] Available from: http://genomebiology.com/2015/16/1/113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanjul-Fernández M, Folgueras AR, Cabrera S, López-Otín C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta BBA - Mol Cell Res. 2010 Jan;1803(1):3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D’Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000 Oct 1;106(7):857–66. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26(1):139. [PubMed] [Google Scholar]
- 40.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009 Sep;15(9):1072–6. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 41.Muschler J, Streuli CH. Cell-Matrix Interactions in Mammary Gland Development and Breast Cancer. Cold Spring Harb Perspect Biol. 2010 Oct 1;2(10):a003202–a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuurhof A, Bont L, Hodemaekers HM, de Klerk A, de Groot H, Hofland RW, et al. Proteins involved in extracellular matrix dynamics are associated with respiratory syncytial virus disease severity. Eur Respir J. 2012 Jun;39(6):1475–81. doi: 10.1183/09031936.00012311. [DOI] [PubMed] [Google Scholar]
- 43.Hu J, Van den Steen PE, Sang Q-XA, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007 Jun;6(6):480–98. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: An overview. Mol Cell Biochem. 2003;253:269–85. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 45.Gaffney J, Solomonov I, Zehorai E, Sagi I. Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 2015 May;44–46:191–9. doi: 10.1016/j.matbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007 Apr;211(1):19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- 47.Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19:623–9. doi: 10.1016/s0945-053x(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 48.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007 Mar;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim Biophys Acta BBA - Mol Cell Res. 2010 Jan;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutton M, Willenbrock F, Brocklehurst K, Murphy G. Kinetic analysis of the mechanism of interaction of full-length TIMP-2 and gelatinase A: evidence for the existence of a low-affinity intermediate. Biochemistry (Mosc) 1998;37(28):10094–10098. doi: 10.1021/bi980616p. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. AJP Lung Cell Mol Physiol. 2014 Apr 15;306(8):L709–25. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008 Oct 15;112(8):3455–64. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 53.Marchant DJ, Bellac CL, Moraes TJ, Wadsworth SJ, Dufour A, Butler GS, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014 Apr 28;20(5):493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 54.Hynes RO. The Extracellular Matrix: Not Just Pretty Fibrils. Science. 2009 Nov 27;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman S, Patel Y, Murray J, Patel KV, Sumathipala R, Sobel M, et al. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005;6(1):1. doi: 10.1186/1471-2121-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, et al. Heparin-II Domain of Fibronectin Is a Vascular Endothelial Growth Factor-Binding Domain: Enhancement of VEGF Biological Activity by a Singular Growth Factor/Matrix Protein Synergism. Circ Res. 2006 Oct 13;99(8):853–60. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013 Feb 13;14(3):166–80. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ten Dijke P, Arthur HM. Extracellular control of TGFβ signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007 Nov;8(11):857–69. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 59.Kanzaki T, Olofsson A, Moren A, Wernstedt C, Hellman U, Miyazono K, et al. TGF-BI Binding Protein: A Component of the Large Latent Complex of TGFBI with-Multiple Repeat - Sequences. Cell. 1990;61:1051–61. doi: 10.1016/0092-8674(90)90069-q. [DOI] [PubMed] [Google Scholar]
- 60.Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996;15(2):245. [PMC free article] [PubMed] [Google Scholar]
- 61.Dubail J, Apte SS. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 2015 May;44–46:24–37. doi: 10.1016/j.matbio.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Hinz B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015 Sep;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Verrecchia F, Chu M-L, Mauviel A. Identification of Novel TGF-/Smad Gene Targets in Dermal Fibroblasts using a Combined cDNA Microarray/Promoter Transactivation Approach. J Biol Chem. 2001 May 18;276(20):17058–62. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 64.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host Responses in Tissue Repair and Fibrosis. Annu Rev Pathol Mech Dis. 2013 Jan 24;8(1):241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012 Jul 6;18(7):1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA. TRANSFORMING GROWTH FACTOR-β REGULATION OF IMMUNE RESPONSES. Annu Rev Immunol. 2006 Apr;24(1):99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 67.Travis MA, Sheppard D. TGF-β Activation and Function in Immunity. Annu Rev Immunol. 2014 Mar 21;32(1):51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li MO, Flavell RA. TGF-β: A Master of All T Cell Trades. Cell. 2008 Aug;134(3):392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008 May 8;453(7192):236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li MO, Wan YY, Flavell RA. T Cell-Produced Transforming Growth Factor-β1 Controls T Cell Tolerance and Regulates Th1- and Th17-Cell Differentiation. Immunity. 2007 May;26(5):579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Huttenlocher A, Horwitz AR. Integrins in Cell Migration. Cold Spring Harb Perspect Biol. 2011 Sep 1;3(9):a005074–a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007 Sep;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 73.Gaylo A, Schrock DC, Fernandes NRJ, Fowell DJ. T Cell Interstitial Migration: Motility Cues from the Inflamed Tissue for Micro- and Macro-Positioning. Front Immunol [Internet] 2016 Oct 14;:7. doi: 10.3389/fimmu.2016.00428. [cited 2016 Nov 14] Available from: http://journal.frontiersin.org/article/10.3389/fimmu.2016.00428/full. [DOI] [PMC free article] [PubMed]
- 74.Lämmermann T, Sixt M. Mechanical modes of “amoeboid” cell migration. Curr Opin Cell Biol. 2009 Oct;21(5):636–44. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20(2):167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 76.Richter M, Ray SJ, Chapman TJ, Austin SJ, Rebhahn J, Mosmann TR, et al. Collagen Distribution and Expression of Collagen-Binding 1 1 (VLA-1) and 2 1 (VLA-2) Integrins on CD4 and CD8 T Cells during Influenza Infection. J Immunol. 2007 Apr 1;178(7):4506–16. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- 77.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin v 6 binds and activates latent TGF 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 78.Huang X-Z, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133(4):921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meliopoulos VA, Van de Velde L-A, Van de Velde NC, Karlsson EA, Neale G, Vogel P, et al. An Epithelial Integrin Regulates the Amplitude of Protective Lung Interferon Responses against Multiple Respiratory Pathogens. In: Klein SL, editor. PLOS Pathog. 8. Vol. 12. 2016. Aug 9, p. e1005804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jolly L, Stavrou A, Vanderstoken G, Meliopoulos VA, Habgood A, Tatler AL, et al. Influenza Promotes Collagen Deposition via v 6 Integrin-mediated Transforming Growth Factor Activation. J Biol Chem. 2014 Dec 19;289(51):35246–63. doi: 10.1074/jbc.M114.582262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunninghake G, Davidson J, Rennard S, Szapiel S, Gadek J, Crystal R. Elastin Fragments Attract Macrophage Precursors to Diseased Sites in Pulmonary Emphysema. Science. 212(4497):925–7. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 82.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct 1;66(4):859–62. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99(3):870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brassart B, Fuchs P, Huet E, Alix AJP, Wallach J, Tamburro AM, et al. Conformational Dependence of Collagenase (Matrix Metalloproteinase-1) Up-regulation by Elastin Peptides in Cultured Fibroblasts. J Biol Chem. 2001 Feb 16;276(7):5222–7. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 85.Fahem A, Robinet A, Cauchard JH, Duca L, Soula-Rothhut M, Rothhut B, et al. Elastokine-mediated up-regulation of MT1-MMP is triggered by nitric oxide in endothelial cells. Int J Biochem Cell Biol. 2008;40(8):1581–96. doi: 10.1016/j.biocel.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 86.Toupance S, Brassart B, Rabenoelina F, Ghoneim C, Vallar L, Polette M, et al. Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin Exp Metastasis. 2012 Jun;29(5):511–22. doi: 10.1007/s10585-012-9467-3. [DOI] [PubMed] [Google Scholar]
- 87.Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest. 2016 Sep 1;126(9):3176–84. doi: 10.1172/JCI83147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taddese S, Weiss AS, Jahreis G, Neubert RHH, Schmelzer CEH. In vitro degradation of human tropoelastin by MMP-12 and the generation of matrikines from domain 24. Matrix Biol. 2009 Mar;28(2):84–91. doi: 10.1016/j.matbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for Macrophage Elastase for Cigarette Smoke–Induced Emphysema in Mice. Science. 1997;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 90.Houghton AM. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006 Mar 1;116(3):753–9. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pfister RR, Haddox JL. A neutrophil chemoattractant is released from cellular and extracellular components of the alkali-degraded cornea and blood. Invest Ophthalmol Vis Sci. 1996;37(1):230–237. [PubMed] [Google Scholar]
- 92.Pfister RR, Haddox JL, Sommers CI, Lam K-W. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1995;36(7):1306–1316. [PubMed] [Google Scholar]
- 93.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, et al. A novel peptide CXCR ligan derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–23. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 94.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, et al. A Novel Proteolytic Cascade Generates an Extracellular Matrix-Derived Chemoattractant in Chronic Neutrophilic Inflammation. J Immunol. 2008 Apr 15;180(8):5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akthar S, Patel DF, Beale RC, Peiró T, Xu X, Gaggar A, et al. Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat Commun. 2015 Sep 24;6:8423. doi: 10.1038/ncomms9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, et al. A Critical Role for LTA4H in Limiting Chronic Pulmonary Neutrophilic Inflammation. Science. 2010 Oct 1;330(6000):90–4. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koshikawa N. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB J [Internet] 2003 Dec 19; doi: 10.1096/fj.03-0584fje. [cited 2016 Nov 16]; Available from: http://www.fasebj.org/cgi/doi/10.1096/fj.03-0584fje. [DOI] [PubMed]
- 98.Pirilä E, Sharabi A, Salo T, Quaranta V, Tu H, Heljasvaara R, et al. Matrix metalloproteinases process the laminin-5 γ2-chain and regulate epithelial cell migration. Biochem Biophys Res Commun. 2003 Apr;303(4):1012–7. doi: 10.1016/s0006-291x(03)00452-2. [DOI] [PubMed] [Google Scholar]
- 99.Roychaudhuri R, Hergrueter AH, Polverino F, Laucho-Contreras ME, Gupta K, Borregaard N, et al. ADAM9 Is a Novel Product of Polymorphonuclear Neutrophils: Regulation of Expression and Contributions to Extracellular Matrix Protein Degradation during Acute Lung Injury. J Immunol. 2014 Sep 1;193(5):2469–82. doi: 10.4049/jimmunol.1303370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, et al. Neutrophil Elastase Cleaves Laminin-332 (Laminin-5) Generating Peptides That Are Chemotactic for Neutrophils. J Biol Chem. 2008 Apr 11;283(15):9513–22. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding B-S, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-Derived Angiocrine Signals Induce and Sustain Regenerative Lung Alveolarization. Cell. 2011 Oct;147(3):539–53. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt A-K, et al. Versican in inflammation and tissue remodeling: The impact on lung disorders. Glycobiology. 2015 Mar 1;25(3):243–51. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang D, Liang J, Noble PW. Hyaluronan as an Immune Regulator in Human Diseases. Physiol Rev. 2011 Jan 1;91(1):221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J Immunol. 2006 Jul 15;177(2):1272–81. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 105.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, et al. Hyaluronan Contributes to Bronchiolitis Obliterans Syndrome and Stimulates Lung Allograft Rejection through Activation of Innate Immunity. Am J Respir Crit Care Med. 2014 Mar;189(5):556–66. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, et al. ADAMTS Metalloproteases Generate Active Versican Fragments that Regulate Interdigital Web Regression. Dev Cell. 2009 Nov;17(5):687–98. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016 doi: 10.1182/blood-2016-03-705780. blood–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Solomonov I, Zehorai E, Talmi-Frank D, Wolf SG, Shainskaya A, Zhuravlev A, et al. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc Natl Acad Sci. 2016 Sep 27;113(39):10884–9. doi: 10.1073/pnas.1519676113. [DOI] [PMC free article] [PubMed] [Google Scholar]