Abstract
Purpose
While stereotactic body radiation therapy (SBRT) can reduce tumor volumes in metastatic renal cell carcinoma (mRCC) patients, little is known regarding the immunomodulatory effects of high-dose radiation in the tumor microenvironment. The main objectives of this pilot study were to assess the safety and feasibility of nephrectomy following SBRT treatment of mRCC patients and analyze the immunological impact of high-dose radiation.
Experimental Design
Human RCC cell lines were irradiated and evaluated for immunomodulation. In a single-arm feasibility study, mRCC patients were treated with 15 Gray (Gy) SBRT at the primary lesion in a single fraction followed 4 weeks later by cytoreductive nephrectomy. RCC specimens were analyzed for tumor-associated antigen (TAA) expression and T cell infiltration. The trial has reached accrual (ClinicalTrials.gov identifier: NCT01892930).
Results
RCC cells treated in vitro with radiation had increased TAA expression compared to untreated tumor cells. Fourteen patients received SBRT followed by surgery and treatment was well tolerated. SBRT-treated tumors had increased expression of the immunomodulatory molecule calreticulin and TAA (CA9, 5T4, NY-ESO-1, and MUC-1). Ki67+ proliferating CD8+ T cells and FOXP3+ cells were increased in SBRT-treated patient specimens in tumors and at the tumor-stromal interface compared with archived patient specimens.
Conclusions
It is feasible to perform nephrectomy following SBRT with acceptable toxicity. Following SBRT, patient RCC tumors have increased expression of calreticulin, TAA, as well as a higher percentage of proliferating T cells compared to archived RCC tumors. Collectively, these studies provide evidence of immunomodulation following SBRT in mRCC.
Keywords: Tumor-associated antigens, SBRT, clinical trial, renal cell carcinoma
INTRODUCTION
Renal cell carcinoma represents 3% of adult malignancies and approximately one quarter of patients present with metastatic disease (1). Metastatic renal cell carcinoma (mRCC) is especially difficult to treat with an approximate 10% 5 year overall survival (2). Immunotherapeutic approaches including conventional cytokine therapy (3), recently developed immune checkpoint inhibitor strategies (4), and dendritic cell vaccination (5) have shown promise in the treatment of mRCC with a subset of patients having complete, durable responses following therapy.
The emphasis of most immunotherapeutic regimens is to increase the number and improve the cytolytic function of tumor-specific T cells. This focus largely overlooks the tumor intrinsic barriers to effective T cell-mediated killing including T cell trafficking to the tumor microenvironment (6), presence of immunosuppressive cells (7), and expression of immunogenic antigens by tumors cells (8). mRCC is characterized as a heavily lymphocyte infiltrated tumor type with low and heterogeneous expression of tumor-associated antigens (TAA) (9, 10).
Diminished TAA expression is a well-characterized mechanism of immune evasion employed within the tumor microenvironment (8). Radiation-induced inflammation in tumors has the potential to overcome this impediment by releasing pro-inflammatory mediators, upregulating TAA, and increasing T cell infiltration (11). A growing body of evidence has also demonstrated that sublethal doses of radiation can induce expression of calreticulin and CD80, leading to increased cytotoxic lymphocyte activity following irradiation of tumor cells (12–14). Preclinical tumor models and in vitro data utilizing other tumor types have revealed that radiation therapy can increase TAA expression (13, 15). While RCC appears resistant to conventional fractionated radiation therapy (16), recent publications have shown that high doses delivered with modern stereotactic body radiation therapy (SBRT) can produce local control with limited toxicity (17, 18). Treatment of patients with SBRT in combination with conventional high-dose IL-2 treatment has demonstrated partial and complete objective responses in melanoma and mRCC patients that correlate with increased proliferating T cells in peripheral blood (17). While dozens of clinic trials are currently investigating the combination of radiation therapy with immunotherapy for treatment of malignancies (19), the isolated contributions of high-dose radiation in preconditioning patient tumors for effective antitumor responses remain unknown.
The capacity of SBRT to control growth of primary tumors and the findings that resection of primary tumors may improve overall survival of mRCC patients (20), led us to consider whether a combination of these treatments would be advantageous. First, we performed in vitro studies to explore the effects and timing of immune changes caused by high-dose irradiation as a surrogate for SBRT. These studies informed the design of a first in-human pilot trial to evaluate the feasibility and safety of combining single fraction SBRT with cytoreductive nephrectomy.
PATIENTS AND METHODS
Cell culture sample preparation and treatment
These studies were performed according to a protocol approved by the Roswell Park Cancer Institute Institutional Review Board (IRB). A498 human kidney carcinoma cells (ATCC, Manassas, VA) were cultured at 37°C in 75 cm2 flasks (Corning Inc.) at 200,000 cells/flask in RPMI 1640 supplemented with 0.05 mmol/L of 2-mercaptoethanol, 10% fetal bovine serum (ThermoFisher Scientific), 1% Penicillin streptomycin, 1 mmol/L of sodium pyruvate, 20 mmol/L of HEPES, 1% non-essential amino acids, and 2 mmol/L of L-glutamine (Corning Inc.). Culture media was refreshed every other day. A Shepherd Mark I Model 68, 4000 Ci Cesium 137 source irradiator (J.L. Shepherd and Associates), was used to irradiate cells, with a total dose rate of 1200 or 1600 cGy. Cells were trypsinized with 0.125% Trypsin EDTA (ThermoFisher Scientific). ELISPOT assays were performed using NY-ESO-1 specific T cell clones generated as described previously (21). A498 target cells previously treated with or without radiation were plated at a 1:1 ratio with NY-ESO-1-specific CD8+ T cells in 96 well plates (EMD Millipore) and later evaluated for IFN-ɣ secretion using C.T.L. ImmunoSpot Analyzer (Cellular Technologies).
Patients
We report results of a single center, IRB approved pilot study (ClinicalTrials.gov identifier: NCT01892930). All patients provided written informed consent, and the study was conducted in accordance to all applicable local regulatory requirements and laws. Eligible patients were at least 18 years of age, had mRCC, and were deemed fit for cytoreductive surgery to the primary tumor site. The exclusion criteria included: any chemotherapy, any radiotherapy, or investigational agent within 30 days prior to enrollment or radiation to the primary tumor at any point prior to treatment.
Patients with mRCC received 15 Gy SBRT to the primary tumor on Day 1 followed by nephrectomy on Day 29 (± 3 days). This dose was chosen based on our recent experience with this treatment being well tolerated in inoperable renal cell carcinoma patients (18). Patients were followed for protocol related toxicity 30 ± 3 days following surgery or until resolution of any toxicity. Thereafter, patients were followed as clinically indicated. An interim analysis for safety was conducted after the first 5 patients were treated. The study was then allowed to proceed. The data safety monitoring board of Roswell Park Cancer Institute reviewed this study annually.
Stereotactic body radiation therapy (SBRT) and nephrectomy
SBRT was delivered as previously described (18). Briefly, a stereotactic body immobilization system (BodyFIX, Medical Intelligence) was used with a 4-dimensional CT to assess tumor motion with respiration. Tumor delineation was carried out in all respiratory phases, creating an internal target volume and then a 5-mm isotropic expansion was used to create a planning target volume (PTV). Treatment was carried out with a Varian True-Beam linear accelerator (Varian Medical Systems). Volumetric modulated arc therapy with 6 MV photons was used. On the day of treatment, cone-beam CT was obtained following setup on the treating machine, and appropriate shifts were made.
Archival Tumor Specimens
Archival tumor specimens were obtained from patients who underwent cytoreductive nephrectomy for RCC and had given written consent for research use of a portion of the pathologic specimen. An independent broker, a person not associated with this research study, linked demographic and follow-up data with specimens to allow analysis. Archival specimens utilized in flow cytometry experiments were from 9 women and 7 men, resected between 2011 and 2015. Median age for this group was 60 years (range 41–72) (Supplementary Table 1). The control cohort utilized in immunohistochemistry analysis consisted of 2 women and 9 men with a median age of 69 years (range 45–80) (Supplementary Table 1).
Patient sample preparation
Under sterile conditions, RCC patient tumor samples were minced into small pieces and exposed to collagenase/hyaluronidase (collagenase 1 mg/mL, hyaluronidase 0.1 mg/mL, Sigma-Aldrich) digestive solution for 120 minutes. Samples were filtered through 100 μm cell strainers, washed with PBS, and stored at −80°C.
Flow cytometry
Cells were isolated, washed in PBS, and stained with Zombie UV™ Fixable Viability Kit for 10 minutes (Cat# 423108 2.5:100 BioLegend). Flow cytometric analysis was performed after staining with antibodies (Supplementary Table 2). NY-ESO-1 was detected with rat anti-mouse IgG1 secondary antibody (cat# 550083 PE 1:100, BD Biosciences). For intracellular staining, cells were washed and incubated with Fixation/Permeabilization Solution Kit (Cat# 554714, BD Biosciences). Cells were stained for expression of intracellular NY-ESO-1 and labeled with PE secondary antibody and fixed in 2% Formaldehyde solution.
Flow cytometric analysis was performed on BD LSR Fortessa flow cytometer (BD Biosciences). For adjustment, control cells were stained with each individual fluorochrome to serve as compensation controls. The outcome was analyzed using FlowJo_V10 cytometry software.
Immunohistochemistry analysis
Immunohistochemistry assays for Ki67 & CD8 doublestain, FOXP3, CD68, PD-L1, CD31, and Vimentin were performed by the Pathology Network Shared Resource at Roswell Park Cancer Institute (see Supplementary Table 3 for antibody information). Sections (4–5 μm) were placed on charged slides and dried at 60°C for one hour, then cooled to room temperature, deparaffinized in three changes of xylene, and rehydrated using graded alcohols. For antigen retrieval, slides were submerged in either Target Retrieval Solution, pH 9 (Dako) or EDTA buffer, pH 8 (ThermoFisher Scientific) and heated in a steamer (Supplementary Table 3), followed by a cooldown at room temperature for 20 minutes. Endogenous peroxidases were quenched with aqueous 3% H2O2 for 10 minutes and washed with PBS/T. Slides were loaded onto a DAKO autostainer (Dako) and serum-free protein block was applied for 5 minutes, blown off, and then primary antibody was applied (Supplementary Table 3). An irrelevant isotype-matched antibody was applied on replicate slides instead of primary antibody as a negative control. The EnVision+ Peroxidase system or G2 System/AP (Dako), PowerVision system (Leica), and/or the Vectastain Elite ABC kit (Vector Laboratories) and corresponding chromogens were used for visualization (Supplementary Table 3). Lastly, slides were counterstained with hematoxylin, dehydrated, cleared and cover slipped.
Slides were digitally scanned using Aperio ScanScope (Aperio Technologies, Inc.) with 20× bright-field microscopy. Once scanned, Aperio ImageScope version 11.2.0.780 (Aperio Technologies, Inc.) was used to view images for analysis. Slide image data fields were populated, images were examined for quality and amended as necessary. Annotation layers were created for each image representing tumor and interface regions with analysis performed on an average of 4 fields per tumor. Areas of target cells for image analysis were circled with care taken to avoid areas with staining artifacts or red blood cells using the negative free form pen.
Proliferating (Ki67+) CD8+ T cells were quantified using Aperio’s Image analysis tools as follows. Since Ki67 is expected to be present in the nuclei of many cell types in the tumor microenvironment, Genie software was first trained to recognize CD8+ T lymphocytes in order to limit Ki67 analysis to this cell population. Once training was complete, the fully trained Genie was saved, and applied as a classifier prior to the basic nuclear algorithm. This combined approach provided the total number of CD8+ lymphocytes and the percentage of Ki67+, proliferating CD8+ T cells within the area of analysis.
Statistical analyses
The primary purpose of this study was to demonstrate the feasibility of conducting a nephrectomy in patients receiving SBRT for mRCC. Eligible patients who had nephrectomy were considered evaluable for statistical analysis. SBRT complication rates were quantified as the binomial proportion of evaluable patients with at least one surgical complication related to SBRT. The plausible upper limit for the true SBRT complication rate was estimated using a one-sided 95% Clopper-Pearson confidence interval. Comparisons of SBRT-treated patient specimens with archival samples were performed using independent sample Student t tests. Overall survival was defined as the number of months between SBRT initiation and death from any cause. Patients who were still alive at the time of analysis were censored at the date of last follow-up. The overall survival distribution was described using the Kaplan-Meier estimator. Throughout, p-values less than 0.05 were deemed statistically significant. The p-values have not been adjusted for multiplicity. All statistical analyses were done using PRISM v6.07 (GraphPad Software).
RESULTS
Irradiation increases immune recognition of human RCC cells in vitro
Initial in vitro studies focused on the sensitivity of human RCC A498 cells to radiation-induced immune modulation. We found a higher percentage of tumor cells expressed calreticulin, CD80, and HSP70 on live A498 cells following treatment with either 12 Gy or 16 Gy compared to untreated RCC cells (Fig. 1A). These changes were consistent when evaluating mean fluorescence intensity (MFI) (Supplementary Fig. S1A) and occurred as early as 24 h after radiation treatment. A498 displayed an increased percentage of cells expressing CD86 and increased CD54 MFI (Supplementary Fig. S1B) following radiation. Irradiation of A498 cells also increased the percentage of cells expressing TAA as early as 24 h (NY-ESO-1), with all TAA (CA9, 5T4, MUC-1, and intracellular NY-ESO-1) expressed at higher levels at 96 h post treatment with either radiation dose (Fig. 1B). MFI of CA9, MUC-1, and intracellular NY-ESO-1 were also increased on A498 cells treated with radiation (Supplementary Fig. S2A). Since calreticulin has been shown to bind NY-ESO-1 protein at the cell surface (22), we evaluated membrane expression of NY-ESO-1 and determined that radiation increased surface expression of NY-ESO-1 as early as 72 h after treatment (Supplementary Fig. S2B). Importantly, as studies have shown radiation can increase autofluorescence (23), we found a minimal contribution of non-specific staining for any isotype control antibody and fluorochrome combination (Supplementary Fig. S2C).
Figure 1. Radiation increases expression of tumor-associated antigens and immune recognition by antigen-specific T cells.

A498 cells were treated or untreated with radiation. Immunomodulatory markers (A) and tumor-associated antigens (B) were measured at indicated times by flow cytometry. Data (mean ± s.e.m.) are from ≥ 3 independent experiments. (C) 96 h after treatment, A498 tumor cells were cocultured with expanded NY-ESO-1 specific CD8 T cells and monitored for IFN-ɣ secretion by ELISPOT assays. One representative experiment out of two is shown (mean ± s.e.m.). *P < 0.05 vs. 0 Gy; unpaired two-tailed Student’s t-test.
To directly assess if TAA-specific CD8+ T cells were more responsive to irradiated RCC tumor cells expressing higher levels of TAA, we performed coculture experiments with NY-ESO-1 specific CD8+ T cells and evaluated IFN-ɣ secretion by ELISPOT. In these experiments, more antigen-specific CD8+ T cells secreted IFN-ɣ when cultured with A498 cells that were irradiated 96 h prior to coculture (Fig. 1C).
Study Cohort
A total of 16 patients were enrolled between July 2013 and November 2015 (Table 1). The median age at diagnosis was 63.9 years (52–75). Patients were treatment naïve prior to SBRT therapy, but many received systemic treatment following nephrectomy (Table 1). SBRT was successfully performed in all patients per study specifications. Patient #2 (intracranial bleeding from a small, previously unknown brain metastasis) died prior to surgery. This prompted the requirement of brain imaging prior to SBRT to rule out immune-mediated intracranial side effects. Patient #11 had known spinal disease (outside of SBRT field), developed cord compression 12 days following SBRT, and therefore did not have nephrectomy. She died 12 months after SBRT. The remaining 14 patients underwent nephrectomies. Twelve patients had clear cell renal cell carcinoma while Patient #4 was found to have a high grade papillary urothelial carcinoma and Patient # 8 showed chromophobe RCC. Since tumor type can influence TAA expression and infiltration, we limited our remaining analysis to patients with clear cell histology.
Table 1.
Patient characteristics and response
| Patient | Gender | PTV cm3 | Metastatic Site | Progressed | Heng Score | Pathologic T Stage | Survival Time (Months) | Adjuvant Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 619 | Adrenal | N | 1 | 2A | 42+ | Clinical Trial agent, Adrenalectomy |
| 2 | F | 891 | Lung, psoas | N | 4 | NA | 1 | None |
| 3 | F | 637 | Lung | N | 3 | 3A | 4 | None |
| 5 | M | 355 | Retroperitoneal | N | 1 | 3A | 4 | None |
| 6 | F | 501 | Adrenal | Y | 2 | 4 | 35+ | Pazopanib, Bevacizumab alternating trial |
| 7 | M | 129 | Adrenal | Y | 2 | 3A | 20 | RT, Pazopanib |
| 9 | M | 37 | Lung, Retroperitoneal | N | 2 | 1A | 24+ | Sutent |
| 10 | F | 664 | Lung, Liver | N | 1 | 3A | 3 | None |
| 11 | F | 306 | Bone | Y | 1 | NA | 12 | RT, Sutent, Nivolumab |
| 12 | F | 105 | Retroperitoneal | N | 2 | 1 | 20+ | Sutent, Nivolumab |
| 13 | F | 891 | Bone | Y | 2 | 2 | 18+ | Pazopanib, Nivolumab, RT, Sorafenib |
| 14 | M | 360 | Bone, Retroperitoneal | N | 1 | 3A | 17+ | Pazopanib, RT, Nivolumab |
| 15 | F | 381 | Bone | Y | 2 | 2A | 17+ | Pazopanib, RT, Nivolumab |
| 16 | F | 1241 | Lung | N | 2 | 3A | 16+ | Pazopanib, Nivolumab |
Notes:
NA = Not applicable because patients 2 and 11 did not undergo surgery following SBRT.
(+) denotes still alive.
PTV = Planning Target Volume for radiation.
Patients 4 and 8 were removed due to final pathology other than clear cell cancer.
The median PTV volume for SBRT was 441 cc (37–1241) for clear cell patients. Respiratory gating to treat the tumor only at pre-specified points in the respiratory cycle and active breath hold techniques were both allowed. A 15 Gy dose was prescribed to the PTV. The maximum percent dose 2 cm from the PTV ranged from 57 to 89 and generally increased with increasing PTV size. The ratio of the intermediate dose spillage at 50% of the prescription dose volume and the PTV, (R50%) was measured to describe the dose gradient. The average R50% was 3.67 (2.97 – 4.32.). Two-week post SBRT CT scans were performed. There were no significant changes from baseline in any scan.
Intraoperatively, there was a noticeable degree of perinephric fibrosis. This made the dissection of the retroperitoneal space a bit more challenging at times. Kocherizing the duodenum was difficult for patient #1, but no intraoperative complications were encountered.
Three patients had partial nephrectomies and 11 had total nephrectomies. Blood loss averaged 100 cc. The planned type of nephrectomy was not changed following SBRT. There were no post-surgical complications in the 14 patients who received surgery. Using a 1-sided 95% Clopper Pearson confidence limit, the true SBRT complication rate is likely less than 0.23.
Treatment related adverse events are shown (Table 2). One patient had a grade 3 anemia. The most common adverse events were nausea and vomiting occurring in 44% and 38% of patients respectively. 4 of 16 patients (25%) suffered grade 2 acute toxicity. 1 and 2 year overall survival estimates are 71% and 48% respectively and all patients still alive have been followed for a minimum of 17 months (Table 1).
Table 2.
Number of patients with adverse events following treatment
| Adverse Event | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| System Organ Class | Specific Term | |||
| Cardiac disorders | Tachycardia | 0 | 2 | 0 |
| Gastrointestinal disorders | Any AE - Maximum | 7 | 3 | 0 |
| Abdominal pain | 2 | 2 | 0 | |
| Constipation | 1 | 1 | 0 | |
| Diarrhea | 1 | 0 | 0 | |
| Nausea | 7 | 0 | 0 | |
| Vomiting | 5 | 1 | 0 | |
| General disorders and administration site conditions | Any AE - Maximum | 2 | 0 | 0 |
| Fatigue | 2 | 0 | 0 | |
| Edema peripheral | 1 | 0 | 0 | |
| Pyrexia | 1 | 0 | 0 | |
| Investigations | Any AE - Maximum | 3 | 0 | 1 |
| Alanine aminotransferase increased | 1 | 0 | 0 | |
| Aspartate aminotransferase increased | 1 | 0 | 0 | |
| Blood creatinine increased | 3 | 0 | 0 | |
| Hemoglobin decreased | 0 | 0 | 1 | |
| Metabolism and nutrition disorders | Any AE - Maximum | 4 | 2 | 0 |
| Decreased appetite | 3 | 0 | 0 | |
| Hypercalcemia | 0 | 1 | 0 | |
| Hyperkalemia | 0 | 1 | 0 | |
| Hypocalcaemia | 2 | 0 | 0 | |
| Hypomagnesaemia | 0 | 1 | 0 | |
| Musculoskeletal and connective tissue disorders | Any AE - Maximum | 1 | 1 | 0 |
| Flank pain | 0 | 1 | 0 | |
| Myalgia | 1 | 0 | 0 | |
| Nervous system disorders | Dizziness | 1 | 0 | 0 |
| Renal and urinary disorders | Hematuria | 1 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | Dyspnea | 1 | 0 | 0 |
| Vascular disorders | Hypotension | 0 | 1 | 0 |
| Injury, poisoning and procedural complications | Radiation skin injury | 0 | 1 | 0 |
| ANY AE - Maximum Grade Seen Total (percentage) | 6 (37.5) | 4 (25) | 1 (6) | |
Surface expression of immunomodulatory molecules and tumor-associated antigens in archival control and study cohort tumors
CD45 and viability stains were utilized throughout analysis to identify live tumor cells (CD45−) or leukocytes (CD45+) within single cell suspensions (Supplementary Fig. S3A–B). Tumor cell surface expression of calreticulin was increased in samples resected from clear cell RCC patients treated by SBRT (Fig. 2A). The expression of surface molecules CD80, HSP70, MHC I, and CD86 were unchanged when comparing SBRT treated patient tumor cells to the archival cohort (Fig. 2B–C, Supplementary Fig. S4A–B). CD54+ (ICAM-1) expression decreased in SBRT treated samples compared to controls (Supplementary Fig. S4C).
Figure 2. Tumors resected from clear cell RCC patients treated with SBRT have increased levels of calreticulin.
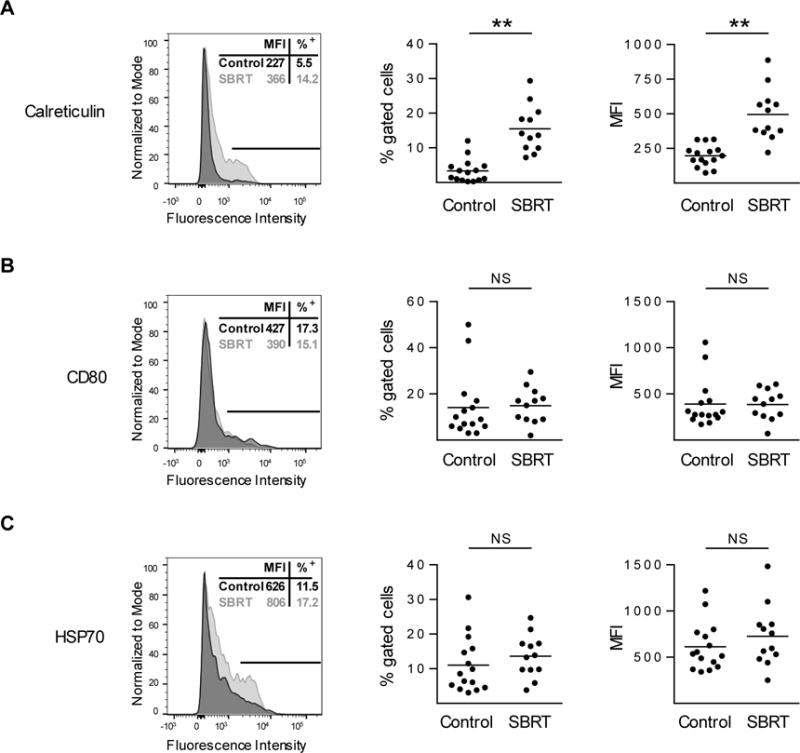
Control (archived nephrectomy) or SBRT (radiation plus nephrectomy) RCC patient tumors were evaluated for calreticulin (A), CD80 (B), and HSP70 (C) by flow cytometry. Single parameter flow histograms of representative samples are shown (left, MFI and percentage positive is indicative of sample shown). Line indicates portion of sample that is positive for marker. Percent positive (middle) and MFI (right) values are shown wherein horizontal lines indicate mean. n ≥ 12 per condition. **P < 0.01; NS, not significant; unpaired two-tailed Student’s t-test.
Analysis of TAA expression (CA9, MUC1, 5T4, and NY-ESO-1) by flow cytometry showed increased expression in SBRT treated specimens compared to the control cohort, both in terms of the percentage of tumor cells expressing TAA, and in the expression level of TAA (Fig. 3A–D). Further analysis demonstrated that surface expression of NY-ESO-1 was increased in SBRT samples compared to archived control samples (Supplementary Fig. S4D).
Figure 3. Tumors resected from clear cell RCC patients treated with SBRT have increased levels of tumor-associated antigens.
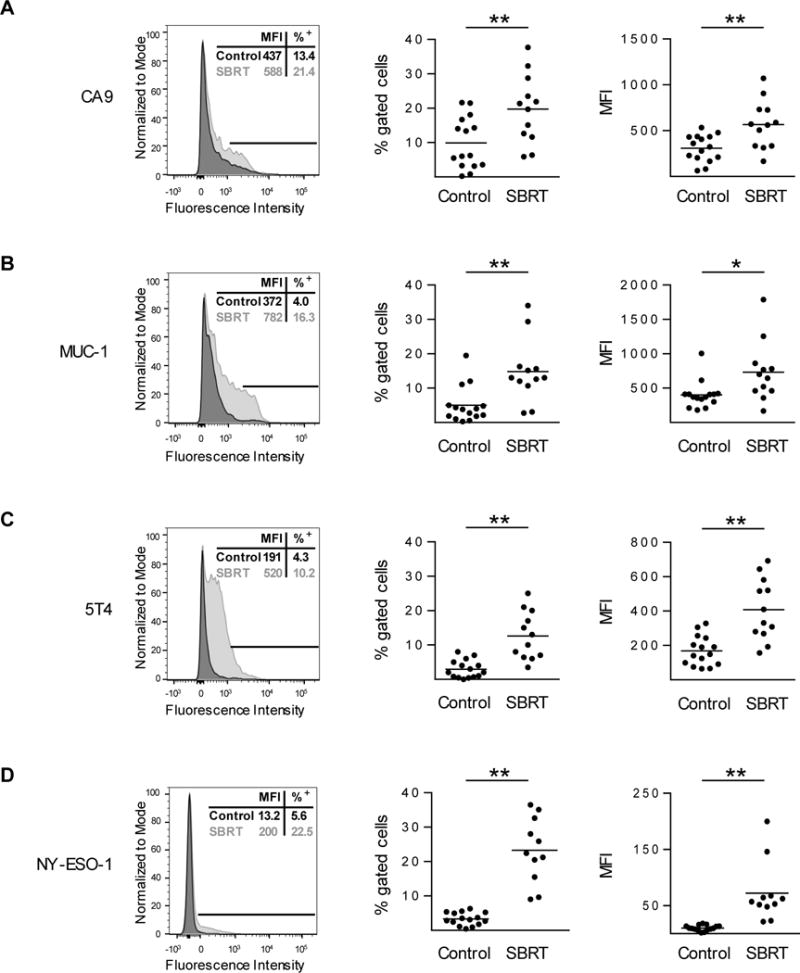
Control (archived nephrectomy) or SBRT (radiation plus nephrectomy) RCC patient tumors were evaluated for CA9 (A), MUC-1 (B), 5T4 (C), and intracellular NY-ESO-1 (D) by flow cytometry. Single parameter flow histograms of representative samples are shown (left, MFI and percentage positive is indicative of sample shown). Line indicates portion of sample that is positive for the marker. Percent positive (middle) and MFI (right) values are shown wherein horizontal lines indicate mean. n ≥ 11 per condition. *P < 0.05, **P < 0.01; unpaired two-tailed Student’s t-test.
Increased immune infiltration in SBRT treated RCC tumors compared to control cohort
To evaluate CD8+ T cell infiltration within intratumoral and peritumoral regions following SBRT treatment, we performed two-color immunohistochemistry staining of nephrectomy specimens for CD8+ T cells and Ki67 as a marker of proliferation. We observed no differences in CD8+ T cell infiltration within the tumor or at the tumor-stromal interface following SBRT compared to control nephrectomy specimens (Fig. 4A–B). However, when we focused our analysis on proliferating cells, we observed that the density of Ki67+ CD8+ cells was increased in SBRT samples compared to control (Fig. 4A–B). Similar to our findings by IHC that CD8+ density was not increased, characterization of the T cell infiltrate by flow cytometry demonstrated no change in the number of CD4+ or CD8+ T cells in resected tumors (Supplementary Fig. S5A). However, the percentage of CD4+ T cells that expressed the immune checkpoint protein PD-1 was increased in SBRT-treated specimens (Supplementary Fig. S5A). Further investigation into the immune cell infiltrate showed increased FOXP3+ cell and CD68+ macrophage accumulation in SBRT treated samples (Fig. 4C, Supplementary Fig S5B).
Figure 4. Tumors resected from SBRT-treated clear cell RCC patients have higher density of proliferating CD8+ T cells and FOXP3+ cells.
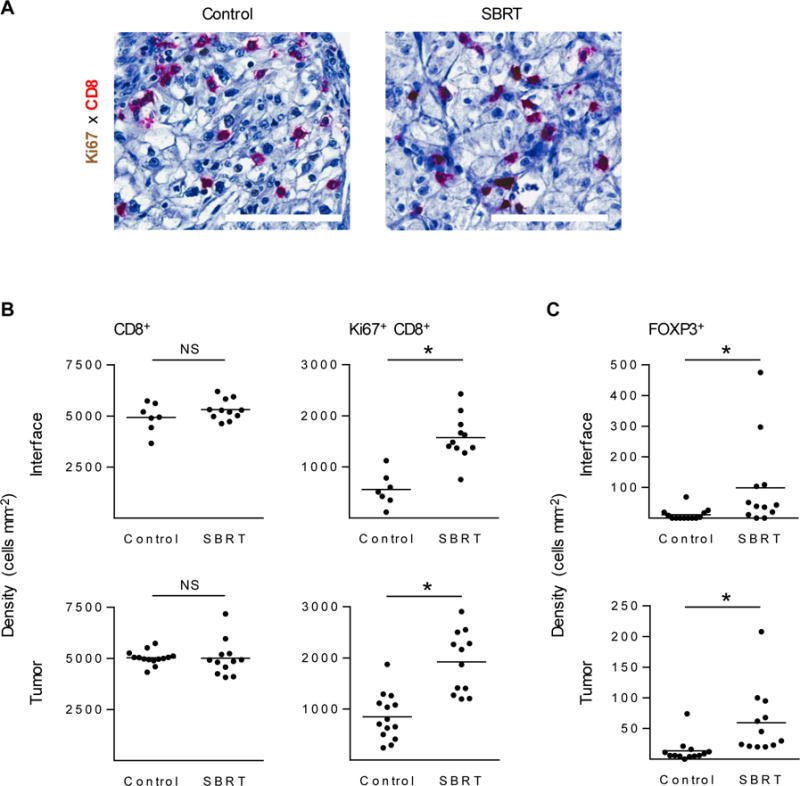
(A) IHC of CD8+ T cells (red) and Ki67+ (brown) in either control archival samples (left) or SBRT-treated (right) resected patient RCC tumors. Scale bars: 100 μm. Total CD8+ T cells numbers and Ki67+ CD8+ T cells were quantified by Aperio image analysis software (B). FOXP3+ cell density was evaluated in control and SBRT samples (C). n ≥ 7 patient samples per condition. *P < 0.05; NS, not significant; unpaired two-tailed Student’s t-test. Horizontal lines denote mean.
We performed additional analysis to investigate the effects of SBRT on the tumor stroma. We evaluated vascular density in SBRT-treated tumors and observed no difference (Supplemental Fig. S5C). However, expression of vimentin, a common fibroblast marker which can be expressed on endothelial cells and RCC, was increased in SBRT samples compared to archived controls (Supplemental Fig. S5D). Immunohistochemistry analysis of patient biopsy samples showed no change in PD-L1 expression or any correlation of PD-L1 with Ki67+ CD8+ density (Supplementary Fig. S5E) as previously described (24).
DISCUSSION
This unique prospective pilot trial demonstrates that single fraction, 15 Gy, SBRT followed by nephrectomy for mRCC is: 1) feasible and well tolerated and 2) induced tumor intrinsic changes (increased calreticulin and TAA expression) and increased the percentage of proliferating CD8+ T cells in primary tumors.
Overall, SBRT followed by nephrectomy was safe. The single grade 3 treatment-related adverse event occurred in a patient with pre-existing history of anemia who received a transfusion following SBRT. Grade 2 acute toxicity occurred in only 25% of patients. There were no grade 3 or higher surgical complications and the qualitative clinical assessment of the surgeons was that SBRT did not make surgery appreciably more difficult. There are 2 published reports, with a total of 4 patients, of histological examination of kidney tissue following SBRT. Onishi et al. reported autopsy data on a patient treated with 60 Gy in 10 fractions who died 2.5 years later of unrelated causes showing that some viable renal cancer cells remained (25). Ponski et al performed surgery in 3 patients 8 weeks after 16 Gy was delivered in 4 fractions and showed a complete response in 1 patient (26). Consistent with our findings that SBRT prior to surgery was well tolerated, there were no acute or late toxicities within the first year of follow-up.
However, it must be noted that 2 patients (intracranial bleed and cord compression) on this trial did not receive their planned surgery due to tumor progression soon after, but far distant from the irradiated area. These may have been random events of disease progression and were officially coded as such. Yet, the patient with initial cord compression from known vertebral body metastases remained alive with good function for one year following neurosurgical decompression. Notably, similar events (described as pseudo-progression) have been seen with Nivolumab in a small number of mRCC patients (27) and a variety of other immune agents as summarized by Chiou and Burrato (28). It is therefore also possible that disease progression events noted in this trial are partly the result of swelling from infiltration of immune cells.
SBRT has been shown, in preclinical models, to induce both stimulating and suppressive immune effects within the tumor microenvironment (7). The balance between stimulation and suppression may depend on tumor type, radiation dose and number of fractions (14), as well as the time of observation. Clinically, a wide range of SBRT fractions (1–10) and doses (18 to 60 Gy) for inoperable RCC have been reported in the literature (29). In this study, 15 Gy in 1 fraction was utilized due to 3 factors: 1) clinical experience with this dose being well tolerated in our inoperable patients (18), 2) radiobiological data suggesting vascular effects with this dose (30), and 3) in vitro experiments (Fig. 1) showing that this dose has immunomodulatory effects.
Experiments performed in vitro found significant upregulation of both immunomodulatory markers (calreticulin, CD80, and HSP70, Fig. 1A) and TAAs (MUC1, NY-ESO-1, CA9, and 5T4, Fig. 1B) following radiation. These findings are consistent with data that radiation-induced tumor cell death can promote antitumor immunity through surface expression of calreticulin and secretion of immunogenic proteins (31). Radiation therapy has also been shown to increase expression of calreticulin (12, 32), while enhancing expression of costimulatory molecules (15, 33) and tumor-associated antigens (TAA) (15) on live tumor cells. The finding of increased IFN-ɣ secretion from NY-ESO-1 specific T cells following coculture with an irradiated human renal tumor cell line (Fig. 1C) is consistent with data showing that irradiation can improve tumor cell sensitivity to effector CD8+ T cell-mediated cytolysis (34).
Consistent with our in vitro findings that surface expression of calreticulin can be induced on RCC cells surviving radiation treatment (Fig. 1A), we found an increase in calreticulin expression on samples resected from patients treated by SBRT (Fig. 2A). Contrary to our in vitro data, the expression of previously identified radio-inducible surface molecules CD80, HSP70, MHC I,(33, 35, 36) as well as CD86 and PD-L1 were unchanged when comparing SBRT treated patient tumor cells to the archival cohort (Fig. 2 B–C, Supplementary Fig. S4A–B, Supplementary Fig. S5E). Unexpectedly, expression of CD54 (ICAM-1) was decreased on SBRT treated human tumor (Supplementary Fig. S5C), which is in contrast to previous reports showing radiation can increase CD54 expression on tumor cell lines (15).
Immune stimulation results from 2 basic mechanisms in preclinical models: 1) direct killing of tumor cells by radiation increases the availability and cross-presentation of TAA to CD8+ T cells (12, 15) and 2) immune changes resulting from cytokine production, particularly interferon gamma (IFN-γ). Radiation doses as low as 10 Gy have been shown to increase TAA expression in numerous cell lines (15, 34), but few studies have addressed TAA expression in human tumors treated in vivo (37). Furthermore, previous findings utilizing conventional radiation have shown RCC to be refractory to treatment (16). We selected the TAA CA9, MUC-1, 5T4, and NY-ESO-1 which are overexpressed in RCC patient tumors compared to normal tissues (38–40) and were increased by radiation in our in vitro studies (Fig. 1B).
Our finding of NY-ESO-1 and MUC-1 upregulation in mRCC patient tumors following 15 Gy SBRT (Fig. 3) is supported by published in vitro studies showing an upregulation of these tumor-associated antigens in multiple non renal cell carcinoma cell lines following ionizing radiation (13, 37). Similarly, the tumor-associated antigen CA9 was found to be elevated following SBRT in our study (Fig. 3). This upregulation is a novel finding and is particularly significant since CA9 is a known prognostic factor in RCC; in a meta-analysis of 15 studies with 2611 RCC patients, low expression was associated with poor disease-specific survival (41).
We observed higher levels of proliferating CD8+ T cells and FOXP3+ T regulatory cells (Tregs) without changes in overall CD8+ T cell numbers or tumor size from baseline, suggesting that presence of additional mechanisms preventing CD8+ T cell infiltration and cytolytic function (Fig. 4B–C, Supplementary Fig. S5A). Lower intratumoral ratios of CD8+ T cells to Tregs may indicate poor prognosis (42, 43), providing rationale for strategic inhibition of Tregs with SBRT to promote antitumor responses. Studies have also demonstrated that increased presence of intratumoral proliferating CD8+ T cells occur in responding patients to immune checkpoint inhibition (24), providing additional support for a combined SBRT and immune checkpoint blockade strategy which has been the subject of numerous investigations (43, 44). Previous studies demonstrating that a single dose of radiation can induce durable immune responses to antigen that are still observed weeks after treatment (45), also suggest that increased Ki67+ CD8+ intratumoral T cells may indicate a sustained systemic immune response.
Gene expression analysis and immunohistochemical studies have characterized RCC as a heavily lymphocyte infiltrated tumor type with high levels of cytolytic activity (10, 46). However unlike other tumor types, abundant CD8+ T cell infiltration is associated with shorter overall survival in RCC (10). Conversely a high proliferative capacity of intratumoral CD8+ T cells, evaluated by Ki67 staining, is associated with longer survival in RCC (10). Park et al. found a similar increase in tumor reactive CD8+ T cells in murine models of renal cell cancer and melanoma treated with SBRT; moreover, they found that this effect was enhanced in mice treated with PD-1 blockade and in PD-1 knockout mice (47). Recent human studies have shown that higher levels of intratumoral proliferating T cells are prognostic indicators for response to immune checkpoint inhibition strategies. Immune checkpoint blockade has also been shown to increase intratumoral proliferating T cells in responding patients (24, 43). Interestingly, tyrosine kinase inhibitors may also improve the proliferative capacity of intratumoral lymphocytes in RCC patients (48), and thus may provide another treatment option to bolster endogenous antitumor immune responses.
A significant limitation of this study is the single observation of patient tumors 4 weeks after SBRT treatment. This snapshot of the tumor microenvironment following radiation does not reflect the dynamic process that may have occurred earlier following treatment. As others have noted in preclinical murine models and in vitro studies, increased immune infiltration and expression of immunomodulatory molecules including MHC I occurs early following radiation before returning to baseline levels (11, 33). Furthermore, while FOXP3+ T regulatory cell and macrophage infiltration were increased at this time point, these cells may be functioning in an anti-inflammatory role weeks after a local immune response was triggered. The observation of the tumor microenvironment at this single time point (28 days after SBRT) does not allow this investigation to delineate early and late responses to treatment and the plasticity of the immune cell subsets within the tumor microenvironment. Specifically, intratumoral macrophages could have protumorigenic, angiogenic, and immunosuppressive function within RCC tumors leading to immune dysfunction (49, 50). Conversely, macrophages within RCC can also promote cytotoxic function (50). The impact of radiation on the behavior of these cells within the RCC lesions is an open area of investigation. The extension of our findings on immunomodulation to patient survival are also limited by the number of patients, lack of a pretreatment biopsy for comparison, as well as the varied treatments following enrollment in this trial (Table 1).
In conclusion, this trial is the first to demonstrate that nephrectomy following SBRT to the primary tumor is safe and feasible. These data demonstrate for the first time that SBRT can make significant changes to the immune landscape within primary RCC lesions, which may impact the efficacy of immunotherapies.
Supplementary Material
Translational Relevance.
Metastatic renal cell carcinoma (mRCC) is a lethal disease that, in some instances, can be treated long-term with immune-mediated strategies. Effective antitumor responses require intratumoral T cell infiltration, detection of tumor-associated antigens (TAA), and initiation of tumor cell lysis. Immunotherapy of RCC is challenged by poor TAA expression. Radiation increases TAA in multiple tumor types, but data in mRCC are scarce. The advent of stereotactic body radiation therapy (SBRT) allows delivery of radiation to well-defined areas with limited collateral damage. We performed a clinical trial to assess the feasibility of surgery following SBRT and analyze the intratumoral immune landscape after SBRT. We found that cytoreductive nephrectomy was feasible following SBRT and show for the first time that patient tumors treated with SBRT have increased TAA expression and intratumoral Ki67+ T cell infiltration. The immunomodulatory effects of SBRT provide rationale for combining SBRT with immune-activating approaches including checkpoint inhibition and vaccination.
Acknowledgments
The authors would like to thank Angela Omilian for assistance during setup of human IHC studies and Michelle Appenheimer for critical reading of the manuscript.
Financial support: Research reported here was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 to the University at Buffalo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work also supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Flow and Image Cytometry as well as Biostatistics Shared Resources. Research funding also provided by NIH R01CA140622 (S. Abrams), the Sklarow Foundation, Elsa Kreiner Memorial Fund, Fraternal Order of Eagles, and RPCI Friends of Urology (T. Schwaab and J. Muhitch).
Footnotes
Trial registration ID: NCT01892930
Conflict of interest statement: The authors of this manuscript report no relationship to disclose.
Data in this manuscript were in part presented at a podium session at the American Urological Association Meeting, May 15 – 19, 2015, New Orleans, LA.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Wahlgren T, Harmenberg U, Sandstrom P, Lundstam S, Kowalski J, Jakobsson M, et al. Treatment and overall survival in renal cell carcinoma: a Swedish population-based study (2000–2008) Br J Cancer. 2013;108(7):1541–9. doi: 10.1038/bjc.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113(2):293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol. 2016;34(8):833–42. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 5.Schwaab T, Schwarzer A, Wolf B, Crocenzi TS, Seigne JD, Crosby NA, et al. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and IFN-{alpha}2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res. 2009;15(15):4986–92. doi: 10.1158/1078-0432.CCR-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121(10):3846–59. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Wolf K, Vermaelen K, De Meerleer G, Lambrecht BL, Ost P. The potential of radiotherapy to enhance the efficacy of renal cell carcinoma therapy. Oncoimmunology. 2015;4(10):e1042198. doi: 10.1080/2162402X.2015.1042198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I, et al. 5-aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin Cancer Res. 2002;8(8):2690–5. [PubMed] [Google Scholar]
- 10.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132–6. [PubMed] [Google Scholar]
- 11.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 12.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–16. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell-Mediated Killing. Int J Radiat Oncol Biol Phys. 2016;95(1):120–30. doi: 10.1016/j.ijrobp.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 16.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34(1):251–66. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 17.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2–tumor and immunological responses. Sci Transl Med. 2012;4(137):137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 18.Hanzly M, Creighton T, Mix M, Zeeck K, Fung-Kee-Fung S, Singh AK, et al. Stereotactic Body Radiotherapy for the Treatment of Renal Tumors. Urol Case Rep. 2014;2(5):147–9. doi: 10.1016/j.eucr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden EB, Formenti SC. Radiation therapy and immunotherapy: growing pains. Int J Radiat Oncol Biol Phys. 2015;91(2):252–4. doi: 10.1016/j.ijrobp.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Heng DY, Wells JC, Rini BI, Beuselinck B, Lee JL, Knox JJ, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66(4):704–10. doi: 10.1016/j.eururo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaki J, Tsuji T, Luescher IF, Shiku H, Mineno J, Okamoto S, et al. Direct tumor recognition by a human CD4(+) T-cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses. Sci Rep. 2015;5:14896. doi: 10.1038/srep14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng G, Aldridge ME, Tian X, Seiler D, Zhang X, Jin Y, et al. Dendritic cell surface calreticulin is a receptor for NY-ESO-1: direct interactions between tumor-associated antigen and the innate immune system. J Immunol. 2006;177(6):3582–9. doi: 10.4049/jimmunol.177.6.3582. [DOI] [PubMed] [Google Scholar]
- 23.Schaue D, Ratikan JA, Iwamoto KS. Cellular autofluorescence following ionizing radiation. PLoS One. 2012;7(2):e32062. doi: 10.1371/journal.pone.0032062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi H, Kawasaki T, Zakoji H, Yoshida T, Komiyama T, Kuriyama K, et al. Renal cell carcinoma treated with stereotactic radiotherapy with histological change confirmed on autopsy: a case report. BMC Res Notes. 2014;7:270. doi: 10.1186/1756-0500-7-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponsky LE, Mahadevan A, Gill IS, Djemil T, Novick AC. Renal radiosurgery: initial clinical experience with histological evaluation. Surg Innov. 2007;14(4):265–9. doi: 10.1177/1553350607310546. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33(31):3541–3. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swaminath A, Chu W. Stereotactic body radiotherapy for the treatment of medically inoperable primary renal cell carcinoma: Current evidence and future directions. Can Urol Assoc J. 2015;9(7–8):275–80. doi: 10.5489/cuaj.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 31.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 32.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133(3):624–36. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 35.Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, et al. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol. 2011;101(1):109–15. doi: 10.1016/j.radonc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 36.Vereecque R, Buffenoir G, Gonzalez R, Cambier N, Hetuin D, Bauters F, et al. gamma-ray irradiation induces B7.1 expression in myeloid leukaemic cells. Br J Haematol. 2000;108(4):825–31. doi: 10.1046/j.1365-2141.2000.01967.x. [DOI] [PubMed] [Google Scholar]
- 37.Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H, et al. gamma-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One. 2011;6(11):e28217. doi: 10.1371/journal.pone.0028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffiths RW, Gilham DE, Dangoor A, Ramani V, Clarke NW, Stern PL, et al. Expression of the 5T4 oncofoetal antigen in renal cell carcinoma: a potential target for T-cell-based immunotherapy. Br J Cancer. 2005;93(6):670–7. doi: 10.1038/sj.bjc.6602776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dannenmann SR, Hermanns T, Bransi A, Matter C, von Boehmer L, Stevanovic S, et al. Spontaneous peripheral T-cell responses toward the tumor-associated antigen cyclin D1 in patients with clear cell renal cell carcinoma. Cancer Immunol Res. 2013;1(5):288–95. doi: 10.1158/2326-6066.CIR-13-0113. [DOI] [PubMed] [Google Scholar]
- 40.Fujita K, Denda K, Yamamoto M, Matsumoto T, Fujime M, Irimura T. Expression of MUC1 mucins inversely correlated with post-surgical survival of renal cell carcinoma patients. Br J Cancer. 1999;80(1–2):301–8. doi: 10.1038/sj.bjc.6690355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Z, Liao G, Li Y, Zhou S, Zou H, Fernando S. Prognostic value of carbonic anhydrase IX immunohistochemical expression in renal cell carcinoma: a meta-analysis of the literature. PLoS One. 2014;9(11):e114096. doi: 10.1371/journal.pone.0114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res. 2017;23(6):1388–1396. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190(11):5874–81. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res. 2015;3(6):610–9. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guislain A, Gadiot J, Kaiser A, Jordanova ES, Broeks A, Sanders J, et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother. 2015;64(10):1241–50. doi: 10.1007/s00262-015-1735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41(5):815–29. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Santoni M, Massari F, Amantini C, Nabissi M, Maines F, Burattini L, et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2013;62(12):1757–68. doi: 10.1007/s00262-013-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


