Abstract
Background
Carotid plaque area is a strong predictor of cardiovascular events. High homocysteine levels, which are associated with plaque formation, can result from inadequate intake of folate and vitamin B12. Now that folic acid fortification is widespread in North America, vitamin B12 has become an important determinant of homocysteine levels. We sought to determine the prevalence of low serum levels of vitamin B12, and their relation to homocysteine levels and carotid plaque area among patients referred for treatment of vascular disease since folic acid fortification of enriched grain products.
Methods
We evaluated 421 consecutive new patients with complete data whom we saw in our vascular disease prevention clinics between January 1998 and January 2002. We measured total carotid plaque area by ultrasound and determined homocysteine and serum vitamin B12 levels in all patients.
Results
The patients, 215 men and 206 women, ranged in age from 37 to 90 years (mean 66 years). Most were taking medications for hypertension (67%) and dyslipidemia (62%). Seventy-three patients (17%) had vitamin B12 deficiency (vitamin B12 level < 258 pmol/L with homocysteine level > 14 μmol/L or methylmalonic acid level > 271 nmol/L). The mean area of carotid plaque was significantly larger among the group of patients whose vitamin B12 level was below the median of 253 pmol/L than among those whose vitamin B12 level was above the median: 1.36 (standard deviation [SD] 1.27) cm2 v. 1.09 (SD 1.0) cm2; p = 0.016.
Conclusions
Vitamin B12 deficiency is surprisingly common among patients with vascular disease, and, in the setting of folic acid fortification, low serum vitamin B12 levels are a major determinant of elevated homocysteine levels and increased carotid plaque area.
Elevated plasma total homocysteine levels are a strong, graded independent risk factor for stroke and myocardial infarction.1,2 Mechanisms by which homocysteine may cause vascular disease include a propensity for thrombosis and impaired thrombolysis, increased production of hydrogen peroxide, endothelial dysfunction and increased oxidation of low-density lipoproteins and Lp(a) lipoproteins.3
Folic acid fortification of enriched cereal grain products began in North America in March 1996 and was made mandatory in 1998. Fortification has reduced the number of neural tube defects by half,4 which is clearly a beneficial outcome, but so far it has had little impact on cardiovascular mortality.5
Carotid plaque area is a strong predictor of cardiovascular events.6 High homocysteine levels, which are associated with plaque formation, can result from inadequate intake of folate and vitamin B12. Now that folic acid fortification is widespread, vitamin B12 has become an important determinant of homocysteine levels.7 We sought to determine the prevalence of low serum levels of vitamin B12, and their relation to homocysteine levels and carotid plaque area among patients referred for treatment of vascular disease since folic acid fortification of enriched grain products.
Methods
The subjects of this study were all consecutive new patients referred to 1 of us (JDS) for secondary vascular prevention in either the Premature Atherosclerosis Clinic or the Stroke Prevention Clinic of the University Campus of the London Health Science Centre in London, Ont., between January 1998 (after folic acid fortification had begun) and January 2002 (coinciding with JR's time in our research unit). During that time, 520 patients were referred. A chart review showed that 421 patients had complete data, including measurement of carotid plaque areas, serum vitamin B12 levels, plasma total homocysteine levels, blood pressure, smoking (pack-years), serum cholesterol levels, triglyceride levels, high-density lipoprotein cholesterol levels and calculated low-density lipoprotein cholesterol levels. The data used for analysis were abstracted from the clinical records; except for the measurement of serum methylmalonic acid levels in a subgroup from the Stroke Prevention Clinic, all investigations were done as part of patient care.
Vitamin B12 levels were measured on the Advia Centaur Analyzer (Bayer Diagnostics, Walpole, Mass.) by competitive immunoassay using direct chemiluminescent technology, in which vitamin B12 from the patient sample competes with labelled vitamin B12 for a limited amount of purified intrinsic factor. Plasma total homocysteine was measured by high-performance liquid chromatography with electrochemical detection, which had been standardized against reference methods in both Oregon and Cleveland.8,9
Serum methylmalonic acid, the specific metabolic indicator of vitamin B12 deficiency, was measured in the last 50 patients included in the study, using stable isotope dilution gas chromatography–mass spectrometry.10 In this subgroup, there were no patients with severe renal impairment; the lowest serum creatinine level was 47 μmol/L; 90% had creatinine levels greater than 60 μmol/L, and the mean was 86.7 (standard deviation [SD] 23) μmol/L. Creatinine levels were measured in 224 patients, including all those in whom methylmalonic acid levels were measured.
Carotid plaque area was measured with high resolution duplex ultrasonography, using an ATL 5000 HDI (Advanced Technology Laboratories, Seattle, Wash.).6 Plaque was defined as a local thickening of the intima exceeding 1 mm in thickness. Measurements were made in magnified longitudinal views of each plaque seen in the right and left common, internal and external carotid arteries. The plane in which each plaque was measured was chosen by scanning around the artery until the view showing the largest extent of that plaque was obtained. The image was then frozen and magnified, and the plaque was measured by tracing around the perimeter with a cursor on the screen. The microprocessor in the scanner then displayed the cross-sectional area of the plaque. The operator repeated the process for every plaque seen, until all visible plaque were measured. The sum of cross-sectional areas of all plaques seen between the clavicle and the angle of the jaw was taken as total plaque area. Intraobserver reliability (intraclass correlation) was 0.94 for repeat measurements; interobserver reliability was 0.85.
All 50 patients whose methylmalonic acid levels were measured attended the Stroke Prevention Clinic and consented to a protocol approved by the University of Western Ontario ethics review board. The results from clinical practice were exempted by the ethics board because they involved only a chart review.
We analyzed the relation of serum vitamin B12 and homocysteine levels to carotid plaque area. We defined vitamin B12 deficiency as a serum vitamin B12 level of less than 258 pmol/L with homocysteine levels of 14 μmol/L or more or methylmalonic acid levels of 271 nmol/L or more.11 The median vitamin B12 level in our population was 253 pmol/L, which is very close to the level indicating deficiency; we therefore used analysis of variance (ANOVA) to compare means of baseline characteristics of the study population to determine differences between patients whose vitamin B12 level was above the median and those whose level was below. The study population was large enough to permit analysis of relations between carotid plaque area, vitamin B12 levels and homocysteine levels by quartiles, so this was done to explore the range of values of these parameters. A 2-sample t test was used to examine sex differences in mean vitamin B12 levels, and multiple regression was used to adjust for age and sex because both are determinants of homocysteine levels. We used multiple regression analysis to evaluate adjusted effects on carotid plaque, controlling for age, sex, homocysteine levels, vitamin B12 levels and, where this was measured, serum creatinine levels.
Multiple regression with forced entry of age, sex, creatinine levels, homocysteine levels and vitamin B12 was carried out to evaluate the relative contribution of creatinine and homocysteine to carotid plaque area. The distribution of the dependent variable, plaque area, was normalized by a cubic root transformation.
Results
The study subjects included 421 vascular patients whose ages ranged from 37 to 90 years (mean 65.63 [SD 10.76] years); 49% were female; 67.1% were taking medication for hypertension and 61.5% for dyslipidemia.
Table 1 shows important study population characteristics by median vitamin B12 level. Vitamin B12 deficiency was found in 73 cases (17.3%). Among those 50 patients whose methylmalonic acid levels were measured, 39% exceeded 2 standard deviations for the reference range and 20% exceeded 3 standard deviations, which confirmed metabolic vitamin B12 deficiency.
Table 1
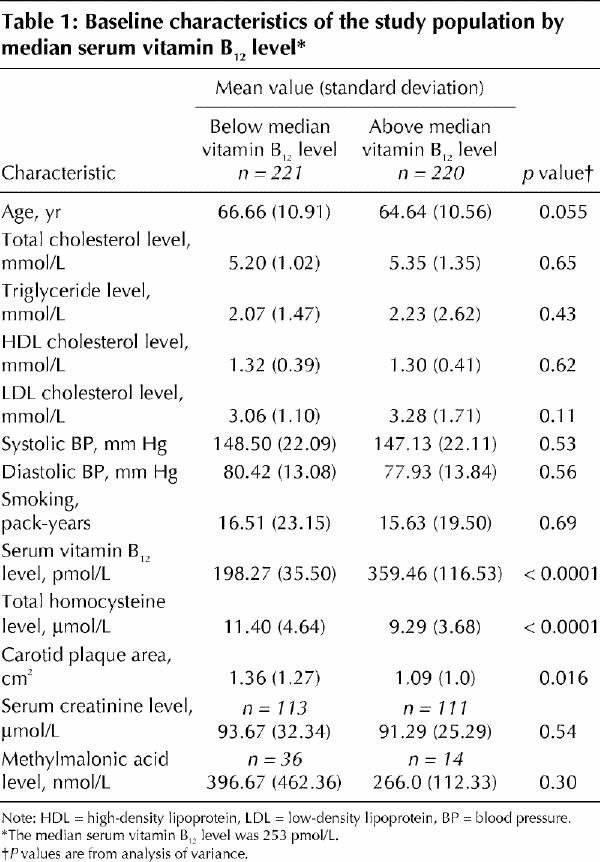
Plasma homocysteine levels were higher at lower levels of vitamin B12 for all age groups (p < 0.001). There was no difference in levels of vitamin B12 (p = 0.43) associated with sex; patients whose vitamin B12 levels were in the lower stratum were slightly but not significantly older (66.66 [SD 10.91] v. 64.64 [SD 10.56] years; p = 0.055).
Age and vitamin B12 were found to be significant predictors of homocysteine levels (both p < 0.001), but sex was not; the R2 was 0.14; β coefficients were 0.26 for age and –0.22 for vitamin B12, which indicated that, independent of age and sex, vitamin B12 accounted for an additional 22% of the explained variance of homocysteine levels.
Analysis of the relations between carotid plaque area, vitamin B12 levels and homocysteine levels by quartiles showed that homocysteine levels fell significantly as vitamin B12 levels rose (Fig. 1) and that, above the median vitamin B12 level of 253 pmol/L, there was significantly more carotid plaque (Fig. 2). Carotid plaque area increased markedly above levels of homocysteine that would usually be regarded as low (7.7 μmol/L), but there was a ceiling effect at a levels that would usually be regarded as normal (9.6 μmol/L) (Fig. 3).
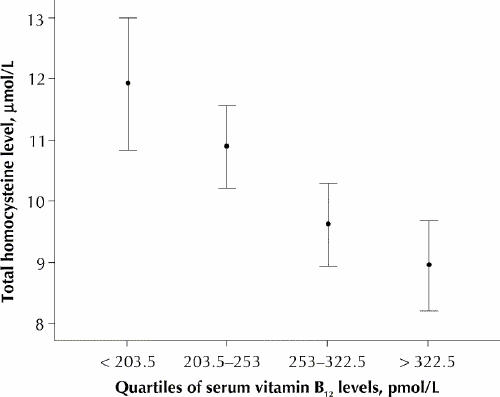
Fig. 1: Total homocysteine by quartiles of serum vitamin B12 levels. Error bars represent 95% confidence intervals.
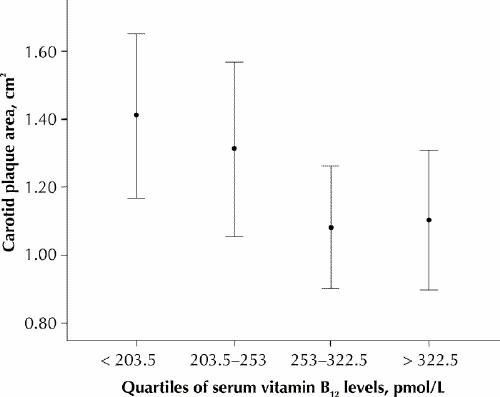
Fig. 2: Carotid plaque area by quartiles of serum vitamin B12 levels. Error bars represent 95% confidence intervals. Carotid plaque area is significantly related to a median split of vitamin B12 (p = 0.012).
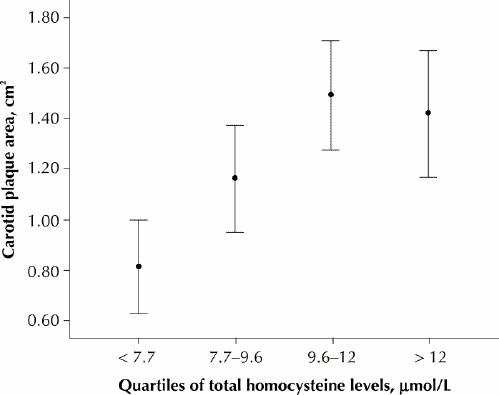
Fig. 3: Carotid plaque area by quartiles of plasma total homocysteine levels. Error bars represent 95% confidence intervals.
Among the 224 patients whose serum creatinine levels were measured, homocysteine accounted for a higher proportion of the explained variance in plaque area than either creatinine or vitamin B12 (Table 2.
Table 2
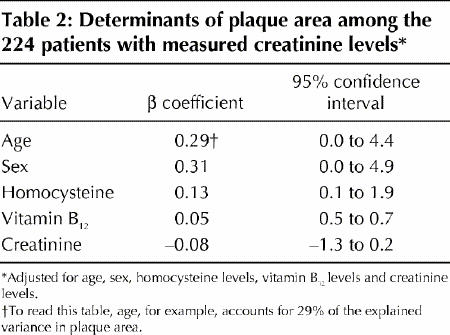
The effect of vitamin B12 levels and creatinine levels on carotid plaque appeared to be related to their effect on homocysteine levels. In a simple Pearson correlation, creatinine (r = 0.331, p < 0.001) and vitamin B12 (r = 0.297, p < 0.001) both correlated with homocysteine, and homocysteine (r = 0.147, p = 0.028) correlated with plaque area, but creatinine (r = 0.054, p = 0.43) and vitamin B12 (r = 0.03, p = 0.65) did not.
Interpretation
We found that 17.3% of our patients had vitamin B12 deficiency; this may be an underestimate, since only 10% of the study group had methylmalonic acid levels measured. Half of our patients had vitamin B12 levels below 258 pmol/L, a cut-off point below which levels have been shown to be associated with a high prevalence of vitamin B12 deficiency, defined by elevated homocysteine or methylmalonic acid levels.11,12,13
Carotid plaque area was associated with elevated homocysteine levels and low vitamin B12 levels. The strong relation between quartiles of vitamin B12 and homocysteine shown in Fig. 1 supports the finding by Quinlivan and associates7 that, since folic acid fortification, vitamin B12 has become the main nutritional determinant of homocysteine levels. The striking reciprocity of vitamin B12, homocysteine and plaque area shown in Fig. 2 and Fig. 3 suggests a key role for vitamin B12 in the treatment of elevated homocysteine levels for vascular disease. Our findings also suggest that “folate therapy,” the term commonly used for vitamin therapy for lowering homocysteine levels, is no longer appropriate.
Inspection of Fig. 3 raises the possibility of a threshold or ceiling effect of homocysteine on carotid plaque, which may indicate that target levels for homocysteine should be lower. It is possible that the null effect of the VISP trial may be explained in part by the failure to sufficiently reduce homocysteine levels. It may be necessary in future to individualize treatment using multiple daily doses of B vitamins or other therapies such as betaine14 or thiols15,16 to achieve target homocysteine levels.
The results of the VISP trial3 showed that a modest reduction of total homocysteine levels (by 1.5 to 2.0 μmol/L) using folic acid, vitamin B6 and vitamin B12 after nondisabling cerebral infarction had no effect on vascular outcomes. Possible reasons for this result include the improvement in outcomes in the low-dose arm due to folic acid fortification, which negated the effect of high-dose folic acid, and problems with the handling of vitamin B12: the high-dose group received only 400 mcg daily, whereas Rajan and associates17 have shown that 1000 mcg daily is required for adequate absorption among elderly patients; furthermore, the low-dose group received the recommended daily intake of vitamin B12 (6 mcg), and in both treatment arms those with low vitamin B12 levels received injections of vitamin B12. Differences in results between the coronary angioplasty trials reported by Schnyder and associates18 (in which patients benefitted from vitamin therapy for homocysteine levels) and that of Lange and associates19 (in which they did not) may be because of the difference in vitamin B12 doses (400 mcg daily v. 40 mcg daily respectively). A number of trials are underway to determine whether treatment of vascular disease with folic acid, vitamin B6 and vitamin B12 reduces stroke.20,21
An important weakness of our study was that we did not have data on serum folate levels. Folate was not measured because the study was based on measurements performed as part of patient care, and folate deficiency has essentially been eliminated by folic acid fortification: Selhub and associates22 reported that, since fortification, the incidence of serum folate levels of less than 3 nmol/L has been reduced from 22% to less than 2% in the Framingham population, and in the VISP trial, less than 1% had levels below 6.7 nmol/L (unpublished data).
Among the 224 patients whose creatinine levels were measured, creatinine levels were found to be as strong a predictor of homocysteine levels as were vitamin B12 levels. Among the 10% of patients whose methylmalonic acid levels were measured, creatinine levels were also as strong a predictor of homocysteine levels than were vitamin B12 levels, and vitamin B12 levels were a stronger predictor of methylmalonic acid levels than were creatinine levels. These findings suggest that creatinine may be a stronger predictor than or as strong a predictor as vitamin B12 of homocysteine levels and are worthy of further investigation.
Acknowledgments
We thank Rose Freitas for coordinating the methylmalonic acid sampling.
Footnotes
This article has been peer reviewed.
Contributors: Julie Robertson carried out the initial data collection and preliminary analyses and wrote a first report of the study for a project during medical school at the University of Western Ontario. Francesco Iemolo collected and entered further data and carried out further analyses during a sabbatical leave at Western. Sally Stabler and Robert Allen provided intellectual input and contributed the assays of methylmalonic acid. David Spence conceived of the study, supervised the conduct of the study and had primary responsibility for revisions of the article. All of the authors participated in the writing and revising of the manuscript.
The study was funded by the Stroke Prevention and Atherosclerosis Research Centre, Robarts Research Institute, and by the laboratory of Sally Stabler and Robert Allen.
Competing interests: None declared for Julie Robertson and Francesco Iemolo. Sally Stabler and Robert Allen and the University of Colorado hold patents in the use of homocysteine to diagnose vitamin B12 and folate deficiency. The university has formed a company to assay homocysteine and other metabolites. David Spence has received vitamin tablets and matching placebo from Pan American Laboratories for a clinical trial funded by the Canadian Institutes of Health Research for which he is principal investigator.
Correspondence to: Dr. J. David Spence, Stroke Prevention and Atherosclerosis Research Centre, 1400 Western Rd., London ON N6G 2V2; fax 519 663-3018; dspence@robarts.ca
References
- 1.Graham IM, Daly L, Refsum H, Robinson K, Brattstrom LE, Ueland PM, et al. Plasma homocysteine as a risk factor for vascular disease. JAMA 1997;277:1775-81. [DOI] [PubMed]
- 2.Nygård O, Nordehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230-6. [DOI] [PubMed]
- 3.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering plasma total homocysteine to prevent recurrent stroke, myocardial infarction, and death in ischemic stroke patients: results of the Vitamin Intervention for Stroke Prevention (VISP) randomized trial. JAMA 2004;291(5):565-75. [DOI] [PubMed]
- 4.McDonald SD, Ferguson S, Tam L, Lougheed J, Walker MC. The prevention of congenital anomalies with periconceptional folic acid supplementation. J Obstet Gynaecol Can 2003;25(2):115-21. [DOI] [PubMed]
- 5.Anderson JL, Jensen KR, Carlquist JF, Bair TL, Horne BD, Muhlestein JB. Effect of folic acid fortification of food on homocysteine-related mortality. Am J Med 2004;116:158-64. [DOI] [PubMed]
- 6.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33(12):2916-22. [DOI] [PubMed]
- 7.Quinlivan EP, McPartlin J, McNulty H, Ward M, Strain JJ, Weir DG, et al. Importance of both folic acid and vitamin B12 in reduction of risk of vascular disease. Lancet 2002;359(9302):227-8. [DOI] [PubMed]
- 8.Malinow MR, Sexton G, Averbuch M, Grossman M, Wilson DL, Upson B. Homocyst(e)inemia in daily practice: levels in coronary artery disease. Coron Artery Dis 1990;1:215-20.
- 9.Jacobsen DW, Gatautis VJ, Green VJ, Robinson K, Savon SR, Secic M, et al. Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate levels in healthy subjects. Clin Chem 1994;40:873-81. [PubMed]
- 10.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism 1993;42:978-88. [DOI] [PubMed]
- 11.Stabler SP, Lindenbaum J, Allen RH. The use of homocysteine and other metabolites in the specific diagnosis of vitamin B12 deficiency. J Nutr 1996;126:1266S-72S. [DOI] [PubMed]
- 12.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994;60(1):2-11. [DOI] [PubMed]
- 13.Naurath HJ, Joosten E, Riezler R, Stabler SP, Allen RH, Lindenbaum J. Effects of vitamin B12, folate, and vitamin B6 supplements in elderly people with normal serum vitamin concentrations. Lancet 1995;346(8967):85-9. [DOI] [PubMed]
- 14.Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr 2003;133(12):4135-8. [DOI] [PubMed]
- 15.House AA, Eliasziw M, Urquhart BL, Freeman DJ, Spence JD. Dimercaptosuccinic acid for the treatment of hyperhomocysteinemia in hemodialysis patients: a placebo-controlled, double-blind, randomized trial. Am J Kidney Dis 2004;44:689-94. [PubMed]
- 16.Spence JD, Cordy P, Kortas C, Freeman D. Effect of usual doses of folate supplementation on elevated plasma homocyst(e)ine in hemodialysis patients: No difference between 1 and 5 mg daily. Am J Nephrol 1999;18:405-10. [DOI] [PubMed]
- 17.Rajan S, Wallace JI, Brodkin KI, Beresford SA, Allen RH, Stabler SP. Response of elevated methylmalonic acid to three dose levels of oral cobalamin in older adults. J Am Geriatr Soc 2002;50(11):1789-95. [DOI] [PubMed]
- 18.Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, Eberli FR, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med 2001;345(22):1593-1600. [DOI] [PubMed]
- 19.Lange H, Suryapranata H, De Luca G, Borner C, Dille J, Kallmayer K, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 2004;350(26):2673-81. [DOI] [PubMed]
- 20.Clarke R, Collins R. Can dietary supplements with folic acid or vitamin B6 reduce cardiovascular risk? Design of clinical trials to test the homocysteine hypothesis of vascular disease. J Cardiovasc Risk 1998;5(4):249-55. [PubMed]
- 21.Spence JD, Howard VJ, Chambless LE, Malinow MR, Pettigrew LC, Stampfer M, et al. Vitamin Intervention for Stroke Prevention (VISP) trial: rationale and design. Neuroepidemiology 2001;20(1):16-25. [DOI] [PubMed]
- 22.Selhub J, Jacques PF, Bostom AG, Wilson PW, Rosenberg IH. Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification. Public Health Rev 2000;28(1-4):117-45. [PubMed]


