Abstract
Background
Basins used for patient bathing have been shown to be contaminated with multidrug resistant organisms (MDRO) and have prompted the evaluation of alternatives to soap and water bathing methods.
Methods
We conducted a prospective, randomized, open-label interventional crossover study to assess the impact of replacing traditional bath basins with pre-packaged washcloths on the incidence of hospital-associated infections (HAI), MDRO and, secondarily, on rates of skin deterioration. Unit-wide use of disposable washcloths over an 8-month period was compared to an 8-month period of standard care using basins.
Results
A total of 2,637 patients were included from two medical surgical units at a single tertiary medical center, contributing 16,034 patient days. During the study period, there were a total of 33 unit-acquired infections, the rates of which were not statistically different between study phases (IRR=1.05; 95% C.I. 0.50–2.23; p=0.88). However, occurrence of skin integrity deterioration was significantly less in the intervention group (OR 0.44, 95% CI 0.22–0.88; p=0.02).
Conclusions
While we were unable to demonstrate a significant reduction in HAI or MDRO acquisition, we found a decrease in skin deterioration with the use of disposable washcloths and confirmed earlier findings of MDRO contamination of wash basins.
Keywords: Bathing, Washcloths, hospital-associated infections, skin integrity
Introduction
Hospital-associated infections (HAI) are responsible for over 700,000 infections1 and an estimated $9.8 billion2 annually in the United States. The hospital environment has been increasingly recognized as an important reservoir for nosocomial pathogens and a facilitator of the transfer of multi-drug resistant organisms (MDROs) between patients3. Although bathing of patients is routinely performed to improve hygiene, evidence suggests that this practice in some instances might contribute to the spread of hospital pathogens. Standard bathing practice of patients involves the use of a plastic basin, standard soap, water, wash cloths and towels. In particular, bath basins, commonly used in intensive care unit settings, have been shown to be contaminated with nosocomial pathogens, including MDROs in patient care settings. Previous research has shown alarmingly high bath basin contamination rates ranging from 62% to 98%4, 5 and 62% in a multicenter study. However, the degree to which bath basin contamination contributes to incident HAI in patients is unclear.
Beyond the potential of MDRO transmission from contaminated basins, soap and water bathing itself has been associated with skin deterioration in hospitalized patients6. Effective alternatives to traditional basin-based bathing may have the added benefit of reducing skin deterioration and maintaining the integrity of the skin as an important barrier to infection. Skin deterioration can lead to pressure ulcers, which are costly to both the patient and hospital and are categorized as a hospital-acquired condition by Centers for Medicare and Medicaid services and can impact reimbursement7. There are numerous prepackaged bathing products on the market, yet studies examining their benefits over traditional bathing are few8.
In this randomized crossover trial, we aimed to assess the impact of replacing traditional bath basins with pre-packaged washcloths on the incidence of hospital-associated infections and, secondarily, on rates of skin deterioration.
Methods
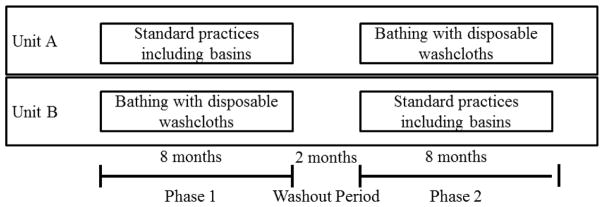
This was a prospective, randomized, open-label, interventional study with a cross-over design. The study was conducted at blinded, a 268 bed hospital in blinded, from January 10, 2012 through July 14, 2013. All study procedures were approved by the blinded Institutional Review Board and a waiver of informed consent was obtained prior to the initiation of all study procedures. The study was conducted on two medical-surgical units (Units A and B) from 01/10/2012 to 7/14/2013. During the study period, Unit A was a 20-bed unit and was staffed by 15 nursess and 2 patient-care assistants. Unit B was a 15-bed unit and was staffed by 15 nursess and no patient-care assistants. An 8-month intervention period with implementation of bathing with disposable washcloths was compared to an 8-month control period where pre-existing, standard bathing practices protocols were used. The order of the intervention period versus the control period for each of the units was selected randomly. Each intervention period was separated by a two-month washout period (Figure 1) during which standard bathing procedures were followed.
Figure 1.
Flow chart of study intervention periods, by unit
During the intervention period, nursing staff used disposable washcloths (Comfort Bath® Cleansing Washcloths) for patient cleaning instead of standard-of-care soap and water bathing. Patients who were bed-bound, but able to bathe themselves, were trained in either basin-based bathing or bathing using a pre-packaged washcloth, depending on the intervention period and unit. Patients who were ambulatory and could shower independently, did not partake in either intervention. Patients and staff were trained to use pre-packaged washcloths for routine standard of care bathing intervals for the entire intervention period. Specific number of days are frequency of bathing varied by patient based on standard practice. During intervention periods with the pre-packaged washcloths, nursing staff were directed to remove plastic basins from the unit. Training prior to the intervention period as conducted by industry personnel and was overseen by the study principal investigator (blinded). During the study period, study personnel performed walk rounds on each study unit two times/week to make certain that bath basins were not present on the intervention unit; and that Comfort Bath® Cleansing Washcloths were not present on the standard-of-care unit.
Control periods involved the use of standard-of-care bathing protocols using a plastic basin, bar soap and washcloths. Although changes in incontinence care were not part of the intervention, Comfort Shield® Barrier Cream Cloths were routinely used for incontinence care during both control and intervention periods.
Data collection and analysis
During the 18-month study period, data including demographics, microbiologic test results, comorbid conditions, and clinical outcomes were collected from medical records as patients were admitted to and discharged from study units. Skin integrity was assessed through review of daily nursing notes. All patients were evaluated for risk of pressure ulcer development at admission to the unit and on a daily basis using the Braden Scale9; assessments were performed more frequently when warranted clinically. Patients with a Braden Scale score <12 were evaluated every shift. Skin deterioration was defined as a worsening of skin integrity since admission to the unit. Study personnel categorized infections according to standard National Healthcare Safety Network (NHSN) criteria10. All infections meeting NHSN criteria were confirmed through review with an infectious diseases epidemiologist (blinded) and an intensivist. In addition, surveillance of clinical cultures during the study periods was performed for the following MDROs: methicillin-resistant Staphylococcus aureus (MRSA); vancomycin-resistant enterococcus (VRE); extended-spectrum β-lacamase-producing Eshcerichia coli or Klebsiella spp.(ESBL); carbapenem-resistant enterobacteriaceae (CRE); Acinetobacter baumannii; Pseudomonas aeruginosa; and Clostridium difficile. Clinical cultures of individuals with infection were collected at the discretion of the treating physician following standard clinical practice. No screening cultures were collected. Data on clinical cultures was obtained from admission through two days following discharge. Infections were categorized as “unit-acquired” if they were diagnosed at least three days following admission to the unit, through two days following discharge.
Laboratory Methods
During the study period, environmental swabs were collected from plastic basins located in rooms of patients with a clinical culture positive for an MDRO with a stay of at least three days or more following the collection of the positive clinical culture. Environmental swabs were collected by wiping twice on all inside surfaces including the edge of the bath basins. Samples were sent immediately to the clinical laboratory for qualitative microbial analysis. Bath basin isolates were identified to the species level by the Detroit Medical Center Clinical Laboratory and antibiotic susceptibilities were determined by Microscan (Siemens, Germany). Selective culturing techniques were used as follows: MRSA: screening on non-selective sheep blood agar media followed by Cefoxitin disk screening for Oxacillin resistance and Microscan ID and minimum inhibitory concentration (MIC) confirmation; VRE: screening on non-selective sheep blood agar media followed by Microscan ID and MIC confirmation; ESBL/CRE: screening for Gram negative colonies on MacConkey agar characteristic for particular suspected Gram negative organism followed by Microscan ID confirmation (ESBL’s further confirmed by Microscan ESBL confirmatory wells, CRE’s further confirmed by modified Hodge testing if MIC criteria met for possible CRE (>8 ug/ml for ceftriaxone of ceftazidime; and >=2 ug/ml for ertapenem); A. baumannii: screening for Gram negative colonies on MacConkey agar characteristic for particular suspected Gram negative organism followed by Microscan ID confirmation; C. difficile: anaerobic culture and phenotypic screening followed by ID confirmation with the Remel rapID ANA II system (Thermo Fisher, Lenexa, KS).
In cases where the same MDRO was recovered from the basin and the patient’s clinical culture, molecular typing was performed to compare the two isolates. DNA was extracted from bacterial culture using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Strain typing was performed using spa typing for S. aureus11 and multi-locus sequence typing for other species using published methods12, 13. Sequence type was determined using DNAGear for S. aureus14 and the Institut Pasteur website for K. pneumoniae (www.pasteur.fr/mlst) and the Acinetobacter baumannii website (pubmlst.org/abaumannii/).
Statistical Methods
Incidence rate ratios and exact 95% confidence intervals were calculated (Stata 13). Characteristics of patients admitted during standard practice and intervention phases were compared using logistic regression. General estimating equations were used to compare incidence of skin deterioration in the two study groups, accounting for correlation by clinical unit (SAS 9.3).
Results
A total of 2,637 patients were admitted during the study periods for a final sample size of 16,034 patient days. The total number of patients admitted, the total patient days and the overall patient demographics were similar between the two study arms, with the exception of the proportion of patients >65 years of age (Table 1). The mean age of patients was 57 years (SD: 17), and the majority of patients were male and African American. Median length of stay was the same in both groups (4 days; interquartile range 3–6). The proportion of admitted patients with chronic underlying conditions was similar between the two study arms, and diabetes was the most commonly reported condition (Table 1). Study groups differed somewhat with respect to the presence of chronic skin ulcers on admission (n= 17.3% in the bath basin study arm vs n= 14.8% in the pre-packaged washcloth arm). However, this difference was not statistically significant (p=0.09). Most study patients (76%) were initially admitted to study units; the remainder of the study population arrived following transfer from intensive care unit (ICU) care.
Table 1.
Characteristics of Patients Admitted to Study Units, by Study Phase
| Washcloth N=1318 |
Bath Basin N=1319 |
OR (95% CI) | p | |
|---|---|---|---|---|
| Age >65 years, n(%) | 409 (31) | 618 (47) | 0.5 (0.4, 0.6) | <0.01 |
| Male gender, n(%) | 701 (53) | 720 (55) | 0.9 (0.8, 1.1) | 0.47 |
| Non home residence, n(%) | 208 (16) | 231 (18) | 0.9 (0.7, 1.1) | 0.23 |
| Partially or fully dependent functional status, n(%) | 248 (19) | 255 (19) | 1.0 (0.8, 1.2) | 0.74 |
| McCabe Score of rapidly fatal, n(%) | 33 (3) | 26 (2) | 1.3 (0.7, 2.2) | 0.36 |
| Peripheral vascular disease, n(%) | 86 (7) | 59 (4) | 1.5 (1.05, 2.1) | 0.02 |
| Diabetes mellitus, n(%) | 437 (33) | 409 (31) | 1.1 (0.9, 1.3) | 0.24 |
| Renal disease, n(%) | 242 (18) | 201 (15) | 1.3 (1.02, 1.6) | 0.03 |
| Chronic skin ulcers, n(%) | 196 (15) | 229 (17) | 0.8 (0.7, 1.03) | 0.08 |
| Hospitalization ≤ 3 months prior to admission to the study units, n(%) | 542 (41) | 554 (42) | 1.0 (0.8, 1.1) | 0.65 |
| ICU stay during current hospitalization prior to admission/transfer to the study unit, n(%) | 316 (24) | 303 (23) | 1.0 (0.9, 1.3) | 0.5 |
| ICU stay ≤ 3 months prior to admission to study unit, n(%) | 149 (11) | 152 (12) | 1.0 (0.8, 1.3) | 0.86 |
| Chronic hemodialysis, n(%) | 65 (5) | 65 (5) | 1.00 (0.7, 1.4) | 0.99 |
| Presence of indwelling devices, n(%) | 314 (24) | 342 (26) | 0.9 (0.7, 1.1) | 0.21 |
| Antibiotics for ≥ 48 hours in the prior 3 months, n(%) | 438 (33) | 879 (67) | 0.2 (0.2, 0.3) | <0.01 |
| Death during hospitalization, n(%) | 31 (2) | 30 (2) | 1.0 (0.6, 1.8) | 0.9 |
| Weighted Charlson Comorbidity Index, Median (IQR) | 1(1–3) | 1 (0–3) | --- | 0.09 |
| Length of admission prior to study phase, median (IQR) | 0 (0–1) | 0 (0–1) | --- | 0.33 |
IQR= Inter-Quartile Range (25 to 75), OR=Odds Ratio, ICU=Intensive Care Unit
During the study period, there were a total of 33 unit-acquired infections, including 6 device-related infections, 6 bloodstream infections (3 CLABSI), 4 wound infections and 12 urinary tract infections (including 3 catheter-associated infections) among patients admitted to study units (Table 2). Eleven cases of C. difficile were identified overall. A total of 65 unit-acquired MDROs were recovered on the study units, including 24 occurrences of MRSA and 20 occurrences of P. aeruginosa (Table 3). The incidence of unit-acquired HAIs between study groups was not significantly different (Table 2). Likewise, no statistical difference was found in the incidence of specific MDROs acquired on unit (Table 3). Four patients had corresponding MDROs isolated from both their clinical culture and the associated bath basin: MRSA (two pairs), carbapenem-resistant Klebsiella pneumonia (one pair), and A. baumannii (one pair) (Table 4). Both pairs of MRSA isolates had corresponding spa types, t002 for one pair and t681 for the second pair. Multilocus sequence typing determined a matching sequence type for both KPC isolates (ST258). The paired A. baumannii isolates were found to have different sequence types, neither of which matched a known type (Table 4).
Table 2.
Hospital Associated Infections (HAI), by Study Phase
| Infections, Bath Basin Phase (rate/1000 patient days)) N=7981 patient days |
Infections, Washcloth Phase (rate/1000 patient days)) N=8053 patient days |
IRR (95% CI) | p | |
|---|---|---|---|---|
| Total HAI | 16 (2.0) | 17 (2.1) | 1.05 (0.50–2.23) | 0.88 |
| Device Infections | 0 (0) | 6 (0.7) | --- | --- |
| Total BSI | 2 (0.3) | 4 (0.5) | 1.98 (0.28–21.9) | 0.46 |
| CLABSI | 0 | 3 (0.4) | --- | |
| BSI, non-CLABSI | 2 (0.3) | 1 (0.1) | 0.50 (0.008– 9.52) | 0.62 |
| Wound | 3 (0.4) | 1 (0.1) | 0.33 (0.006– 4.11) | 0.37 |
| SSI | 3 (0.4) | 0 (0) | --- | |
| Wound, non-SSI | 0 | 1 (0.1) | --- | |
| Total UTI | 6 (0.8) | 6 (0.7) | 0.99 (0.26–3.71) | 0.99 |
| CAUTI | 0 (0) | 3 (0.4) | --- | --- |
| UTI, non-CAUTI | 6 (0.8) | 3 (0.4) | 0.50 (0.08–2.32) | 0.34 |
| CDAD | 5 (0.6) | 6 (0.7) | 1.19 (0.30–4.93) | 0.79 |
IQR: Inter-Quartile Range; AIDS: Acquired Immunodeficiency Syndrome; BSI: Blood Stream Infections; CLABSI: Central Line Associated Blood Stream Infections; SSI: Surgical Site Infections; UTI: Urinary Tract Infections; CAUTI: Catheter Associated Urinary Tract Infection; CDAD: Clostridium Difficile Associated Disease; IRR: Incidence Rate Ratio.
Table 3.
Multi-drug Resistant Organisms (MDROs) Detected, by Study Phase
| Infections, Bath Basin Phase (rate/1000 patient days) N=7981 patient days |
Infections, Washcloth Phase (rate/1000 patient days) N=8053 patient days |
IRR (95% CI) | p | |
|---|---|---|---|---|
| Total MDROs | 40 (5.0) | 25 (3.1) | 0.62 (0.36–1.05) | 0.06 |
| Methicillin-resistant Staphylococcus aureus | 12 (1.5) | 12 (1.5) | 0.99 (0.41–2.41) | 0.98 |
| Vancomycin-resistant Enterococcus spp | 5 (0.6) | 2 (0.2) | 0.39 (0.04–2.42) | 0.28 |
| Acinetobacter baumannii | 7 (0.9) | 2 (0.2) | 0.28 (0.03–1.49) | 0.11 |
| Extended-spectrum beta-lactamase producing Klebsiella pneumonia | 3 (0.4) | 2 (0.2) | 0.66 (0.06–5.77) | 0.68 |
| Pseudomonas aeruginosa | 13 (1.6) | 7 (0.9) | 0.53 (0.18–1.44) | 0.18 |
IRR: Incidence Rate Ratio.
Table 4.
DNA analysis of Clinical and Bath basin isolates
| Source | Isolate | spa type | MLST | |
|---|---|---|---|---|
| 1 (a) | Bath Basin | Methicillin-resistant S. aureus | 2 | --- |
| 1 (b) | Clinical Culture | Methicillin-resistant S. aureus | 2 | --- |
| 2 (a) | Bath Basin | Methicillin-resistant S. aureus | 681 | --- |
| 2 (b) | Clinical Culture | Methicillin-resistant S. aureus | 681 | --- |
| 3 (a) | Bath Basin | Extended-spectrum beta-lactamase producing Klebsiella pneumonia | --- | ST258 |
| 3 (b) | Clinical Culture | Extended-spectrum beta-lactamase producing Klebsiella pneumonia | --- | ST258 |
| 4 (a) | Bath Basin | Acinetobacter baumannii | --- | No Typea |
| 4 (b) | Clinical Culture | Acinetobacter baumannii | --- | No Typea |
The alleles identified for patient 4 did not match any previously-identified type in the Pubmlst database.
Results for the MLST loci were 1,12,1,2,4,98,5 for the bath basin isolate and 10,12,3,33,2,66,3 for the clinical culture.
Occurrence of skin integrity deterioration was significantly less among patients bathed with pre-packaged washcloths (n=33; 2.5%) as compared to patients bathed using standard plastic basins (n=75; 5.5%) (OR 0.44, 95% CI 0.22–0.88; 0.02). After controlling for the presence of chronic skin ulcers at the time of unit admission, the association between use of pre-packaged washcloths and skin preservation was unchanged.
Discussion
Eliminating bathing methods using water and bath basins has been hypothesized to be a method for reducing environmental contamination and reducing hospital-acquired infections5, 8, 15. In this study, while we were unable to demonstrate a statistically significant reduction in HAI or MDRO acquisition associated with basin elimination, we were able to demonstrate four instances where the same MDRO pathogen was isolated from a clinical patient culture and the basin of the same patient following standard-of-care (basin) bathing. Identified MDRO included MRSA, in two instances, carbapenem-resistant K. pneumonia, and A. baumannii. In three of these instances, the paired isolates had identical molecular types, suggesting transfer from patient to basin or vice versa. These findings raise concern about the role of bath basins as reservoirs for hospital-acquired MDROs.
Another notable finding was the significant decrease in skin deterioration associated with use of the pre-packaged cloths in lieu of basins. Traditional bathing practices may drive skin deterioration, potentially due to changes in skin pH, drying effects of traditional cleansers16, 17 and friction18. Pre-packaged cloths are significantly softer than standard, reusable cloths. The cleansing solution is pH-balanced to match the acid mantle of the skin and contains additional emollients and humectants. Thus the cloths may be less likely to produce irritation during cleaning.
There were a couple of limitations to this study, one of which was the relatively small size of the study and the fact that this was not a randomized controlled, blinded study. It is possible that unobserved differences between the study groups (i.e. differences in underlying skin condition other than skin ulcers or in specific clinical practices) may have affected study results. Another limitation was that Comfort Shield® Barrier Cream Cloths were used for perineal care on study units during all study periods. Ideally, these cloths would have only been used on units during the intervention period where bathing with disposable washcloths was performed and on study units practicing standard care, cleaning the perineum would have been performed using soap, water, and washcloths. However, due to nursing preference, the Barrier Cream Cloths were used by both units during all study periods. There were a few episodes where basins appeared on units during the period when bathing was supposed to be exclusively conducted using disposable washcloths. Although these episodes were infrequent (n=3) and routine rounding was conducted by study personnel, it is possible that there might have been additional lapses in study protocol. Given the challenges of changing routine care practices, these limitations were unavoidable. As is the case with many product-based interventions, increased cost of the intervention over existing practice is a consideration. While a cost-effectiveness analysis was not performed, it can be noted that the health system where this study was conducted did not ultimately implement permanent use of the product, limiting our ability to assess the long term impact on clinical care.
HAIs and skin deterioration occurring during hospitalization are increasingly becoming important quality metrics and are being tied to reimbursement7. Eliminating unnecessary nosocomial pathogen and MDRO is a practical approach to reduction of HAIs. By eliminating bath basins, and utilizing a pre-packaged cloth for patient bathing, hospitals cannot only reduce environmental reservoirs for MDROs but decrease the risk for skin deterioration and improve safety for patients.
Highlights.
Basins used for patient bathing may become contaminated with resistant organisms.
Disposal washcloths had no effect on prevention of infection in a randomized trial.
Skin deterioration was less among patients bathed by disposable washcloth.
Acknowledgments
Financial support. This work was supported by an investigator-initiated grant from Sage Inc to blinded. blinded is supported by the National Institutes of Health (grant no. blinded). Potential conflicts of interest. blinded reports having received grant support from Sage Inc.
Footnotes
Findings have been previously presented in part at IDWeek 2014, Philadelphia, PA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–46. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 3.Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL, Umscheid CA. Cleaning Hospital Room Surfaces to Prevent Health Care-Associated Infections: A Technical Brief. Ann Intern Med. 2015;163:598–607. doi: 10.7326/M15-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson D, Lineweaver L, Maze LM. Patients’ bath basins as potential sources of infection: a multicenter sampling study. Am J Crit Care. 2009;18:31–8. 41. doi: 10.4037/ajcc2009968. discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 5.Marchaim D, Taylor AR, Hayakawa K, et al. Hospital bath basins are frequently contaminated with multidrug-resistant human pathogens. Am J Infect Control. 2012;40:562–4. doi: 10.1016/j.ajic.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Bryant RA, Rolstad BS. Examining threats to skin integrity. Ostomy Wound Manage. 2001;47:18–27. [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. 2015 [cited 2016 7/17/2016]. Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/hospitalacqcond/hospital-acquired_conditions.html.
- 8.Larson EL, Ciliberti T, Chantler C, et al. Comparison of traditional and disposable bed baths in critically ill patients. Am J Crit Care. 2004;13:235–41. [PubMed] [Google Scholar]
- 9.Mortenson WB, Miller WC, Team SR. A review of scales for assessing the risk of developing a pressure ulcer in individuals with SCI. Spinal Cord. 2008;46:168–75. doi: 10.1038/sj.sc.3102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Shopsin B, Gomez M, Montgomery SO, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–63. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43:4382–90. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–82. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FAL-T, Brunel AS, Bouzinbi N, Corne P, Banuls AL, Shahbazkia HR. DNAGear--a free software for spa type identification in Staphylococcus aureus. BMC Res Notes. 2012;5:642. doi: 10.1186/1756-0500-5-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John LD. Nosocomial infections and bath water: any cause for concern? Clin Nurse Spec. 2006;20:119–23. doi: 10.1097/00002800-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Mason SR. Type of soap and the incidence of skin tears among residents of a long-term care facility. Ostomy Wound Manage. 1997;43:26–30. [PubMed] [Google Scholar]
- 17.Birch S, Coggins T. No-rinse, one-step bed bath: the effects on the occurrence of skin tears in a long-term care setting. Ostomy Wound Manage. 2003;49:64–7. [PubMed] [Google Scholar]
- 18.Voegeli D. The effect of washing and drying practices on skin barrier function. J Wound Ostomy Continence Nurs. 2008;35:84–90. doi: 10.1097/01.WON.0000308623.68582.d7. [DOI] [PubMed] [Google Scholar]