Abstract
Adults with Down syndrome (DS) have a high incidence of Alzheimer’s disease (AD), providing a unique opportunity to explore the early, preclinical stages of AD neuropathology. We examined change in brain amyloid-β accumulation via the PET tracer [11C] Pittsburgh compound B (PiB) across two data collection cycles, spaced 3 years apart, and decline in cognitive functioning in 58 adults with DS without clinical AD. PiB retention increased in the anterior cingulate gyrus, precuneus cortex, parietal cortex and anterior ventral striatum. Across the two cycles, 14 (27.5%) participants were consistently PiB+, 31 (60.8%) were consistently PiB−, and 6 (11.7%) converted from PiB− at Cycle 1 to PiB+ at Cycle 2. Increased global amyloid-β was related to decline in verbal episodic memory, visual episodic memory, executive functioning, and fine motor processing speed. Participants who were consistently PiB+ demonstrated worsening of episodic memory, whereas participants who were consistently PiB− evidenced stable or improved performance. Amyloid-β accumulation may be a contributor to or biomarker of declining cognitive functioning in preclinical AD in DS.
Keywords: Down syndrome, Alzheimer’s disease, PiB, amyloid-β, mild cognitive impairment, dementia, amyloid
Down syndrome (DS), estimated to occur in 1 in 691 live births (Parket et al., 2006), is a developmental disability most commonly due to a third copy of chromosome 21. Adults with DS evidence ‘accelerated aging’ (Horvath et al., 2015; Patterson & Cabelof, 2012), including earlier onset and increased incidence of Alzheimer’s disease (AD). Indeed, nearly all adults with DS evidence neuropathology of AD by their fourth decade of life (Mann & Esiri, 1989; Wisniewski, Wisniewski, & Wen, 1985) and more than half of adults with DS in their 60s exhibit clinical symptoms of AD (Coppus et al., 2006; McCarron et al., 2014). The early onset and increased incidence of AD in adults with DS is attributed to the overproduction of amyloid-β due to the triplication of chromosome 21, which contains the gene for the amyloid precursor protein (APP) (Wiseman et al., 2015). The accumulation of amyloid-β plaques, followed by neurofibrillary tangles of the protein tau, is an early event in the pathogenesis leading to clinical AD years to decades later (Hardy & Higgins, 1992). However, whether amyloid-β accumulation is a contributor to or a relevant biomarker of subtle declines in cognitive functioning during the transitionary stage (mild cognitive impairment [MCI]), prior to the clinical onset of AD, is unclear. The current study examined the impact of amyloid-β accumulation across two data collection cycles (3 years apart) on declines in cognitive functioning in 58 adults with DS without clinical AD at Cycle 1.
Longitudinal studies on the general population have examined amyloid-β accumulation, via the positron emission tomography (PET) tracer [11C] Pittsburgh compound B (PiB), during the later-stages of AD and found a consistent worsening of cognitive functioning based on initial PiB retention (indicating higher amyloid-β accumulation) (Kadir et al., 2012; Villemagne et al., 2011; Yau et al., 2015). However, findings regarding PiB retention and cognitive functioning during the earlier-stages of AD (MCI) are varied. Some studies report that higher PiB retention was associated with poorer memory and executive functioning performance (Mormino et al., 2009; Rowe et al., 2010; Wolk et al., 2009) while other studies found no association (Aizenstein et al., 2008; Forsberb et al., 2010; Jack et al., 2008; Sperling et al., 2009; Storandt et al., 2009).
In the DS population, there is some evidence that neocortical amyloid-β accumulation, as assessed via the PET tracer PiB is associated with lower cognitive performance. However, conclusions are limited due to cross-sectional designs (Annus et al., 2016; Handen et al., 2012; Hartley et al., 2014; Nelson et al., 2011), small sample sizes (Handen et al., 2012), and restricted neuropsychological batteries of cognitive functioning (Nelson et al., 2011). In the largest cross-sectional study, and the one with the most extensive battery of directly-administered neuropsychological measures, Hartley et al. (2014) found a negative association between neocortical PiB retention and verbal and visual episodic memory, executive functioning, and expressive language in 63 adults with DS (aged 30–50 years) who did not exhibit clinical signs of AD. A handful of studies have also examined neocortical amyloid-β accumulation in the preclinical stages of AD in DS using the PET tracer Florbetapir (Rafii et al., 2015; Sabbagh et al., 2015). In a sample of 12 adults with DS without clinical AD, Florbetapir-PET was not significantly associated with cognitive functioning (Rafii et al., 2015). Chronological age was strongly associated with PiB - or Florbetapir-PET retention in several previous cross-sectional studies (Annus et al., 2016; Hartley et al., 2014; Nelson et al., 2011; Sabbagh et al., 2015), such that the effect of normative age-related decline may have been indistinguishable from the effect of amyloid-β accumulation.
The current study builds on the Hartley et al. (2014) study by examining change in global PiB retention using PET-PIB in relation to subtle declines in cognitive functioning across two time points (3 years apart) in the same sample. In addition to assessing neocortical PiB retention, we examined PiB retention in the striatum, as it is the brain region with the earliest amyloid-β accumulation in the DS population (Annus et al., 2016; Lao et al., 2016). PiB retention was evaluated as both a continuous variable and dichotomous variable by categorizing adults with DS as PiB + versus PiB−, in line with previous studies (Annus et al., 2016; Hartley et al., 2014; Nelson et al., 2011; Lao et al., 2016). Analyses were conducted with and without controlling for chronological age to separate out the effects of normative aging from those of amyloid-β accumulation. An increase in global PiB retention from Cycle 1 to Cycle 2 was hypothesized to be associated with a decline in cognitive functioning, particularly verbal and visual episodic memory, executive functioning and expressive language, in adults with DS. Moreover, adults with DS who converted from PiB− to PiB+ or who were consistently PiB+ across the study cycles were predicted to experience greater declines in cognitive functioning relative to adults with DS who were consistently PiB− across study cycles.
Methods
Participants
Participants were part of a longitudinal study consisting of 81 adults with DS at Cycle 1. There were two study sites: [removed for review] and [removed for review]. The Internal Review Board at both study sites reviewed and approved the study. Consent or assent for study participation was obtained from all adults with DS. Proxy consent was obtained from caregivers who served as legal guardians. Inclusion criteria included being ≥ 30 years, genetic testing indicating trisomy 21, mental age ≥ 2.5 years, at least minimal verbal communication (3 word utterances), no medical condition that contraindicated brain imaging, no medical/psychiatric condition impairing cognition, and not having received a diagnosis of AD or other dementia. The Dementia Scale for Down syndrome (DSDS; Geyde, 1995) was conducted with caregivers to verify that participants did not exhibit dementia. All but two participants scored in the asymptomatic range (< 3 Cognitive Cutoff Score) on the DSDS at Cycle 1. The two participants above this cutoff (both score of 3) were judged to not have AD (based on clinical case consensus review using information from a directly-administered dementia screen and caregiver interview), but thought to have MCI. None of the participants took memory enhancement/AD medications at Cycle 1. Of the 81 adults with DS at Cycle 1, 58 had neuropsychological data at Cycle 2 (2 medical condition precluded imaging, 5 declined, 5 could not be reached, and 11 did not reach Cycle 2 window but will be followed in later study) and are included in current analyses. Independent sample t-tests and chi-square statistics indicated no significant differences in race/ethnicity, sex, mental age, or Cycle 1 PiB retention between these 58 participants and the participants without Cycle 2 data. Table 1 presents the socio-demographics of the 58 participants in current analyses. Five participants did not have useable brain imaging scans at both cycles (1 did not complete and 4 had excessive motion); thus 53 participants are in analyses involving change in PiB retention.
Table 1.
Socio-demographics of Adults with Down syndrome at Cycle 1 and Cycle 2
| Cycle 1 | Cycle 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total N = 58* |
PiB− N = 39 |
PiB+ N = 17 |
Total N = 58* |
PiB− N = 32 |
PiB+ N = 22 |
|
| Sex | ||||||
| Male n (%) | 30(51.7) | 17(43.6) | 11(64.7) | 30(51.7) | 16(50.0) | 11(50.0) |
| Age in years, M (SD) | 37.6(6.8) | 34.6(5.4) | 44.6(4.2) | 40.5(7.1) | 36.4(5.4) | 45.9(4.8) |
| Race/Ethnicity, n (%) | ||||||
| White | 57(98.3) | 39(100) | 16(94.1) | 56(98.2) | 32(100) | 20(95.2) |
| American Indian/Alaska Native | 1(1.7) | 0(0) | 1(5.9) | 1(1.7) | 0(0) | 1(4.8) |
| Residence, n (%) | ||||||
| Family | 37 (63.8) | 25(64.1) | 10(58.8) | 36(62.1) | 19(59.4) | 14 (63.6) |
| Group home | 7 (12.1) | 3(7.7) | 4(23.5) | 6(10.3) | 2(6.3) | 4(18.2) |
| Supported apartment | 9(15.5) | 7(17.9) | 2(11.8) | 8(13.8) | 6(18.8) | 2(9.1) |
| Independently | 4(6.9) | 3(7.7) | 1(5.9) | 5(8.6) | 4(12.5) | 0(0) |
| Other | 1 (1.7) | 1(2.6) | 0(0) | 3(5.2) | 1(3.1) | 2(9.1) |
| Employment, n (%) | ||||||
| Full or part time | 19(32.8) | 13(33.3) | 5(29.4) | 20(34.5) | 10(31.3) | 9(40.9) |
| Full or part time with support | 13(22.4) | 10(25.6) | 3(17.6) | 11(19.0) | 9(28.1) | 1(4.5) |
| Supported workshop | 14(24.1) | 8(20.5) | 5(29.4) | 13(22.4) | 7(21.9) | 4(18.2) |
| Volunteer | 6(10.3) | 3 (7.7) | 3(17.6) | 5(8.6) | 2(6.3) | 3(13.6) |
| Day treatment or not employed | 6(10.3) | 5(12.8) | 1 (5.9) | 9(15.5) | 4(12.5) | 5(22.7) |
| Mental Age in years, M (SD) | 5.5 (1.3) | 5.6(1.1) | 5.5(1.6) | 5.8(2.7) | 6.4(3.3) | 5.1(1.4) |
| Dementia Symptoms | ||||||
| DSDS - CCS, n (%) 3 or above | 2(3.4%) | 1(2.6%) | 1(5.9%) | 5(8.6)* | 1(3.1) | 3(13.6) |
| Medications | ||||||
| Hypothyroidism | 31(53.4) | 23(59.0) | 7(41.2) | 36(63.2) | 22(71.0) | 11(50.0) |
| Hypertension | 3(5.2) | 2(5.1) | 1(5.9) | 4(6.9) | 2(6.3) | 2(9.1) |
| Antipsychotic | 7(12.1) | 3(7.7) | 4(23.5) | 6(10.3) | 2(6.3) | 4(18.2) |
| Antidepressant/anti-anxiety | 16 (27.6) | 11(28.2) | 5(29.4) | 18(31.0) | 8(25.0) | 9(40.9) |
| Mood/Behavior stabilizer | 1 (1.7) | 0(0) | 1(5.9) | 2(3.4) | 0(0.0) | 2(9.1) |
| Narcotic Pain reliever | 1 (1.7) | 0(0) | 1(5.9) | 2(3.4) | 1(3.1) | 1(4.5) |
| Cholesterol | 7(12.1) | 3(7.7) | 3(17.6) | 8(13.8) | 2(6.3) | 4(18.2) |
| Memory enhancer/Alzheimer’s | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Other | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
Note.
Total number does not equal sum of PiB- and PiB+ given that PiB status could only be determined on 56 participants at Cycle 1 and 54 participants at Cycle 2. Mental age was assessed using the Stanford-Binet, Fifth Edition Abbreviated Battery (Roid, 2003), DSDS-CCS = Down Syndrome Dementia Scale (Gedye, 1995) – Cognitive Cutoff.
Procedure
Participants completed two cycles of data collection between 2010 and 2015, spaced 3 years apart (range: 2.1 to 4.3 years). Each cycle consisted of Day 1 neuropsychological evaluation (3–4 hours) and Day 2 brain imaging scans (3 hours). Day 2 was performed within 5 months of Day 1 (M = 20.8 days, SD =57.3 days). Participants were evaluated at two sites; examiners underwent training and cross-site validation. Examiners were blind to imaging results, caregiver interview data, and Cycle 1 neuropsychological scores.
Neuropsychological Evaluation
Adaptive behavior
The Vineland Adaptive Behavior Scales, 2nd Edition (Sparrow et al., 2005), was completed by caregivers. The General Adaptive Composite score assessed adaptive functioning and has strong psychometric properties (Sparrow et al., 2005).
Dementia screens
The DSDS is a caregiver-interview screen for dementia in adults with DS that has a specificity rate of 0.90 and a sensitivity rate of 0.85 (Gedye, 1995). The Severe Impairment Battery Short Form (SIB; Saxton et al., 2005) is a 26-item direct assessment of cognitive impairments indicative of dementia.
Verbal learning and memory
The Cued Recall Test (Zimmerli & Devenny, 1995) assesses verbal learning and episodic memory. The Free Recall (number of objects correctly recalled during the free recall trials), Free and Cued Recall (number of objects correctly recalled in free recall and cued recall trials), and Cued Recall Intrusion (number of incorrect responses in the cued recall trials) scores are sensitive to dementia in DS (Zimmerli & Devenny, 1995). The Wechsler Memory Scale, 4th Edition (WISC-IV; Wechsler, 2004) Story Recall Logical Memory I and Logical Memory II assess immediate and delayed recall of verbal information and are sensitive to memory decline in DS (Brugge et al., 1994).
Visual memory
The Visual Memory subtests of the Rivermead Behavioral Memory Test for Children (RBMT; Wilson et al., 1991) assesses visual episodic memory and have been used in DS (Hartley et al., 2014).
Attention and processing speed
The Wechsler Intelligence Scales for Children-Revised (WISC-R; Wechsler, 1974) Digits Forward (sum of the number of digits in trials remembered correctly) has been used with adults with DS (Devenny et al., 2005). The Corsi Block Tapping Forward (sum of the number of digits in trials remembered correctly) measures visuospatial memory (Shapiro et al., 1992). The NEPSY Visual Attention subtest assesses visual attention (Visual Attention Accuracy) and processing speed (Visual Attention Time) and is appropriate in DS (Heller et al., 2006).
Executive and working memory
The Stroop Dog and Cat Task (Ball et al., 2008), is a modified Stroop task of executive functioning shown to identify memory changes in adults with DS (Nash & Snowling, 2008). The Cat Dog Switch Error score is the number of errors made in the switch trial. The Cat Dog Switch Time score is the switch trial time minus the initial trial time. The WISC-IV (Wechsler, 2004) Digit Span Backwards (sum of the number of digits in trials remembered correctly) and Corsi Span Backward (sum of the number of digits in trials remembered correctly) assess short-term working memory and are valid in DS (Devenny et al., 2005).
Visuospatial construction
The Developmental Test of Visual-Motor Integration, 5th Edition (VMI; Berry et al., 2004) assesses visual-motor integration skills. The Purdue Pegboard (Bega, 1969) assesses fine motor functioning speed (Purdue Single and Both Time) and executive functioning (Both Hands score). The WISC-IV (Wechsler, 2004) Block Design and Haxby extension (Haxby, 1989) assesses visuospatial construction and are sensitive to dementia in DS (Shapiro et al., 1992).
Language
The NEPSY-2nd Edition (Korkman et al., 2007) Word Generation Semantic Fluency subtest is a valid measure of verbal fluency in DS (Devenny et al., 2005). The Expressive-One Word Picture Vocabulary Test (Brownell, 2000) assesses expressive language. The Peabody Picture Vocabulary Test-4 (PPVT; Dunn & Dunn, 2007) assesses receptive language. Both of these measures have been found to be sensitive to language impairments in individuals with intellectual disability (Ypsilanti, et al., 2005).
Neuroimaging
Magnetic resonance imaging
Structural T1-weighted 3 T MRI scans using GE Medical Systems (site name) or Siemens Magnetom Trio (site name) scanners were used to acquire high resolution volumetric spoiled gradient or MPRAGE sequence. MRI data was used for PET-MRI registration, region definition, and magnetic resonance-guided correction of PET data for atrophy-related CSF dilution.
Positron emission tomography
11C-PiB was synthesized at high specific activity (>2000 mCi/μmol). A nominal dose of 15 mCi of radiotracer was injected by bolus (20–30 s) through an intravenous catheter. Following a 40-min uptake, a 30-min PET acquisition (5 minute frames) was conducted, followed by a 6–10 minute transmission scan to correct for attenuation of annihilation radiation. Siemens ECAT EXACT HR + PET scanners were operated in 3D mode. The data were reconstructed using filtered back-projection and corrected for deadtime, normalization, scatter, and radioactive decay.
Image processing
PET-MRI registration followed automatic methods (Minoshima et al., 1993). Images were re-oriented along the anterior–posterior commissure (AC-PC line). Between-frame motion of PET data was corrected on frame-by-frame basis. PiB retention was expressed as standardized uptake value ratio (SURV) during 50–70 minute post-injection, using the cerebellar grey matter as the reference region. Regions of interest (ROI) were defined using T1W MRI and transferred to PET data for sampling over single and multiple transverse planes (Rosario et al., 2011) for the six brain regions: frontal cortex, anterior cingulate gyrus, parietal cortex, lateral temporal cortex, and precuneus cortex, and also the striatum (anterior ventral region [AVS]). A composite index of Global PiB representing an average of the six brain regions was also calculated for measuring changes in amyloid burden between imaging cycles. Two-component magnetic resonance-based CSF correction corrected for the partial volume effect of expanded CSF spaces accompanying normal aging and disease-related cerebral atrophy on PiB retention (Meltzer et al., 1999).
PiB+ versus PiB−
Using sparse k-means clustering with resampling (Cohen et al., 2013), PiB+ was defined as exceeding the cut-off in one (or more) of the six regions in the global PiB. Cut-off points (SUVR): frontal cortex = 1.71, anterior cingulate gyrus = 1.78, parietal cortex = 1.63, lateral temporal cortex = 1.50, precuneus cortex = 1.73, and AVS = 1.48 [46].
Data Analysis Plan
Distributions of variables and histograms of residuals were reviewed to assess normalcy of data; there was a normal distribution of data without skew. Although multiple analyses were conducted, an alpha of p ≤ 0.05 was used for statistical significance given that small declines in cognitive functioning are anticipated during the transitionary stage of AD. Across cycles, four participants had some missing neuropsychological data due to lack of understanding and/or complying with instructions. At Cycle 1, floor effects (i.e., lowest possible score) occurred in Story Recall Logical Memory (n = 10, 17.9%), Digit Span Backwards (n = 10, 17.9%), Corsi Span Backward (n = 9, 16.4%), and Rivermead Picture Recognition (n = 4, 7.1%). Information on PiB retention change across cycles is provided elsewhere (Lao et al., under review).
Analyses first examined within-person differences on neuropsychological measures from Cycle 1 to Cycle 2 without controlling for chronological age (paired sample t-tests), and then controlling for chronological age (paired sample t-test with covariate). Analyses then examined the association between change in neuropsychological measures (from Cycle 1 to Cycle 2) and change in both global and AVS-only PiB retention (from Cycle 1 to Cycle 2) as a continuous variable, without controlling for chronological age (Pearson correlations) and then controlling for chronological age (multiple linear regressions). The AVS was analyzed individually because this region reveals the earliest presence of elevated PiB binding in the DS population (Annus et al., 2016; Hartley et al., 2014; Nelson et al., 2011; Lao et al., 2016). Finally, one-way analyses of variance (ANOVAs) and Bonferroni-corrected post-hoc comparisons examined differences in neuropsychological measures by PiB categorization status (consistently PiB−, consistently PiB+, and converted from PiB− to PiB+). These analyses were re-run controlling for chronological age using analyses of covariance (ANCOVAs).
Results
Across Cycle 1 to Cycle 2, participants evidenced significant increases in PiB retention in four of the six brain regions: anterior cingulate gyrus, precuneus cortex, parietal cortex and AVS (Table 2). Across cycles, 14 (27.5%) participants were consistently PiB+, 31 (60.8%) participants were consistently PiB−, and 6 (11.7%) participants converted from PiB− at Cycle 1 to PiB+ at Cycle 2. No participant reverted from PiB+ at Cycle 1 to PiB− at Cycle 2.
Table 2.
Means and Standard Deviation for regional PiB retention
| Cycle 1 | Cycle 2 | Cycle 1 –Cycle 2 Difference | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | p-Value | |
| Anterior Ventral Striatum | 1.41 | 0.49 | 1.59 | 0.60 | 0.00 |
| Anterior Cingulate Gyrus | 1.45 | 0.31 | 1.51 | 0.39 | 0.007 |
| Frontal Cortex | 1.39 | 0.31 | 1.43 | 0.40 | 0.067 |
| Lateral Temporal Cortex | 1.34 | 0.22 | 1.36 | 0.30 | 0.389 |
| Precuneus Cortex | 1.35 | 0.29 | 1.45 | 0.38 | 0.000 |
| Parietal Cortex | 1.31 | 0.23 | 1.37 | 0.32 | 0.002 |
| Global | 1.37 | 0.29 | 1.45 | 0.38 | 0.000 |
Note. See Cohen et al., 2013 for information about cutoffs. Global is the average of the 6 separate regions of interest volumes.
At Cycle 2, five participants received a DSDS score above the Cognitive Cutoff Score (scores of 3, 3, 4, 4, and 5) indicative of possible dementia. Based on clinical case consensus review using information from dementia screens (SIB and DSDS) and caregivers (Vineland and behavioral/medical history), but without knowledge of PiB retention, three of these participants were deemed to have clinical AD. One converted from PiB− to PiB+ and two were consistently PiB+ across the cycles.
Table 3 displays the means and standard deviations for neuropsychological measures at Cycle 1 and Cycle 2 and the results of the paired sample t-tests examining within-person change across the cycles. Across the sample, there were significant within-person declines on Vineland, Verbal Fluency Number, and Purdue Pegboard Single Hand, and Purdue Pegboard Both Hands, and an increase in Cued Recall Intrusions. Estimated change in neuropsychological measures from Cycle 1 to Cycle 2 when controlling for chronological age is also displayed in Table 3. When chronological age was a co-variate, the same pattern emerged with the addition of significant within-person declines in Free and Cued Recall, Expressive One Word, and VMI (Table 3).
Table 3.
Change in Neuropsychological Measures From Cycle 1 to Cycle 2 in Adults with Down syndrome
| No Control for Age | Controlling for Age | ||||
|---|---|---|---|---|---|
|
| |||||
| Cycle 1 | Cycle 2 | ||||
|
| |||||
| Measure | Mean (SD) | Mean (SD) | t - value | Estimate (SE) | t - value |
| Vineland | 183.67 (47.65) | 170.17 (49.81) | −3.67** | −15.61 (3.96) | −3.94*** |
| Free Recall | 16.57 (6.15) | 17.81 (6.47) | 1.54 | 0.25 (0.70) | 0.35 |
| Free and Cued Recall | 33.02 (5.37) | 31.73 (5.34) | −1.76 | −1.94 (0.63) | −3.08** |
| Cued Recall Intrusions | 2.21 (3.29) | 3.19 (3.81) | 2.00* | 1.57 (0.45) | 3.52*** |
| Block Design | 27.43 (9.08) | 26.79 (9.88) | −0.74 | −1.63(0. 86) | −1.90+ |
| Severe Impairment Battery | 46.64 (3.62) | 46.38(4.29) | −0.57 | −0.48 (0.49) | −0.99 |
| Visual Attention Time | 82.59 (41.49) | 86.50 (49.81) | 0.70 | 10.18 (5.46) | 1.86 + |
| Visual Attention Accuracy | 18.00 (3.14) | 18.09 (3.08) | 0.16 | −0.29 (0.42) | −0.69 |
| Verbal Fluency Number | 24.25 (9.06) | 20.80 (9.15) | −2.93** | −4.30 (1.10) | −3.92*** |
| Verbal Fluency Repetition | 2.49 (2.53) | 3.05 (3.37) | 1.33 | 0.43 (0.44) | 0.97 |
| Purdue Pegboard - Single Hands | 14.93 (3.41) | 13.93 (3.73) | −2.70** | −1.55 (0.33) | −4.97*** |
| Purdue Pegboard - Both Hands | 5.43 (1.94) | 4.56 (1.89) | −4.25*** | −1.02 (0.20) | −5.12*** |
| Story Recall Initial | 2.25(2.07) | 2.64 (2.26) | 1.68 | 0.17 (0.23) | 0.73 |
| Story Recall Initial - Delayed | 3.25 (2.79) | 3.52 (3.06) | 0.55 | 0.17 (0.28) | 0.61 |
| Expressive One Word | 75.73 (24.18) | 72. 89 (24.59) | −1.93 + | −4.70 (1.42) | −3.31** |
| PPVT age equivalent | 97.14 (40.13) | 94.39 (39.11) | −0.90 | −4.33 (3.10) | −1.40 |
| Rivermead Picture Recognition | 6.06 (3.27) | 5.79 (3.50) | −0.68 | −0.57 (0.41) | −1.39 |
| VMI | 17.36 (2.89) | 16.89 (2.88) | −1.36 | −0.68 (0.32) | −2.10* |
| Cat Dog Switch Errors | 2.02 (3.79) | 2.07 (3.75) | 0.10 | 0.48 (0.46) | 1.04 |
| Cat Dog Switch Time | 10.04 (8.45) | 9.69 (9.57) | −0.22 | −0.55 (1.38) | −040 |
| Corsi Forward | 11.93 (7.98) | 12.32 (7.95) | 0.38 | −0.25 (0.96) | −0.26 |
| Corsi Backward | 4.04 (4.13) | 3.75 (4.65) | −0.53 | −0.68 (0.70) | −0.97 |
| Digits Span Forward | 11.63 (6.99) | 11.89 (7.65) | 0.36 | −0.25 (0.76) | −0.34 |
| Digits Span Backward | 4.89 (4.98) | 4.96 (5.61) | 0.13 | 0.35 (0.73) | 0.48 |
Note.
p < .10;
p ≤.05;
p ≤ .10;
p ≤.001. When controlling for chronological age, estimate represents change for a participant at age 40 years with mean Cycle 1 score. As follow-up, analyses were re-run excluding individuals with floor level (i.e., lowest possible score) scores at Cycle 1 as these individuals would not be able to show decline. The pattern of significant results remained the same.
Correlations between change in neuropsychological measures (from Cycle 1 to Cycle 2) and change in PiB retention as a continuous variable (from Cycle 1 to Cycle 2) are presented in Table 4. Correlations were conducted for global PiB retention and then AVS PiB retention alone. Across the two cycles, worsening of performance in Free and Cued Recall Total, Cued Recall Intrusions, Block Design Total, Purdue Pegboard Single Hands, and Rivermead Picture Recognition was significantly associated with an increase in global PiB retention. Table 4 also presents estimates for the association between change in neuropsychological measures (from Cycle 1 to Cycle 2) and change in PiB retention controlling for chronological age. Findings for global PiB retention were the same with and without controlling for chronological age (Table 4). In regard to AVS, worsening of performance in Purdue Pegboard Single Hands was significantly associated with an increase in AVS PiB retention when not controlling for chronological age. However, when controlling for chronological age, a worsening of performance in the VMI and Cat Dog Switch Time were significantly associated with an increase in AVS PiB retention (Table 4).
Table 4.
Associations between Cycle 1 to Cycle 2 Change in Neuropsychological Measures and Change in PiB Retention
| Striatum Change | Global Change | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| No Control for Age | Controlling for Age | No Control for Age | Controlling for Age | |||
|
| ||||||
| r | Estimate (SE) | t-value | r | Estimate (SE) | t-value | |
| Vineland | 0.01 | 0.85 (21.83) | 0.04 | −0.11 | −45.21 (31.81) | −1.42 |
| Free Recall | −0.24+ | 2.26 (3.64) | 0.62 | −0.21 | −2.49 (4.97) | −0.50 |
| Free and Cued Recall | −0.20 | 0.08 (3.33) | 0.02 | −0.34* | −10.93 (4.22) | −2.59** |
| Cued Recall Intrusions | 0.16 | 1.16 (2.43) | 0.48 | 0.35** | 10.27 (2.96) | 3.47*** |
| Block Design | −0.23 | −3.77 (4.81) | −0.78 | −0.32* | −14.30 (6.17) | −2.32* |
| Severe Impairment Battery | −0.05 | 0.63 (2.76) | 0.23 | −0.14 | −3.70 (3.66) | −1.01 |
| Visual Attention Time | 0.02 | 4.18 (28.01) | 0.15 | −0.07 | 10.62 (39.26) | 0.27 |
| Visual Attention Accuracy | −0.07 | 1.06 (2.61) | 0.41 | −0.10 | −2.28 (3.53) | 0.27 |
| Verbal Fluency Number | −0.22 | −7.51 (6.38) | −1.18 | −0.10 | −7.68 (8.62) | −0.89 |
| Verbal Fluency Repetition | −0.05 | 2.50 (2.51) | 1.00 | −0.04 | 5.68 (3.32) | 1.71+ |
| Purdue Pegboard - Single Hands | −0.36** | −1.80 (1.79) | −1.00 | −0.43** | −5.20 (2.34) | −2.23* |
| Purdue Pegboard - Both Hands | 0.05 | 1.29 (1.15) | 1.12 | −0.01 | 0.17 (1.60) | 0.10 |
| Story Recall Initial | −0.11 | −0.04 (1.41) | −0.03 | −0.03 | −0.01 (1.96) | −0.01 |
| Story Recall Initial – Delayed | −0.15 | −2.94 (2.54) | −1.16 | −0.13 | −3.64 (3.33) | −1.09 |
| Expressive One Word | −0.17 | −4.92 (8.43) | −0.58 | −0.19 | −8,85 (11.38) | −0.78 |
| PPVT age equivalent | 0.02 | −8.37 (18.68) | −0.45 | −0.03 | −17.11 (25.18) | −0.68 |
| Rivermead Picture Recognition | −0.22 | 0.21 (2.13) | 0.10 | −0.33* | −5.46 (2.65) | −2.06* |
| VMI | −0.19 | −3.40 (1.64) | −2.07* | −0.14 | −3.69 (2.25) | −1.64 |
| Cat Dog Switch Errors | 0.14 | −2.24 (2.57) | −0.87 | 0.13 | 1.74 (3.46) | 0.50 |
| Cat Dog Switch Time | 0.24+ | 17.40 (8.10) | 2.15* | 0.13 | 10.55 (11.32) | 0.93 |
| Corsi Forward | 0.18 | 9.04 (5.84) | 1.55 | 0.18 | 11.89 (7.81) | 1.52 |
| Corsi Backward | −0.10 | −4.31 (4.29) | −1.00 | −0.00 | −0.82 (5.24) | −0.16 |
| Digits Forward | 0.03 | 2.57 (4.80) | 0.54 | −0.13 | −3.73 (6.27) | −0.59 |
| Digits Backward | −0.02 | −5.07 (4.01) | −1.27 | 0.06 | −1.16 (5.20) | −0.22 |
Note.
= p < .10;
= p ≤ .05;
p ≤ .01;
p ≤ .001. As follow-up, analyses were re-run excluding individuals with floor level (i.e., lowest possible score) scores at Cycle 1 as these individuals would not be able to show decline. The pattern of significant results remained the same.
Table 5 displays the means and standard deviations for neuropsychological measures in Cycle 1 and Cycle 2 by PiB categorization status (consistently PiB−, consistently PiB+, and converted from PiB− to PiB+). One-way ANOVAs indicated a significant difference among PiB categorization status groups in amount of change in Free Recall Total, Free and Cued Recall Total, Cued Recall Intrusions, Block Design, Purdue Pegboard Single Hand, and Corsi Forward (Table 5). Bonferroni post-hoc comparisons (interpreted at p <.05) indicated that the consistently PiB− group exhibited either no change or improvement from Cycle 1 to Cycle 2, whereas the consistently PiB+ group exhibited worsening of performance. The group that converted from PiB− to PiB+ did not significantly differ from the other groups. Based on descriptive statistics, the group that converted from PiB− to PiB+ had a pattern in-between the other groups; on average, they exhibited improvement on Free Recall Total, Block Design, and Corsi Forward from Cycle 1 to Cycle 2 but a worsening of performance on Free and Cued Recall Total, Cued Recall Intrusions, and Purdue Pegboard Single Hands. Table 5 also displays results of the one-way ANCOVAs controlling for chronological age in examining change in neuropsychological measures by PiB categorization status group. There was a significant group difference in Free and Cued Recall Total and Cued Recall Intrusions. Figures 1 and 2 display change from Cycle 1 to Cycle 2 in these two measures by PiB categorization status group. There was also trend-level group difference in Block Design. Bonferroni post-hoc comparisons (interpreted at p <.05) indicated that the consistently PiB− group exhibited no change or improvement from Cycle 1 to Cycle 2, whereas the consistently PiB+ group exhibited worsening of performance. There was not a significant group difference in Free Recall, Purdue Pegboard Single Hands, or Corsi Forward when controlling for chronological age.
Table 5.
Change in Cognitive Functioning by PiB Category Group (consistently PiB−, consistently PiB+, and converted PiB− to PiB+) from Cycle 1 to Cycle 2.
| PiB − to PiB − | PiB + to PiB+ | PiB − to PiB + | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | No Control for Age | Controlling for Age | ||||
|
| |||||||||||
| Measure | N | Mean (SD) | Mean (SD) | N | Mean (SD) | Mean (SD) | N | Mean (SD) | Mean (SD) | F value | F value |
| Vineland | 31 | 190.97 (49.00) | 181.29 (48.78) | 13 | 171.77 (43.85) | 159.69 (54.89) | 6 | 164.17 (26.70) | 141.50 (25.87) | 0.71 | 0.81 |
| Free Recall | 31 | 17.68 (6.15) | 20.13 (5.17) | 12 | 14.17 (5.25) | 12.50 (6.67) | 6 | 14.00 (6.90) | 18.00 (7.43) | 3.39* | 1.78 |
| Free and Cued recall | 31 | 33.19 (6.36) | 33.87 (3.00) | 12 | 32.50 (4.27) | 27.83 (5.97) | 5 | 34.20 (2.05) | 31.80 (6.14) | 6.13** | 5.69** |
| Cued Recall intrusion | 31 | 1.94 (3.15) | 1.65 (1.96) | 12 | 2.75 (4.27) | 6.25 (4.71) | 5 | 1.80 (2.05) | 2.20 (2.28) | 6.44** | 5.84** |
| Block Design Total | 31 | 29.77 (9.61) | 30.45 (8.90) | 14 | 27.29 (7.73) | 23.29 (9.08) | 6 | 22.33 (7.17) | 25.83 (8.38) | 4 77** | 2.33+ |
| Severe Impairment Battery | 31 | 47.10 (3.38) | 47.32 (3.02) | 14 | 45.71 (3.58) | 45.14 (5.35) | 6 | 47.17 (4.07) | 47.33 (3.08) | 0.26 | 0.27 |
| Visual Attention Time | 31 | 71.06 (34.70) | 71.87 (43.42) | 14 | 104.57 (43.22) | 110.21 (43.08) | 6 | 87.33 (47.31) | 81.33 (37.76) | 0.26 | 0.28 |
| Visual Attention Accuracy | 31 | 18.16 (3.08) | 18.65 (1.52) | 14 | 17.36 (4.07) | 16.86 (5.48) | 6 | 19.50 (0.84) | 18.50 (1.87) | 0.47 | 0.17 |
| Verbal Fluency Number | 31 | 24.84 (8.29) | 22.94 (8.97) | 13 | 21.38 (8.35) | 18.00 (8.91) | 6 | 27.33 (12.61) | 19.00 (7.27) | 1.46 | 0.65 |
| Verbal Fluency Repetitions | 31 | 2.35 (2.43) | 2.71 (2.75) | 13 | 2.69 (3.15) | 3.46 (4.98) | 6 | 3.00 (2.53) | 3.33 (1.51) | 0.09 | 1.12 |
| Purdue Pegboard - Single Hands | 30 | 15.37 (3.74) | 15.23 (3.11) | 13 | 14.54 (2.47) | 12.46 (3.38) | 6 | 15.67 (3.83) | 14.00 (3.29) | 3.23* | 0.42 |
| Purdue Pegboard - Both Hands | 30 | 5.93 (1.89) | 4.90 (1.84) | 13 | 5.15 (1.86) | 4.23 (1.88) | 6 | 5.17 (1.60) | 4.83 (1.72) | 0.54 | 1.03 |
| Story Recall Initial | 31 | 2.58 (2.17) | 3.16 (1.90) | 14 | 1.57 (2.06) | 1.71 (2.13) | 6 | 2.50 (1.52) | 3.00 (2.68) | 0.34 | 0.03 |
| Story Recall Initial - Delayed | 31 | 3.68 (2.96) | 3.94 (2.98) | 14 | 3.00 (2.88) | 2.71 (3.02) | 6 | 2.83 (1.72) | 3.50 (2.59) | 0.18 | 0.00 |
| Expressive One Word | 31 | 80.77 (25.18) | 80.58 (22.33) | 14 | 68.00 (22.05) | 63.07 (25.16) | 6 | 68.83 (18.16) | 63.17 (21.48) | 1.37 | 0.39 |
| PPVT age equivalent | 31 | 104.68 (46.84) | 104.13 (43.08) | 14 | 90.43 (28.17) | 85.93 (32.42) | 6 | 93.00 (27.48) | 84.50 (34.60) | 0.37 | 0.35 |
| Rivermead Picture Recognition | 31 | 6.45 (3.42) | 5.97 (3.55) | 14 | 4.29 (3.24) | 4.14 (3.44) | 6 | 7.33 (3.08) | 6.17 (3.66) | 0.38 | 0.82 |
| VMI | 31 | 17.87 (3.03) | 17.71 (2.84) | 14 | 17.21 (2.99) | 16.21 (2.49) | 6 | 16.17 (1.47) | 15.33 (1.51) | 0.79 | 2.06 |
| Cat Dog Switch Errors | 30 | 1.00 (2.39) | 0.83 (1.98) | 14 | 2.29 (4.03) | 3.21 (5.77) | 6 | 4.33 (6.74) | 1.50 (1.76) | 1.78 | 0.86 |
| Cat Dog Switch Time | 30 | 9.63 (8.11) | 9.50 (9.80) | 14 | 13.07 (10.47) | 9.71 (11.13) | 6 | 7.83 (6.49) | 12.50 (7.18) | 1.00 | 0.40 |
| Corsi Forward | 31 | 13.58 (8.76) | 14.71 (7.28) | 14 | 12.21 (7.08) | 9.43 (6.56) | 6 | 6.83 (6.37) | 13.50 (11.64) | 3.28* | 1.74 |
| Corsi Backward | 31 | 4.26 (3.93) | 5.06 (5.38) | 13 | 4.31 (4.33) | 2.38 (2.43) | 6 | 4.00 (6.20) | 2.33 (4.08) | 2.43+ | 2.83+ |
| Digits Forward | 31 | 13.00 (7.65) | 14.03 (8.51) | 14 | 11.00 (6.42) | 9.86 (5.45) | 6 | 7.67 (3.83) | 10.17 (7.08) | 1.12 | 0.43 |
| Digits Backward | 31 | 5.94 (5.40) | 5.97 (6.49) | 14 | 3.57 (4.88) | 3.64 (3.59) | 6 | 4.33 (3.20) | 5.83 (5.31) | 0.31 | 1.92 |
Note.
= p < .10;
= p ≤ .05;
p ≤ .01;
p ≤ .001.
F values for overall ANOVA or ANCOVA using the three groups for change in neuropsychological measure across cycles. Bonferroni pairwise post-hoc comparisons indicated PiB− to PiB− group differed from PiB+ to PiB+ group at p <.05 in all significant analyses. As a follow-up, analyses were re-run excluding individuals with floor level (i.e., lowest possible score) scores at Cycle 1 as these individuals would not be able to show decline. The pattern of significant results remained the same.
Figure 1.
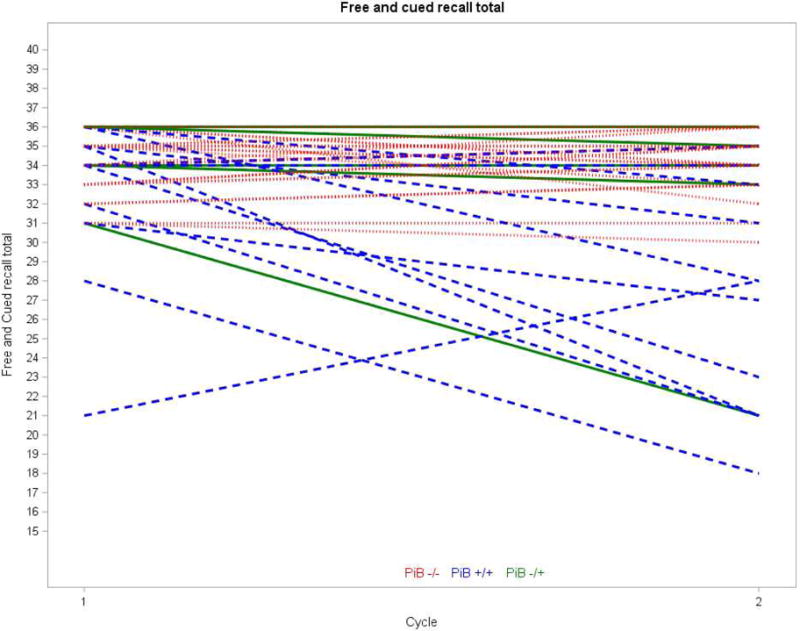
Change from Cycle 1 to Cycle 2 in Free and Cued Recall Total score by PiB Categorization group. The one participant who had a floor level (i.e., lowest possible score) score at Cycle 1 was removed.
Figure 2.
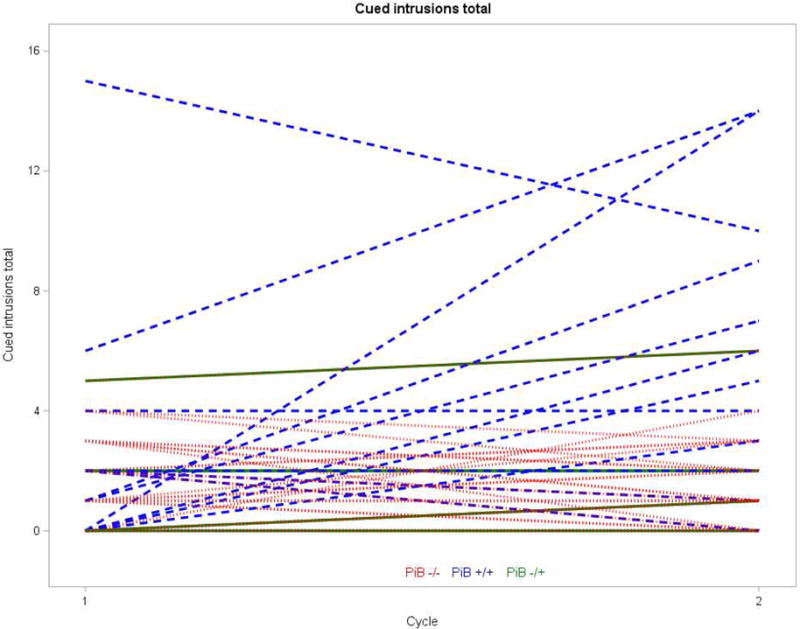
Change from Cycle 1 to Cycle 2 in Cued Intrusions Total score by PiB Categorization group.
Discussion
The current study provides the first longitudinal examination of the association between amyloid-β accumulation and declines in cognitive functioning prior to the clinical onset of AD in adults with DS. The study also builds on previous cross-sectional studies in DS (e.g., Annus et al., 2016; Nelson et al., 2011) by including an extensive neuropsychological battery. Across the 3 years, adults with DS evidenced increased amyloid-β accumulation in the AVS and across the neocortex (details see Lao et al., under review).
Overall, an increase in global amyloid-β across the 3-year period was related to subtle declines in verbal episodic memory (Free and Cued Recall Total), visual episodic memory (Rivermead Picture Recognition), visuospatial construction (Block Design), and fine motor processing speed (Purdue Pegboard Single Hands). This pattern remained after controlling for chronological age; thus, an increase in global amyloid-β is related to decreased cognitive function in these areas beyond normative aging. After controlling for normative age-related declines, an increase in amyloid-β in the AVS was associated with declines in executive functioning (Cat Dog Switch Time) and visuospatial construction (VMI), in line with the role of the striatum in inhibiting responses, mental flexibility, and motor performance (Liljehom & O’Doherty, 2012; Mattfelt et al., 2011).
Given evidence from the general population (Villemagne et al., 2013) that amyloid-β accumulation may have little impact on cognitive decline prior to reaching a threshold level (i.e., PiB+), we also examined PiB retention as a dichotomous variable (PiB− versus PiB+). After controlling for chronological age, adults with DS who were consistently PiB+ demonstrated a worsening of performance in episodic memory – remembered less information (Free Recall Total) and made more recall errors (Cued Recall Intrusions) – whereas adults with DS who were consistently PiB− evidenced stable or improved performance. Thus, difficulties remembering newly learned information (e.g., what to buy at store or who is visiting tomorrow) may be important early indicators that an adult with DS is on the pathway to clinical AD. Across the 3-years, six adults with DS converted from PiB− to PiB+ based on global amyloid-β (neocortical regions and striatum). This translates into a conversion rate of 19% (6 out of the 32 PiB− participants at Cycle 1) over three years. These participants exhibited a pattern that was in-between that of participants who were consistently PiB− and participants who were consistently PiB+.
Our longitudinal findings are consistent with findings in the general population showing a link between neocortical amyloid-β accumulation and memory and executive functioning declines (Mormino et al., 2009; Rowe et al., 2010). In addition, our findings are consistent with cross-sectional findings based on Cycle 1 using this sample (Hartley et al., 2014) in that both analyses indicated a negative association between global PiB retention and verbal and visual episodic memory and executive functioning performance. However, the current longitudinal findings did not indicate an association between expressive language declines and global amyloid-β accumulation. Although, there was an average-level decrease in expressive language (Expressive One Word) across the 3-years, this appeared to be a normative age-related decline. Similarly, in the current analyses, there were declines in adaptive behavior (Vineland), verbal fluency (Verbal Fluency Number), and fine motor processing (Purdue Pegboard) across the 3-years, but these also appeared to be normative age-related declines and were not associated with amyloid-β accumulation.
It is important to note that the large majority (15 of 17) of adults with DS in our sample who were PiB+ at both cycles of data collection remained pre-symptomatic for AD based both on clinical judgement and caregiver report. Indeed, only three adults with DS were deemed to have clinical AD at Cycle 2; two were consistently PiB+ and one converted from PiB− to PiB+. This highlights that declines in cognitive functioning were mild and did not have marked impacts on everyday lives. Yet, these declines may provide meaningful early markers of AD relevant for early screening and as outcomes of interest in therapeutic trials aimed at delaying or preventing clinical AD. Findings may also have relevance to other populations involving amyloid-β overproduction (e.g., autosomal dominant AD) or amyloid-β accumulation in the AVS (e.g., higher Braak neurofibrillary stages; Beach et al., 2012), which has also been shown to correlate with cognitive declines (Wolf et al., 1999).
There were strengths to the current study. We included a relatively large sample of adults with DS and a rigorous neuropsychological evaluation to capture fine-grained declines and understand the specific domains of cognition affected by amyloid-β accumulation. We conducted analyses with and without controlling for chronological age in order to separate normative age-related declines from those associated with the early stages of AD. There were also limitations. An alpha value of .05 was used for significance to allow for the detection of mild cognitive declines. This strategy increases risk of type 2 errors; findings from current study are exploratory until replicated. The current study is not representative of adults with DS who are non-verbal or who have a mental age equivalent of less than 2.5 years. Moreover, floor level effects occurred in some of the neuropsychological measures in a subset of participants (4.1% – 17.9%), meaning it was not possible to detect potential declines across cycles for these participants. Longer-term longitudinal studies with multiple data collection cycles are needed to tease apart time-order causal pathways, and are currently ongoing. Future studies should examine change in brain structure in relation to declining cognitive functioning and amyloid-β accumulation. Moreover, studies should examine whether increased amyloid-β accumulation is associated with subtle changes in emotional and behavioral functioning in AD, as there is evidence that these domains are also altered in the earliest stages of AD in DS (Ball et al., 2006). In the current study, anti-depressant medication usage increased (29% to 41%) in participants who were consistently PiB+, potentially signaling increased depressive symptoms.
In summary, the current study provides the first longitudinal investigation of early change in biomarkers of amyloid-β accumulation in adults with DS who initially did not have clinical AD. Findings provide important information about the association between early AD neuropathology and cognitive decline, beyond the effects of chronological age. Findings also identify cognitive measures that are sensitive to AD biomarkers in the early preclinical AD stage, and thus may be useful outcomes for therapeutic trials or early AD screening tools in DS.
Highlights.
Amyloid-β increased throughout neocortex and in striatum
Increased global amyloid-β related to decline in cognitive functioning across 3 years
PET PiB relevant biomarker of early, preclinical decline in cognitive functioning in DS.
Acknowledgments
GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. WK is a co-inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. All other authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The research is funded by the National Institute on Aging (R01AG031110 to B.H. and B.C.; U01AG051406 to B.H., W.K., and B.C.) and the Eunice Kennedy Schriver National Institute of Child Health and Human Development (P30 HD03352 to A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstien HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annus T, Wilson LR, Hong YT, Acosta-Cabronero J, Fryer TD, Cardemas-Blanco A, et al. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dement. 2016;12(15):538–545. doi: 10.1016/j.jalz.2015.07.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Holland AJ, Hon J, Huppert FA, Treppner P, Watson PC. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: findings from a prospective population-based study. Ger Psychiatry. 2006;21(7):661–673. doi: 10.1002/gps.1545. [DOI] [PubMed] [Google Scholar]
- Ball SL, Holland AJ, Treppner P, Watson PC, Huppert FA. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. Brit J Clin Psychol. 2008;47:1–29. doi: 10.1348/014466507X230967. [DOI] [PubMed] [Google Scholar]
- Beach TG, Li S, Walker DG, et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer’s disease: Implications for amyloid imaging. J Alz Dem. 2012;28(4):869–876. doi: 10.3233/JAD-2011-111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE, Buktenica NA, Beery NA. The Beery-Buktenica developmental test of visual-motor integration. 5th. Bloomington, MN: Pearson; 2004. [Google Scholar]
- Brownell R. The expressive one-word vocabulary test. East Moline, IL: LinguiSystems, Inc.; 2000. [Google Scholar]
- Brugge KL, Nichols SL, Salmon DP, Hill LR, Delis DC, Aaron L, et al. Cognitive impairment in adults with Down’s syndrome: similarities to early cognitive changes in Alzheimer’s disease. Neurology. 1994;44:232–8. doi: 10.1212/wnl.44.2.232. [DOI] [PubMed] [Google Scholar]
- Coppus A, Evenhuis H, Verberne GJ, Visser F, van Gool P, Eikelenboom P, et al. Dementia and mortality in persons with Down’s syndrome. J Intellect Disabil Res. 2006;50:768–777. doi: 10.1111/j.1365-2788.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, et al. Classification of amyloid-positivity in controls: Comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–215. doi: 10.1016/j.neuroimage.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenny DA, Wegiel J, Schupt N, Jenkins E, Zigman W, Krinsky-McHale SJ, et al. Dementia of the Alzheimer’s Type and accelerated aging in down syndrome. Sci Aging Knowl Environ. 2005;14:1–14. doi: 10.1126/sageke.2005.14.dn1. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody picture vocabulary test. 4th. San Antonio, TX: NCD Pearson, Inc.; 2007. [Google Scholar]
- Forsberg A, Almkvist O, Engler H, Wall A, Langstrom B, Nordberg A. High PiB retention in Alzheimer’s disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010;7(1):56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- Gedye A. Dementia Scale for Down’s Syndrome: Manual. Vancouver, BC: Gedye Research and Counseling; 1995. [Google Scholar]
- Handen BL, Cohen AD, Channamalappa U, Bulova P, Cannon SQ, Cohen WI, et al. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimer Dement. 2012;8:496–501. doi: 10.1016/j.jalz.2011.09.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–194. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hartley SL, Handen BL, Devenny DA, Hardison R, Mihaila I, Price JC, et al. Cognitive functioning in relation to brain amyloid-β in healthy adults with Down syndrome. Brain. 2014;137:2556–2563. doi: 10.1093/brain/awu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV. Neuropsychological evaluation of adults with Down’s syndrome: patterns of selective impairment in non-demented old adults. J Ment Defic Res. 1989;33:193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Heller JH, Spiridigliozzi GA, Crissman BG, Sullivan JA, Eells RL, Li J, et al. Safety and efficacy of rivastigmine in adolescents with Down Syndrome: A preliminary 20-week, open-label study. J Child Adolesc Psychopharmacol. 2006;16(6):755–765. doi: 10.1089/cap.2006.16.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Garagnani P, Bacalini MG, Pirazzini C, Salvioli S, Gentilini D, et al. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir A, Almkvist O, Forsberg A, Wall A, Engler H, Långström B, et al. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer’s disease. Neurobiol Aging. 2012;33(1):198.e1–14. doi: 10.1016/j.neurobiolaging.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY-II. San Antonio, TX: Harcourt Assessment Inc.; 2007. [Google Scholar]
- Lao PJ, Betthauser TJ, Hilmer AT, Price J, Klunk W, Mihaila I, et al. The effects of normal aging on amyloid-β deposition in a population of nondemented adults with Down syndrome as imaged by [11C] PIB. Alzheimer Dement. 2016;12(4):380–90. doi: 10.1016/j.jalz.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao PJ, Betthauser TJ, Tudorascu D, Mihalia I, Bulova PD, Hartley SL, et al. Longitudinal changes in amyloid PET and volumetric MRI in the non-demented Down syndrome population. Alzheimer Demen Under Review [Google Scholar]
- Liljeholm M, O’Doherty JP. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci. 2012;16(9):467–475. doi: 10.1016/j.tics.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci. 1989;89:169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CEL. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learning & Memory. 2011;18(11):703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron M, McCallion P, Reilly E, Mulryan N. A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2014;58:61–70. doi: 10.1111/jir.12074. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40:2053–65. [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–9. [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Alzheimer’s Disease Neuroimaging Initiative. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash HN, Snowling MJ. Semantic and phonological fluency in children with Down syndrome: Atypical organization of language or less efficient retrieval strategies? Cog Neuropsych. 2008;25(5):690–703. doi: 10.1080/02643290802274064. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Siddarth P, Kepe V, Scheihel KE, Huang SC, Barrio JR, et al. Positron emission tomography of brain OE4-amyloid and tau levels in adults with Down syndrome. Arch Neurol. 2011;68:768–74. doi: 10.1001/archneurol.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, CanField R, Rickard Y, Wang RE, Meyer R, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Patterson D, Cabelof DC. Down syndrome as a model of DNA polymerase beta haploinsufficiency and accelerated aging. Mech Ageing and Dev. 2012;133(4):133–137. doi: 10.1016/j.mad.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Rafii MS, Wishnek H, Brewer JB, et al. The down syndrome biomarker initiative (DSBI) pilot: Proof of concept for deep phenotyping of Alzheimer’s disease biomarkers in down syndrome. Frontiers Behav Neurosci. 2015;9:239–243. doi: 10.3389/fnbeh.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario BL, Weissfeld LA, Lymon CM, Mathis CA, Klunk WE, Berginc MD, et al. Inter-rater reliability of manual and automated region-of-interest delineation for PIB PET. Neuroimage. 2011;55(3):933–941. doi: 10.1016/j.neuroimage.2010.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Chen K, Rogers J, Fleisher AS, Liebsack C, Bandy D, et al. Florbetapir PET, FDG PET, and MRI in Down syndrome individuals with and without Alzheimer’s dementia. Alz & Dem. 2015;11(8):994–1004. doi: 10.1016/j.jalz.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J, Kastango KB, Hugonot-Diener L, Boller F, Verny M, Sarles CE, et al. Development of a short form of the severe impairment battery. Am J Geriat Psychiat. 2005;13:999–1005. doi: 10.1176/appi.ajgp.13.11.999. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Haxby JV, Grady CL. Nature of mental retardation and dementia in Down syndrome: Study with PET, CT, and neuropsychology. Neurobiol Aging. 1992;13(6):723–734. doi: 10.1016/0197-4580(92)90096-g. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales. 2nd. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Aβ deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega A. Use of Purdue pegboard and finger tapping performance as a rapid screening test for brain damage. J of Clin Psych. 1969;25:255–8. doi: 10.1002/1097-4679(196907)25:3<255::aid-jclp2270250306>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of amyloid-β and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–9. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler intelligence scale for children revised. New York: Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Fourth Edition: Administration and scoring manual. San Antonio: The Psychological Corporation; 2004. [Google Scholar]
- Wilson B, Ivani-Chalian CF, Aldrich F. Rivermead behavioral memory test for children. Bury St Edmunds, U.K.: Thames Valley Test Co; 1991. [Google Scholar]
- Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VLJ, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16(9):564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13:226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–68. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WY, Tudorascu DL, McDade EM, Ikonomovic S, James JA, Minhas D, et al. Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2015;14(8):804–813. doi: 10.1016/S1474-4422(15)00135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilanti A, Grouios G, Alevriadou A, Tsapkini K. Expressive and receptive vocabulary in children with Williams and Down syndromes. J Intellect Disabil Res. 2005;49:353–64. doi: 10.1111/j.1365-2788.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- Zimmerli E, Devenny DA. Paper presented at the Gatlinburg conference on research and theory in mental retardation and developmental disabilities. Gatlinburg, TN: 1995. Mar 11–14, Cued recall as a screen for dementia in the MR population. [Google Scholar]


