Abstract
Prostate-specific membrane antigen (PSMA) is a membrane protein that is overexpressed manifold in prostate cancer and provides an attractive target for molecular therapy. Near infrared photoimmunotherapy (NIR-PIT) is a highly selective tumor treatment that employs an antibody-photo-absorber conjugate (APC). Here, we describe the efficacy of NIR-PIT, using a fully human IgG1 anti-PSMA monoclonal antibody (mAb), conjugated to the photo-absorber, IR700DX, in a PSMA expressing PC3 prostate cancer cell line. Anti-PSMA-IR700 showed specific binding and cell-specific killing was observed after exposure of the cells to NIR in vitro. In the in vivo study, anti-PSMA-IR700 showed high tumor accumulation and high tumor-background ratio. Tumor-bearing mice were separated into 4 groups: (1) no treatment; (2) 100 μg of anti-PSMA-IR700 i.v.; (3) NIR light exposure; (4) 100 μg of anti-PSMA-IR700 i.v., NIR light exposure was administered. These were performed every week for up to 3 weeks. Tumor growth was significantly inhibited by NIR-PIT treatment compared with the other control groups (p < 0.001), and significantly prolonged survival was achieved (p < 0.0001 vs other control groups). More than two thirds of tumors were cured with NIR-PIT. In conclusion, the anti-PSMA antibody is suitable as an APC for NIR-PIT. Furthermore, NIR-PIT with the anti-PSMA-IR700 antibody is a promising candidate of the treatment of PSMA-expressing tumors and could be readily translated to humans.
Keywords: near infrared photoimmunotherapy, PSMA, prostate cancer, monoclonal antibodies, molecular imaging
Introduction
Prostate cancer is the most common cancer in men, with 161,360 estimated new cases and it is the third leading cause of cancer-related death among men in the United States (US), with 26,730 deaths estimated in 2017 (1). Localized prostate cancer typically is treated with surgery or radiation, and recurrent disease can be controlled temporarily with radiation often combined with androgen ablation (2). However, many prostate cancers eventually become hormone refractory and then rapidly progress (3). Hormone-refractory or androgen-independent metastatic prostate cancer is usually lethal. Therefore, it is important that the clinical trajectory of prostate cancer be intercepted as early in the course of the disease as possible (4, 5). New molecularly targeted therapies are urgently needed for this task.
Monoclonal antibody (mAb) therapies have shown considerable value in the treatment of many malignant tumors (6–9). As monotherapy, mAbs can either block receptors needed for growth or induced antibody dependent complement-mediated cytotoxicity (ADCC). Antibodies can also be used to deliver drugs or therapeutic radioisotopes. However, for antibodies to be most effective a distinct antigen must be commonly expressed on the cancer cell surface.
Prostate specific membrane antigen (PSMA) is a well-established cell membrane marker of prostate cancer and therefore is a plausible target for molecular therapy. PSMA is a type 2 integral membrane glycoprotein, also known as glutamate carboxypeptidase II (GCPII) or folate hydrolase 1 (FOLH1) (10–12) and it was originally discovered in the androgen-dependent LNCap human prostatic adenocarcinoma cell line (13). PSMA is expressed in nearly all prostate cancers, and expression is highest in poorly differentiated, metastatic and hormone-refractory cases (14–17).
Compared with other therapies antibody directed phototherapy has several advantages over conventional therapies because it is minimally invasive and can be used repeatedly without limitation of the total dose or treatment resistance (18). In the past, antibodies were conjugated to very hydrophobic photosensitizers in conventional photodynamic therapy (PDT). As a consequence of their hydrophobicity, these conjugates tended to be nonspecific and limited by toxicity.
Near infrared photoimmunotherapy (NIR-PIT) is a newly developed cancer treatment that employs a highly targeted mAb-photo-absorber conjugate (APC). The photo-absorber, IRDye700DX (IR700, silica-phthalocyanine dye), is a highly hydrophilic dye, differentiating it from prior hydrophobic dyes used in PDT (19). The first-in-human phase 1 trial of NIR-PIT in patients with inoperable head and neck cancer targeting epidermal growth factor receptor (EGFR) was approved by the US Food and Drug Administration (FDA), and started in June 2015 (https://clinicaltrials.gov/ct2/show/NCT02422979) and finished in August 2016. In this trial, patients were injected with cextuximab-IR700 conjugate, (referred to as RM1929 in the study), that binds to target EGFR molecules on the cell membrane of head and neck cancers. About 24 hours later the tumor is exposed to NIR light by means of a laser at a wavelength of 690 nm which is absorbed by IR700. NIR-PIT induces nearly immediate necrotic cell death rather than apoptotic cell death which is most commonly induced by other cancer therapies (20).
NIR-PIT has been shown to be effective with a variety of different antibodies but has not been previously tested with fully human anti-PSMA antibody (21–26). In this study, we investigated anti-PSMA antibody-IR700 as a candidate APC for NIR-PIT. Using a PSMA expressing PC3 cell line in vitro binding, in vivo tumor accumulation and intratumoral distribution were evaluated. NIR-PIT was then performed with anti-PSMA-IR700 in vitro and repeated NIR-PIT was performed in a tumor-bearing mouse model in vivo.
Materials and Methods
Reagents
Water soluble, silica-phthalocyanine derivative, IRDye 700DX NHS ester was obtained from LI-COR Biosciences (Lincoln, NE, USA). A fully human IgG1 anti-human prostate-specific membrane antigen (PSMA) was kindly provided by Progenics Pharmaceuticals, Inc. (Tarrytown, NY, USA, Patent; US 8114965 B2) (27). All other chemicals were of reagent grade.
Synthesis of IR700-conjugated anti-PSMA
Conjugation of dyes with mAb was performed according to a previous report (19). In brief, anti-PSMA mAb (1.0 mg, 6.7 nmol) was incubated with IR700 NHS ester (65.1 μg, 33.3 nmol) in 0.1 M Na2HPO4 (pH 8.6) at room temperature for 1 h. The mixture was purified with a Sephadex G25 column (PD-10; GE Healthcare, Piscataway, NJ, USA). The protein concentration was determined with Coomassie Plus protein assay kit (Thermo Fisher Scientific Inc, Rockford, IL, USA) by measuring the absorption at 595 nm with UV-Vis (8453 Value System; Agilent Technologies, Santa Clara, CA, USA). The concentration of IR700 was measured by absorption at 689 nm to confirm the number of fluorophore molecules per mAb. The synthesis was controlled so that an average of two IR700 molecules was bound to a single antibody. We abbreviate IR700 conjugated to anti-PSMA mAb as anti-PSMA-IR700. As a quality control for the conjugate, we performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Conjugate was separated by SDS-PAGE with a 4–20% gradient polyacrylamide gel (Life Technologies, Gaithersburg, MD, USA). A standard marker (Crystalgen Inc., Commack, NY, USA) was used as a protein molecular weight marker. After electrophoresis at 80 V for 2.5 h, the gel was imaged with a Pearl Imager (LI-COR Biosciences, Lincoln, Nebraska, USA) using a 700 nm fluorescence channel. We used diluted anti-PSMA antibody as a control. The gel was stained with Colloidal Blue staining to determine the molecular weight of conjugate.
Cell culture
PC3-PSMA+ (PC3pip) cell line generated by transduction of PC3 cells using VSV-G pseudotyped lentiviral vector expressing human PSMA and a control blank-vector transfected PC3 cell line, PC3-PSMA- (PC3flu) (28, 29) were used for PSMA targeting studies with anti-PSMA-IR700. Both cells were established at the Cleveland Clinic Foundation. A luciferase stably expressed PC3pip cell line was also established with the transfection of pGL4.51 luc2/CMV/Neo Vector (Promega, Madison, WI, USA). High luciferase expression was confirmed with 10 passages. We abbreviate this cell line as PC3pip-luc. Cells were grown in RPMI 1640 (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Life Technologies) in tissue culture flasks in a humidified incubator at 37°C in an atmosphere of 95% air and 5% carbon dioxide.
Flow cytometry
To verify in vitro anti-PSMA-IR700 binding, fluorescence from cells after incubation with anti-PSMA-IR700 was measured using a flow cytometer (FACS Calibur, BD BioSciences, San Jose, CA, USA) and CellQuest software (BD BioSciences). PC3flu and PC3pip-luc cells (2 × 105) were seeded into 12 well plates and incubated for 24 h. Medium was replaced with fresh culture medium containing 3 μg/mL of anti-PSMA-IR700 and incubated for 6 h at 37°C. To validate the specific binding of the conjugated antibody, excess antibody (30 μg) was used to block 3 μg of anti-PSMA-IR700.
Fluorescence microscopy
To detect the antigen specific localization and effect of NIR-PIT, fluorescence microscopy was performed (BX61; Olympus America, Inc., Melville, NY, USA). Ten thousand PC3flu and PC3pip-luc cells were seeded on cover-glass-bottomed dishes and incubated for 24 h. anti-PSMA-IR700 was then added to the culture medium at 3 μg/mL and incubated for 6 h at 37°C. After incubation, the cells were washed with phosphate buffered saline (PBS). The filter set to detect IR700 consisted of a 590–650 nm excitation filter, a 665–740 nm band pass emission filter. Transmitted light differential interference contrast (DIC) images were also acquired.
In vitro NIR-PIT
The cytotoxic effects of NIR-PIT with anti-PSMA-IR700 were determined by flow cytometric propidium iodide (PI) (Life Technologies) staining, which can detect compromised cell membranes. Two hundred thousand PC3flu and PC3pip-luc cells were seeded into 12 well plates and incubated for 24 h. Medium was replaced with fresh culture medium containing 3 μg/ml of anti-PSMA-IR700 and incubated for 6 h at 37°C. After washing with PBS, PBS was added, and cells were irradiated with a red light-emitting diode (LED), which emits light at 690 ± 20nm wavelength (L690-66-60; Marubeni America Co., Santa Clara, CA, USA) at a power density of 50 mW/cm2 as measured with an optical power meter (PM 100, Thorlabs, Newton, NJ, USA). Cells were scratched 1 h after treatment. PI was then added in the cell suspension (final 2 μg/mL) and incubated at room temperature for 30 min, followed by flow cytometry. Each value represents mean ± standard error of the mean (SEM) of five experiments.
For bioluminescence imaging (BLI), either two hundred thousand PC3pip-luc cells were seeded into 12 well plates or twenty million PC3pip-luc cells were seeded onto a 10 cm dish; both were pre-incubated for 24 h. After replacing the medium with fresh culture medium containing 3 μg/mL of anti-PSMA-IR700, the cells were incubated for 6 h at 37˚C in a humidified incubator. After washing with PBS, phenol-red-free culture medium was added. Then, cells were exposed with a LED or a NIR laser which emits light at 690 ± 5nm wavelength (BWF5-690-8-600-0.37; B&W TEK INC., Newark, DE, USA). The output power density in mW/cm2 was measured with an optical power meter (PM 100, Thorlabs, Newton, NJ, USA). For luciferase activity, 150 μg/mL D-luciferin-containing media (Gold Biotechnology, St. Louis, MO, USA) was administered to PBS-washed cells 1 h after NIR-PIT which were analyzed on a BLI system (Photon Imager; Biospace Lab, Paris, France),. Regions of interest (ROIs) were placed on each entire well, and the luciferase activity (photons/min) was then calculated using M3 Vision Software (Biospace Lab).
Animal and tumor models
All in vivo procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), US National Research Council, and approved by the local Animal Care and Use Committee. Six to eight week old female homozygote athymic nude mice were purchased from Charles River (NCI-Frederick, Frederick, MD, USA). During the procedure, mice were anesthetized with inhaled 3–5% isoflurane and/or via intraperitoneal injection of 1 mg of sodium pentobarbital (Nembutal Sodium Solution, Ovation Pharmaceuticals Inc., Deerfield, IL, USA). In order to determine tumor volume, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were measured with an external caliper. Tumor volumes were based on caliper measurements and were calculated using the following formula; tumor volume = length × width2 × 0.5. Body weight was also measured. Mice were monitored daily for their general health. The presence of skin necrosis or toxicity attributable to the anti-PSMA-IR700 was evaluated with the observation of skin color and general health, including weight loss and appetite loss. Tumor volumes were measured three times a week until the tumor volume reached 2000 mm3, whereupon the mice were euthanized with inhalation of carbon dioxide gas.
In vivo fluorescence imaging studies
PC3pip-luc cells (2 × 106) were injected subcutaneously in the right dorsum of the mice. Tumors were studied after they reached volumes of approximately 50 mm3. Serial ventral and dorsal fluorescence images of IR700 were obtained with a Pearl Imager using a 700 nm fluorescence channel before and 0, ½, 1, 2, 3, 4, 5, 6, 9, 12, 24, 48, 72, 96, 120, 144, and 168 hours after i.v. injection of 100 μg of anti-PSMA-IR700 via the tail vein. Pearl Cam Software (LICOR Biosciences, Lincoln, NE, USA) was used for analyzing fluorescence intensities. ROIs were placed on the tumor and liver. ROIs were also placed in the adjacent non-tumor region as background (left dorsum and lower abdomen). Average fluorescence intensity of each ROI was calculated. TBRs (fluorescence intensities of target/fluorescence intensities of background) were also calculated (n = 10).
In vivo NIR-PIT
PC3pip-luc cells (2 × 106) were injected subcutaneously in the right dorsum of the mice. Tumors were studied after they reached volumes of approximately 50 mm3. To examine the therapeutic effect of in vivo NIR-PIT on PC3pip-luc tumors, tumor-bearing mice were randomized into 4 groups of at least 10 animals per group for the following treatments: (1) no treatment (control); (2) 100 μg of anti-PSMA-IR700 i.v., no NIR light exposure (anti-PSMA-IR700 i.v.); (3) NIR light exposure only, NIR light exposure was administered at 50 J/cm2 on day 1 and 100 J/cm2 on day 2 (NIR light exposure); (4) 100 μg of anti-PSMA-IR700 i.v., NIR light exposure was administered at 50 J/cm2 on day 1 and 100 J/cm2 on day 2 after injection (NIR-PIT). These therapies were performed every week for up to 3 weeks. Serial fluorescence images, as well as white light images, were obtained using a Pearl Imager with a 700 nm fluorescence channel.
For in vivo BLI, D-luciferin (15 mg/mL, 200 μL) was injected intraperitoneally and the mice were analyzed on a BLI system (Photon Imager) for luciferase activity (photons/min). ROIs were set on the entire tumors to quantify the luciferase activities. ROIs were also placed in the adjacent non-tumor region as background. Average luciferase activity of each ROI was calculated. To clarify therapeutic effect luciferase activity of the tumor before NIR-PIT set to 100%.
Histological analysis
To detect the antigen-specific micro-distribution in the tumor, fluorescence microscopy was performed. Tumor xenografts were excised from mice without treatment, 24 h after injection of anti-PSMA-IR700 (anti-PSMA-IR700 i.v.) and 24 h after NIR-PIT. Fluorescence images, as well as white light images, of extracted tumors were obtained using a Pearl Imager with a 700 nm fluorescence channel. Then, extracted tumors were frozen with optimal cutting temperature (OCT) compound (SAKURA Finetek Japan Co., Tokyo, Japan) and frozen sections (10 μm thick) were prepared. Fluorescence microscopy was performed using the BX61 microscope with the following filters: excitation wavelength 590 to 650 nm, emission wavelength 665 to 740 nm long pass for IR700 fluorescence. DIC images were also acquired. To evaluate histological changes, light microscopy study was also performed using Olympus BX61. Extracted tumors were also placed in 10% formalin and serial 10 μm slice sections were fixed on glass slide with hematoxylin and eosin (H&E) staining.
Statistical analysis
Data are expressed as means ± standard error of mean (SEM) from a minimum of five experiments, unless otherwise indicated. Statistical analyses were carried out using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA). For multiple comparisons, a one-way analysis of variance (ANOVA) followed by the Tukey’s correction for multiple comparisons was used. The cumulative probability of survival based on volume (2000 mm3) were estimated in each group with a Kaplan-Meier survival curve analysis, and the results were compared with use of the log-rank test. Student’s t test was used to compare the treatment effects with that of control. A p-value of < 0.05 was considered statistically significant.
Results
In vitro characterization of PC3flu and PC3pip-luc cell lines
As defined by SDS-PAGE, anti-PSMA-IR700 and non-conjugated control anti-PSMA mAb showed an identical molecular weight, around 150 kDa, and fluorescence intensity was confirmed in the band of anti-PSMA-IR700 (Fig. 1A). After a 6 h incubation with anti-PSMA-IR700, PC3pip-luc cells showed high fluorescence signal, which was confirmed with flow cytometry (Fig. 1B) and fluorescence microscopy (Fig. 1C). On the other hand, PC3ful cells showed no fluorescence signal. Fluorescence signal in PC3pip-luc cells was completely blocked by adding excess anti-PSMA mAb (Fig. 1C), indicating that anti-PSMA-IR700 specifically binds to PSMA on the cells.
Figure 1. Confirmation of PSMA expression as a target for NIR-PIT in PC3 cells, and evaluation of in vitro NIR-PIT.

(A) Validation of anti-PSMA-IR700 by SDS-PAGE (left: Colloidal Blue staining, right: fluorescence). Diluted anti-PSMA mAb was used as a control. (B) Expression of PSMA in PC3flu and PC3pip-luc cells was evaluated by FACS. After 6 h of anti-PSMA-IR700 incubation, PC3pip-luc cells showed high fluorescence signal. Fluorescence in PC3pip-luc cells was completely blocked by adding excess PSMA. On the other hand, PC3flu cells showed no fluorescence signal. (C) Differential interference contrast (DIC) and fluorescence microscopy images of PC3flu and PC3pip-luc cells after incubation with anti-PSMA-IR700 for 6 h. High fluorescence intensities were shown in only PC3pip-luc cells. Necrotic cell death was observed upon excitation with NIR light (after 15min) in PC3pip-luc cells. Scale bars = 20 μm. (D) Bioluminescence imaging (BLI) of a 10 cm dish demonstrated that luciferase activity in PC3pip-luc cells decreased in a NIR-light dose-dependent manner. (E) Luciferase activity in PC3pip-luc cells was measured, which also decreased in a NIR-light dose-dependent manner (n = 5, *p < 0.05 vs. untreated control, **p < 0.01 vs. untreated control, by Student’s t test). (F) Membrane damage of PC3flu cells induced by NIR light exposure was measured with the dead cell count using propidium iodide (PI) staining. No membrane damage was observed in PC3flu cells after NIR light exposure. (G) Membrane damage of PC3pip-luc cells induced by NIR-PIT was measured with the dead cell count using PI staining, which increased in a light dose dependent manner (n = 5, **p < 0.01, vs. untreated control, by Student’s t test). There was no significant cytotoxicity associated with NIR light exposure alone in the absence of anti-PSMA-IR700 and with anti-PSMA-IR700 alone without NIR light exposure.
In vitro NIR-PIT
Immediately after exposure, NIR light exposure induced cellular swelling, bleb formation, and rupture of vesicles representing necrotic cell death was observed in PC3pip-luc cells (Supplementary Video). Most of these morphologic changes were observed within 15 min of light exposure (Fig. 1C), indicating rapid induction of necrotic cell death. On the other hand, PC3flu cells showed no obvious change after NIR light exposure. Bioluminescence showed a decrease of luciferase activity in a light dose dependent manner (Fig. 1D). BLI also showed significant decreases of luciferase activity in NIR-PIT treated cells (Fig. 1E). Based on incorporation of PI, although no NIR-PIT effects were shown in PC3flu cells (Fig. 1F), percentage of cell death increased in a light dose dependent manner in PC3pip-luc cells (Fig. 1G). Over 80% of PC3pip-luc cells died when exposed to 8 J of NIR light. There was no significant cytotoxicity associated with NIR light exposure alone in the absence of anti-PSMA-IR700 and with anti-PSMA-IR700 alone without NIR light exposure.
In vivo fluorescence imaging studies
The fluorescence intensity of anti-PSMA-IR700 in PC3pip-luc tumor showed high intensities within 1 day after anti-PSMA-IR700 injection but this decreased gradually over the following days (Fig. 2A, 2B). On the other hand, target-to-background ratio (TBR) of anti-PSMA-IR700 in tumor and liver was high immediately after APC injection, following which the TBR did not change for several days (Fig. 2C). TBR of anti-PSMA-IR700 was high in tumor due to specific APC binding to PSMA expressing PC3pip-luc cells, while TBR was high in liver likely due to non-specific accumulation of anti-PSMA-IR700 conjugate as the liver is not known to express PSMA. To obtain the maximal therapeutic effect, the tumor fluorescence caused by binding of the APC should be high in tumor and low in background. Tumor fluorescence was high after APC injection, while fluorescence signal of background including liver decreased beginning 9 hours after APC injection. Thus, we used 1 day of incubation with APC to get the maximal difference between tumor and background normal tissue.
Figure 2. In vivo fluorescence imaging of PC3pip-luc tumor.

(A) In vivo anti-PSMA-IR700 fluorescence real-time imaging of tumor-bearing mice (right dorsum). The tumor showed high fluorescence intensity after injection and the intensity was gradually decreased over days. Most of the excess agent was excreted to the urine immediately after injection. ROIs were placed on the tumor and liver, then ROIs were also placed in the adjacent non-tumor region as background (left dorsum; blue circle and lower abdomen; green circle). (B) Time course of NIR fluorescence signal of IR700 in tumors and livers (n = 10). The IR700 fluorescence intensity of tumor and liver showed high intensities within 1 day after anti-PSMA-IR700 injection but this decreased gradually over days. (C) Time course of NIR fluorescence signal of TBR in tumors and livers (n = 10). TBR of tumor and liver showed high within four days after anti-PSMA-IR700 injection, then the TBR was gradually decreased over the following days.
In vivo NIR-PIT
The treatment and imaging regimen is shown in Fig. 3A. One day after injection of anti-PSMA-IR700, the tumors showed higher fluorescence intensity than did the tumor with no anti-PSMA-IR700. After exposure to 50 J/cm2 of NIR light, IR700 tumor fluorescence signal decreased due to dying cells and partial photo-bleaching, while the IR700 fluorescence gradually decreased over the following days in tumors receiving anti-PSMA-IR700 but not NIR light exposure (Fig. 3B). NIR-PIT resulted in decreases in bioluminescence (Fig. 3C). Luciferase activity significantly decreased after NIR-PIT (p < 0.0001 vs. other control groups) (Fig. 3D). In contrast, luciferase activity of tumor in other control groups showed an increase due to rapid tumor growth. Tumor growth was significantly inhibited in the NIR-PIT treatment group compared with the other control groups (p < 0.001) (Fig. 3C), and significantly prolonged survival was achieved in the NIR-PIT group (p < 0.0001 vs other control groups) (Fig. 3D). Surprisingly, more than two thirds of tumors were cured with this regimen of NIR-PIT. No significant therapeutic effect was observed in the control groups, including those receiving anti-PSMA-IR700 i.v. only or in mice receiving NIR light exposure only. There was no skin necrosis or toxicity attributable to the anti-PSMA-IR700 in any group.
Figure 3. In vivo effect of NIR-PIT for PC3pip-luc tumor.
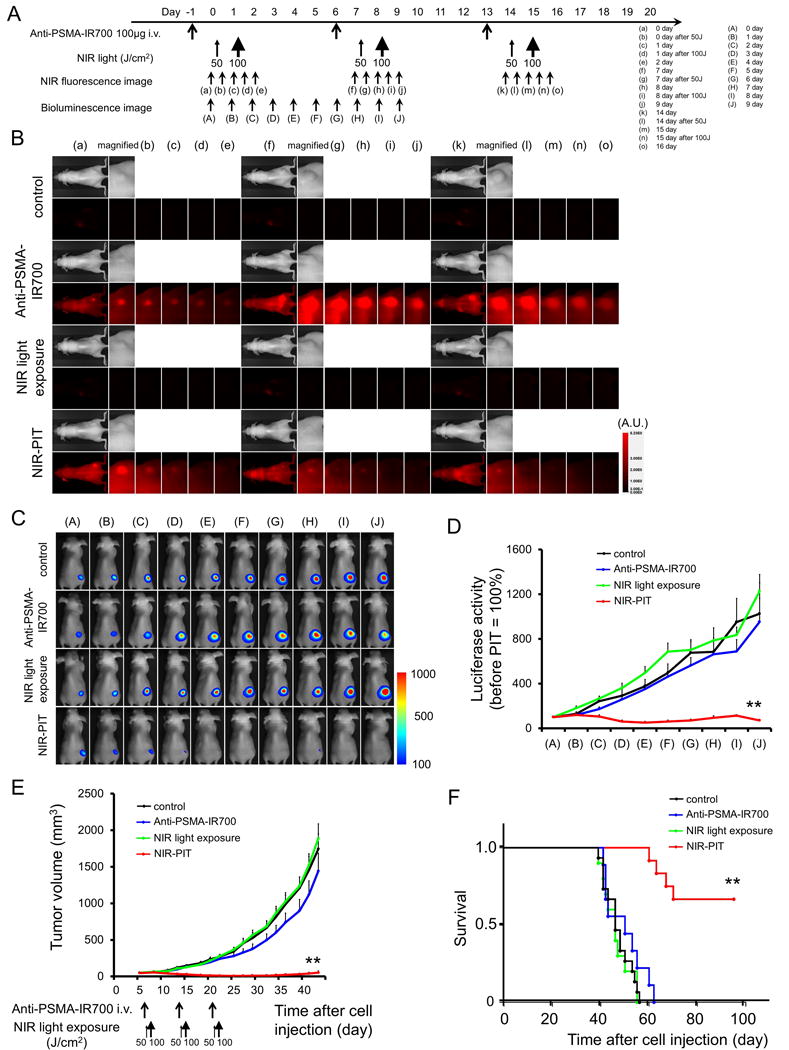
(A) NIR-PIT regimen. Fluorescence and bioluminescence images were obtained at each time point as indicated. (B) In vivo fluorescence real-time imaging of tumor-bearing mice in response to NIR-PIT. The tumor treated by NIR-PIT showed decreasing IR700 fluorescence after NIR-PIT. (C) In vivo BLI of tumor bearing mice in response to NIR-PIT. Before NIR-PIT, tumors were approximately the same size and exhibited similar BLI signals. The tumor treated by NIR-PIT showed decreasing luciferase activity after NIR-PIT. (D) Quantitative luciferase activity (before NIR-PIT is set to 100) showed a significant decrease in NIR-PIT tumors (n ≧ 10, **p < 0.001 vs. other groups, by Tukey’s test with ANOVA). Luciferase activity of tumor in other control groups showed an increase due to rapid tumor growth. (E) Tumor growth was significantly inhibited in the NIR-PIT treatment group with anti-PSMA-IR700 (n ≧ 10, **p < 0.001 vs other control groups, Tukey’s test with ANOVA). (F) Significantly prolonged survival was observed in the NIR-PIT treatment group with anti-PSMA-IR700 (n ≧ 10, **p < 0.0001 vs other control groups, by Log-rank test).
Histological Analysis
The treatment and imaging regimen is shown in Figure 4A. High fluorescence intensity was shown in tumors 24 h after anti-PSMA-IR700 injection compared with that in control tumors. The majority of fluorescence signal in tumors disappeared 24 h after NIR-PIT in resected tumor (Fig. 4B). In frozen histologic specimens, high fluorescence intensity was also shown in tumors 24 h after anti-PSMA-IR700 injection, and the signal decreased 24 h after NIR-PIT (Fig. 4C). H&E staining of NIR-PIT treated PC3pip-luc tumors revealed diffuse necrosis and micro-hemorrhage, with scattered clusters of live but damaged tumor cells, while no obvious damage was observed in the tumor receiving only anti-PSMA-IR700 but no light (Fig. 4D).
Figure 4. In vivo histological fluorescence distribution and histological NIR-PIT effect.

(A) The regimen of NIR-PIT and imaging is shown. (B) Fluorescence images of resected PC3pip-luc tumors. White light images (upper) and IR700 fluorescence image (lower). High fluorescence intensity was shown in PC3pip-luc tumor 24 h after injection of anti-PSMA-IR700, but the fluorescence decreased 24 h after NIR-PIT. (C) Differential interference contrast (DIC) and fluorescence microscopy images of PC3pip-luc tumor xenografts. High fluorescence intensity was shown in PC3pip-luc tumor 24 h after injection of anti-PSMA-IR700, but the fluorescence decreased 24 h after NIR-PIT. Scale bars = 100 μm. (D) Resected tumor stained with hematoxylin and eosin (H&E). A few scattered clusters of damaged tumor cells were seen within a background of diffuse cellular necrosis and micro-hemorrhage after NIR-PIT, while no obvious damage was observed after anti-PSMA-IR700 alone with NIR light exposure. White scale bars = 100 μm. Black scale bars = 20 μm.
Discussion
PSMA is the prototypic cell-surface marker of prostate cancer and provides an attractive target for mAb targeted therapies. The first mAb that received FDA approval for clinical use in prostate cancer was capromab pendetide, a murine, 111In-labeled, mAb (7E11) directed to PSMA (13, 30). However, results were disappointing since the mAb targeted an internal domain of PSMA (31, 32). Several mAbs targeting the external domain of PSMA are currently undergoing clinical investigation in prostate cancer. Though there was some potential clinical utility of radiolabeled mAb for imaging and diagnostic purposes (33), therapeutic radioisotopic labeling of the mAb have proven disappointing (7, 34).
The conjugate anti-PSMA-IR700 proved to be an effective agent for treating a PSMA expressing prostate cancer model with NIR-PIT. Unlike other mAb mediated therapies, NIR-PIT with anti-PSMA-IR700 led to rapid cell death in vitro and tumor growth reduction and survival improvement in vivo. Thus, anti-PSMA-IR700 could be an effective platform for NIR-PIT in PSMA expressing tumors. IR700 conjugation minimally alters the antibody due to the small size and hydrophilic nature of the IR700 dye (19). As a result, these APCs show high target accumulation with minimal distribution in normal tissue and minimal binding to non-target expressing cells. The effectiveness of NIR-PIT has been demonstrated in a variety of different tumor types with a variety of antibodies (19, 21–26, 35).
The anti-PSMA antibody-IR700 conjugate achieved adequate tumor TBRs as shown in Figure 2, indicating that it may be practical for clinical application during surgical, endoscopic or trans-needle procedures because of its high TBR on the PSMA expressing tumors. Efficient binding and distribution of the antibody in the tumor are important for APCs to be effective as agents for NIR-PIT. This also holds for antibody-toxin or antibody-drug conjugates since, to be effective, the drugs and toxins must be internalized after cell binding. Our results showed that anti-PSMA antibody bound to PSMA on cells specifically and was internalized within 6 hours of incubation in PSMA expressing cancer cells as shown in Figure 1. These results suggest that anti-PSMA antibody has favorable characteristics for an antibody-drug conjugate.
Rapid and massive cancer cell killing adjacent to tumor vessels with NIR-PIT leads to an immediate increase in vascular permeability after therapy (36–38). The delivery of various nano-sized or macromolecular drugs into a NIR-PIT treated tumor increases up to 24-fold compared with that in a control non-treated tumor immediately after the initial NIR light exposure, a phenomenon that is known as the super enhanced permeability and retention (SUPR) effect induced by NIR-PIT (36–38). After the first NIR-PIT, circulating APCs can enter the treated tumor bed with greater permeability and penetration than prior to NIR-PIT. There, they bind homogeneously to the surviving fraction of cancer cells (39). Therefore, the second exposure to NIR light can further enhance therapeutic effects of NIR-PIT (40). Thus, we chose the current therapeutic regimen with a single injection of the APC and two light exposures to maximize therapeutic effectiveness.
Fractionated dosing of the APC with repeated light exposure is also likely to increase effectiveness. For instance, in a model utilizing EGFR-targeted NIR-PIT repeated dosing of the APC and NIR light dosing was reported to improve the therapeutic effect compared to a single shot approach (40, 41). Therefore, we investigated this regimen of repeated APC and NIR light exposure. This proved to be an effective therapy for treating a PSMA expressing prostate cancer model. Looking forward to NIR-PIT use in humans, splitting the APC dose and using repeated light exposures will reduce toxicity of drugs because NIR light exposure by itself is harmless. Therefore, repeated NIR-PIT will optimize efficacy without increasing toxicity.
In cancer therapy, anticancer drugs often fail because heterogeneous cellular density and vascularity, increased interstitial pressure and other structural barriers imposed by the extracellular matrix prevent the drug from reaching its target in sufficient concentrations to be effective (42, 43). Moreover, naturally occurring tumors usually are phenotypically and functionally heterogeneous (44, 45). Repeated NIR-PIT with additional various types of APC or delivery of higher doses of non-targeted anticancer drugs by taking advantage of the SUPR effect are good strategies to improve the therapeutic effect of cancer (38).
Another aspect of NIR-PIT is its immunogenic nature. Cells treated with NIR-PIT undergo rapid volume expansion leading to rupture of the cell membrane and extrusion of cell contents into the extracellular space (20). Thus, NIR-PIT induces nearly immediate immunogenic cell death rather than apoptotic cell death which is induced by most other cancer therapies (46–48). As the immune effects of NIR-PIT are currently unknown it is difficult to know whether this will be of benefit to patients, although it is anticipated that immunogenic cell death will augment the therapeutic effect of NIR-PIT as it selectively kills target cells while non-target cells immediately adjacent, including effector immune cells, show no toxic effects (19). In one specific example, targeting of local regulatory T cells with NIR-PIT (35), induced a rapid antitumor immune activation and regression of the PIT-treated tumor. Moreover, NIR-PIT could enable activated cytotoxic effector cells to attack other tumors of a similar nature located distant from the NIR-PIT treated lesion.
While NIR-PIT shows highly selective cytotoxicity, and NIR light exposure can be easily applied to superficial tumors, an obvious limitation is the inability to deliver NIR light to the tumor located deep in the tissue such as bone. Skin, fat and other organs will absorb NIR light before it reaches the tumor. There are several potential solutions to this problem. For instance, NIR light exposure could be delivered to a tumor and to adjacent structures while the tissues are still exposed during a surgical resection, thus treating residual tumor in the tumor margin or in regional lymph nodes. Such procedures have been proposed in the past with PDT (49, 50); however, we believe that NIR-PIT would be much more effective with lower toxicity than PDT. Alternatively, fiber optic light probes could be placed within or nearby tumor using endoscopes, laparoscopes, catheters or image guided percutaneous needles. This would enable lesions such as prostate cancer recurrences or regional lymph nodes to be effectively treated as the depth of penetration of NIR light is at least 2 cm. For instance, it is entirely feasible that locally recurrent prostate cancer adjacent to the urethra could be treated with NIR-PIT via a transparent transuretheral catheter.
Conclusion
The fully human anti-PSMA antibody-IR700 conjugate showed accumulation in PSMA-expressing prostate cancer cells. Subsequent NIR-PIT using anti-PSMA-IR700 induced remarkable therapeutic responses after repeated NIR-PIT cycles in a PSMA-expressing prostate cancer and cured more than two third of cancers. Thus, NIR-PIT utilizing PSMA as the targeting antigen for the anti-PSMA-IR700might be successful new treatment modality for prostate cancer especially at diagnosis, initial treatment and at the time of locoregional recurrence in the pelvis.
Supplementary Material
Implications.
Near infrared photoimmunotherapy (NIR-PIT) using a fully human anti-PSMA-IR700 conjugate showed potential therapeutic effects against a PSMA-expressing prostate cancer that is readily translated to humans.
Acknowledgments
Grant Support
All authors were supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Abbreviations
- ANOVA
one-way analysis of variance
- ADCC
antibody dependent complement-mediated cytotoxicity
- APC
antibody-photo-absorber conjugate
- BLI
bioluminescence imaging
- DIC
differential interference contrast
- EGFR
epidermal growth factor receptor
- FDA
Food and Drug Administration
- H&E
hematoxylin and eosin
- IR700
IRDye700DX
- LED
light-emitting diode
- mAb
monoclonal antibodies
- NIR
near-infrared
- OCT
optimal cutting temperature
- PBS
phosphate buffered saline
- PDT
photodynamic therapy
- PI
propidium iodide
- PIT
photoimmunotherapy
- PSMA
prostate-specific membrane antigen
- ROI
regions of interest
- SEM
standard error of mean
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SUPR
super enhanced permeability and retention
- TBR
target-to-background ratio
- US
United States
Footnotes
Authors’ Contributions
Conception and design: T. Nagaya, P.L. Choyke, H. Kobayashi
Development of methodology: T. Nagaya, Y. Nakamura, H. Kobayashi
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): T. Nagaya, Y. Nakamura, S. Okuyama, F. Ogata, Y Maruoka, H. Kobayashi
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): T. Nagaya, Y. Nakamura, S. Okuyama, F. Ogata, Y. Maruoka, P.L. Choyke, H. Kobayashi
Writing, review, and/or revision of the manuscript: T. Nagaya, P.L. Choyke, H. Kobayashi
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): T. Nagaya, S. Okuyama, F. Ogata, P.L. Choyke, H. Kobayashi
Study supervision: H. Kobayashi
Conflict of interest: There is no conflict of interest to be claimed for all authors in this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Klein EA, Kupelian PA. Localized prostate cancer: radiation or surgery? Urol Clin North Am. 2003;30:315–30, ix. doi: 10.1016/s0094-0143(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulley J, Dahut WL. Chemotherapy for prostate cancer: finally an advance! Am J Ther. 2004;11:288–94. doi: 10.1097/01.mjt.0000133582.68709.e3. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 7.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2:1289–97. [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Leget GA, Czuczman MS. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10:548–51. doi: 10.1097/00001622-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Barinka C, Sacha P, Sklenar J, Man P, Bezouska K, Slusher BS, et al. Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein Sci. 2004;13:1627–35. doi: 10.1110/ps.04622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chatterjee S, Lisok A, Minn I, Pullambhatla M, Wharram B, et al. A PSMA-targeted theranostic agent for photodynamic therapy. J Photochem Photobiol B. 2016;167:111–6. doi: 10.1016/j.jphotobiol.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Bennett M, Thorpe PE. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. Prostate. 2004;61:1–11. doi: 10.1002/pros.20074. [DOI] [PubMed] [Google Scholar]
- 13.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–35. [PubMed] [Google Scholar]
- 14.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–11. [PubMed] [Google Scholar]
- 16.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 17.Wright GL, Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 18.Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:R61–109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 19.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–91. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa M, Tomita Y, Nakamura Y, Lee MJ, Lee S, Tomita S, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017 doi: 10.18632/oncotarget.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanaoka H, Nakajima T, Sato K, Watanabe R, Phung Y, Gao W, et al. Photoimmunotherapy of hepatocellular carcinoma-targeting Glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond) 2015;10:1139–47. doi: 10.2217/nnm.14.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Hodge JW, et al. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget. 2016 doi: 10.18632/oncotarget.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy of B-cell lymphoma. Mol Oncol. 2016;10:1404–14. doi: 10.1016/j.molonc.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaya T, Nakamura Y, Sato K, Zhang YF, Ni M, Choyke PL, et al. Near infrared photoimmunotherapy with an anti-mesothelin antibody. Oncotarget. 2016;7:23361–9. doi: 10.18632/oncotarget.8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Choyke PL, Kobayashi H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS One. 2014;9:e113276. doi: 10.1371/journal.pone.0113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe R, Hanaoka H, Sato K, Nagaya T, Harada T, Mitsunaga M, et al. Photoimmunotherapy targeting prostate-specific membrane antigen: are antibody fragments as effective as antibodies? J Nucl Med. 2015;56:140–4. doi: 10.2967/jnumed.114.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D, Hopf CE, Malewicz AD, Donovan GP, Senter PD, Goeckeler WF, et al. Potent antitumor activity of an auristatin-conjugated, fully human monoclonal antibody to prostate-specific membrane antigen. Clin Cancer Res. 2006;12:2591–6. doi: 10.1158/1078-0432.CCR-05-2107. [DOI] [PubMed] [Google Scholar]
- 28.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8. [PubMed] [Google Scholar]
- 29.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng KW. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69:1128–41. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn D, Williams RD, Seldin DW, Libertino JA, Hirschhorn M, Dreicer R, et al. Radioimmunoscintigraphy with 111indium labeled CYT-356 for the detection of occult prostate cancer recurrence. J Urol. 1994;152:1490–5. doi: 10.1016/s0022-5347(17)32453-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–34. [PubMed] [Google Scholar]
- 32.Troyer JK, Beckett ML, Wright GL., Jr Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232–42. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Wong WW, Schild SE, Vora SA, Ezzell GA, Nguyen BD, Ram PC, et al. Image-guided radiotherapy for prostate cancer: a prospective trial of concomitant boost using indium-111-capromab pendetide (ProstaScint) imaging. Int J Radiat Oncol Biol Phys. 2011;81:e423–9. doi: 10.1016/j.ijrobp.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 34.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999;14:99–111. doi: 10.1089/cbr.1999.14.99. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med. 2016;8:352ra110. doi: 10.1126/scitranslmed.aaf6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4:81–9. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano. 2013;7:717–24. doi: 10.1021/nn305011p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano K, Nakajima T, Choyke PL, Kobayashi H. The effect of photoimmunotherapy followed by liposomal daunorubicin in a mixed tumor model: a demonstration of the super-enhanced permeability and retention effect after photoimmunotherapy. Mol Cancer Ther. 2014;13:426–32. doi: 10.1158/1535-7163.MCT-13-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Kobayashi H. Improved micro-distribution of antibody-photon absorber conjugates after initial near infrared photoimmunotherapy (NIR-PIT) J Control Release. 2016;232:1–8. doi: 10.1016/j.jconrel.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H. Near-infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate. Bioconjug Chem. 2012;23:604–9. doi: 10.1021/bc200648m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaya T, Sato K, Harada T, Nakamura Y, Choyke PL, Kobayashi H. Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen. PLoS One. 2015;10:e0136829. doi: 10.1371/journal.pone.0136829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hambley TW, Hait WN. Is anticancer drug development heading in the right direction? Cancer Res. 2009;69:1259–62. doi: 10.1158/0008-5472.CAN-08-3786. [DOI] [PubMed] [Google Scholar]
- 43.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 44.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–5. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 45.Nowell PC. Mechanisms of tumor progression. Cancer Res. 1986;46:2203–7. [PubMed] [Google Scholar]
- 46.Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 47.Willingham MC. Cytochemical methods for the detection of apoptosis. J Histochem Cytochem. 1999;47:1101–10. doi: 10.1177/002215549904700901. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler U, Groscurth P. Morphological features of cell death. News Physiol Sci. 2004;19:124–8. doi: 10.1152/nips.01519.2004. [DOI] [PubMed] [Google Scholar]
- 49.Hiroshima Y, Maawy A, Zhang Y, Sato S, Murakami T, Yamamoto M, et al. Fluorescence-guided surgery in combination with UVC irradiation cures metastatic human pancreatic cancer in orthotopic mouse models. PLoS One. 2014;9:e99977. doi: 10.1371/journal.pone.0099977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pass HI, Temeck BK, Kranda K, Thomas G, Russo A, Smith P, et al. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol. 1997;4:628–33. doi: 10.1007/BF02303746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


