Abstract
Variation in regional identity, patterning, and structure of epidermal appendages contributes to skin diversity among many vertebrate groups, and is perhaps most striking in birds. In pioneering work on epidermal appendage patterning, John Saunders and his contemporaries took advantage of epidermal appendage diversity within and among domestic chicken breeds to establish the importance of mesoderm-ectoderm signaling in determining skin patterning. Diversity in chickens and other domestic birds, including pigeons, is driving a new wave of research to dissect the molecular genetic basis of epidermal appendage patterning. Domestic birds are not only outstanding models for embryonic manipulations, as Saunders recognized, but they are also ideal genetic models for discovering the specific genes that control normal development and the mutations that contribute to skin diversity. Here, we review recent genetic and genomic approaches to uncover the basis of epidermal macropatterning, micropatterning, and structural variation. We also present new results that confirm expression changes in two limb identity genes in feather-footed pigeons, a case of variation in appendage structure and identity.
Introduction
Vertebrates exhibit striking anatomical and functional variation in epidermal appendages, including teeth, mammary glands, and structures that cover or are embedded in the skin. The skin is an organism’s major interface with its environment and thus plays key roles in thermoregulation, protection against mechanical and pathogenic assault, osmoregulation, locomotion, camouflage, and communication within and among species. The epidermal appendages that adorn vertebrate skin are particularly important in these roles, and their enormous diversity reflects the breadth of functional and physiological demands in different lineages. Despite largely originating from the same embryonic tissues, the scales of fish and lepidosaurs, the glandular skin of amphibians, the hair of mammals, and the feathers of birds have vastly different mechanical and biological properties.
The cellular and tissue interactions that determine skin morphogenesis can be summarized as a three-stage process (Olivera-Martinez et al., 2004). First, a macropatterning stage determines the prospective epidermal appendage fields. Next, micropattern is established as epidermal appendage location and spacing within these fields is specified. Finally, the appendages themselves form and structural variation becomes apparent. Genetic, developmental, and evolutionary changes that affect these stages result in epidermal appendage variation both among and within major vertebrate lineages. In mammals, for example, hair is an insulative epidermal appendage that typically covers the skin, yet epidermal appendages have been modified into sensory whiskers, horns (e.g., rhinoceros), baleen, and other specialized structures in different species (Hieronymus et al., 2006).
Among vertebrates, epidermal appendages are perhaps most diverse in birds. Feathers serve physiological (insulation), locomotory (flight), and communication roles (structural and pigmentary variation), but these highly derived epidermal structures are not the only skin coverings in avian species. Birds typically have scaled feet, and many have featherless regions of skin, such as the heads and necks of turkey vultures and other carrion feeders. Thus, birds vary in epidermal appendage macropattern, or regional patterning of the skin. Micropattern, including organization of epidermal appendages within a scale or feather tract, varies within and among species as well, as do the final appendage structures that emerge from these tracts in an individual. For example, the large, pennaceous primary feathers on the wings of birds differ from contour and downy feathers of the body. The scales on bird feet are not uniform, either, as rhomboid scutellate scales armor the dorsal side of the foot while round reticulate scales are on the plantar surface, and scale morphology also shows positional differences within each of these broad categories (Lucas and Stettenheim, 1972; Prin et al., 2004). In summary, epidermal appendage patterning can be characterized by at least two levels of organization, plus structural variation among the appendages themselves.
Tissue recombination experiments and the importance of developmental timing
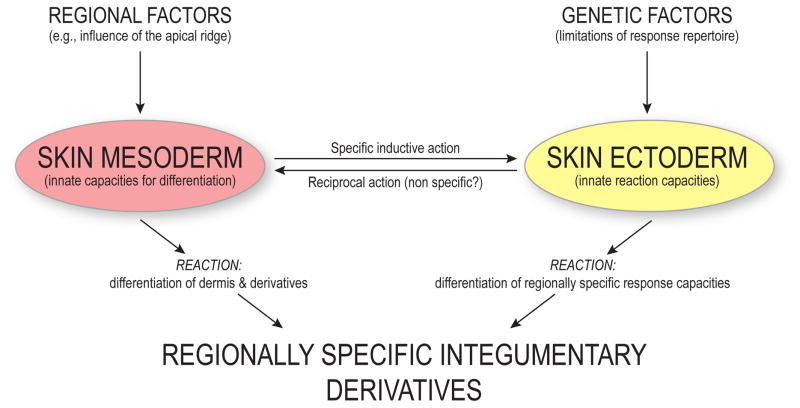
Foundational work by John Saunders, whose achievements we celebrate in this Special Issue, and his contemporaries showed that interactions between the embryonic mesoderm and ectoderm are critical for both macro- and micropatterning of the skin and its appendages (Figure 1). Saunders relied heavily on the domestic chicken as a model for this work because of the accessibility of embryos (availability as well as ease of surgical manipulations) and the phenotypic diversity among breeds and within individuals. In his words, the chicken has “a variety of regionally distinctive integumentary derivatives” such as feathers and scales of different types (Saunders, 1958). For example, the pennaceous feathers of the White Leghorn (tightly linked feather barbs) and Silkie (fluffy due to missing hooklets on barbules; Feng et al., 2014) breeds are so different that he could use feather morphology as a marker in his embryonic tissue transplant experiments (Cairns and Saunders, 1954). Saunders also recognized the value of embryonic tissue transplants between fore- and hindlimb buds to study the roles of the mesoderm and ectoderm in limb-specific epidermal appendage patterning: whichever tissue(s) conferred forelimb epidermal identity should predictably produce feathers when transplanted, and tissues bearing the hindlimb signal should produce scales (Cairns and Saunders, 1954).
Figure 1. Developmental factors and inductive interactions between embryonic mesoderm and ectoderm that determine regionally specific skin patterning.
Redrawn from Saunders (1958).
What developmental events determine patterning of avian skin? Saunders’s experiments led to several key advances in the developmental biology of epidermal appendages. Before revisiting his embryological work, it is useful to recall the context of his experiments. Prior to Saunders’s work, Lillie and Wang (Lillie and Wang, 1941; Wang, 1943) showed that the regional specification of feather morphology is a property of the epidermis. Epidermal tissue transplanted from the breast to saddle (dorsal body) region, for example, results in breast feathers on the saddle. In contrast, transplants of dermal papillae – mesoderm-derived, proliferative structures at the bases of the recessed feather follicles – from one body region to another produced feathers that were appropriate to the host site, not the donor body region. Together, these results showed that the adult epidermis has a distinct regional identity that obtains little or no positional information from the underlying dermis. However, these experiments did not reveal how the adult epidermis obtained this regionally specific information in the first place.
To determine the tissue-level signals that determined epidermal patterning, Saunders and Cairns (Cairns and Saunders, 1954; Saunders, 1947, 1948) performed a series of tissue recombination experiments in chicken embryos. Grafts of thigh mesoderm to the wing produced feathers in normally apterous (featherless) regions, and these ectopic feathers were morphologically similar to feathers of the thigh tract. The ectoderm that covered the graft came from the host region, demonstrating that the mesoderm provided instructive signals to form thigh feathers (Cairns and Saunders, 1954). Embryonic feather buds of the chick arise in a predictable sequence (Holmes, 1935), and these ectopic feathers erupted at the same time as normal thigh feathers, but earlier than the surrounding wing feathers. Thus, mesodermal signals impacted macropattern (feather tract location), micropattern (specific aspects of tract formation such as placode spacing), structural variation within a tract, and the developmental timing of feather eruption within a tract.
Embryological manipulations performed at different developmental time points yielded different outcomes, suggesting a sequence of events that determined skin appendage morphogenesis. Based on transplantation experiments of dermis and epidermis in embryonic chicken wings, Saunders and Gasseling (1957) concluded that delimitation of a feather tract location was the earliest event, followed by specification of feather identity (i.e., specific morphology within a tract) and the time of emergence of individual feathers. Next, the direction of outgrowth relative to the body axis was determined, and lastly, dorsoventral polarity was specified. Spatial distribution, sequence of emergence, and anteroposterior polarity of feathers could be modified by recombination and transplantation experiments, and transplants of “uncommitted” tissues at earlier stages (e.g., stages 17–19) resulted in fewer tract modifications than those done at later stages (e.g., stages 22–24). However, dorsoventral polarity always remained fixed (Saunders and Gasseling, 1957).
Transplants of distal hindlimb mesoderm also yielded dramatic outcomes. For instance, subapical mesoderm transplanted from leg to wing buds resulted in foot-like structures in the wing, including scaled and clawed digits (Cairns and Saunders, 1954; Saunders et al., 1959), further demonstrating that the identity of epidermal structures depends on mesoderm signaling during early embryogenesis. Subsequent work showed that the leg epidermis similarly relies on mesodermal induction and can potentially form either scales or feathers, depending on signals received from the dermis (Amprino and Camosso, 1959; Rawles, 1963; Sengel, 1958). The timing of these interactions is also critical: early in development, recombination of foot mesoderm with feather-forming ectoderm (or administration of retinoic acid) yields feathers, but scales are induced if these experiments are done after approximately day 12 of incubation (Dhouailly et al., 1980; Hughes et al., 2011; Rawles, 1963; Sengel, 1990). This temporal sensitivity likely corresponds to the timing of scale placode formation, whose fate becomes determined around day 12 (Dhouailly et al., 1980). Interestingly, experimentally-induced feathers on the feet of chickens typically form on scales rather than completely replacing them, suggesting that epidermal identity is not completely transformed (Dhouailly et al., 1980).
The dermis generally exerts a dominant signal over the epidermis to determine skin appendage identity, especially early in development (Sengel, 1990). Nevertheless, the ectoderm is an “active partner” in the specification of local epidermal identity (Cairns and Saunders, 1954), and dermal derivatives of the mesoderm (e.g., dermal papillae) cannot form properly without it (Saunders and Weiss, 1950; Weiss and Matoltsy, 1957). In a striking example of the primacy of ectodermal signaling, Zwilling (1955) showed that duck hindlimb mesoderm transplanted into chick embryos could induce digit webbing, but chick mesoderm could not inhibit webbing in ducks. These and other experiments confirm that epidermal appendage identity depends on more than signaling from the mesoderm alone, and that a timing-dependent conversation between the dermis and epidermis is critical for normal development (Dhouailly, 1973, 1975; Prin and Dhouailly, 2004; Saunders, 1958; Sengel, 1957).
Molecular approaches to skin development and diversity: developmental genetics and genomics
The classic embryology studies of the mid-20th century inspired decades of further research on mesoderm-ectoderm interactions to understand the evolution and development of epidermal appendages. Much of this work focused on the chick as a model system due to the remarkable regional variation in epidermal patterning within a single organism (Prin and Dhouailly, 2004; Rawles, 1963; Saunders, 1958). Classical embryological approaches were augmented by molecular developmental studies, including the discovery of specific genes involved in epidermal appendage development and disruption, most of which focus on particular genes or pathways of a priori interest and characterize their roles in development. Many excellent reviews summarize recent progress in this field, including a landmark volume edited by Chuong (1998). Other recent work and reviews tackle the molecular developmental basis of macropatterning of appendage distribution in different body regions (Chang et al., 2015; Lin et al., 2006), micropatterning determinants of epidermal placode spacing (Houghton et al., 2005; Hughes et al., 2011; Lin et al., 2009), and the evolution and development of structural variation among epidermal appendages within and among species (Chen et al., 2015; Dhouailly, 2009; Musser et al., 2015; Prin and Dhouailly, 2004; Prin et al., 2004; Sawyer et al., 2005).
A complementary and fruitful approach to understanding epidermal appendage development and variation is to use genomic mapping, which seeks to identify DNA sequence variants that are associated with different phenotypes. These approaches were once the purview of canonical model organisms in genetics and genomics, especially mice and humans, but recent precipitous drops in sequencing costs make association mapping possible in a variety of organisms. Following the lead of Saunders and others, some of these recent genomic studies have focused on the vast epidermal diversity within and among domestic avian species. Species such as chickens and pigeons were domesticated thousands of years ago and hundreds of breeds have been subjected to intense artificial selection to generate dramatic diversity in epidermal macropatterning, micropatterning, and appendage structure (Bartels, 2003; Domyan and Shapiro, 2016; Price, 2002). Despite the dramatic phenotypic differences that often characterize different breeds – including differences that resemble the magnitude of variation often observed among species or even genera – these strains are typically interfertile and genomically similar, thereby enabling traditional breeding experiments and whole-genome resequencing approaches to map the genes that underlie morphological diversity (Domyan and Shapiro, 2016). Due to genetic barriers to hybridization and genomic dissimilarity among species, similar approaches are usually not feasible for interspecific comparisons. Thus, domestic avian species have served for decades as critical models for understanding cell, tissue, and gene expression components of epidermal variation, and they can now also be used to explore the genomic basis of this diversity.
Genomic studies of epidermal appendage development and variation are potentially transformative for this field. Genomic mapping can home in on loci that are under selection for particular traits rather than examining a priori candidates. Once a causal genomic region is identified, small numbers of candidate genes and variant alleles can then be tested for their roles in development. Hence, these forward genetic approaches can potentially discover loci that control epidermal appendage variation among populations and species, and can nominate genes for further study that reverse genetic approaches might miss. Here, we review recent progress to uncover the genomic basis of epidermal appendage patterning and structure. We also report new gene expression data on two key determinants of limb identity, and thus epidermal identity, in domestic pigeons (Domyan et al., 2016).
Variation in epidermal macropatterning
Scaleless
A diverse collection of epidermal macropatterning phenotypes has resulted from intense artificial selection in domestic avian species, especially in chickens and pigeons. Perhaps one of the most dramatic examples of variation in macropatterning is the scaleless (sc locus) trait in chicken, a recessive mutation that causes a nearly complete lack of feathers and scales on the body due to a failure of placode formation during embryogenesis. For more than 50 years, researchers have utilized sc/sc mutant chickens as a model to study tissue interactions during skin development; dermal-epidermal recombination experiments using wild type and sc/sc tissue led to the discovery that the scaleless phenotype results from an inability of the epidermis to sustain a response to dermal patterning signals (Abbott and Asmundson, 1957; Brotman, 1977; Dhouailly and Sawyer, 1984; Houghton et al., 2007; Sengel and Abbott, 1963). Despite a strong mechanistic understanding of the developmental events that are altered during epidermal morphogenesis in sc/sc chickens, the molecular identity of sc was identified only recently. Using a genome-wide association mapping strategy, Wells et al. (2012) identified a single 1.25-Mb genomic region that was highly differentiated between sc/sc and sc/+ chickens. Subsequent targeted sequencing in this region demonstrated that a single nonsense mutation in FGF20 resided on all sc chromosomes, but was absent from 264 chromosomes from wild type chickens representing diverse breeds (Wells et al., 2012). The FGF20 mutation is predicted to result in a truncated protein that lacks the highly conserved C-terminal region.
To understand the functional role of FGF20 in skin development, Wells et al. (2012) examined its expression during embryonic development. They found that FGF20 mRNA is expressed in developing feather placodes and restricted to the epithelial component of the skin, corroborating the results of tissue recombination experiments that demonstrated that the sc/sc phenotype is caused by a defect in the epidermis (Brotman, 1977; Houghton et al., 2007; Sengel and Abbott, 1963; Wells et al., 2012). Although FGF signaling is known to play an important role in ectodermal appendage formation, the mechanism by which FGF20 influences epidermal macropatterning remains an open question.
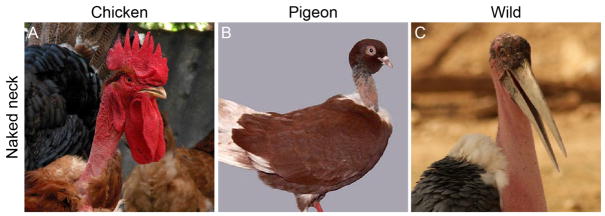
Naked neck
The Naked neck (Na locus) phenotype is another remarkable example of altered macropattern that is found in domestic avian species such as chicken and pigeon, and is mirrored in several wild bird species (Figure 2A–C) (Bartels, 2003; Mou et al., 2011). In contrast to the complete loss of ectodermal appendages in sc/sc chickens, the Na phenotype is characterized by loss of feathers specifically on the neck due to failed feather placode development (Classen and Smyth, 1977; Mou et al., 2011). To identify the genetic basis of Na in chickens, Mou and colleagues performed linkage analyses and recombination mapping to refine a previously identified causative locus to a 770-kb genomic region on chromosome 3 that contains five annotated genes (Mou et al., 2011; Pitel et al., 2000). No coding changes were found; however, a large insertion that was likely transposed from chromosome 1 was identified at the Na locus (Mou et al., 2011).
Figure 2. Variation in epidermal macropatterning in domestic and wild avian species.
(A–C) Naked neck phenotypes in the Transylvanian Naked Neck chicken (A), Romanian Naked Neck pigeon (B), and Mantou stork (C). Photo credit for A, Juan Komún; B, Dina Mergeani; C, Balaji.
Of the five candidate genes within the Na locus, only BMP12 was differentially expressed between wild type and Na embryonic skin (Mou et al., 2011). Importantly, differential BMP12 expression was not observed in internal organs from wild type and Na embryos, suggesting that the chromosome 1 insertion includes a cis-regulatory mutation that specifically increases skin expression of BMP12 (Mou et al., 2011). During avian epidermal development, other BMP molecules regulate feather bud formation (Chen et al., 2015; Harris et al., 2002; Harris et al., 2004); genetic mapping and follow-up developmental work show that BMP12 also functions as an inhibitor of feather tract formation (Mou et al., 2011). Interestingly, the magnitude of this inhibitory effect is not uniform throughout the embryo: wild type neck skin explants were more sensitive to BMP12 levels compared to body skin explants (Mou et al., 2011), suggesting that regional differences in BMP12 sensitivity confine the Na phenotype to the neck region in domestic chickens. This differential response might be due to regional differences in retinoic acid levels (Mou et al., 2011). With a molecular basis for the chicken naked neck phenotype in hand, it will be interesting to determine if altered BMP expression also causes naked neck-like phenotypes in wild avian species.
Variation in epidermal micropatterning
Head crests (pigeon)
Subsequent to the developmental determination of skin regions destined to become feathered, scaled or bare skin, the periodic micropatterning of epidermal appendages is established (Mou et al., 2011; Olivera-Martinez et al., 2004). Although the precise mechanisms of epidermal micropatterning remain poorly understood, evidence from a combination of embryological, molecular and computational modeling experiments indicates a requirement for reaction-diffusion (e.g., interactions between spatially expressed activating and inhibitory molecules) and planar cell polarity signaling (Chen et al., 2015; Chen and Chuong, 2012; Mou et al., 2011). In our lab, a genomic approach to understanding the basis of feathered head crests in domestic pigeons has provided new information about the molecular mechanisms that underlie epidermal micropatterning.
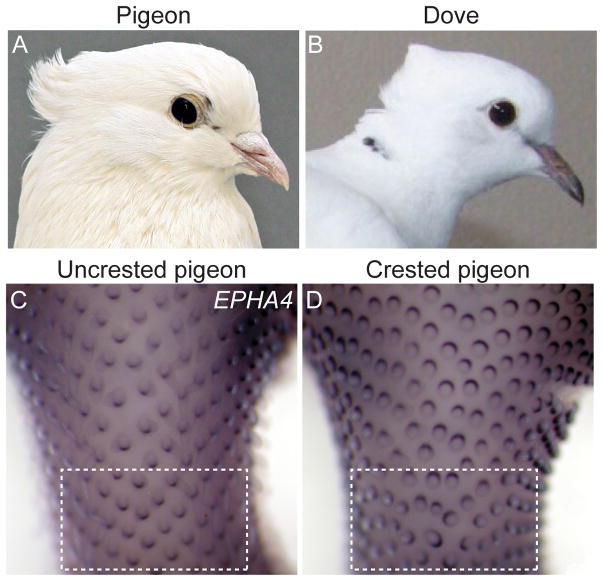
Many wild and domestic bird species display head crests, an ornamental feature in which feathers on the head and/or neck vary in length, structure or polarity of growth (Baptista et al., 2009; Bartels, 2003; Price, 2002). In both domestic pigeons and ringneck doves, crest (cr locus) is a recessive trait characterized by a regional reversal in growth polarity such that feathers grow in a cranial rather than caudal direction on the back of the head and neck (Figure 3A–B) (Levi, 1986; Shapiro et al., 2013; Vickrey et al., 2015). Examination of feather placode polarity in crested and uncrested domestic pigeon embryos suggests that disruption of epidermal polarity occurs early in development prior to feather outgrowth (Shapiro et al., 2013). In addition to altered polarity within individual feather placodes, feather placodes at the boundary of the crest-forming region are less organized (Figure 3C–D, and see figure 3 in (Shapiro et al., 2013). In the neck skin of uncrested pigeons, feather placodes are approximately equidistant from one another, as predicted by reaction-diffusion patterning mechanisms (Chen et al., 2015; Mou et al., 2011). In contrast, in crested pigeons, periodic patterning of feather placodes is often disrupted at the boundary that separates normal and abnormally polarized placodes. Together, these data indicate that an alteration in epidermal micropatterning contributes to pigeon head crests.
Figure 3. Head crests in domestic pigeons and ringneck doves are an example of epidermal micropattern variation.
(A) Indian fantail pigeon with peak crest. (B) Crested ringneck dove. (C–D) Developing feather placodes on the neck and occipital head in HH36 uncrested and crested pigeon embryos (modified from Shapiro et al., 2013). In uncrested pigeon embryos, feather placodes are precisely ordered, equally spaced, and generally form a diamond pattern. In crested pigeon embryos, feather placodes within the crest-forming region of skin are reversed in polarity relative to neighboring placodes, as indicated by localization of the polarity marker EPHA4. At the posterior border of the crest-forming region (outlined by a white box), placode patterning appears disorganized in crested pigeons. Photo credit for A, Sydney Stringham; B, modified from Vickrey et al. (2015).
To determine the genetic basis of head crests in domestic pigeons, whole genome sequences from a panel of crested and uncrested pigeons representing diverse breeds were used to calculate allele frequency differentiation and extended haplotype homozygosity – indicators of selection on a genomic region – across the pigeon genome (Shapiro et al., 2013). In addition, we searched for non-synonymous coding changes associated with this phenotype. Together, these analyses pointed to a single SNP within the coding sequence of the receptor tyrosine kinase gene Ephrin receptor B2 (EPHB2) that was highly differentiated between crested and uncrested pigeons (Shapiro et al., 2013). EPHB2 is a member of the Ephrin family of receptor tyrosine kinases that regulate a diverse set of developmental processes, including tissue patterning, morphogenesis, and feather development. However, the role of EPHB2 in epidermal morphogenesis and feather development is not well understood (Suksaweang et al., 2012). The EPHB2 SNP in pigeon (crpigeon allele) causes a nonsynonymous mutation that is predicted to result in an arginine to cysteine amino acid substitution in the EPHB2 intracellular kinase domain. This change, in turn, likely disrupts ATP catalysis and abrogates kinase activity (Shapiro et al., 2013).
As noted above, some domestic ringneck doves display head crests that are phenotypically similar to the pigeon head crest. Interspecies complementation tests between crested ringneck doves and crested pigeons produce hybrid offspring with head crests (Miller and Demro, 2011), suggesting a similar genetic basis for the cr phenotype in these two species. To test this possibility, Vickrey et al. (2015) compared DNA sequences from the kinase-encoding domain of EPHB2 of crested and uncrested ringneck doves and pigeons. Intriguingly, crested doves harbor a single nucleotide substitution in this region, but at a different site than the crpigeon allele. The crested dove SNP (crdove allele) is located upstream of the crpigeon mutation and associates nearly perfectly with the head crest phenotype (one exception was likely due to incomplete penetrance) (Vickrey et al., 2015). Like the crpigeon allele, crdove is predicted to cause an amino acid substitution that alters the EPHB2 catalytic site, thus abrogating kinase function (Vickrey et al., 2015). Functional assays performed in bacteria demonstrated that both the crpigeon and crdove mutations reduce or inhibit EPHB2 function (Vickrey et al., 2015). In summary, different mutations in the same gene in two species underlie similar epidermal micropatterning phenotypes. In addition, the cr phenotype in columbids (pigeons and doves) illustrates that variation in epidermal micropatterning can include alterations in placode dorso-ventral polarity, a change that Saunders and colleagues never observed in tissue grafting experiments (Saunders and Gasseling, 1957).
Head crests are also observed in certain breeds of chicken and duck. Despite a shared nomenclature, head crest morphology is distinct in chickens and ducks relative to the columbids described above; in chickens and ducks, head crests are composed of elongated feathers on the top of the head that have normal growth polarity. Perhaps not surprisingly, a different genetic mechanism is implicated in chicken head crests, as described below.
Variation in epidermal appendage structure
While much of epidermal appendage variation in birds is attributable to changes in macropattern or micropattern, in many instances epidermal appendage structure itself is the source of variation (Figure 4). In birds, scales and feathers serve distinct physiological functions and are an example of striking variation in epidermal appendage structure. In addition, in many wild bird species, eye-catching variations in feather length or shape are part of extraordinary male displays that play an important role in sexual selection. Here, we focus our discussion on several examples of variation in epidermal appendage structure and morphology that have resulted from long-term selective breeding in domestic avian species. In addition to structural variation, several recent studies elucidate the genetic and genomic basis of plumage color variation in avian populations, and this important topic is addressed elsewhere (Bed’hom et al., 2012; Domyan et al., 2014; Gunnarsson et al., 2011; Hellström et al., 2010; Lopes et al., 2016; Mundy et al., 2016; Oh et al., 2016).
Figure 4. Variation in feather structure in domestic and wild avian species.
(A–C) Silky feathers are present on the bodies of Silkie chickens (A) and silky fantail pigeons (B), as well as some wild avian species, including the emu (C). (D–F) Curled feathers are present on regions of the body in Frizzle chickens (D), Frillback pigeons (E) and some wild avian species, including on the head of the yellow-knobbed curassow (F). (G–I) A variety of domestic and wild avians display foot feathering (ptilopody), including the Cochin chicken breed (G), fairy swallow pigeon (H), and the snowy owl (I). Photo credit for A, sandyseek; B, Kathleen Winder; C, Benjamint444; D, Celeste Tittle; E, Los Angeles Pigeon Club; F, Jim Capaldi; G, Deann Barrera; H, Sydney Stringham; I, Rachel Jacklyn Bilodeau.
Head crests (chicken)
Despite being described with the same nomenclature, the chicken crest (Cr) phenotype is distinct from pigeon head crests at both the morphological and genetic level. As described above, pigeon and dove head crests are recessive traits in which feather polarity is reversed. In contrast, the chicken Cr phenotype is an incompletely dominant trait in which a small cluster of feathers on the top of the head is elongated, but these feathers grow with the same polarity as other body feathers (Bartels, 2003; Wang et al., 2012). Archaelogical evidence and written records suggest that Cr was one of the earliest differentiated phenotypes within domestic chicken breeds (Brothwell, 1979). Today, the Cr trait characterizes diverse chicken breeds from all over the world.
Genetic mapping experiments placed the Cr locus within a ~900-kb linkage group, but the causative gene(s) remained unknown (Kerje, 2004; Wang et al., 2012). To determine the molecular identity of Cr, Wang et al. (2012) employed comparative genomic analyses, as well as genome-wide mapping in populations derived from crested and uncrested breeds of chicken. These approaches identified two SNPs near HOXC8 that showed no recombination with Cr. Although the precise mutation that underlies Cr was not confirmed in this study, Cr is probably a cis-acting regulatory mutation that alters the spatial expression pattern of HOXC8, as HOXC8 mRNA was detected in the cranial skin of crested chickens but not wild type chickens throughout embryonic development (Wang et al., 2012). This misexpression has the phenotypic consequence of transforming head feathers into body-like feathers, and underlying structures are affected as well. In particular, Cr is associated with cranial hernias that result from incomplete closure of the dorsal skull (Brothwell, 1979; Fisher, 1934; Wang et al., 2012). Interestingly, several breeds of crested domestic duck also display abnormal skull and brain morphology (Frahm et al., 2001). Identification of the genetic basis of head crest in domestic ducks would directly test whether these morphological similarities are rooted in mutations in similar genes in these two distantly related avian species.
Silky-feather
In chickens, the silky-feather phenotype is characterized by the replacement of contour feathers with soft feathers composed of unconnected barbs on the body of juvenile and adult birds (Figure 4A) (Bartels, 2003). Silky-feather was first described by Marco Polo in the 13th century and has since become fixed in several chicken breeds, including the modern Silkie breed (Feng et al., 2014). Classical genetics experiments demonstrate that silky-feather is caused by a single recessive allele at the hookless locus (Dunn and Jull, 1927). In birds homozygous for hookless, silky-feather results from a failure in hooklet formation, the structure responsible for holding feather vane barbs together in pennaceous feathers (Feng et al., 2014). To determine the molecular basis of silky-feather, Feng et al. (2014) employed SNP association mapping and identity-by-descent (IBD) analysis to refine a locus previously identified by QTL mapping. Additional whole-genome analyses of unrelated Silkie chickens identified a single shared 21.7-kb haplotype that overlapped completely with the candidate region in the F2 mapping population (Feng et al., 2014).
Within the minimal haplotype, a single C to G transversion (called PDSS2(-103C-G)) was perfectly associated with silky-feather (Feng et al., 2014). PDSS2(-103C-G) is located immediately upstream of the transcription start site of Prenyl (decaprenyl) diphosphate synthase subunit 2 (PDSS2), which encodes an enzyme required for Coenzyme Q biosynthesis and mitochondrial respiration. The PDSS2(-103C-G) mutation causes a reduction in PDSS2 expression in embryonic and postnatal skin, presumably through a disruption in transcription factor binding affinity at the PDSS2 promoter (Feng et al., 2014). The role of PDSS2 and mitochondrial respiration in feather development remains an open question. The PDSS2(-103C-G) haplotype is present in all silky-feather chicken breeds, suggesting a single origin of the phenotype, followed by the spread of this trait via introgression among breeds (Feng et al., 2014).
The silky-feather phenotype is not unique to chickens. As noted by Darwin (1868), certain breeds of domestic pigeon such as the fantail, as well as some ringneck doves, display a phenotype reminiscent of chicken silky-feather (Figure 4B). However, in these two species of columbid, the silky trait is likely the result of a different mutation, as distinct modes of inheritance and feather morphology are observed in silky pigeons and doves relative to silky-feather chickens (Feng et al., 2014; Sell, 2012; Yokouchi et al., 1991). Identifying the genetic underpinnings of silky in pigeons and doves could provide new clues about the range of molecular pathways that regulate feather morphogenesis across distantly related avian species.
Frizzle
Variation in feather structure is also observed in Frizzle chickens, in which the adult contour feathers curl outward and upward to give birds a characteristic “frizzled” appearance (Figure 4D) (Bartels, 2003; Ng et al., 2012). Frizzle has been selected in a variety of domestic chicken breeds and this phenotype is caused by a single incompletely dominant factor that causes abnormal feather rachis structure (Hütt, 1930; Landauer and Dunn, 1930). To determine the molecular origins of Frizzle, Ng et al. (2012) performed linkage analysis using whole-genome SNP data from a series of half-sibling crosses and found a single haplotype containing a large keratin gene cluster that was associated with the phenotype. Considering the critical structural roles of keratin proteins in feathers, Ng et al. searched for mutations in 14 candidate keratin genes and discovered a single variant in the α-keratin gene KRT75 coding sequence that segregated perfectly with Frizzle in the mapping population. The variant, an 84-bp deletion in KRT75 that activates a cryptic splice site and causes a 23-amino-acid deletion in a conserved region of the K75 protein, was also present in additional unrelated Frizzle chickens but was never found in wild type birds. The spatiotemporal expression pattern of KRT75 is not altered in Frizzle embryonic or adult feathers, suggesting that the curved rachis that defines the Frizzle phenotype is the result of a defect in K75 protein function. Retroviral-mediated misexpression of mutant KRT75 in regenerating feather follicles produced feathers with a curved rachis (Ng et al., 2012), thus providing functional evidence that the KRT75 coding mutation is the causative mutation in Frizzle chickens.
As is the case for the silky-feather phenotype, curled feathers are not unique to Frizzle chickens. Among domestic pigeons, the Frillback breed displays unusual curled feathers, particularly on the wing shields, back and feet (Figure 4E) (Levi, 1986). The results of breeding experiments performed in the 1930s suggest that Frillback is an incomplete dominant trait caused by two non-allelic genes, designated Cu1 and Cu2 (Sell, 2012). Although the identity of the Cu genes is not known, comparative analysis of keratin gene sequences from Frillback and wild type pigeon breeds could provide a reasonable starting point to discover the genetic basis of feather curling in Frillback pigeons. Identification of the Cu genes will provide another direct test of whether the same or different molecular mechanisms produce similar feather structural variation in different species.
Ptilopody
Avian foot feathering (ptilopody) is another important example of variation in epidermal appendage structure (Figure 4G–I) (Lucas and Stettenheim, 1972; Rawles, 1960; Sawyer et al., 2005). Although the distal hindlimb of most modern wild birds is scaled, several species (e.g., ptarmigan, snowy owl, golden eagle) display heavily feathered feet (Figure 4I). Likewise, in some breeds of domestic pigeon and chicken, the scaled epidermis covering the metatarsus and toes is replaced by feather-forming epidermis (Figure 4G–H) (Bartels, 2003). Interestingly, paleontological evidence suggests that feathered feet represent the ancestral epidermal appendage in birds and their immediate dinosaurian relatives, and that scutellae on the dorsal foot are actually a derived feature in modern birds (Foth et al., 2014; Godefroit et al., 2014; Hu et al., 2009; Prin and Dhouailly, 2004; Xu et al., 2003; Zheng et al., 2013). Developmental manipulations, too, point to a “ubiquitous feather message” (Dhouailly, 1978) in the development of avian integument, and disruption of this signal could be necessary for scale formation (Dhouailly et al., 1980). Indeed, foot feathers are often associated with scales during normal development in chickens, and recombination experiments demonstrate that the dermis of the distal hindlimb can transmit both feather and scale-inducing signals early in development (Rawles, 1963).
Extensive classical breeding experiments in both pigeons and chickens have demonstrated that foot feathering is controlled by a relatively small number of genetic loci of large effect (Doncaster, 1912; Hollander, 1937; Levi, 1986; Somes, 1992; Wexelsen, 1934). In more recent work, Dorshorst et al. (2010) and Sun et al. (2015) used SNP-phenotype associations to identify genomic regions associated with a variety of morphological features in chickens, including ptilopody. Dorshorst et al. (2010) identified one quantitative trait locus (QTL) of major effect for ptilopody in Silkie chickens, while Sun et al. (2015) identified two major QTL and two minor QTL in an F2 population derived from Beijing-You and commercial broiler chickens. Both studies identified a QTL on chromosome 13 that has a major effect on foot feathering in chickens, raising the possibility that a shared molecular mechanism contributes to the trait in different breeds, but the molecular identity of this and other ptilopody loci in chickens remains unknown.
To better understand the mechanisms that regulate the decision between scale and feather fate in the developing limb epidermis, we recently combined classical genetic, developmental, and genomic approaches to identify the molecular basis of foot feathering in domestic pigeons (Domyan et al., 2016). Two genomic loci of major effect were identified in a QTL mapping experiment using an F2 population derived from feather-footed and scale-footed founder breeds (Domyan et al., 2016). In parallel, probabilistic whole-genome scans of allele frequency differentiation (pFst) were performed on a panel of scale-footed and feather-footed pigeons representing diverse breeds. pFst analyses led to the identification of two genomic regions that are strongly associated with foot feathering and overlap with the two major QTL identified in the mapping cross (Domyan et al., 2016). Thus, independent QTL mapping (one feather-footed breed) and whole-genome sequencing approaches (many breeds) converged on the same two loci.
One of the differentiated regions contains PITX1, which encodes a homeodomain protein that is a critical determinant of hindlimb identity and outgrowth (Duboc and Logan, 2011; Logan and Tabin, 1999; Szeto et al., 1999). The second region contains TBX5, a gene that encodes a T-box transcription factor required for forelimb outgrowth and identity (Duboc and Logan, 2011; Hasson et al., 2007; Hasson et al., 2010; Minguillon et al., 2005; Rallis et al., 2003; Rodriguez-Esteban et al., 1999; Takeuchi et al., 1999). Analyses of PITX1 and TBX5 expression in the hindlimb of scale-footed and feather-footed pigeon embryos revealed that PITX1 expression is reduced in feather-footed hindlimbs relative to scale-footed hindlimbs, while TBX5 – which is normally expressed in forelimb but not hindlimb mesenchyme – is ectopically expressed in feather-footed hindlimbs (Domyan et al., 2016). Allele-specific expression assays demonstrated that both of these effects are the result of cis-regulatory mutations (Domyan et al., 2016). In addition to a change in epidermal appendage fate, cis-regulatory mutations in PITX1 and TBX5 cause abnormal skeletal, muscle, and tendon patterning in feather-footed pigeon breeds, suggesting widespread effects on mesoderm derivatives and signaling (Domyan et al., 2016).
Together, reduced PITX1 and ectopic TBX5 expression confer a partial transformation of the hindlimb to a forelimb-like identity in feather-footed pigeons, demonstrating that, at least in domestic pigeons, epidermal appendage variation can occur through a fundamental change in regional developmental fate. This study also suggests that TBX5 is potentially under more modular cis-regulatory control in the limbs than was previously thought, and that modular expression changes can have localized effects on limb morphology (Domyan et al., 2016).
Although the molecular identities of the chicken foot feathering genes are unknown, PITX1 and TBX5 are located within two of the chicken ptilopody QTL identified by Dorshorst et al. (2010) and Sun et al. (2015). To determine if misregulation of PITX1 and TBX5 also occurs in feather-footed chickens, and therefore represents molecular convergence of foot feathering mechanisms in chickens and pigeons, we examined the expression patterns of PITX1 and TBX5 in the embryonic hindlimb of two feather-footed chicken breeds (Cochin and Silkie). In embryos from both feather-footed breeds, TBX5 is ectopically expressed in the developing hindlimb in a spatially restricted domain similar to that observed in feather-footed pigeons (Domyan et al., 2016). We did not observe a change in hindlimb PITX1 expression at the developmental stages examined (Domyan et al., 2016); however, it is possible that in feather-footed chickens, PITX1 expression levels are altered at a different developmental timepoint than in pigeons. Follow-up work in both species at additional developmental stages represents an important avenue of future research.
PITX1 expression is reduced in grouse-footed pigeon
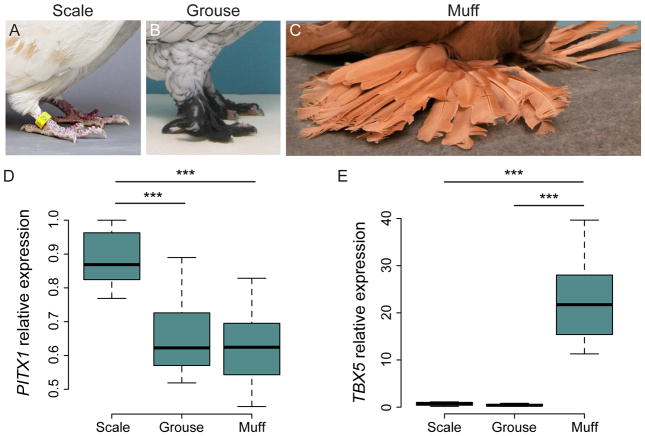
Among feather-footed pigeon breeds, the amount and type of feathers that form on the foot is variable (Figure 5). Classical breeding experiments have demonstrated that two loci produce distinct foot feathering phenotypes: the grouse phenotype (gr locus), present in several pigeon breeds, is a recessive trait characterized by short feathers that tightly cover the metatarsus and toes (Figure 5B). Alternatively, the Slipper phenotype (Sl locus), which is observed almost exclusively in just two breeds (English and Pigmy pouters), is incompletely dominant and displays feathers on the metatarsus and toes. In combination, gr and Sl produce the synergistic muff phenotype (Figure 5C), in which extensive feathering covers the hindlimb and long flight-like feathers decorate the posterior toes (Doncaster, 1912; Hollander, 1937; Levi, 1986; Wexelsen, 1934).
Figure 5. PITX1 expression is reduced in the embryonic hindlimb of grouse-footed pigeons.
(A–C) Foot feathering phenotypes in domestic pigeon breeds. The metatarsus and foot of most pigeon breeds are covered with scaled epidermis (A). The grouse phenotype (B) is characterized by short feathers that tightly cover the metatarsus and digits. Pigeons with the muff phenotype (C) display extensive feathering on the hindlimb, as well as long flight-like feathers on the posterior toes. (D–E) qRT-PCR analyses of PITX1 and TBX5 expression in HH25 pigeon hindlimb buds. Boxes span 1st to 3rd quartiles, bars extend to minimum and maximum values, black line denotes median. For scale-footed embryos, n=4 sets of hindlimb buds, grouse n=4, muff n=10. *, p<0.05; **, p<0.01; ***, p<0.001. Photo credit for A, Sydney Stringham; B–C, modified from (Stringham et al., 2012).
Our previous work provided genomic evidence that the cis-regulatory mutation affecting PITX1 expression is gr, while the mutation that causes ectopic TBX5 in the hindlimb is the Sl allele (Domyan et al., 2016). However, due to unavailability of embryos from grouse breeds, we were previously only able to show that expression of both PITX1 and TBX5 is altered in muff embryos. Nevertheless, we predicted that hindlimbs of embryos with only gr (but not Sl) alleles should only display a decrease in PITX1 and not ectopic expression of TBX5. To test this prediction, we recently obtained embryos from the grouse-footed Oriental Frill breed and measured PITX1 and TBX5 expression in Hamburger-Hamilton stage 25 (HH25; Hamburger and Hamilton, 1951) hindlimb buds by qRT-PCR. Consistent with our model (Domyan et al., 2016), PITX1 expression is reduced to a level similar to muff embryonic hindlimbs, while TBX5 is not ectopically expressed (Figure 5D–E). Together, these data confirm that gr is associated with a cis-regulatory change in PITX1 alone, while muff results from changes in both PITX1 and TBX5. Although the expression levels of PITX1 and TBX5 in hindlimbs of Sl embryos remain an open question, we predict that Sl is associated with an expression change only in TBX5.
Muffs and beard
The Muffs and beard (Mb) trait is found in a variety of chicken breeds and is characterized by small tufts of elongated feathers that surround the face and beak (Bartels, 2003; Guo et al., 2016). By combining genetic mapping, genomic association tests, and IBD analyses, Guo et al. (2016) identified a 48-kb region as the Mb candidate locus. Within this genomic interval, Guo et al. discovered and characterized a complex genomic structural variant (SV) composed of three unique copy number variants (CNVs), which they predicted was the underlying cause of the Mb phenotype. The Mb SV includes seven annotated genes; to determine which of these genes underlies the Mb phenotype, Guo et al. performed quantitative expression analyses in dorsal and facial skin from wild type (+/+), Mb/+, and Mb/Mb chickens at three developmental stages (embryonic, postnatal, and adult). Some degree of differential expression was observed for five of the genes within the Mb SV. However, differential expression of HOXB8 was the most pronounced at all developmental stages and correlated best with Mb genotype, suggesting that ectopic HOXB8 expression in facial skin causes elongated feathers near the face and beak of Mb chickens (Guo et al., 2016). Although further work is required to determine the specific functional consequence of ectopic HOXB8 expression in neck skin, this study demonstrates that complex genomic SVs can contribute to epidermal appendage variation.
Coincidentally, both Mb and Cr phenotypes in chickens are the result of mutations that cause ectopic expression of HOX gene products in regions of the developing skin where elongated feathers will form. Perhaps this is not surprising considering that spatially restricted HOX gene expression is critical for conferring regional body plan identity during embryonic development. Therefore, just as expression changes in limb identity genes are associated with the replacement of scaled with feathered epidermis in pigeons (Figure 5; Domyan et al., 2016), expression changes in HOX genes can result in epidermal appendages with ectopic regional identities.
Initial glimpses into regulatory pathways
The developmental basis of regional specificity and structure of epidermal appendages represents a longstanding biological problem that intrigued Saunders and his contemporaries. In combination with the examples described above, extensive embryological experiments have provided important information about specific genes involved in epidermal patterning (reviewed in Chen et al., 2015 and Chuong, 1998). However, the molecular pathways that regulate epidermal appendage variation remain poorly understood. To identify regulatory pathways involved in the feather vs. scale fate decision, Chang et al. (2015) compared transcriptome microarray datasets from feather-forming dorsal skin and scale-forming metatarsal skin harvested from chicken embryos at two developmental stages associated with the uncommitted or committed state. The authors used cosine similarity analysis to identify genes that are differentially expressed in feather vs. scale forming epidermis and mesenchyme (Chang et al., 2015). In addition to the identification of genes with known roles in epidermal development, the authors discovered that genes involved in calcium signaling are significantly upregulated in developing scale-forming epithelium relative to feather-forming epithelium (Chang et al., 2015). Although substantial functional experimentation is required to define the role of calcium signaling in the feather vs. scale fate decision, this research highlights the prospect of using genome-wide gene expression analyses to elucidate the molecular networks that regulate epidermal appendage fate.
Discussion
Millennia of selective breeding have produced stunning variation in epidermal appendage patterning and structure in domestic birds (Bartels, 2003; Darwin, 1859, 1868; Price, 2002). Hobbyists maintain diverse breeds of birds that are equivalent to mutant lines of mice, zebrafish, or other canonical laboratory organisms, and these avian resources are largely untapped as models for the molecular basis of variation. Saunders and others recognized the value of domestic chickens as embryological models to understand epidermal development, and modern molecular genetics and genomics are enabling discoveries of the specific genes and mutations that underlie classical feather, scale, and skin traits in this species. The same molecular techniques are allowing similar discoveries in other non-canonical model species such as domestic pigeons.
Thus far, the results of genomic studies of skin variation in birds largely corroborate results from classical embryological experiments. For example, tissue recombination experiments indicated that the primary defect in scaleless chickens is epidermal (Sengel and Abbott, 1963), and genomic analyses point to epidermally-expressed FGF20 as the causative mutation in this phenotype (Wells et al., 2012). Likewise, classical experiments determined that the modified hooklets of silkie chicken feathers was an epidermal defect (Cairns and Saunders, 1954; Danforth, 1929), and this is supported by the identification of a mutation in PDSS2, which is expressed in feather epithelium (Feng et al., 2014).
Similarly, developmental properties attributed to the mesoderm are linked to mesodermally-expressed genes. In the case of ptilopody, numerous tissue recombination and gene misexpression experiments previously demonstrated that mesoderm-derived dermis plays an instructive role in the decision between scaled or feathered epidermis. Consistent with these findings, genetic crosses and genomic mapping approaches implicate the mesodermally-expressed transcription factors PITX1 and TBX5 in feather-footed domestic pigeons and chickens (Domyan et al., 2016). The head crests of certain chicken breeds probably result from a re-specification of cranial to dorsal body skin identity, and this phenotype is linked to ectopic expression of HOXC8, a mesodermally-expressed transcription factor (Kanzler et al., 1997; Wang et al., 2012) (HOXB8, which is linked to the muffs and beard phenotype in chickens, is expressed in both mesodermal and ectodermal skin derivatives; Guo et al., 2016; Hughes et al., 2011). However, the pigeon head crest maps to EPHB2, which is expressed throughout the skin mesenchyme and at the mesenchymal-epithelial interface (Suksaweang et al., 2012). Thus, even in the absence of classical tissue recombination experiments, genomic data suggest that the reversed polarity of head and neck feathers of crested pigeons probably results from altered mesoderm-to-ectoderm signaling. The early embryonic appearance of the phenotype (Shapiro et al., 2013) further suggests that this change must affect early events in placode development, when the ectoderm is sensitive to signaling from underlying dermal tissues (Hughes et al., 2011; Rawles, 1963; Sengel, 1990).
A notable limitation of these studies is that they are often restricted to simple or oligogenic traits. In some cases, practical considerations are a limiting factor: phenotypes that are controlled by many loci of modest phenotypic effect are difficult to detect without very large cross sizes, which can be challenging to generate and maintain for relatively large-bodied organisms such as chickens or pigeons. Hence, QTL and association mapping studies are often biased toward simpler traits. In other cases, biological factors are confounding: traits that are found only in certain breeds are difficult to map by association studies because individuals within a breed are often very closely related. Thus, many members of the population will be similar throughout the genome, not just at the loci controlling particular traits of interest, thereby making it difficult to identify specific loci associated with a trait. Furthermore, identification of a genetic variant that is associated with a trait of interest does not by itself reveal the developmental consequences of a mutation. Because inherited variants are be present in every cell throughout ontogeny, so they have the potential to affect the earliest stages of skin morphogenesis. However, genotype-phenotype associations alone are not enough to explain how novel traits are generated in the embryonic skin, as they alone do not inform us about timing of signaling between mesodermal and ectodermal derivatives. These mechanistic issues are important for follow-up studies and remain the purview of developmental biology.
Despite these limitations, we are learning that epidermal appendage variation arises from diverse genomic alterations. Point mutations, genomic structural variants, protein-coding changes, and non-coding regulatory variation all contribute to derived phenotypes. Some traits entail changes at structural genes such as keratins, while others involve transcription factors that confer regional identity to the skin and other embryonic structures. Our focus here has been on feathers and scales, but because of developmental and genetic conservation with other vertebrates, studies of patterning and mesoderm-ectoderm signaling in birds contribute to our understanding of skin development in general (Dhouailly, 1973, 1975, 2009). Experiments in domestic chickens were critical for uncovering tissue-level developmental events that pattern the epidermis and its appendages, and these and other domestic avian species hold similar promise for discovering the genes and specific mutations that contribute to morphogenesis, variation, and disease.
Materials and methods
RNA isolation and cDNA synthesis
Pairs of hindlimb buds from HH25 racing homer (scale), Oriental frill (grouse) and English trumpeter (muff) pigeon embryos were dissected and stored in RNAlater (ThermoFisher Scientific). Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and on-column DNase treatment was performed using the RNase-Free DNase Set (Qiagen). cDNA was synthesized according to the manufacturer’s protocol using Oligo(dT)12–18 primer and M-MLV Reverse Transcriptase (ThermoFisher Scientific).
Quantitative real-time PCR
To assay PITX1 and TBX5 gene expression, intron-spanning primers for pigeon PITX1, TBX5, and ACTB (for normalization) were used. Primer sequences were published previously (Domyan et al., 2016). Amplicons from scale (n=4), grouse (n=4) and muff (n=10) HH25 hindlimb bud cDNA were generated using a CFX96 qPCR instrument and iTaq Universal Sybr Green Supermix (Bio-Rad). qRT-PCR data in Figure 5 represent the results of three experimental replicates. Statistical analysis was performed in R (R_Development_Core_Team, 2008) using a Pairwise Wilcox Test.
Acknowledgments
We thank Lee Niswander for the opportunity to contribute to this Special Issue, and Anna Vickrey and Emily Maclary for comments and discussion on the manuscript. This work was supported by grants from the National Institutes of Health (R01GM115996) and National Science Foundation (CAREER DEB1149160) to M.D.S. All images in Figures 2–5 are reprinted with permission from the artist or under the Creative Commons Generic License. M.D.S. thanks John Saunders for his mentorship and collaboration during the 1996 Woods Hole Embryology course, an experience that changed the path of his career.
Literature Cited
- Abbott UK, Asmundson VS. Scaleless, An Inherited Ectodermal Defect in the Domestic Fowl. Journal of Heredity. 1957;48:63–70. [Google Scholar]
- Amprino R, Camosso M. Feather formation in heterotopically grafted terminal parts of the leg bud in chicken embryos. 1959;142:533–551. doi: 10.1002/jez.1401420125. [DOI] [PubMed] [Google Scholar]
- Baptista LF, Martinez JG, Horblit HM. Darwin’s pigeons and the evolution of the columbiforms: recapitulation of ancient genes. Acta zoológica mexicana. 2009;25:719–741. [Google Scholar]
- Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J Exp Zool B Mol Dev Evol. 2003;298:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- Bed’hom B, Vaez M, Coville JL, Gourichon D, Chastel O, Follett S, Burke T, Minvielle F. The lavender plumage colour in Japanese quail is associated with a complex mutation in the region of MLPH that is related to differences in growth, feed consumption and body temperature. BMC genomics. 2012;13:442. doi: 10.1186/1471-2164-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothwell D. Roman evidence of a crested form of domestic fowl, as indicated by a skull showing associated cerebral hernia. Journal of Archaeological Science. 1979;6:291–293. [Google Scholar]
- Brotman HF. Epidermal-dermal tissue interactions between mutant and normal embryonic back skin: Site of mutant gene activity determining abnormal feathering is in the epidermis. Journal of Experimental Zoology. 1977;200:243–257. doi: 10.1002/jez.1402000206. [DOI] [PubMed] [Google Scholar]
- Cairns JM, Saunders JW. The influence of embryonic mesoderm on the regional specification of epidermal derivatives in the chick. Journal of Experimental Zoology. 1954;127:221–248. [Google Scholar]
- Chang KW, Huang NA, Liu IH, Wang YH, Wu P, Tseng YT, Hughes MW, Jiang TX, Tsai MH, Chen CY, Oyang YJ, Lin EC, Chuong CM, Lin SP. Emergence of differentially regulated pathways associated with the development of regional specificity in chicken skin. BMC Genomics. 2015;16:22. doi: 10.1186/s12864-014-1202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Foley J, Tang PC, Li A, Jiang TX, Wu P, Widelitz RB, Chuong CM. Development, regeneration, and evolution of feathers. Annu Rev Anim Biosci. 2015;3:169–195. doi: 10.1146/annurev-animal-022513-114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chuong CM. Patterning skin by planar cell polarity: the multi-talented hair designer. Exp Dermatol. 2012;21:81–85. doi: 10.1111/j.1600-0625.2011.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM. Molecular Basis of Epithelial Appendage Morphogenesis. R.G. Landes Company; Georgetown, Texas: 1998. [Google Scholar]
- Classen HL, Smyth JR. The Influence of Pea Comb and Naked Neck on Apteria Width in Domestic Fowl. Poultry Science. 1977;56:1683–1685. [Google Scholar]
- Danforth DH. Bantam genetics: distribution of traits in a Sebright-Mille Fleur cross. J Hered. 1929;20:573–582. [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection. John Murray; London: 1859. [Google Scholar]
- Darwin C. The Variation of Plants and Animals under Domestication. John Murray; London: 1868. [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. Journal of embryology and experimental morphology. 1973;30:587–603. [PubMed] [Google Scholar]
- Dhouailly D. Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux’ Archiv. 1975;177:323–340. doi: 10.1007/BF00848183. [DOI] [PubMed] [Google Scholar]
- Dhouailly D. Feather-forming capacities of the avian extra-embryonic somatopleure. Journal of embryology and experimental morphology. 1978;185:195–200. [PubMed] [Google Scholar]
- Dhouailly D. A new scenario for the evolutionary origin of hair, feather, and avian scales. J Anat. 2009;214:587–606. doi: 10.1111/j.1469-7580.2008.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhouailly D, Hardy MH, Sengel P. Formation of feathers on chick foot scales: a stage-dependent morphogenetic response to retinoic acid. Journal of embryology and experimental morphology. 1980;58:63–78. [PubMed] [Google Scholar]
- Dhouailly D, Sawyer RH. Avian scale development. XI. Initial appearance of the dermal defect in scaleless skin. Dev Biol. 1984;105:343–350. doi: 10.1016/0012-1606(84)90291-4. [DOI] [PubMed] [Google Scholar]
- Domyan ET, Guernsey MW, Kronenberg Z, Krishnan S, Boissy RE, Vickrey AI, Rodgers C, Cassidy P, Leachman SA, Fondon JW, 3rd, Yandell M, Shapiro MD. Epistatic and combinatorial effects of pigmentary gene mutations in the domestic pigeon. Curr Biol. 2014;24:459–464. doi: 10.1016/j.cub.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Kronenberg Z, Infante CR, Vickrey AI, Stringham SA, Bruders R, Guernsey MW, Park S, Payne J, Beckstead RB, Kardon G, Menke DB, Yandell M, Shapiro MD. Molecular shifts in limb identity underlie development of feathered feet in two domestic avian species. Elife. 2016;5:e12115. doi: 10.7554/eLife.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Shapiro MD. Pigeonetics takes flight: Evolution, development, and genetics of intraspecific variation. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.1011.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncaster L. Notes on inheritance of colour and other characters in pigeons. Journal of Genetics. 1912;2:89–98. [Google Scholar]
- Dorshorst B, Okimoto R, Ashwell C. Genomic regions associated with dermal hyperpigmentation, polydactyly and other morphological traits in the Silkie chicken. J Hered. 2010;101:339–350. doi: 10.1093/jhered/esp120. [DOI] [PubMed] [Google Scholar]
- Duboc V, Logan MP. Regulation of limb bud initiation and limb-type morphology. Dev Dyn. 2011;240:1017–1027. doi: 10.1002/dvdy.22582. [DOI] [PubMed] [Google Scholar]
- Dunn LC, Jull MA. On the inheritance of some characters op the silky fowl. Journal of Genetics. 1927;19:27–63. [Google Scholar]
- Feng C, Gao Y, Dorshorst B, Song C, Gu X, Li Q, Li J, Liu T, Rubin CJ, Zhao Y, Wang Y, Fei J, Li H, Chen K, Qu H, Shu D, Ashwell C, Da Y, Andersson L, Hu X, Li N. A cis-regulatory mutation of PDSS2 causes silky-feather in chickens. PLoS Genet. 2014;10:e1004576. doi: 10.1371/journal.pgen.1004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Crest and Hernia in Fowls Due to a Single Gene Without Dominance. 1934. [DOI] [PubMed] [Google Scholar]
- Foth C, Tischlinger H, Rauhut OW. New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature. 2014;511:79–82. doi: 10.1038/nature13467. [DOI] [PubMed] [Google Scholar]
- Frahm HD, Rehkamper G, Werner CW. Brain alterations in crested versus non-crested breeds of domestic ducks (Anas platyrhynchos f.d.) Poult Sci. 2001;80:1249–1257. doi: 10.1093/ps/80.9.1249. [DOI] [PubMed] [Google Scholar]
- Godefroit P, Sinitsa SM, Dhouailly D, Bolotsky YL, Sizov AV, McNamara ME, Benton MJ, Spagna P. Dinosaur evolution. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science. 2014;345:451–455. doi: 10.1126/science.1253351. [DOI] [PubMed] [Google Scholar]
- Gunnarsson U, Kerje S, Bed’hom B, Sahlqvist AS, Ekwall O, Tixier-Boichard M, Kämpe O, Andersson L. The Dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell & Melanoma Research. 2011;24:268–274. doi: 10.1111/j.1755-148X.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- Guo Y, Gu X, Sheng Z, Wang Y, Luo C, Liu R, Qu H, Shu D, Wen J, Crooijmans RP, Carlborg O, Zhao Y, Hu X, Li N. A Complex Structural Variation on Chromosome 27 Leads to the Ectopic Expression of HOXB8 and the Muffs and Beard Phenotype in Chickens. PLoS Genet. 2016;12:e1006071. doi: 10.1371/journal.pgen.1006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Harris MP, Linkhart BL, Fallon JF. Bmp7 mediates early signaling events during induction of chick epidermal organs. Dev Dyn. 2004;231:22–32. doi: 10.1002/dvdy.20096. [DOI] [PubMed] [Google Scholar]
- Hasson P, Del Buono J, Logan MPO. Tbx5 is dispensable for forelimb outgrowth. Development. 2007;134:85–92. doi: 10.1242/dev.02622. [DOI] [PubMed] [Google Scholar]
- Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, Mohun TJ, Logan MP. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18:148–156. doi: 10.1016/j.devcel.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström AR, Sundström E, Gunnarsson U, Bed’Hom B, Tixier-Boichard M, Honaker CF, Sahlqvist AS, Jensen P, Kämpe O, Siegel PB, Kerje S, Andersson L. Sex-linked barring in chickens is controlled by the CDKN2A/B tumour suppressor locus. Pigment Cell & Melanoma Research. 2010;23:521–530. doi: 10.1111/j.1755-148X.2010.00700.x. [DOI] [PubMed] [Google Scholar]
- Hieronymus TL, Witmer LM, Ridgely RC. Structure of White Rhinoceros (Ceratotherium simum) Horn Investigated by X-ray Computed Tomography and Histology With Implications for Growth and External Form. J Morphol. 267:1172–1176. doi: 10.1002/jmor.10465. [DOI] [PubMed] [Google Scholar]
- Hollander WF. PhD Thesis. University of Wisconsin; 1937. Hereditary Interrelationships of Certain Factors in Pigeons; p. 67. [Google Scholar]
- Holmes A. The pattern and symmetry of adult plumage units in relation to the order and locus of origin of the embryonic feather papillae. American Journal of Anatomy. 1935;56:513–537. [Google Scholar]
- Houghton L, Lindon C, Morgan BA. The ectodysplasin pathway in feather tract development. Development. 2005;132:863–872. doi: 10.1242/dev.01651. [DOI] [PubMed] [Google Scholar]
- Houghton L, Lindon CM, Freeman A, Morgan BA. Abortive placode formation in the feather tract of the scaleless chicken embryo. Dev Dyn. 2007;236:3020–3030. doi: 10.1002/dvdy.21337. [DOI] [PubMed] [Google Scholar]
- Hu D, Hou L, Zhang L, Xu X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature. 2009;461:640–643. doi: 10.1038/nature08322. [DOI] [PubMed] [Google Scholar]
- Hughes MW, Wu P, Jiang TX, Lin SJ, Dong CY, Li A, Hsieh FJ, Widelitz RB, Chuong CM. In search of the Golden Fleece: unraveling principles of morphogenesis by studying the integrative biology of skin appendages. Integrative biology: quantitative biosciences from nano to macro. 2011;3:388–407. doi: 10.1039/c0ib00108b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütt F. The genetics of the fowl. I. The inheritance of the Frizzled plumage. Journal of Genetics. 1930:22. [Google Scholar]
- Kanzler B, Prin F, Thelu J, Dhouailly D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev Dyn. 1997;210:274–287. doi: 10.1002/(SICI)1097-0177(199711)210:3<274::AID-AJA8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kerje S. The Dominant white, Dun and Smoky Color Variants in Chicken Are Associated With Insertion/Deletion Polymorphisms in the PMEL17 Gene. Genetics. 2004;168:1507–1518. doi: 10.1534/genetics.104.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer W, Dunn L. The “Frizzle” characters of fowls: Its Expression and Inheritance. Journal of Heredity. 1930;21:291–305. [Google Scholar]
- Levi WM. The Pigeon. Levi Publishing Co., Inc; Sumter, S.C: 1986. [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the feather. V. Experimental morphogenesis. Physiol Zool. 1941;14:103–133. [Google Scholar]
- Lin CM, Jiang TX, Baker RE, Maini PK, Widelitz RB, Chuong CM. Spots and stripes: pleomorphic patterning of stem cells via p-ERK-dependent cell chemotaxis shown by feather morphogenesis and mathematical simulation. Dev Biol. 2009;334:369–382. doi: 10.1016/j.ydbio.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Current opinion in cell biology. 2006;18:730–741. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Tabin CJ. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science. 1999;283:1736–1739. doi: 10.1126/science.283.5408.1736. [DOI] [PubMed] [Google Scholar]
- Lopes RJ, Johnson JD, Toomey MB, Ferreira MS, Araujo PM, Melo-Ferreira J, Andersson L, Hill GE, Corbo JC, Carneiro M. Genetic Basis for Red Coloration in Birds. Curr Biol. 2016;26:1427–1434. doi: 10.1016/j.cub.2016.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR. Avian Anatomy - Integument. Washington, D.C: 1972. [Google Scholar]
- Miller WJ, Demro JR. Allelism of crested traits in Columba livia and Streptopelia risoria. Iowa State Pigeon Assoc Bull. 2011:6–7. [Google Scholar]
- Minguillon C, Del Buono J, Logan MP. Tbx5 and Tbx4 are not sufficient to determine limb-specific morphologies but have common roles in initiating limb outgrowth. Dev Cell. 2005;8:75–84. doi: 10.1016/j.devcel.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Mou C, Pitel F, Gourichon D, Vignoles F, Tzika A, Tato P, Yu L, Burt DW, Bed’hom B, Tixier-Boichard M, Painter KJ, Headon DJ. Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering. PLoS Biol. 2011;9:e1001028. doi: 10.1371/journal.pbio.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy NI, Stapley J, Bennison C, Tucker R, Twyman H, Kim KW, Burke T, Birkhead TR, Andersson S, Slate J. Red Carotenoid Coloration in the Zebra Finch Is Controlled by a Cytochrome P450 Gene Cluster. Curr Biol. 2016;26:1435–1440. doi: 10.1016/j.cub.2016.04.047. [DOI] [PubMed] [Google Scholar]
- Musser JM, Wagner GP, Prum RO. Nuclear beta-catenin localization supports homology of feathers, avian scutate scales, and alligator scales in early development. Evol Dev. 2015;17:185–194. doi: 10.1111/ede.12123. [DOI] [PubMed] [Google Scholar]
- Ng CS, Wu P, Foley J, Foley A, McDonald ML, Juan WT, Huang CJ, Lai YT, Lo WS, Chen CF, Leal SM, Zhang H, Widelitz RB, Patel PI, Li WH, Chuong CM. The chicken frizzle feather is due to an alpha-keratin (KRT75) mutation that causes a defective rachis. PLoS Genet. 2012;8:e1002748. doi: 10.1371/journal.pgen.1002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D, Son B, Mun S, Oh MH, Oh S, Ha J, Yi J, Lee S, Han K. Whole Genome Re-Sequencing of Three Domesticated Chicken Breeds. Zoolog Sci. 2016;33:73–77. doi: 10.2108/zs150071. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Viallet JP, Michon F, Pearton DJ, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004;48:107–115. doi: 10.1387/ijdb.15272376. [DOI] [PubMed] [Google Scholar]
- Pitel F, Berge R, Coquerelle G, Crooijmans RP, Groenen MA, Vignal A, Tixier-Boichard M. Mapping the naked neck (NA) and polydactyly (PO) mutants of the chicken with microsatellite molecular markers. Genet Sel Evol. 2000;32:73–86. doi: 10.1186/1297-9686-32-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TD. Domesticated birds as a model for the genetics of speciation by sexual selection. Genetica. 2002;116:311–327. [PubMed] [Google Scholar]
- Prin F, Dhouailly D. How and when the regional competence of chick epidermis is established: feathers vs. scutate and reticulate scales, a problem en route to a solution. Int J Dev Biol. 2004;48:137–148. doi: 10.1387/ijdb.15272378. [DOI] [PubMed] [Google Scholar]
- Prin F, Logan C, D’Souza D, Ensini M, Dhouailly D. Dorsal versus ventral scales and the dorsoventral patterning of chick foot epidermis. Dev Dyn. 2004;229:564–578. doi: 10.1002/dvdy.20007. [DOI] [PubMed] [Google Scholar]
- R_Development_Core_Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Rallis C, Bruneau BG, Del Buono J, Seidman CE, Seidman JG, Nissim S, Tabin CJ, Logan MP. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- Rawles ME. The integumentary system, Biology and Comparative Physiology of Birds. Academic Press; New York and London: 1960. pp. 189–240. [Google Scholar]
- Rawles ME. Tissue Interactions in Scale and Feather Development as Studied in Dermal-Epidermal Recombinations. Journal of embryology and experimental morphology. 1963;11:765–789. [PubMed] [Google Scholar]
- Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398:814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- Saunders JW. An experimental study of the distribution, orientation, and tract specificity of feather germs in the wing of the chicken embyro. Anatomical Record. 1947;99:647. [PubMed] [Google Scholar]
- Saunders JW. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. Journal of Experimental Zoology. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Saunders JW. Inductive specificity in the origin of integumentary derivatives in the fowl. In: McElroy WD, Glass B, editors. Chemical Basis of Development. The Johns Hopkins Press; Baltimore: 1958. pp. 239–254. [Google Scholar]
- Saunders JW, Gasseling MT. The origin of pattern and feather germ tract specificity. Journal of Experimental Zoology. 1957;135:503–528. [Google Scholar]
- Saunders JW, Gasseling MT, Cairns JM. The differentiation of prospective thigh mesoderm grafted beneath the apical ectodermal ridge of the wing bud in the chick embryo. Dev Biol. 1959;1:281–301. [Google Scholar]
- Saunders JW, Weiss P. Effects of removal of ectoderm on the origin and distribution of feather germs in the wing of the chick embryo. Anatomical Record. 1950;108:581. [PubMed] [Google Scholar]
- Sawyer RH, Rogers L, Washington L, Glenn TC, Knapp LW. Evolutionary origin of the feather epidermis. Dev Dyn. 2005;232:256–267. doi: 10.1002/dvdy.20291. [DOI] [PubMed] [Google Scholar]
- Sell A. Pigeon Genetics: Applied Genetics in the Domestic Pigeon. Sell publishing; Achim, Germany: 2012. [Google Scholar]
- Sengel P. Analyse expérimentale du développement in vitro des germes plumaires de l’embryon de poulet. Experientia. 1957;13:177–182. [Google Scholar]
- Sengel P. La différenciation de la peau et des germes plumaires de l’embryon de poulet en culture in vitro. L’Année Biologique. 1958;62:29–52. [Google Scholar]
- Sengel P. Pattern formation in skin development. Int J Dev Biol. 1990;34:33–50. [PubMed] [Google Scholar]
- Sengel P, Abbott UK. In vitro studies with the Scaleless mutant: Interactions during feather and scale differentiation. Journal of Heredity. 1963;54:255–262. doi: 10.1093/oxfordjournals.jhered.a107261. [DOI] [PubMed] [Google Scholar]
- Shapiro MD, Kronenberg Z, Li C, Domyan ET, Pan H, Campbell M, Tan H, Huff CD, Hu H, Vickrey AI, Nielsen SC, Stringham SA, Hu H, Willerslev E, Gilbert MT, Yandell M, Zhang G, Wang J. Genomic diversity and evolution of the head crest in the rock pigeon. Science. 2013;339:1063–1067. doi: 10.1126/science.1230422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somes RG. Identifying the Ptilopody (Feathered Shank) Loci of the Chicken. Journal of Heredity. 1992;83:230–234. doi: 10.1093/oxfordjournals.jhered.a111200. [DOI] [PubMed] [Google Scholar]
- Stringham SA, Mulroy EE, Xing J, Record D, Guernsey MW, Aldenhoven JT, Osborne EJ, Shapiro MD. Divergence, convergence, and the ancestry of feral populations in the domestic rock pigeon. Curr Biol. 2012;22:302–308. doi: 10.1016/j.cub.2011.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksaweang S, Jiang TX, Roybal P, Chuong CM, Widelitz R. Roles of EphB3/ephrin-B1 in feather morphogenesis. Int J Dev Biol. 2012;56:719–728. doi: 10.1387/ijdb.120021rw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu R, Zhao G, Zheng M, Sun Y, Yu X, Li P, Wen J. Genome-Wide Linkage Analysis Identifies Loci for Physical Appearance Traits in Chickens. G3 (Bethesda) 2015;5:2037–2041. doi: 10.1534/g3.115.020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto DP, Rodriguez-Esteban C, Ryan AK, O’Connell SM, Liu F, Kioussi C, Gleiberman AS, Izpisua-Belmonte JC, Rosenfeld MG. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13:484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Matsumoto K, Vogel-Hopker A, Naitoh-Matsuo M, Ogura K, Takahashi N, Yasuda K, Ogura T. Tbx5 and Tbx4 genes determine the wing/leg identity of limb buds. Nature. 1999;398:810–814. doi: 10.1038/19762. [DOI] [PubMed] [Google Scholar]
- Vickrey AI, Domyan ET, Horvath MP, Shapiro MD. Convergent Evolution of Head Crests in Two Domesticated Columbids Is Associated with Different Missense Mutations in EphB2. Mol Biol Evol. 2015;32:2657–2664. doi: 10.1093/molbev/msv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. The morphogenetic functions of the epidermal and dermal components of the papilla in feather regeneration. Physiol Zool. 1943;16:325–350. [Google Scholar]
- Wang Y, Gao Y, Imsland F, Gu X, Feng C, Liu R, Song C, Tixier-Boichard M, Gourichon D, Li Q, Chen K, Li H, Andersson L, Hu X, Li N. The crest phenotype in chicken is associated with ectopic expression of HOXC8 in cranial skin. PLoS One. 2012;7:e34012. doi: 10.1371/journal.pone.0034012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P, Matoltsy AG. Absence of wound healing in young chick embryos. Nature. 1957;180:854. doi: 10.1038/180854a0. [DOI] [PubMed] [Google Scholar]
- Wells KL, Hadad Y, Ben-Avraham D, Hillel J, Cahaner A, Headon DJ. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genomics. 2012;13:257. doi: 10.1186/1471-2164-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexelsen H. Types of leg feathering in pigeons. Hereditas. 1934;18:192–198. [Google Scholar]
- Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Sasaki H, Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991;353:443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhou Z, Wang X, Zhang F, Zhang X, Wang Y, Wei G, Wang S, Xu X. Hind wings in Basal birds and the evolution of leg feathers. Science. 2013;339:1309–1312. doi: 10.1126/science.1228753. [DOI] [PubMed] [Google Scholar]
- Zwilling E. Ectoderm-mesoderm relationship in the development of the chick embryo limb bud. Journal of Experimental Zoology. 1955;128:423–441. [Google Scholar]