Abstract
Motoric cognitive risk (MCR) syndrome is a newly described pre-dementia syndrome characterized by the presence of cognitive complaints and slow gait, which is associated with increased risk of conversion to dementia. The underlying biological mechanisms for MCR have not yet been established. Neuroinflammation mediated through cytokines plays a pivotal role in the pathogenesis of dementia. Hence, our objective was to prospectively examine whether variations in cytokine genes (CRP, IFNG, IL1A, IL1B, IL4, IL6, IL10, IL18, TNF and IL12A) play a role in MCR incidence in 530 community-dwelling Ashkenazi Jewish adults age 65 and older without MCR or dementia at baseline enrolled in the LonGenity study. Over a median follow-up of 2.99 years, 70 participants developed MCR. Single nucleotide polymorphisms (SNPs) in the transcriptional regulatory regions of cytokine IL10, rs1800896 (Hazard ratio adjusted for age, gender and education (aHR) 1.667; 95% CI 1.198–2.321) and rs3024498 (aHR 1.926; 95% CI 1.315–2.822), were associated with incident MCR. Functional analysis using in-silico approaches indicated associated SNP rs3024498 'C' allele being the local expression quantitative trait locus (eQTL). Associated alleles of both the SNPs, rs1800896 and rs3024498 were implicated with over expression of IL10 gene. None of the variants in the neuroinflammatory pathway studied were associated with incident mild cognitive impairment syndrome. These observations support a role for the IL10 gene in dementia pathogenesis by increasing risk of developing MCR in older adults.
Keywords: Inflammation, genetics, dementia, mild cognitive impairment syndrome, cognition, epidemiological study
1. Introduction1
Inflammation mediated through pro and anti-inflammatory cytokines is postulated to play a pivotal role in the pathogenesis of dementia syndromes such as Alzheimer’s disease (AD) as well as vascular dementia in older adults (Akiyama et al., 2000; Cacquevel et al., 2004; Heneka et al., 2015; Rogers et al., 1996). Expression studies show significant differences in cytokine profiles in AD and vascular dementia; a meta-analysis of 40 studies of cytokines in AD patients found higher serum concentrations of interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-1β, IL-12 and IL-18 in these individuals compared to healthy controls (Swardfager et al., 2010). Accumulation of macrophage like cells in the brain (microglia) around amyloid β-plaques is a hallmark of AD (Thériault et al., 2015). Binding of amyloid β to the microglia induces expression of pro-inflammatory cytokines namely IL-1β, TNFα, IL-6 and IL-18 (Wang et al., 2015). Furthermore, inflammatory mediators produced by microglia play a role in increasing the production of amyloid β precursor protein (APP) and its conversion to Aβ-42 protein (Heneka et al., 2015), which leads to worsening cognitive status. Over expression of IL-1β, TNFα and IL-6 was reported in vascular dementia patients (Wada-Isoe et al., 2004; Zuliani et al., 2007). Inflammation and oxidative stress play a significant role in neurovascular dysfunction leading to hypoxia or ischemia, and contributing to cognitive impairment in vascular dementia (Iadecola, 2013).
Genetic association studies also point towards the key role of pro and anti-inflammatory cytokine genes in dementia pathogenesis (McGeer and McGeer, 2001; Mun et al., 2016). Genetic variants in IL1A, IL1B, IL6, TNF, IL10, TGFB, IL1RN, IL18 and IL8 in the inflammatory pathway are the most studied in this context, and were associated with dementia (Bertram et al., 2007; Bertram and Tanzi, 2011; Di Bona et al., 2008; Di Bona et al., 2012; Li et al., 2013; Qi et al., 2012). More importantly, these studies have shown association with the genetic variants in the regulatory regions, mainly promoters of these genes (Bertram et al., 2007; Bertram and Tanzi, 2011; Di Bona et al., 2008; Di Bona et al., 2012; Li et al., 2013; Qi et al., 2012). Promoter region polymorphisms −889C/T (rs1800587) in IL1A (Li et al., 2013), −174G/C (rs1800795), −572C/G (rs1800796) and −597G/A (rs1800797) in IL6 (Dai et al., 2012; Qi et al., 2012) as well as −308 G/A (rs1800629) in TNFA and −1082 A/G (rs1800896) in IL10 gene (Di Bona et al., 2012) are associated with dementia. These observations point towards possible regulatory mechanisms involved at the genetic level in the expression of these genes, which might be controlled by other risk factors associated with dementia.
Motoric cognitive risk (MCR) syndrome is a pre-dementia syndrome in older individuals that is characterized by presence of cognitive complaints and slow gait (Verghese et al., 2014a; Verghese et al., 2012). Individuals with MCR are at high risk for transitioning to dementia, both AD (Verghese et al., 2014a) and vascular dementia (Verghese et al., 2012) even after accounting for other established dementia risk factors as well as overlap with other pre-dementia syndromes such as the Mild Cognitive Impairment syndrome (MCI) (Verghese et al., 2014a). MCR is common in older populations; a pooled analysis of 26,802 older adults from 17 countries showed a prevalence of MCR of 9.7% (Verghese et al., 2014a). An age and sex adjusted incidence of MCR of 65.2/1000 person years was reported in four US-based cohorts (Verghese et al., 2014b).
As MCR was described recently, the underlying biology has not yet been established. Elevated levels of inflammatory markers have been linked to risk of developing the main components of MCR; cognitive complaints (McAfoose and Baune, 2009; Trollor et al., 2012; Wilson et al., 2002) as well as slow gait (Verghese et al., 2011). Hence, we hypothesized that inflammatory gene variants would increase the risk of developing MCR in older adults. To examine the role of inflammatory pathways in the pathogenesis of MCR, we conducted a prospective cohort study in 530 community-residing Ashkenazi Jewish (AJ) seniors participating in the LonGenity Study (Eny et al., 2014; Roshandel et al., 2016). Establishing biological underpinnings of the MCR syndrome may provide new insights into preventive strategies to reduce the burden of dementia.
2. Materials and Methods
2.1 LonGenity cohort
The LonGenity study, established in 2007, recruited a cohort of Ashkenazi Jewish (AJ) adults age 65 and older, who were defined as either OPEL (having at least one parent who lived to age 95 or older) or OPUS (neither parent survived to age 95). The goal of the LonGenity study is to identify genotypes associated with longevity and their association with successful aging. Study participants were systematically recruited using public records such as voter registration lists or through contacts at synagogues, community organizations and advertisements in Jewish newspapers in the New York City area. Potential participants were contacted by telephone to assess interest and eligibility. AJ adults age 65 and above were invited to our research center for further evaluation. Exclusion criterion included diagnosis of dementia (previous physician diagnosed dementia, impairment on the telephone Memory Impairment Screen (Lipton et al., 2003) or diagnosed at consensus case conferences (Lipton et al., 2003)) as well as severe visual or hearing impairments that interfere with study assessments. Participants received detailed medical history evaluation and cognitive testing at baseline as well as at annual follow-up visits. All participants signed written informed consents for study assessment and genetic testing prior to enrollment. The Einstein institutional review board approved the study protocol.
A total of 886 individuals were enrolled in the LonGenity study between October 2008 and January 2016. We excluded 98 individuals who did not complete quantitative gait or cognitive complaint assessments as well as 169 who did not consent or complete genetic testing. Prevalent cases of dementia (n = 6) and MCR (n = 83) were also excluded from this prospective analysis. Hence, the eligible sample for this analysis included 530 participants without MCR or dementia at baseline, who consented to genetic studies, and had quantitative gait assessments (Verghese et al., 2007).
2.2 MCR syndrome
MCR syndrome was diagnosed in participants based on established criteria (Verghese et al., 2014a; Verghese et al., 2014b; Verghese et al., 2012). In brief, MCR adapts definitions of MCI (Petersen, 2011); substituting the objective cognitive impairment criterion based on cognitive tests used in MCI with the criterion of slow gait speed. MCR is defined as presence of subjective cognitive complaints and slow gait in older individuals without dementia or mobility disability. Cognitive complaints were reported by participants based on responses to standardized questions as a part of the Health Self-Assessment Questionnaire and from the Geriatric Depression Scale (GDS) (Verghese et al., 2014b). Gait speed was measured using an 8.5 meter long computerized walkway with embedded pressure sensors (GAITRite; CIR Systems, PA). The GAITRite system is widely used in clinical and research settings, and excellent reliability has been reported in our and other centers (Brach et al., 2008; Holtzer et al., 2012). Participants were asked to walk on the walkway at their normal pace in a quiet well-lit room wearing comfortable footwear and without any attached monitors. Slow gait was defined, as described in the LonGenity cohort (Verghese et al., 2014a; Verghese et al., 2014b), as walking speed one standard deviation (SD) or more below age and sex specific means. Strengths of the MCR construct are that slow gait is defined objectively, independent of clinical gait evaluations that may be prone to variable sensitivity and specificity as well as being examiner dependent. Though slow gait is multifactorial in nature (Verghese et al., 2016), slow gait predicts cognitive decline irrespective of the underlying etiology (Verghese et al., 2007). Subjective cognitive complaints are associated with reduced cognitive function and increased dementia risk (Ronnlund et al., 2015; Schmand et al., 1996). MCR has improved predictive validity for dementia compared to its individual components of subjective cognitive complaints and slow gait (Verghese et al., 2014a).
2.3 MCI
Non-demented participants with self-reported cognitive complaints (assessed using the same questionnaires as MCR) and either a score ≤24 on the Free Recall portion of the Free and Cued Selective Reminding Test score (memory) or a score of ≥1.5 SD below the mean on the Digit Symbol Substitution test (score of ≤37) were classified as MCI. The MCR and MCI syndromes were proposed to identify older individuals at risk of developing dementia. As the MCR syndrome definition is adapted from the MCI definition, some overlap is expected in patients who meet both criteria, though MCR is defined without using cognitive tests. In our multi-country study(Verghese et al., 2014a), clinical overlap between MCR and MCI cases was only 39%; indicating that either syndrome applied alone failed to identify a large pool of at-risk seniors. MCR predicted incident dementia and cognitive decline even after accounting for the concomitant presence of MCI in multiple cohorts(Verghese et al., 2014a; Verghese et al., 2012).
2.4 Covariates
Presence or absence of depression, diabetes, heart failure, hypertension, myocardial infarction, strokes, Parkinson’s disease, chronic obstructive lung disease, and arthritis was used to calculate a global health score (range 0 to 9) as previously described (Verghese et al., 2007).
2.5 Selection of variants and genotyping
We targeted genes in the inflammatory pathway for this analysis based on the functional significance and association with dementia reported in earlier studies (Bertram et al., 2007; Bertram and Tanzi, 2011; Di Bona et al., 2008; Di Bona et al., 2012; Li et al., 2013; Qi et al., 2012; Verghese et al., 2011). Accordingly, we selected CRP, IFNG, IL1A, IL1B, IL4, IL6, IL10, IL18, TNF and IL12A genes; 62 SNPs were selected from these genes spanning from 5’ promoter to 3’UTR based on functional significance and tagging status. We had most of the functionally significant SNPs of these genes genotyped in our cohort including SNPs that were associated with dementia; rs1205 of CRP, rs1800587 and rs17561 in IL1A, rs16944 of IL1B, rs1800796 and rs1800797 in IL6, rs1800629 in TNFA and rs1800896 in IL10.
2.6 Statistical analysis
Baseline characteristics of participants were compared using descriptive statistics (Table 1). All the studied SNPs were analyzed for departure from Hardy Weinberg equilibrium using Haploview 4.2 (Barrett et al., 2005). Cox proportional hazard models were used to compute hazard ratios (HR) with 95% confidence intervals (CI) to predict incident MCR syndrome based on selected SNPs in the genes of the neuroinflammatory pathway. All models were adjusted for age, gender, and education years. Time scale was follow-up time in years to incident MCR diagnosis or final contact. Results were adjusted for multiple testing using False discovery rate (FDR) correction (Dabney et al., 2010). FDR q-value less than 0.05 were considered significant. Proportional hazards assumptions of all models were tested graphically and analytically, and were adequately met. All the statistical analyses were carried out using SPSS software (version 23; IBM Corporation).
Table .1.
Baseline clinical characteristics of LonGenity cohort for MCR analysis.
| Variables | LonGenity |
|---|---|
| OPUS/OPEL | 292/238 |
| Age, mean ± SD, y | 75.13±6.26 |
| Women, % | 54.30% |
| Education, mean y | 17.55±2.63 |
| Gait speed, mean, cm/s | 113.86±17.86 |
| Slow gait cut scores for MCR, cm/s | |
| Men 60 –74 y | 101.90 |
| Men ≥75 y | 85.30 |
| Women 60–74 y | 97.40 |
| Women ≥75 y | 76.70 |
| Medical illnesses | |
| Global Health Score, mean ± SD | 1.013±0.93 |
| Heart disease, % | 8.50 |
| Stroke% | 3.00 |
| Diabetes% | 8.50 |
| Parkinson disease, % | 0.80 |
| Depression, % | 19.70 |
| Arthritis, % | 38.50 |
| Hypertension, % | 45.40 |
| Strokes, % | 3.00 |
| Chronic obstructive lung disease, % | 2.80 |
| Cognitive tests | |
| FCSRT free recall, mean (SD) | 33.17±5.27 |
| DSST, mean (SD) | 60.28 ±14.36 |
FCSRT: Free and cued selective reminding test
DSST: Digit symbol substitution test
Additional sensitivity analyses were conducted. We studied the association of the selected SNPs with incident MCI to address possible overlap in risk factors between MCI and MCR. Familial longevity status showed variation in the MCR incidence with 16.09% and 9.7% cases reported respectively in OPUS and OPEL. Hence, the analysis was further adjusted for OPUS/OPEL status to account for the study design. We also examined whether the SNPs that were found to be significantly associated with incident MCR in the primary analysis were associated with the individual MCR criterion, incident slow gait and incident cognitive complaints.
Linkage Disequilibrium (LD) plots were generated using Haploview 4.2 (Barrett et al., 2005). Haplotype blocks were defined based on the Gabriel criteria (Gabriel et al., 2002). Haplotype analysis were performed using SNPStats software (Solé et al., 2006).
Functional prediction of the associated variants was carried out using various in-silico approaches. Genotype-Tissue Expression portal (GTEx, http://www.gtexportal.org/home/) was used to determine the significant expression quantitative trait loci (eQTL) for SNPs associated with MCR (Consortium, 2015). The eQTL analyses for associated SNPs were performed based on data retrieved from whole blood as eQTL was not observed for the associated SNPs in the brain regions in GTEx. Further, expression profile of the associated genes in the brain region and eQTL of the associated SNPs were evaluated using BRAINEAC (Ramasamy et al., 2014) to determine the significance of our finding with respect to brain. BRAINEAC provides expression profile and eQTL of SNPs from 10 brain regions of 133 human subjects free from neurological disorders. We further attempted to predict functional significance of the associated SNPs using previous functional studies as well as prediction softwares. Regulome DB (http://regulomedb.org/) based on Encyclopedia of DNA Elements (ENCODE) project (Boyle et al., 2012) was used to identify functional effects of the identified SNPs in the association and eQTL analyses. Functional Single Nucleotide Polymorphism (F-SNP); a web-based tool that integrates 16 databases and bioinformatic tools to uncover the functional effect of the SNPs (Lee and Shatkay, 2008) and FuncPred (FuncPred; http://snpinfo.niehs.nih.gov/cgi-bin/snpinfo/snpfunc.cgi) were used to predict the functional effects of associated variants.
3. Results
3.1 Study population
Of the 530 eligible individuals, 292 were OPUS and 238 were OPEL. Over a median follow-up time of 2.99 years (SD=2.02), 70 out of the 530 individuals developed MCR. The ages of the participants ranged from 61.90 to 94.70 years (mean 75.13±6.26 years), and 54.30% were women. The mean age of education was 17.55±2.63 years. Demographic and clinical characteristics are summarized in Table 1.
3.2 MCR
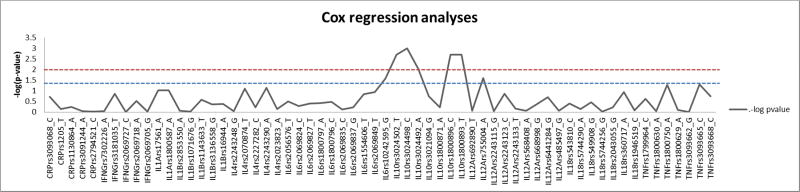
The 62 SNPs selected from 10 genes were found to be in accordance with Hardy- Weinberg expectation (p≥ 0.05). SNPs in the IL10 gene were associated with risk of developing incident MCR (Figure: 1). The most significant association was observed with rs3024498 in the 3’ untranslated region (UTR) with ‘C’ allele associated with an increased risk of incident MCR (HR=1.926; 95%CI=1.315–2.822; p value=0.001, q-value=0.0305). The next significant association was found with ‘G’ allele of rs1800896 in the promoter region (HR = 1.667; 95%CI= 1.198–2.321; p value=0.002, q-value=0.0305). Other SNPs rs3024502, rs1800893 and rs3024492 which fall in the same Linkage disequilibrium (LD) block in the IL10 gene were found to be associated with incident MCR (Table: 2). Other SNPs in the CRP, IFNG, IL1A, IL1B, IL4, IL6, IL18, TNF and IL12A genes were not associated with incident MCR (Table: 2).
Figure 1.
Cox regression survival analysis with studied SNPs in regard with MCR. SNPs were plotted against −log(p value)
Table .2.
Effect of studied SNPs on overall survival in additive model with MCR. All the models were adjusted for Age, Gender and Education
| SNP | HR; 95% CI; p | SNP | HR; 95% CI; p |
|---|---|---|---|
| CRPrs3093068_C | 1.388; 0.853–2.259; 0.187 | IL6rs10242595_G | 1.472; 1.048–2.067; 0.026 |
| CRPrs1205_T | 1.058; 0.763–1.469; 0.735 | IL10rs3024502_T | 1.680; 1.209–2.334; 0.002* |
| CRPrs1130864_A | 0.902; 0.624–1.303; 0.582 | IL10rs3024498_C | 1.926; 1.315–2.822; 0.001* |
| CRPrs3091244_A | 0.977; 0.702–1.358; 0.888 | IL10rs3024492_A | 1.795; 1.157–2.784; 0.009 |
| CRPrs2794521_C | 0.981; 0.648–1.483; 0.926 | IL10rs3021094_G | 0.672; 0.374–1.209; 0.185 |
| IFNGrs7302226_A | 0.973; 0.650–1.456; 0.895 | IL10rs1800871_A | 0.899; 0.601–1.344; 0.603 |
| IFNGrs3181035_T | 1.442; 0.890–2.338; 0.137 | IL10rs1800896_G | 1.667; 1.198–2.321; 0.002* |
| IFNGrs2069727_C | 1.001; 0.711–1.409; 0.994 | IL10rs1800893_T | 1.667; 1.198–2.321; 0.002* |
| IFNGrs2069718_A | 1.189; 0.854–1.657; 0.305 | IL12Ars692890_T | 0.964; 0.668–1.393; 0.847 |
| IFNGrs2069705_G | 1.017; 0.691–1.498; 0.931 | IL12Ars755004_A | 1.791; 1.076–2.982; 0.025 |
| IL1Ars17561_A | 1.346; 0.950–1.908; 0.095 | IL12Ars2243115_G | 1.025; 0.637–1.651; 0.919 |
| IL1Ars1800587_A | 1.346; 0.950–1.908; 0.095 | IL12Ars2243123_C | 1.385; 0.903–2.123; 0.135 |
| IL1Brs2853550_A | 1.040; 0.634–1.706; 0.877 | IL12Ars2243133_T | 0.894; 0.520–1.538; 0.687 |
| IL1Brs1071676_G | 0.983; 0.668–1.445; 0.929 | IL12Ars568408_A | 0.953; 0.546–1.662; 0.865 |
| IL1Brs1143633_T | 1.230; 0.853–1.744; 0.267 | IL12Ars668998_G | 0.866; 0.615–1.221; 0.413 |
| IL1Brs3136558_G | 1.163; 0.799–1.691; 0.430 | IL12Ars6441284_G | 1.263; 0.884–1.804; 0.200 |
| IL1Brs16944_A | 0.868; 0.614–1.228; 0.424 | IL12Ars485497_G | 1.033; 0.741–1.439; 0.849 |
| IL4rs2243248_G | 0.958; 0.516–1.780; 0.893 | IL18rs543810_C | 0.834; 0.546–1.273; 0.401 |
| IL4rs2070874_T | 1.405; 0.961–2.055; 0.079 | IL18rs5744290_A | 1.296; 0.315–5.330; 0.720 |
| IL4rs2227282_C | 1.103; 0.772–1.576; 0.591 | IL18rs549908_G | 1.207; 0.818–1.781; 0.343 |
| IL4rs2243290_A | 1.455; 0.969–2.185; 0.071 | IL18rs5744256_G | 0.983; 0.621–1.556; 0.942 |
| IL4rs2023823_A | 1.069; 0.752–1.521; 0.710 | IL18rs2043055_G | 0.902; 0.622–1.307; 0.585 |
| IL6rs2056576_T | 0.845; 0.607–1.175; 0.316 | IL18rs360717_A | 1.376; 0.927–2.043; 0.113 |
| IL6rs2069824_C | 0.872; 0.580–1.311; 0.510 | IL18rs1946519_C | 0.960; 0.682–1.351; 0.813 |
| IL6rs2069827_T | 1.384; 0.658–2.913; 0.392 | TNFrs1799964_C | 1.254; 0.864–1.820; 0.234 |
| IL6rs1800797_A | 1.180; 0.812–1.714; 0.385 | TNFrs1800630_A | 0.973; 0.607–1.559; 0.909 |
| IL6rs1800796_C | 0.697; 0.337–1.441; 0.331 | TNFrs1800750_A | 2.311; 0.995–5.369; 0.052 |
| IL6rs2069835_C | 1.080; 0.661–1.765; 0.759 | TNFrs1800629_A | 0.912; 0.473–1.759; 0.783 |
| IL6rs2069837_G | 0.866; 0.516–1.455; 0.587 | TNFrs3093662_G | 1.001; 0.612–1.640; 0.995 |
| IL6rs1554606_T | 1.295; 0.916–1.831; 0.143 | TNFrs3093665_C | 0.372; 0.138–1.003; 0.051 |
| IL6rs2069849_T | 1.559; 0.901–2.700; 0.113 | TNFrs3093668_C | 1.622; 0.800–3.286; 0.180 |
q-value (FDR-corrected P-value) < 0.05
Using different genetic models, we tried to assess the effect of individual genotype, with associated risk allele in homozygous and as well as with heterozygous status, with incident MCR. Recessive model based analysis found homozygous GG genotype of rs1800896 (HR= 2.620; 95%CI= 1.560–4.401; p value=0.00027) and CC genotype of rs3024498 (HR= 4.447; 95%CI= 2.005–9.860; p value=0.00024) to be associated with incident MCR (Table: 3).
Table 3.
Effect of IL10 SNPS rs1800896 and rs3024498 on overall survival, shown for different genetic model. All the models were adjusted for Age, Gender and Education
| SNP | Genetic model | HR | 95%CI | P-value |
|---|---|---|---|---|
| Additive (G) | 1.686 | 1.209–2.350 | 0.002 | |
| rs1800896 | GG vs AG+AA | 2.62 | 1.560–4.401 | 0.00027 |
| GG+AG vs AA | 1.549 | 0.927–2.580 | 0.095 | |
|
| ||||
| Additive (C) | 1.933 | 1.320–2.831 | 0.001 | |
| rs3024498 | CC vs CT+TT | 4.447 | 2.005–9.860 | 0.00024 |
| CC+CT vs TT | 1.84 | 1.138–2.975 | 0.013 | |
3.3. Sensitivity analyses
There were 60 incident MCI cases over the follow-up period (Table: 1). The MCR associated IL10 gene SNPs rs1800896 (HR= 1.047; 95%CI= 0.735–1.493; p value=0.798) and rs3024498 (HR=0.946; 95%CI= 0.577–1.550; p value=0.825) were not associated with incident MCI. Furthermore, we did not find an association of any our other selected variants in the neuroinflammatory pathways with incident MCI (Supplementary Table: 2). Of the 70 incident MCR cases in this study, only 15 (21%) met criteria for either incident or prevalent MCI at the same or earlier waves. The association of the IL10 polymorphisms rs1800896 (HR= 1.758; 95%CI= 1.208–2.560; p value=0.003) and rs3024498 (HR= 1.984; 95%CI= 1.305–3.017; p value=0.001) with incident MCR still remains significant even after removing these 15 MCI cases.
The association of IL10 SNPs, rs1800896 (HR = 1.673; 95%CI= 1.200–2.331; p value=0.002) and rs3024498 (HR =1.896; 95%CI= 1.290–2.786; p value=0.001) with incident MCR in the full model was significant even when adjusted for OPUS/OPEL status to account for the LonGenity study design. Inflammation plays an important role in many complex disorders including arthritis. Even after adjusting the present model with global health score, rs1800896 (HR=1.752; 95%CI=1.247–2.461; p value=0.001) and rs3024498 (HR=2.019; 95%CI=1.369–2.975; p value=0.0004) remained associated with incident MCR.
Over the study follow-up there were 79 incident cases of slow gait and 86 incident cases of cognitive complaints. A significant association was observed between the two IL-10 SNPs that were significant in the primary analysis with incident slow gait; rs1800896 (HR = 1.754; 95%CI= 1.283–2.398; p value=0.0004) and rs3024498 (HR =1.758; 95%CI= 1.207–2.559; p value=0.003). However, rs1800896 (HR = 1.110; 95%CI= 0.814–1.513; p value=0.511) and rs3024498 (HR =1.251; 95%CI= 0.866–1.807; p value=0.233) did not predict incident cognitive complaints.
3.4 Bioinformatics
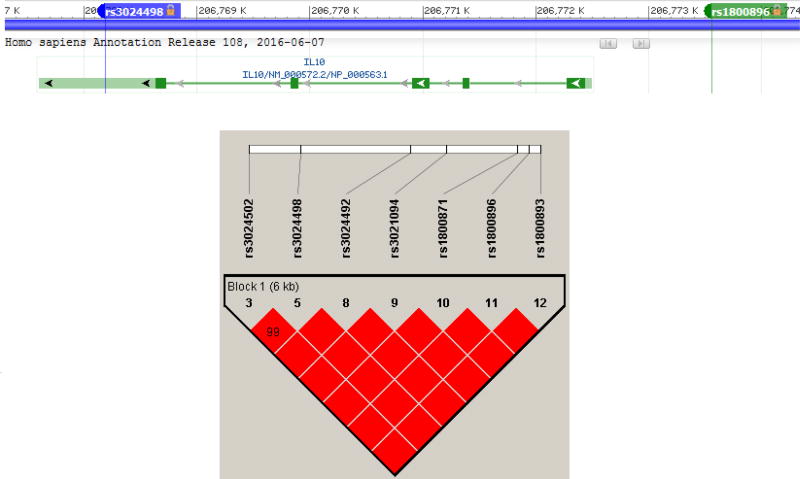
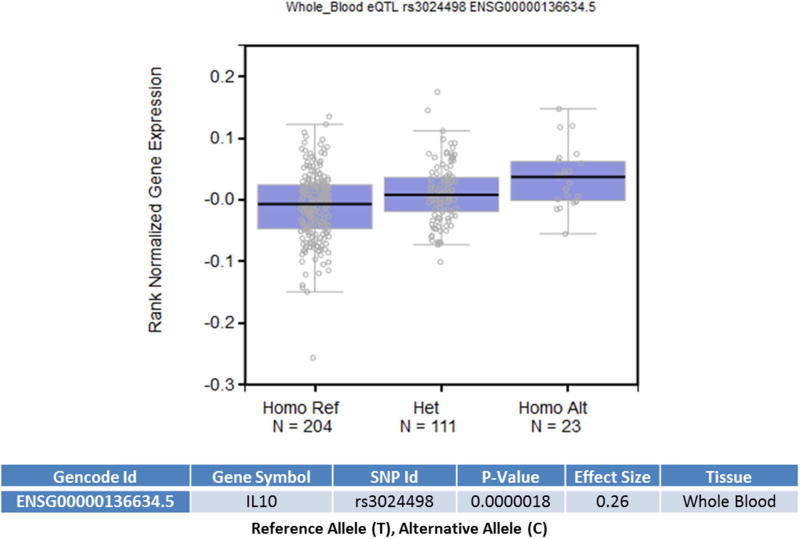
We assessed the functional significance of the associated SNPs in our study. Linkage disequilibrium plot of IL10 gene in our cohort showed presence of associated SNPs in a single LD block (Figure: 2). Haplotype analysis to investigate the combined effect of IL10 SNPs found significant association (p-value =0.006) with haplotype involving risk alleles (TCATGGT) at seven loci combination (20% vs. 13.6%) (Supplementary Table: 1). Further, considering that these SNPs were located in the transcription regulatory regions, we used an in silco approach to determine whether they were local expression quantitative trait loci (eQTL). From the eQTL data available from 338 whole blood samples in GTEx, we determined rs3024498 (p-value= 0.0000018) and rs3024492 (p-value= 0.0000032) to be significant eQTL for IL10 gene. SNP in the 3’ UTR, rs3024498 ‘C’ allele correlates with increased expression of IL10 gene in whole blood (Figure: 3). In silico functional analysis using Regulome DB provided a score of 4, indicating its location in DNase hypersensitive site (DHS) and role in transcriptional factor (TF) binding. However, using FuncPred, we couldn’t find differential binding of miRNA for rs3024498, though it was predicted site for hsa-miR-496. Using BRAINEAC database we observed expressional difference of IL10 gene across different brain regions but we couldn’t verify the effect of the associated SNPs (Supplementary Figure: 1). Earlier expressional studies showed association with the promoter polymorphism rs1800896 with the ‘G’ allele being associated with increased expression of IL10 (Turner et al., 1997; Yılmaz et al., 2005), but data for this SNP were not found in eQTL and other in-silico approaches. In summary, we found significant association of MCR occurrence with SNPs in regulatory regions of IL10 gene involved in regulation of expression, namely rs1800896 in the promoter region and rs3024498 in the 3’UTR.
Figure.2.
Top panel showing location of associated SNPs rs3024498 and rs1800896 located in 3’UTR and Promoter respectively in the IL10 gene. Lower panel shows the linkage disequilibrium (LD) plot of IL10 gene in our cohort.
Figure.3.
Data analyses using the Genotype-Tissue Expression (GTex) portal. eQTL data analysis using GTex portal with the whole blood samples (n=338 total) of rs3024498. Alternative allele (C) is associated with increased expression of IL10 (p= 1.8*10−6).
4. Discussion
The present study was designed to decipher the role of genetic variants in the neuroinflammatory pathway with risk of developing incident MCR in older adults. We uncovered association of SNPs in the regulatory region of IL-10 gene with incident MCR; rs3024498 in the 3’UTR and rs1800896 in the promoter region being the lead SNPs. Using functional analyses, we found associated ‘C’ allele of rs3024498 to be a possible up regulator of IL-10 expression.
IL-10 has an inhibitory action on macrophage as well as Th1 cells involved in production of proinflammatory cytokines; IL-1, IL-6, TNF-A, IL-18 and IFNG (Moore et al., 1993). Interestingly, in-silico approaches and previous functional studies found the associated alleles of rs3024498 in the 3’UTR and rs1800896 in the promoter region to be associated with the over expression of anti-inflammatory cytokine IL-10. Recent studies have suggested several roles for IL-10 in the pathogenesis of dementia (Chakrabarty et al., 2015; Guillot-Sestier et al., 2015). Chakrabarty and colleagues showed that expression of IL-10 in APP transgenic mouse brain leads to increase in amyloid β accumulation and reduction of synaptic proteins culminating in cognitive impairment. IL-10 also leads to overexpression of ApoE, which in turn binds to amyloid β and gets sequestered in the plaques. Moreover, IL-10 along with ApoE suppresses phagocytosis of amyloid β by microglia; further increasing the concentration of amyloid β in brain (Chakrabarty et al., 2015). Guillot-Sestier and colleagues independently reported that IL10 deficiency in APP/PS1 mice promoted amyloid β clearance and preserved synaptic integrity and diminished cognitive deficits (Guillot-Sestier et al., 2015). These studies point towards the proamyloidogenic effect of IL-10 in cognitive decline.
Our findings extend these observations to a possible role for the over expression of anti-inflammatory cytokine IL-10 in the development of a pre-dementia state, possibly through the regulatory 3’UTR and promoter regions. Coordinated regulatory action mediated though these regions are further strengthened by the haplotype association found involving risk alleles of associated IL-10 SNPs spanning these two regions. Earlier functional studies have shown ‘G’ allele of promoter polymorphism rs1800896 to be associated with the over expression of IL-10 (Turner et al., 1997; Yılmaz et al., 2005). There have been reports of over expression of anti-inflammatory IL-10 in patients with dementia (Angelopoulos et al., 2008). Torres and colleagues reported an increase in the number of CD4+ T cells expressing IL-10 in AD compared to controls (Torres et al., 2013). These observations suggest the possibility that expression of IL-10 gene might play a role in dementia pathogenesis by increasing risk of MCR (and not MCI), which may be mediated through transcriptional regulatory regions.
As MCR is a relatively new pre-dementia syndrome, biological investigations are lacking. Expressional and genetic studies in MCI may provide a comparison. A meta-analysis of studies of cytokine levels in MCI failed to show an increase in inflammatory cytokines (IL-1β, IL-3, IL-6, IL-8, IL-10, IL-12, TNF-α and CRP) compared to healthy controls even though significant heterogeneity was observed between the studies (Saleem et al., 2015). However, a study of the proteomic profile in the amnestic subtype of MCI (aMCI) showed an increased expression of IL-10 in aMCI cases compared to normal controls in Mexican Americans (Edwards et al., 2015). IL10 gene variants rs1800896 and rs3024498, which were associated with incident MCR, have not, to our knowledge, been reported to predict incident MCI. However, IL10 gene variants rs1800896 have been associated with AD (Zhang et al., 2011) as well as in the progression from MCI to AD (Arosio et al., 2010). Unlike our study, a meta-analysis by the International Genomics of Alzheimer’s Project (IGAP) showed no significant associations of these polymorphisms in the IL-10 gene with AD (Lambert et al., 2013). The discrepancy in results between our studies might be due to differences in phenotypes and study approaches. Our study focused on incident MCR which is high risk pre-dementia stage for both incident AD as well as incident vascular dementia(Verghese et al., 2014a; Verghese et al., 2012); whereas IGAP focused on prevalent AD that was defined using different criteria in the studies included in the meta-analysis(Lambert et al., 2013).
Our sensitivity analyses suggests that the association of the IL10 polymorphisms with MCR incidence may be via motoric pathways that are markers of cognitive decline (Verghese et al., 2002; Verghese et al., 2007), and may account for the non-overlap in genetic associations of MCR and MCI within these inflammatory pathways. Our results also support examining MCR in addition to MCI as novel potentially modifiable risks may be discovered that could further reduce dementia burden.
The strengths of our study include the systematic cognitive and clinical assessments, longitudinal design and well-characterized homogenous population (Eny et al., 2014; Roshandel et al., 2016). Limitations include absence of IL10 gene expression data to correlate with associated genotype and cohort characteristics. Our findings were based in a relatively homogenous AJ population with high levels of education used successfully for other genetic discoveries (Atzmon et al., 2010; Atzmon et al., 2006; Barzilai et al., 2003; Bergman et al., 2007; Eny et al., 2014; Roshandel et al., 2016) that have then been cross validated in other heterogeneous cohorts. Our findings need to be validated in other more diverse populations. The reported IL-10 gene associations with incident MCR survived adjustment for medical illnesses. However, medical illnesses were ascertained by self-report in our study, which is a limitation. Inflammation plays an important role in many complex disorders, and needs further study. While MCR increases risk of dementia, not all individuals with MCR may convert to dementia. Hence, We plan to continue follow-up in our cohort to test whether IL-10 polymorphisms may increase risk of dementia, and if this association may transition through MCR.
In conclusion, we found significant association of SNPs in the promoter and 3’UTR of IL10 gene with incident MCR, which has a possible effect in IL10 expression. Our findings support a role for IL10 gene in dementia pathogenesis via the MCR pathway. Longitudinal studies in more heterogeneous samples are needed to confirm the causal relationships of the IL10 gene with MCR and dementia.
Supplementary Material
Highlights.
Motoric cognitive risk syndrome is a pre-dementia syndrome associated with increased risk of developing dementia; however, its biological underpinnings have not yet been established.
Neuroinflammation mediated through cytokines may play a pivotal role in the pathogenesis of dementia.
The IL10 gene may play a role in dementia pathogenesis by increasing risk of developing Motoric cognitive risk syndrome in older adults.
Acknowledgments
Funding: This work was supported by grants from NIH R01AG044829 (JV, NB) P01AG021654 (NB), R01 AG 046949 (NB) RO1AG042188 (GA), the Nathan Shock Center of Excellence for the Biology of Aging P30AG038072 (NB), K23AG051148 (SM), Glenn Center for the Biology of Human Aging Paul Glenn Foundation Grant (NB).
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
MCR: Motoric cognitive risk syndrome, IL: Interleukin, TNF-α: tumor necrosis factor alpha
-
We have disclosed all sources of funding in the manuscript. Details are as below:Funding: This work was supported by grants from NIH R01AG044829 (JV, NB) P01AG021654 (NB), R01 AG 046949 (NB) RO1AG042188 (GA), the Nathan Shock Center of Excellence for the Biology of Aging P30AG038072 (NB), K23AG051148 (SM), Glenn Center for the Biology of Human Aging Paul Glenn Foundation Grant (NB).The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
- There are no conflicts of interests to declare.
- The data contained in the manuscript has not been previously published, not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
- All other authors have no conflicts of interest to report in relation to the current article.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL. Inflammation and Alzheimer’s disease. Neurobiology of aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulos P, Agouridaki H, Vaiopoulos H, Siskou E, Doutsou K, Costa V, Baloyiannis S. Cytokines in Alzheimer's disease and vascular dementia. International Journal of Neuroscience. 2008;118(12):1659–1672. doi: 10.1080/00207450701392068. [DOI] [PubMed] [Google Scholar]
- Arosio B, Mastronardi L, Vergani C, Annoni G. Intereleukin-10 promoter polymorphism in mild cognitive impairment and in its clinical evolution. International Journal of Alzheimer’s Disease 2010. 2010 doi: 10.4061/2010/854527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, Siegel G, Bergman A, Huffman DM, Schechter CB. Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proceedings of the National Academy of Sciences. 2010;107(suppl 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Rincon M, Schechter CB, Shuldiner AR, Lipton RB, Bergman A, Barzilai N. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4(4):e113. doi: 10.1371/journal.pbio.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. Jama. 2003;290(15):2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Bergman A, Atzmon G, Ye K, MacCarthy T, Barzilai N. Buffering mechanisms in aging: a systems approach toward uncovering the genetic component of aging. PLoS Comput Biol. 2007;3(8):e170. doi: 10.1371/journal.pcbi.0030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature genetics. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi R. Genetics of Alzheimer’s disease. Neurodegeneration: the molecular pathology of dementia and movement disorders. 2011:51–91. [Google Scholar]
- Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S. Annotation of functional variation in personal genomes using RegulomeDB. Genome research. 2012;22(9):1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, Perera S, Studenski S, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Archives of physical medicine and rehabilitation. 2008;89(12):2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacquevel M, Lebeurrier N, Chéenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer's disease. Current drug targets. 2004;5(6):529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, DiNunno N, Rosario AM, Cruz PE, Verbeeck C. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85(3):519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium G. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney A, Storey JD, Warnes G. qvalue: Q-value estimation for false discovery rate control. R package version. 2010;1(0) [Google Scholar]
- Dai L, Liu D, Guo H, Wang Y, Bai Y. Association between polymorphism in the promoter region of Interleukin 6 (−174 G/C) and risk of Alzheimer’s disease: a meta-analysis. Journal of neurology. 2012;259(3):414–419. doi: 10.1007/s00415-011-6164-0. [DOI] [PubMed] [Google Scholar]
- Di Bona D, Plaia A, Vasto S, Cavallone L, Lescai F, Franceschi C, Licastro F, Colonna-Romano G, Lio D, Candore G. Association between the interleukin-1β polymorphisms and Alzheimer's disease: a systematic review and meta-analysis. Brain research reviews. 2008;59(1):155–163. doi: 10.1016/j.brainresrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Di Bona D, Rizzo C, Bonaventura G, Candore G, Caruso C. Association between interleukin-10 polymorphisms and Alzheimer's disease: a systematic review and meta-analysis. Journal of Alzheimer's Disease. 2012;29(4):751–759. doi: 10.3233/JAD-2012-111838. [DOI] [PubMed] [Google Scholar]
- Edwards M, Hall J, Williams B, Johnson L, O’Bryant S. Molecular Markers of Amnestic Mild Cognitive Impairment among Mexican Americans. Journal of Alzheimer's Disease. 2015;49(1):221–228. doi: 10.3233/JAD-150553. [DOI] [PubMed] [Google Scholar]
- Eny KM, Lutgers HL, Maynard J, Klein BE, Lee KE, Atzmon G, Monnier VM, van Vliet-Ostaptchouk JV, Graaff R, van der Harst P. GWAS identifies an NAT2 acetylator status tag single nucleotide polymorphism to be a major locus for skin fluorescence. Diabetologia. 2014;57(8):1623–1634. doi: 10.1007/s00125-014-3286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Guillot-Sestier M-V, Doty KR, Gate D, Rodriguez J, Leung BP, Rezai-Zadeh K, Town T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85(3):534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor control. 2012;16(1):64. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, DeStefano AL, Bis JC, Beecham GW. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature genetics. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic acids research. 2008;36(suppl 1):D820–D824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B-H, Zhang L-L, Yin Y-W, Pi Y, Guo L, Yang Q-W, Gao C-Y, Fang C-Q, Wang J-Z, Xiang J. Association between interleukin-1α C (− 889) T polymorphism and Alzheimer’s disease: a meta-analysis including 12,817 subjects. Journal of neural transmission. 2013;120(3):497–506. doi: 10.1007/s00702-012-0867-y. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, Crystal HA, Buschke H. Screening for Dementia by Telephone Using the Memory Impairment Screen. Journal of the American Geriatrics Society. 2003;51(10):1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune B. Evidence for a cytokine model of cognitive function. Neuroscience & Biobehavioral Reviews. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Polymorphisms in inflammatory genes and the risk of Alzheimer disease. Archives of neurology. 2001;58(11):1790–1792. doi: 10.1001/archneur.58.11.1790. [DOI] [PubMed] [Google Scholar]
- Moore KW, O'garra A, Malefyt RdW, Vieira P, Mosmann TR. Interleukin-10. Annual review of immunology. 1993;11(1):165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Mun M-J, Kim J-H, Choi J-Y, Jang W-C. Genetic polymorphisms of interleukin genes and the risk of Alzheimer's disease: An update meta-analysis. Meta gene. 2016;8:1–10. doi: 10.1016/j.mgene.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Clinical practice. Mild cognitive impairment. The New England journal of medicine. 2011;364(23):2227. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- Qi H-P, Qu Z-Y, Duan S-R, Wei S-Q, Wen S-R, Bi S. IL-6-174 G/C and -572 C/G polymorphisms and risk of Alzheimer’s disease. PLoS One. 2012;7(6):e37858. doi: 10.1371/journal.pone.0037858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy A, Trabzuni D, Guelfi S, Varghese V, Smith C, Walker R, De T, Coin L, de Silva R, Cookson MR. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nature neuroscience. 2014;17(10):1418–1428. doi: 10.1038/nn.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Webster S, Lue L-F, Brachova L, Civin WH, Emmerling M, Shivers B, Walker D, McGeer P. Inflammation and Alzheimer's disease pathogenesis. Neurobiology of aging. 1996;17(5):681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- Ronnlund M, Sundstrom A, Adolfsson R, Nilsson LG. Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: Evidence from the Betula prospective cohort study. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11(11):1385–1392. doi: 10.1016/j.jalz.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Roshandel D, Klein R, Klein BE, Wolffenbuttel BH, van der Klauw MM, van Vliet-Ostaptchouk JV, Atzmon G, Ben-Avraham D, Crandall JP, Barzilai N. A New Locus for Skin Intrinsic Fluorescence in Type 1 Diabetes also Associated with Blood and Skin Glycated Proteins. Diabetes. 2016:db151484. doi: 10.2337/db15-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Herrmann N, Swardfager W, Eisen R, Lanctôt KL. Inflammatory markers in mild cognitive impairment: a meta-analysis. Journal of Alzheimer's Disease. 2015;47(3):669–679. doi: 10.3233/JAD-150042. [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- Solé X, Guinó E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22(15):1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biological psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Thériault P, ElAli A, Rivest S. The dynamics of monocytes and microglia in Alzheimer’s disease. Alzheimer's research & therapy. 2015;7(1):1. doi: 10.1186/s13195-015-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres KC, Pereira PA, Lima GS, Bozzi IC, Rezende VB, Bicalho MA, Moraes EN, Miranda DM, Romano-Silva MA. Increased frequency of T cells expressing IL-10 in Alzheimer disease but not in late-onset depression patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;47:40–45. doi: 10.1016/j.pnpbp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, Samaras K, Crawford J, Lux O, Kochan NA. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age. 2012;34(5):1295–1308. doi: 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D, Williams D, Sankaran D, Lazarus M, Sinnott P, Hutchinson I. An investigation of polymorphism in the interleukin-10 gene promoter. European journal of immunogenetics. 1997;24(1):1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, Bridenbaugh SA, Buchman AS, Callisaya ML, Camicioli R. Motoric cognitive risk syndrome Multicountry prevalence and dementia risk. Neurology. 2014a;83(8):718–726. doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Ayers E, Barzilai N, Bennett DA, Buchman AS, Holtzer R, Katz MJ, Lipton RB, Wang C. Motoric cognitive risk syndrome Multicenter incidence study. Neurology. 2014b;83(24):2278–2284. doi: 10.1212/WNL.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66(10):1083–1089. doi: 10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. New England Journal of Medicine. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Allali G, Holtzer R, Ayers E. Modifiable risk factors for new-onset slow gait in older adults. Journal of the American Medical Directors Association. 2016;17(5):421–425. doi: 10.1016/j.jamda.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012:gls191. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Isoe K, Wakutani Y, Urakami K, Nakashima K. Elevated interleukin-6 levels in cerebrospinal fluid of vascular dementia patients. Acta neurologica scandinavica. 2004;110(2):124–127. doi: 10.1111/j.1600-0404.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- Wang W-Y, Tan M-S, Yu J-T, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Annals of translational medicine. 2015;3(10) doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Yılmaz V, Yentür SP, Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine. 2005;30(4):188–194. doi: 10.1016/j.cyto.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Tian C, Xiao Y, Li X, He C, Huang J, Fan H. The −1082G/A polymorphism in IL-10 gene is associated with risk of Alzheimer's disease: A metaanalysis. Journal of the neurological sciences. 2011;303(1):133–138. doi: 10.1016/j.jns.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Ranzini M, Guerra G, Rossi L, Munari M, Zurlo A, Volpato S, Atti A, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. Journal of psychiatric research. 2007;41(8):686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.