Abstract
Sarcomas are a rare group of malignant tumors originating from mesenchymal stem cells. Surgery, radiation and chemotherapy are currently the only standard treatments for sarcoma. However, their response rates to chemotherapy are quite low. Toxic side effects and multi-drug chemoresistance make treatment even more challenging. Therefore, better drugs to treat sarcomas are needed. Histone deacetylase inhibitors (HDAC inhibitors, HDACi, HDIs) are epigenetic modifying agents that can inhibit sarcoma growth in vitro and in vivo through a variety of pathways, including inducing tumor cell apoptosis, causing cell cycle arrest, impairing tumor invasion and preventing metastasis. Importantly, preclinical studies have revealed that HDIs can not only sensitize sarcomas to chemotherapy and radiotherapy, but also increase treatment responses when combined with other chemotherapeutic drugs. Several phase I and II clinical trials have been conducted to assess the efficacy of HDIs either as monotherapy or in combination with standard chemotherapeutic agents or targeted therapeutic drugs for sarcomas. Combination regimen for sarcomas appear to be more promising than monotherapy when using HDIs. This review summarizes our current understanding and therapeutic applications of HDIs in sarcomas.
Keywords: Sarcoma, Epigenetic, Histone acetylation, Histone deacetylases inhibitor (HDI)
1. Introduction
Sarcomas are malignant tumors that arise from transformed cells of mesenchymal origin, in contrast to carcinomas which originate from epithelial cells. Sarcomas account for over 20% of all pediatric solid malignancies and less than 1% of all adult solid malignancies1. More than 50 distinct histologic sarcoma subtypes exist and many of these subtypes can occur at any age and are not restricted to a specific location of the body 1. The rarity of this disease combined with the diverse number of subtypes present challenges in formulating a consensus on the treatment of sarcomas. Standard treatment modalities may include surgery and radiation for local control and chemotherapy for adjuvant and palliative therapy. With the progress of modern multimodality therapies, the 5-year survival rate in some types of sarcoma, such as in osteosarcoma, has increased to over 70%. However, progress has slowed over the past 30 years, and efforts to improve outcomes with intensifying regimens or adding novel agents have brought disappointing results. Additionally, for patients with metastatic sarcoma at diagnosis and for those with relapsed disease, outcomes are remarkably poor with 4- to 5-year overall survival rate at less than 20%. Furthermore, current chemotherapeutic drugs are generally not effective in some types of sarcomas, such as chondrosarcoma or chordoma. Therefore, the development of new therapeutic strategies is critical for achieving positive results in sarcoma treatment.
The critical mechanisms that drive sarcoma cell proliferation and growth remain largely unclear. However, an increasing amount of data have shown that histone deacetylases (HDACs) influence diverse cellular processes and contribute to sarcoma growth and progression by multiple mechanisms. HDAC inhibitors (HDAC inhibitors, HDACi, HDIs) are epigenetic drugs that can regulate gene expression through action on histones as well as non-histone proteins without changing the gene sequence. HDIs have emerged over the past 10 years as potential treatment options based on their ability to inhibit tumor growth in vitro and in vivo. The pan-HDI, vorinostat (ZOLINZA™, Merck & Co., Inc.), was the first HDI approved by U.S. Food and Drug Administration (FDA) for patients with cutaneous tumor and peripheral T-cell lymphoma in 2006 2. Also approved were belinostat (BELEODAQ™, Spectrum Pharmaceuticals, Inc.) for relapsed or refractory peripheral T-cell Lymphoma and panobinostat (FARYDAK®, Novartis Pharmaceuticals Co.) for multiple myeloma3, 4. Although single-agent treatment with HDI has demonstrated efficacy in hematologic malignancies, benefits in patients with sarcomas have yet to be realized. The acceptable toxicity profile has led to combinations with other currently approved agents, including chemotherapeutics, radiation, and tyrosine kinase inhibitors, in which HDIs may appear to be a promising modulator in sarcoma treatment. Here, we review the current knowledge of the expression, function of HDAC, preclinical findings and clinical trials using HDIs in sarcoma research and chemotherapy. We also discuss the mechanisms of resistance to HDIs in the treatment of sarcoma.
2. HDAC and HDI in sarcoma
2.1 Definition of histone acetylation and histone deacetylation
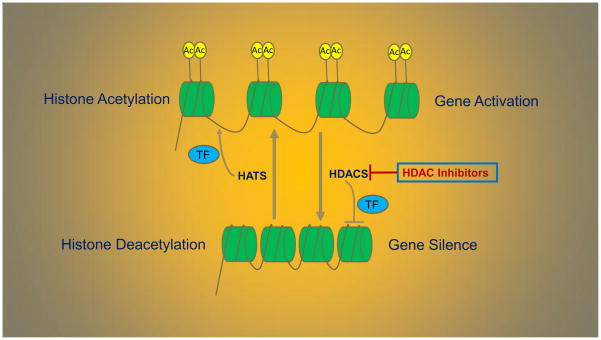
Histone acetylation and deacetylation are reversible processes that are regulated by histone acetyltransferases (HATs) and HDACs. Acetylation removes the positive charge on the histones, thereby decreasing the interaction of the N terminus of histones with the negatively charged phosphate groups of DNA. As a consequence, the chromatin is transformed into a more relaxed state. And in this state, the DNA is more accessible, leading to more transcription factors being able to reach the DNA. Thus, acetylation of histones is known to increase the expression of genes, leading to gene expression in the multiple pathways involved in proliferation, migration, angiogenesis and differentiation. Deacetylation performed by HDACs has the opposite effect. By deacetylating the histone tails, the DNA becomes more tightly wrapped around the histone cores, making it harder for transcription factors to bind to the DNA. This leads to decreased levels of gene expression and is known as gene silencing (Fig. 1). Dysregulation and overexpression of HDACs have been observed in many cancer types including sarcomas. The upregulation of HDACs that inhibit or silence the growth suppressor genes is an important mechanism to promote cancer cell proliferation. As such, HDIs can be used to halt cancer cell growth.
Fig. 1.
The processes of histone acetylation and histone deacetylation that regulated by HATs and HDACs. As a result, acetylation increase levels of gene expression and deacetylation performed by HDACs has the opposite effect.

2.2 Classification of HDACs
To date, eighteen distinct HDACs have been identified and they are classified into four groups based on their structural divergence, namely class I, II, III and IV HDACs (Table 1) 5. They are subdivided into Zn2+-dependent (classes I, II, and IV) which are referred as the ‘classical family’, and nicotinamide adenine dinucleotide (NAD+)-dependent (class III) enzymes 6. Class I HDAC consists of HDACs 1, 2, 3, and 8, that are primarily localized in the nucleus and ubiquitously expressed in all tissues. Class I HDACs have the deacetylase domain located at their N-terminus and carry a variable C-terminus depending on the specific HDAC of the class. Class II HDACs are localized both in the nucleus as well as the cytoplasm. They are divided into class IIa including HDAC 4, 5, 7, 9, and class IIb which is consisted of HDAC 6 and 10. Class II HDACs have the deacetylase domain at the C-terminus with the exception of HDAC 6, which contains two acetylase domains at both the N- and C-termini. Class III HDACs are homologues of yeast silent information regulator 2 proteins and consist of Sirtuins 1–7 7. Sirtuins are distinct from Class I and II HDACs because of their enzymatic dependence on the coenzyme nicotinamide adenine dinucleotide (NAD+-dependent) for deacetylase activity, rather than Zn2+-dependent. HDAC 11 is the only member of class IV HDAC, which has the properties of both class I and class II HDACs 8.
Table 1.
The classification and characteristic of HDACs
| Class | Name | a.a number | Localization | Function | Zn2+/NAD+ |
|---|---|---|---|---|---|
| I classic | HDAC 1 | 482 | Nucleus | Deacetylase | Zn2+-dependent |
| HDAC 2 | 488 | Nucleus | Deacetylase | ||
| HDAC 3 | 428 | Nucleus/cytoplasm | Deacetylase | ||
| HDAC 8 | 377 | Nucleus | Deacetylase | ||
| HDAC 4 | 1084 | Nucleus/cytoplasm | Deacetylase | ||
| IIa classic | HDAC 5 | 1122 | Nucleus/cytoplasm | Deacetylase | Zn2+-dependent |
| HDAC 7 | 991 | Nucleus/cytoplasm | Deacetylase | ||
| HDAC 9 | 1069 | Nucleus/cytoplasm | Deacetylase | ||
| IIb classic | HDAC 6 | 1215 | Mainly cytoplasm | Deacetylase | Zn2+-dependent |
| HDAC10 | 669 | Mainly cytoplasm | Deacetylase | ||
| III Sirtuins (human) | Sirtuin 1 | 747 | Nucleus/cytoplasm | Deacetylase | NAD+-dependent |
| Sirtuin 2 | 389 | Nucleus | Deacetylase | ||
| Sirtuin 3 | 399 | Nucleus/Mitochondria | Deacetylase | ||
| Sirtuin 4 | 314 | Mitochondria | ADP-ribosyltranferase | ||
| Sirtuin 5 | 310 | Mitochondria | Demalonylase Desuccinylase Deacetylase |
||
| Sirtuin 6 | 355 | Nucleus | Demyristoylase Depalmitoylase Weak deacetylase ADP-ribosyltranferase |
||
| Sirtuin 7 | 400 | Nucleus | Weak deacetylase | ||
| IV classic | HDAC11 | 420 | Nucleus/cytoplasm | Deacetylase | Zn2+-dependent |
2.3 Expression and clinical significance of HDACs in sarcomas
HDACs have been closely linked with malignant phenotypes in tumorigenesis. In most sarcoma cases, a high level of HDACs is associated with advanced disease and poor clinical outcomes. For instance, HDACs 1, 4, 6, 7, and 8 have been shown to exhibit a high frequency of strong immunoreactivity and were associated with a lower disease-free survival trend, which may represent potential therapeutic targets for endometrial stromal sarcoma 9. HDAC 2 may also be a predictive therapeutic biomarker of endometrial stromal sarcoma, as its expression is much higher than that of HDAC 1 10. In chondrosarcoma, HDAC 4 represses vascular endothelial growth factor (VEGF) expression by modulating RUNX2 activity, resulting in tumor angiogenesis inhibition 11. HDAC 4 is also one of the targets of miR-1. Upregulation of HDAC 4 contributed to chordoma pathogenesis, and downregulation of miR-1 was likely to be one factor that is involved in this process 12. Besides, Scheipl et al. confirmed the expression of HDAC4 in chordomas and additionally they described the immuno-histochemical expression of HDACs 2–6 in chordoma collective with the strongest expression of HDAC 6 13. Future research is needed to address the role of HDAC6 and the cellular functions mediated by this epigenetic enzyme in chordoma pathogenesis. In osteosarcoma, overexpression of HDAC5 could promote proliferation of cancer cells, due to the ability of upregulating mRNA expression of twist 1, which has been reported as an oncogene 14. β-catenin is an oncogene and a substrate of HDAC 6. In osteosarcoma, rho-associated coiled-coil kinase regulated the stability of β-catenin by preventing TPPP1-mediated inhibition of HDAC 6 activity 15.
Sirtuin 1 is a class III HDAC and it may also serve as a therapeutic predictor biomarker of sarcomas16, 17. Strong Sirtuin 1 expression was observed in leiomyoma, leiomyosarcoma, rhabdomyosarcoma and osteosarcoma 18. Overexpression of Sirtuin 1, DBC1, β-catenin, and cyclin D1 were significantly correlated with higher clinical stage, higher histological grade, increased mitotic counts, and higher potential of distant metastasis. More importantly, the expression of Sirtuin 1 was an independent prognostic indicator for metastasic potency and disease-free survival of sarcoma patients 19. Like HDAC 4, Sirtuin 1 is also one of the targets of some miRNAs. For example, miR-126, miR-133b, miR-204 inhibited the proliferation, migration, invasion and epithelial-mesenchymal transition of osteosarcoma cells via targeting Sirtuin 1 20–22.
2.4 Classification of HDIs
HDIs include a broad class of compounds that can be divided into pan- HDIs and specific HDIs according to their specificity of action (Table 2). Pan-HDIs are compounds that can inhibit the activity of more than one class of HDACs. The most common pan-HDIs are vorinostat, panobinostat, belinostat and trichostatin A. In contrast, specific HDIs work only on one class or a single specific HDAC. For instance, tenovin-6 is a specific class III HDI that inhibits the activity of Sirtuin 1 and Sirtuin 2. Romidepsin (Istodax®, Gloucester Pharmaceuticals Inc.), also known as FK-228, FR901228, and depsipeptide, is a specific class I HDI that has been approved by the U.S. FDA for the clinical treatment of cutaneous T cell lymphoma 23. There are also some novel HDIs, like PCI-48012 and PCI-34051, which specifically inhibit the activity of HDAC 8. Both pan-HDIs and specific HDIs are in development, and there is uncertainty which will be more successful in sarcoma treatment.
Table 2.
Characteristics of HDAC inhibitors in sarcoma
| Drug Name | Molecular Formula | Target HDACs |
|---|---|---|
| Vorinostat(SAHA) | C14H20N2O3 | Pan-HDI of class I and IIa |
| Panobinostat(LBH589) | C21H23N3O2 | Pan-HDI of class I, II, and IV |
| Belinostat(PXD101) | C15H14N2O4S | Pan-HDI of class I, II, and IV |
| Romidepsin(FK-228, FR901228, Depsipeptide) | C24H36N4O6S2 | Specific-HDI of class I, specially HDAC 1,2 |
| Entinostat (MS-275, SNDX-275) | C21H20N4O3 | Specific-HDI of HDAC 1, 3 |
| Valproic acid (VPA, Valproate) | C8H15NaO2 | Pan-HDI of Class I and IIa |
| Abexinostat(PCI-24781) | C21H23N3O5 | Pan-HDI of class I and IIb |
| Pracinostat(SB939) | C20H30N4O2 | Pan-HDI of class I, II and IV |
| Sodium phenybutyrate | C10H11O2Na | Pan-HDI of Class I and IIa |
| Quisinostat(JNJ-26481585) | C21H28Cl2N6O2 | Pan-HDI of class I, II and IV |
| LAQ824 | C22H25N3O3 | Pan-HDI of class I, II, IV |
| Sodium Butyrate | C4H7NaO2 | Pan-HDI of class I, IIa, and IV |
| Trichostatin A (TSA) | C17H22N2O3 | Pan-HDI of class I and II |
| Tenovin-6 | C25H34N4O2S | Specific-HDI of Sirtuin 1,2,3 |
| Pyroxamaide (NSC696085) | C13H19N3O3 | Specific-HDI of HDAC 1 |
| MC1742 | C21H21N3O3S | Pan-HDI of Class I and II |
| MC2625 | / | Pan-HDI of class I, II, IV |
| PCI-34051 | C17H16N2O3 | Specific-HDI of HDAC 8 |
| PCI-48012 | / | Specific-HDI of HDAC 8 |
FDA approved for hematologic neoplasms not sarcomas Po:by mouth i.v.: intravenous infusion
3 Molecular mechanisms of HDIs in sarcoma
3.1 HDIs upregulate tumor suppressor genes and downregulate oncogenes
Unbalance of the tumor suppressor p53 and the oncogene MDM2 feedback loop contributes to tumorigenesis. In osteosarcoma, HDI sodium butyrate inhibited the proliferation of tumor cells by enhancing p53 expression, and conversely, decreasing MDM2 expressing24. HDIs, panobinostat and vorinostat, upregulated the expression of tumor suppressor gene PTEN and p21 in well-differentiated liposarcoma. The same combined treatment resulted in dephosphorylation and depletion of MDM2 and TP53, irrespective of p53 mutational status in MDM2-amplified liposarcoma 25. HDIs have also enhanced the transcriptional function of p53 by directly stabilizing the acetylation of p53. For example, HDI trichostatin A promoted p53–p300 interaction and recruitment of p53 Lys-382 to promoter regions of its target genes p21 and PUMA, consequently upregulating their expression in Ewing sarcoma cells 26.
Polo-like kinase 2 (Plk2) has tumor suppressor functions which were regulated by the tumor microenvironment. Trichostatin A upregulated Plk2 gene expression by directly enhancing GATA-1 acetylation in human osteosarcoma 27. In epithelioid sarcoma, pan-HDIs, vorinostat and entinostat, induced widespread gene expression changes, and among these, EZH2 was significantly downregulated leading to abrogated cell growth in vitro 28. Retinoid X receptors (RXRs) and retinoic acid receptors (RARs) are nuclear receptors that mediate the biological effects of retinoids by their involvement in retinoic acid-mediated gene activation. HDI valproic acid restored the expression of RXRα target genes RARβ, CRABPII and p21, and conversely, repressed the expression of fusion oncogenes, EWS-ERG and EWS-Fli1 in Ewing sarcoma cells 29. Plakoglobin is a member of the catenin protein family and a homologue to β-catenin. Promoter regions (P1–P3) of plakoglobin gene were associated with hypoacetylated H4 histone in embryonal rhabdomyosarcoma 30. HDI trichostatin A activated the Tcf/Lef target promoter partly by upregulation of plakoglobin expression in human fibrosarcoma 31.
Many sarcomas bear fusion oncogenes like SS18-SSX in synovial sarcoma, EWS-Fli1 in Ewing sarcoma and Pax3:Foxo1 in embryonal rhabdomyosarcoma. These sarcomas were more sensitive to HDI treatment than other sarcomas lacking known translocations. The underlying mechanism may be related to HDI’s inhibiting fusion oncogene activity by suppressing gene transcriptional activity, or directly acetylating the fusion oncogene proteins. For example, HDIs, entinostat and romidepsin, decreased the expression of fusion oncoprotein EWS-ATF1 in clear cell sarcoma 32. SS18-SSX while serving as a bridge between activating transcription factor 2 (ATF2) and transducin-like enhancer of split 1 (TLE1), resulted in repression of ATF2 target genes. Besides, the fusion oncoprotein SS18-SSX via TCF/LEF, TLE1 and HDAC interaction leads to an upregulation of AXIN2, which is involved in the WNT pathway but without direction interaction with the pathway 33. Romidepsin significantly suppressed the growth of synovial sarcoma cells compared with that of osteosarcoma, as it impacted SS18-SSX target gene expression by preventing TLE1 complex recruitment 34, 35. Early growth response-1 (EGR1) has been suggested as a tumor suppressor as its overexpression dramatically inhibits tumor cell growth. HDI, romidepsin, reversed SS18-SSX-mediated polycomb silencing of EGR1 in synovial sarcoma 36. In rhabdomyosarcoma, HDI, entinostat, directly suppressed the activity of Pax3:Foxo1 at the transcriptional level. As a result, Pax3:Foxo1-positive embryonal rhabdomyosarcoma was transformed fusion-negative like state in rhabdomyosarcoma 37.
3.2 HDIs induce apoptosis in sarcoma cells
Apoptosis, programmed cell death, is probably one of the most widely-studied subjects among cancer cell biologists 38. Using HDIs to treat sarcomas can not only directly induce apoptosis through the extrinsic death receptor pathway and intrinsic mitochondrial pathway, but also enhances the susceptibility of sarcoma cells to apoptosis (Fig. 2). Fas and TRAIL are cell surface death receptors belonging to the tumor necrosis factor super family, which trigger apoptosis upon ligand binding 39. HDIs vorinostat and sodium butyrate induced tumor cell death and enhanced the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma 40. Additionally, HDIs also increased Fas expression and regulated the localization of Fas on tumor cell membranes. Entinostat, a specific class I HDI, promoted Fas expression by increasing histone acetylation of the Fas gene promoter and mRNA expression 41. HDI valproic acid enhanced human osteosarcoma cell susceptibility to Fas-induced cell death by decreasing the secretion of soluble Fas and increasing the sensitivity to Fas mediated cell death 42. However, another study has demonstrated that entinostat sensitized osteosarcoma cells to Fas L treatment is not mediated through inducing Fas expression on the cell surface, instead lipid rafts are required 43. Cellular FLIP(c-FLIP) is a master anti-apoptotic regulator which can decrease the localization of Fas in lipid rafts. Some sarcoma cells exhibit a phenotype resistant to death receptor-mediated apoptosis by expressing c-FLIP. However, HDIs sensitize such resistant sarcoma cells by directly downregulating c-FLIP expression. For example, HDI romidepsin downregulate c-FLIP by inhibiting generation of FLIP mRNA, rather than stimulating degradation at either the protein or mRNA level 44. In vivo, oral HDI entinostat administered to mice with osteosarcoma lung metastases resulted in decreased c-FLIP in tumor nodules and tumor regression 45.
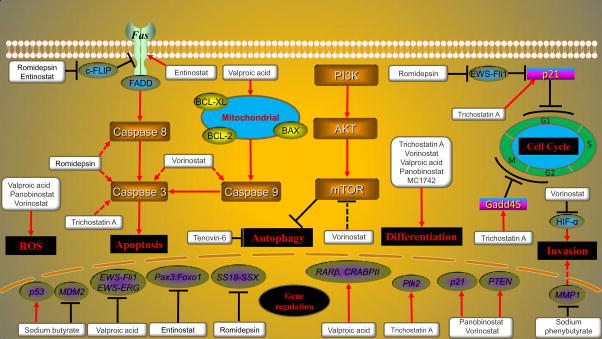
Fig. 2.
Molecular actions of HDIs in sarcoma. HDIs upregulate some tumor suppressor genes and downregulate oncogenes gene expression. HDIs also induce apoptosis by extrinsic death receptor pathway, intrinsic mitochondrial pathway and caspase common pathway. Besides, HDIs cause cell cycle arrest, regulate autophagy, increase ROS generation, reduce invasion ability and induced cell differentiation in sarcomas.
The intrinsic mitochondrial apoptotic pathway is closely regulated by a group of proteins belonging to the BCL-2 family, which can be either pro-apoptotic including BAX, BAK and BOK among others or anti-apoptotic such as BCL-XL and BCL-W 46. Combination treatment of HDI valproic acid and chloroquine for human osteosarcoma cells led to an increase of BAX expression and a decrease of BCL-2 and BCL-XL expression 47. HDIs have also induced apoptosis through common caspase pathway in sarcoma. In addition to upregulating Fas expression, romidepsin also induced apoptosis by activating caspase-8 and caspase-3 in osteosarcoma 48. In osteosarcoma, trichostatin A induced apoptosis of tumor cells partly via the activation of procaspase-3 and cleavage of PARP 49. In uterine sarcoma, vorinostat induced immediate apoptotic cell death as supported by upregulation of caspase-9 as well as activation of caspases-3, caspases-7 and PARP cleavage 50.
HDIs have also induced apoptosis in sarcoma cells through the gene signaling pathway. In synovial sarcoma, PTEN is crucial for EGR1-induced apoptosis. EGR1 reactivation by HDIs promoted cell death in sarcoma by PTEN tumor suppressor 51. HDI plus phosphoinositide 3-kinase (PI3K) inhibitors were identified as specific activators of E2F1/FOXO transcription. Combining the HDI vorinostat with a PI3K inhibitor led to enhanced FOXO-dependent apoptosis in osteosarcoma cells 52. In addition, HDI trichostatin A significantly inhibited the human osteosarcoma cells growth and promoted apoptosis in a dose-dependent manner through p53 signaling pathway activation 53.
3.3 HDIs induce cell cycle arrest in sarcomas
HDAC 1 and 2 directly bind to the promoters of the cell cycle associated p21 gene and negatively regulate its expression 54. HDAC 2-siRNA knockdown led to p21 increment and arrested endometrial stromal sarcoma cell proliferation55. Epigenetically, accumulation of acetylated histones and induction of p21 expression were observed in human rhabdomyosarcoma cells and uterine sarcomas cells exposed to HDACI vorinostat 56, 57. A recent study has shown HDI trichostatin A induced G1 cell cycle arrest in osteosarcoma cells via the p53-independent activation of p21 promoter through the specific Sp1 sites 58. Fusion oncoprotein EWS-Fli1 downregulated the expression of p21 by inhibiting the p300-mediated transactivation of the p21 gene 59. However, HDI romidepsin strongly induced p21 expression by inhibiting the expression of EWS-Fli1 at protein and mRNA levels 60.
Two new HDIs, PCI-34051 and PCI-48012, specifically inhibited the activity of HDAC 8 leading to marked S-phase cell cycle arrest in human malignant peripheral nerve sheath tumors cells 61. In osteosarcoma, vorinostat arrested the cell cycle in G1 and G2/M phase, while HDI sodium butyrate arrested the cell cycle in G2/M phase 62. In chondroma, trichostatin A arrested the cell cycle in G2/M phase but valproic acid arrested the cell cycle in G1 phase 63. Gadd45, a p53-regulator and DNA damage inducible protein, has recently been demonstrated to play a role in the G2-M checkpoint in response to DNA damage 64. Trichostatin A increased gadd45 mRNA and protein levels directly through targeting its promoter without the need of functional p53, leading to G2/M cell cycle arrest in human osteosarcoma cells 65.
3.4 HDIs decrease invasion, metastasis and angiogenesis in sarcomas
HDIs attenuated the expression of hypoxia inducible factor 1 alpha (HIF-1α) that led to a decrease of chordoma cell invasion 66. Invadopodia are specialized membrane protrusions that are associated with degradation of the extracellular matrix in cancer invasiveness and metastasis. In fibrosarcoma, HDAC 6 served as a key participant of hypoxia-induced cell invasion. One study has shown hypoxia induced invadopodia formation in a biphasic manner, which involves the activation of HDAC 6 deacetylase activity by EGFR 67. HDI vorinostat inhibits the metastatic potential of highly metastatic osteosarcoma cells by reducing invadopodia formation and decreasing the expressions of metastasis-associated factors, including mTOR and ALDH gene 68. Furthermore, vorinostat suppresses embryonal rhabdomyosarcoma growth by reducing the self-renewal and migratory capacity of tumor cell in vitro. In this process, EFNB1 is regulated by vorinostat to modulate migratory behavior of embryonal rhabdomyosarcoma cells 69. Matrix metalloproteinase 1 (MMP-1) is involved in the miR-10b and brain-derived neurotrophic factor (BDNF)-mediated chondrosarcoma cell migration and invasion. Treatment with DNA methyltransferase (DNMT) inhibitor 5-aza-dC and HDACI 4-phenylbutyric acid cause markedly reduced of MMP-1 expression, which further suppressed the migratory and invasive capacities of chondrosarcoma cells 70. Overexpression of gelatinase A, also known as MMP-2, is associated with pulmonary metastasis and related to the prognosis of osteosarcoma 71. HDI trichostatin A has acted to control metastatic potential by reducing the ability of metastatic cells to recruit stromal cells to secrete MMP-2 in fibrosarcoma 72.
3.5 HDIs inhibit sarcoma growth through regulating autophagy
The role of autophagy in sarcoma is complex and is likely dependent on sarcoma type-associated genetic context. HDI vorinostat inhibits sarcoma growth that is associated with autophagy upregulation, however, the class III HDI tenovin-6 impairs the autophagy process which induces cell death in sarcoma 73. MTOR is one of the factors involved in the process of upregulating autophagy under vorinostat treatment. In osteosarcoma, vorinostat upregulates autophagy by decreasing mTOR gene expression and increasing LC3 expression 68. In addition, vorinostat significantly inhibits the proliferation of endometrial stromal sarcoma cells via activation of autophagy by decreasing mTOR and phospho-mTOR expression 74. Furthermore, in rat chondrosarcoma cells, vorinostat induces autophagy-associated cell death which was confirmed by the detection of autophagosome-specific protein and specific ultrastructural morphology in the cytoplasm 75.
3.6 HDIs induce reactive oxygen species (ROS) generation in sarcomas
ROS is a double-edged sword in cancer and most of the chemotherapeutic and radiotherapeutic agents kill cancer cells by augmenting ROS stress 76. An increase of ROS generation was also found in sarcoma cells after treatment with HDIs. In osteosarcoma, the cytotoxic effect of the combination treatment of chloroquine and HDI valproic acid trigger the generation of ROS, leading to an increase of the apoptosis related gene expression and a decrease of anti-apoptotis related gene expression 47. Melatonin inhibits osteosarcoma cell growth, targeting Sirtuin 1 signaling partly by increasing the ROS generation. Sirtinol, a known Sirtuin 1 inhibitor, further enhances the antitumor activity of melatonin 77. Pan-HDIs panobinostat and vorinostat induce the generation of ROS, which are regarded as highly effective inhibitors of rhabdomyosarcoma in vitro and in vivo 78. In a later study, combination treatment of HDI quisinostat and proteasome inhibitors lead to elevated endoplasmic reticulum stress, activation of pro-apoptotic effector proteins and increased levels of ROS in synovial sarcoma 79.
3.7 HDIs induce sarcoma cell differentiation
Treating malignant tumor by induction of cell differentiation has been an attractive concept, but clinical development of differentiation-inducing agents to treat malignant tumor, especially for sarcomas, has been limited 80. Long-term treatment with low dose HDI depsipeptide induces cell differentiation in chondrosarcoma 81. HDAC 2 inhibition by valproic acid in endometrial stromal sarcoma cells lead to differentiation and arrested cell proliferation55. Low-dose panobinostat acts predominantly as a potent “differentiating” agent, as differentiation of alternative mesenchymal lineages is induced when treating osteosarcoma cells 82. Notch1- and EphrinB1-mediated pathways are directly regulated by HDACs to inhibit myogenic differentiation and enhance migratory capacity of embryonal rhabdomyosarcoma cells. Epigenetic treatment with trichostatin A or vorinostat can reduce the expressing of Notch1 and EphrinB1 in embryonal rhabdomyosarcoma cells 69. Moreover, a new class I and II HDI, MC1742, promote the sarcoma stem cells to osteogenic differentiation at nontoxic doses 83.
4 Preclinical studies of HDIs in sarcoma
4.1 Sensitizing sarcoma to radiotherapy
HDIs could be radiation sensitizers that augment the response of sarcomas to radiation. Addition of vorinostat to radiotherapy has been shown to induce apoptosis and cause cell cycle arrest through elevated p53, p21, and Fas expression in osteosarcoma cell lines84. Moreover, vorinostat enhances radiation in osteosarcoma and rhabdomyosarcoma cells by inhibiting the expression of radiation-induced DNA repair proteins such as Rad51 and Ku80 85. Heavy ion radiotherapy has been shown certain therapeutic advantages including potentially reducing undesirable side-effects, lower risk of secondary malignancies and improvement of clinical outcome and quality of life when compared with conventional photon radiotherapy. In osteosarcoma xenografts, the combination of vorinostat and heavy ion radiotherapy induce a significant delay of tumor growth through the increased rate of apoptosis, increased expression of p53 and p21, and inhibition of proliferation and angiogenesis compared to osteosarcomas treated with heavy ion radiotherapy only 86.
4.2 Sensitizing sarcoma to chemotherapy and reverse multidrug resistance
HDIs such as vorinostat, quisinostat and abexinostat have been shown to increase drug sensitivity in the cells of specific sarcomas. Vorinostat and doxorubicin synergistically induce apoptosis of fibrosarcoma cells and inhibited fibrosarcoma xenograft growth more than when treated with either agent alone87. HDIs vorinostat and quisinostat sensitize rhabdomyosarcoma cells to doxorubicin-induced apoptosis by changing the ratio of pro- and anti-apoptotic BCL-2 family proteins with the downregulation of MCL-1 and BCL-XL, dephosphorylation of BCL-2 and upregulation of BimEL, thus shifting the balance towards apoptosis 88. In addition, the combination treatment of vorinostat and doxorubicin cooperate to induce caspase activation and caspase-dependent apoptosis 89. Temozolomide is an oral chemotherapeutic drug that methylate DNA primarily at O6-guanine. Vorinostat enhances cytotoxicity of temozolomide by downregulating cyclin D1 to induce G0/G1 cell cycle arrest and promoting apoptosis by cleavage of caspase-3 and PARP in Ewing sarcoma cells90. Doxorubicin and etoposide are both topoisomerase II enzyme inhibitors. Vorinostat antagonistically affects the antiproliferative effect of doxorubicin in majority Ewing sarcoma cells, but synergistically enhances the antiproliferative activity of etoposide91. One explanation of the different combined effects was that the molecular mechanisms of the two chemotherapeutics may be different. Doxorubicin performs DNA double-strand damage repair through pathways other than etoposide. Etoposide and doxorubicin also show different repair kinetics92. The manifestation may also be explained by the different biological background of sarcomas. Some sarcomas are with chromatin translocations resulting in abnormal fusion proteins like Ewing sarcomas, synovial sarcoma, and embryonal rhabdomyosarcoma. The fact was that these sarcomas with fusion oncogenes are more sensitive to HDIs treatment than sarcomas lacking known translocations. Abexinostat, an HDI, exhibits significant anti-sarcoma proliferative activity in vitro, by inducing S phase depletion, G2/M cell cycle arrest, and increasing apoptosis. Superior effects are seen when combined with chemotherapy. An abexinostat-induced reduction in Rad51, a major mediator of DNA double-strand break homologous recombination repair, has been shown as a mechanism underlying abexinostat chemo-sensitization 93. The most effective tumor growth inhibition in vitro was observed with doxorubicin and the HDI vorinostat. In the in vivo xenograft mouse model, the combination treatment of doxorubicin and vorinostat did not reduce the tumor growth94. In this study, xenograft animal models were established by subcutaneously injecting the stable oligoclonal cell cultures from freshly tumor species of two patients into immuno-deficient mice. So, the treatment results in the xenograft model were more reliable than in tumor derived cell cultures, as tumor derived cell cultures may do not reflect the actual treatment condition that is observed in clinical patients. Future in vivo models are required to test HDIs and potential chemotherapeutics for the treatment of sarcomas prior to clinical use.
The roles of HDIs in multi-drug resistant sarcomas have been investigated recently. Abexinostat has a synergistic effect on doxorubicin-induced apoptosis in bone sarcoma cells 95. Instead of influencing the protein expression level of P-gp, abexinostat reverses drug resistance in multidrug-resistant sarcoma cells by inhibiting of Rad51 expression and inducing the Gadd45α expression 96. In doxorubicin-resistant osteosarcoma cells, treatment involving both DNMT inhibitors and HDIs inhibit tumor growth by inducing cell growth arrest and reprograming multidrug resistance osteosarcoma cells to osteoblast differentiation 97.
4.3 Synergistic effect of HDIs on kinase inhibitor pazopanib
Patients with alveolar soft part sarcoma, undifferentiated pleomorphic sarcoma and synovial sarcoma have shown promising clinical outcomes after treatment with pazopanib98. Combination treatment of pazopanib and HDI vorinostat have a synergistic effect on killing sarcoma cells. In vitro, co-treatment increases autophagy, inactivates mTOR and activates AKT in sarcoma cells. Knockdown of autophagy associated Beclin1 or Atg 5 reduces drug combination toxicity. In vivo, treatment of xenografted mice carrying sarcomas with pazopanib and valproic acid results in a greater than additive reduction in tumor volume compared with single treatment with either drug 99.
5 HDIs interact with other agents on sarcoma
5.1 Synergistic effect of HDIs and DNMT inhibitors
DNMT inhibitor is another group of epigenetic drugs that has proven useful for the treatment of cancers. Deregulation of epigenetic silencing by histone acetylation and DNA hyper-methylation might play a fundamental role in the etiology of uterine sarcomas 50. Comparable effects are achieved in osteosarcoma cells using vorinostat and the DNMT inhibitor zebularine, with significantly more pronounced cytotoxicity in cells whose molecular phenotypes are indicative of aggressive biological behavior 100. The HDIs entinostat, trichostatin A, phenylbutyrate, LAQ824 and depsipeptide enhance the anti-neoplastic action of 5-aza-dC on Ewing sarcoma cells (Table 3). Combination of 5-aza-dC and entinostat shows marked synergy, and is correlated with significant reactivation of the expression of two tumor suppressor genes, E-cadherin and tumor suppressor lung cancer-1 101. In addition, targeting of DNMT and HDAC activities is highly effective in preventing formation of Ptch-associated tumors. For example, in a study that adopted heterozygous Ptch knockout mice, 5-aza-dC and HDI valproic acid combination therapy reactivates wild-type Ptch expression by reducing methylation of the Ptch promoter and induction of histone hyperacetylation, effectively preventing rhabdomyosarcoma formation 102.
Table 3.
HDAC inhibitors have synergisitic effect with other moleculars
| HDAC Inhibitor | Combination Therapy | Molecular Name | Effect on tumor inhibition |
|---|---|---|---|
| Trichostatin A | DNMT inhibitor | 5-aza-cdR | Inhibit cell proliferation Reduce cell invasion Reverse multidrug resistance |
| Valproic acid | DNMT inhibitor | 5-aza-cdR | Prevent Ptch-associated tumors formation |
| Entinostat | DNMT inhibitor | 5-aza-cdR | Synergistic antitumor effect |
| Vorinostat | DNMT inhibitor | Zebularine | Synergistic antitumor effect |
| Vorinostat | mTOR/PI3K inhibitor | Ridaforolimus/LY | Synergistic antitumor effect |
| Valproic acid | - | Hydralazine | Enhanced the susceptibility of osteosarcoma cells to Fas-/NK cell-mediated cell death |
| Vorinostat | NK cell | - | Enhance NK cell killing cancer cell |
| Vorinostat | TRAIL | - | Synergistic antitumor effect |
| Valproic acid | - | Chloroquine | Synergistic antitumor effect |
| Quisinostat | Proteasome inhibitor | - | Synergistic antitumor effect |
5.2 HDIs augment NK cell immunotherapy of sarcomas
Recent studies have indicated that HDIs can augment NK cell immunotherapy of sarcomas (Table 3). HDI entinostat enhances NK cell killing of sarcoma cells through upregulation of both NKG2D on human NK cells and its ligands MICA/B expression on sarcoma cells 103. Both hydralazine and HDI valproic acid enhance the susceptibility of osteosarcoma cells to NK cell-mediated cell death, and combination treatment further enhanced the effects. Mechanistically, valproic acid and hydralazine increase the expression of cell-surface MICA and B in osteosarcoma cells, and their combination induces a greater increase in their expression. In addition, valproic acid inhibits the production of both soluble MICA and MICB in osteosarcoma cell lines104. Chemotherapy-resistant Ewing sarcoma exhibits reduced susceptibility to resting NK cells. Pretreatment with HDI entinostat induces the expression of NKG2D ligands in an ATM/ATR-dependent manner and sensitizes chemotherapy-resistant Ewing sarcoma cells for NKG2D-dependent cytotoxicity 105.
5.3 HDIs interact with PI3K and mTOR inhibitors
Vorinostat combined with inhibitors of PI3K and mTOR may represent an efficient therapy option for patients with endometrial stromal sarcoma or synovial sarcoma (Table 3). Single vorinostat treatment induces autophagy in endometrial stromal sarcoma cells via inhibition of mTOR activation and reduces the growth of endometrial stromal sarcoma cell lines by inhibiting protein kinase B AKT and mTOR/p70S6K cascade activation. Combination of vorinostat and PI3K and mTOR inhibitors reduces p-p70S6K/p-4E-BP1 and cell growth to the lowest measured levels in endometrial stromal sarcoma cells lines106. Ridaforolimus is a small-molecule inhibitor of mTOR. In synovial sarcoma cell lines, the combination of ridaforolimus and vorinostat demonstrates in vitro synergism, as vorinostat abrogates ridaforolimus-induced AKT activation which may be a possible mechanism of resistance to mTOR inhibitors 107.
6 Clinical trials of HDIs in sarcoma
Based on the significant anti-sarcoma activities of HDIs as single agents in preclinical studies, beneficial performance of HDIs in clinical treatment for patients with sarcomas is anticipated (Table 4). In one case report, a female with Ewing sarcoma developed inoperable progressive lung metastases and was treated with the panobinostat. During 18 months of treatment, no new lesions appeared 108. In another case of a female with leiomyosarcoma who had progressed through multiple chemotherapeutic agents achieved a partial response to vorinostat treatment 109. A phase II study was conducted to assess the clinical efficacy of monotherapy of oral panobinostat for patients with advanced soft tissue sarcoma. In this cohort study of 47 patients, 17 patients had stable disease and six patients were progression-free at 6 months 110. SB939 is an oral inhibitor of classes 1 and 2 HDAC. In a phase II trial, eligible patients with recurrent or metastatic translocation-associated sarcoma were treated with SB939. Fourteen patients were assessable for response with confirmed specific chromosomal translocations. Among these, 8 patients achieved stable disease with a median duration of 5.4 months. The 3-month progression-free survival rate was 49% 111. Clinical outcomes of vorinostat in patients with locally advanced or metastatic soft tissue sarcomas, that failed first-line treatment with anthracycline-based chemotherapy, was investigated in a phase II trial. Stable disease was seen in a subgroup of 23% of 40 patients. Median progression-free survival was 3.2 months 112. Even though there was a modest clinical outcome of HDI monotherapy for sarcomas, further exploration of combination regimens is warranted.
Table 4.
Clinical trials of HDAC inhitors in sarcoma
| HDAC | Combination therapy | Phase | Number of sarcoma patients | Dose |
|---|---|---|---|---|
| Panobinostat | - | II | 47 | 20–40mg thrice weekly |
| Pracinostat | - | II | 22 | 60 mg/day every other day for 3 of 4 weeks |
| Vorinostat | - | II | 40 | 400 mg po qd for 28 day followed by a treatment-free period of 7 day |
| Abexinostat | Doxorubicin | I | 22 | Abexinostat: 45 mg/m2 twice daily administered on days 1 through 5 Doxorubicin: 75 mg/m2 on day 4 of a 3- week cycle |
| Belinostat | Doxorubicin | I/II | 41 | Belinostat:1000 mg/m2/day Doxorubicin: 75mg/m2 |
| Panobinostat | Epirubicin | I | 21 | Panobinostat: 50 mg Epirubicin:75 mg/m2 |
| Abexinostat | Pazopanib | I | 6 | Abexinostat: 30–45 mg/m2 twice Daily Pazopanib:400–800 mg Daily |
A phase I study evaluated the clinical parameters of oral abexinostat when administered to metastatic sarcoma patients in combination with doxorubicin. The maximum tolerated dose of abexinostat and doxorubicin was significantly higher when patients received granulocyte-colony stimulating factor (G-CSF). More importantly, the maximum tolerated dose of abexinostat and doxorubicin exceeded doses for maximal histone acetylation, so that the effectiveness of this combination would not be limited by inadequate dosing. Clinical benefit of this combination resulted in 7 out of 17 evaluable participants maintained stable disease for at least 5 cycles of chemotherapy 113. Another study evaluated the response rate of belinostat in combination with doxorubicin for patients with soft tissue sarcoma. The combination therapy was well tolerated in these patients. Response rate was moderate but median time to progression was 6.0 months which is superior to some reports using single-agent doxorubicin 114. Clinical outcomes of combining panobinostat and epirubicin for refractory sarcoma patients suggested that the combination treatment is well tolerated and may reverse anthracycline resistance. In this clinical trial, 12 out of 20 patients derived clinical benefit (1 partial response and 11 stable disease, median overall survival 8.3 months), including 8 patients who previously progressed on topoisomerase II therapy 115.
HDIs in combination with multityrosine kinase inhibitor pazopanib, mTOR inhibitor sirolimus, cyclin dependent kinase inhibitor flavopiridol and therapeutic proteasome inhibitor bortezomib were also evaluated in clinical trials. In a latest reported phase I trial, the addition of HDI abexinostat to pazopanib is well tolerated and resulted in durable responses in patients who experienced prior progression during treatment with VEGF inhibitors. Peripheral blood histone acetylation and HDAC2 gene expression were associated with durable response to treatment, which supports epigenetically mediated reversal of treatment resistance 116. In a phase I dose-escalation study of the mTOR inhibitor sirolimus and the HDI vorinostat in patients with advanced malignancy, stable disease was observed in patient with fibromyxoid sarcoma 117. A phase I study was conducted to examine the effect of pulse-dose vorinostat plus flavopiridol treatment in 34 patients, 7 of which were sarcoma patients. Clinical outcomes support the strategy of combining intermittent high dose vorinostat with other anti-cancer agents where higher vorinostat levels may enhance efficacy 118. In a phase I trial of vorinostat and bortezomib, the combination was generally well-tolerated at doses that achieved clinical benefit in relapsed or refractory sarcoma patients. Bortezomib did not affect the pharmacokinetics of vorinostat 119. A later study from the same center was conducted to evaluate an intermittent dosing schedule of vorinostat with bortezomib. Stable disease was found in two pre-treated sarcoma patients 120.
7 Mechanisms of HDIs resistance in sarcoma
Molecular mechanisms inducing resistance to HDIs in sarcomas have not been fully documented. However, understanding the mechanisms of resistance are crucial to overcome this resistance with combination treatments or to develop new HDIs. Combination treatment of HDIs with agents targeting those signaling pathways synergistically induces the death of sarcoma cells. The effect of HDIs, like standard chemotherapeutic agents, can be affected by drug efflux, target overexpression and desensitization, epigenetic silencing, anti-apoptotic or pro-survival mechanisms. Drug efflux is caused partly by overexpression of ATP binding cassette transporter superfamily, which includes P-gp. In fact, romidepsin resistance of osteosarcoma cells was related to P-gp overexpression and MAPK pathway activated by romidepsin. Treatment of romidepsin-resistant clones with the P-gp inhibitor verapamil restored romidepsin sensitivity. Besides, the combined treatment of romidepsin and a MEK inhibitor effectively overcame romidepsin resistance 121. It is also suggested that other epigenetic modulators can lead to resistance to HDIs in sarcomas. The combination of vorinostat and TRAIL synergistically enhanced the intrinsic apoptosis pathway. However, apoptosic resistance was caused by reduced expression of caspase-8 and TRAIL-R1 in sarcoma cells, due to epigenetic silencing by DNA hyper-methylation of gene promoter sequences. Treatment with 5-aza-dC restored gene expression and increased the sensitivity of both cell lines against TRAIL-induced apoptosis 50. Expression levels of pro-apoptotic and anti-apoptotic BCL-2 protein families are also important for sensitivity to HDIs. Indeed, overexpression of anti-apoptotic BCL-2 family proteins BCL-2 and BCL-XL attenuated the activity of HDIs and inhibited the synergistic induction of apoptosis induced by combination treatment of HDIs and chemotherapy agents in sarcoma 88, 89.
Conclusion and prospective
Overall, HDIs display several desirable properties for a novel therapeutic, notably a broad range of biologic effects across cellular pathways including apoptosis, cell cycle, autophagy, cell differentiation and ROS generation in sarcomas. These molecular evidences reveal that HDIs may be a promising treatment agent for patients with sarcomas and lay foundation on the clinical application of HDIs in sarcomas. What’s more, evidences from preclinical studies showed that HDIs can not only sensitize sarcomas to radiotherapy and chemotherapy, but also increase the treatment efficacy when combine chemotherapeutic drugs or other targeted drugs in sarcomas. However, the problem is that HDIs may be too blunt of an instrument to modulate very subtle nuances in cellular signaling. In some subtypes of sarcomas, combination treatment with HDIs and chemotherapy was different between animal models and cell culture models. New and better animal models are required to test HDIs for the treatment of sarcomas prior to clinical use. Another challenge is that sarcomas have diverse subtypes and genetic alterations involve oncogenic somatic mutations in some subtypes and chromosomal translocations in other subtypes. In some context, sarcomas with chromosomal translocations can be more sensitive to HDI treatment than sarcomas lacking known translocations. As HDIs can regulate gene expression either via HDACs or by directly inhibiting these fusion oncogenic fusion proteins, further studies are needed to focus on the gene level of regulation of HDIs in sarcomas. Four HDIs have been approved by the FDA for treatment of hematopoietic neoplasm based on evidence showing benefit from HDIs in vivo and in vitro models, and in clinical trials. However, HDIs as monotherapy do not appear to be effective for sarcomas patients. Compared to HDIs on hematopoietic neoplasm, clinical trials in sarcomas revealed that combination therapy with other therapeutics including chemotherapy drugs and small-molecular inhibitors such as pazopanib, sirolimus, flavopiridol and bortezomib seem more promising than HDIs as monotherapy. An improved understanding of the disease specific targets and the development of appropriate biomarkers will improve the efficacies of HDIs in future human sarcoma trials to reverse drug resistance.
Highlights.
Sarcomas are a diverse group of difficult-to-treat malignant tumors.
Histone deacetylase inhibitors are epigenetic drugs that can inhibit sarcoma growth in vitro and in vivo.
Histone deacetylase inhibitors can increase treatment efficiency when combined with chemotherapeutic drugs.
Histone deacetylase inhibitors represent a promising new class of compounds for the treatment of sarcomas.
Acknowledgments
This work was supported, in part, by the Gattegno and Wechsler funds, the Kenneth Stanton Fund, and the Jennifer Hunter Yates Foundation. Zhenfeng Duan is supported, in part, through a Grant from the Sarcoma Foundation of America (SFA), a Grant from National Cancer Institute (NCI)/National Institutes of Health (NIH), UO1, CA151452-01. This work was also supported by funds from the National Cancer Institute of the National Institutes of Health under Award Number U54CA168512. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Fan Tang is supported by a scholarship from the China Scholarship Council (Award Number 201606240118).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burningham Z, Hashibe M, Spector L, Schiffman JD. The Epidemiology of Sarcoma. Clinical sarcoma research. 2012;2:14. doi: 10.1186/2045-3329-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berenson JR, Hilger JD, Yellin O, Boccia RV, Matous J, Dressler K, et al. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Annals of hematology. 2014;93:89–98. doi: 10.1007/s00277-013-1910-2. [DOI] [PubMed] [Google Scholar]
- 3.Gertz MA. Panobinostat in multiple myeloma. The Lancet Haematology. 2016;3:e552–e3. doi: 10.1016/S2352-3026(16)30169-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee HZ, Kwitkowski VE, Del Valle PL, Ricci MS, Saber H, Habtemariam BA, et al. FDA Approval: Belinostat for the Treatment of Patients with Relapsed or Refractory Peripheral T-cell Lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:2666–70. doi: 10.1158/1078-0432.CCR-14-3119. [DOI] [PubMed] [Google Scholar]
- 5.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. The Biochemical journal. 2003;370:737–49. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunology and cell biology. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- 7.Smith BC, Hallows WC, Denu JM. Mechanisms and molecular probes of sirtuins. Chemistry & biology. 2008;15:1002–13. doi: 10.1016/j.chembiol.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. American journal of translational research. 2011;3:166–79. [PMC free article] [PubMed] [Google Scholar]
- 9.Baek MH, Park JY, Rhim CC, Park Y, Kim KR, Kim JH, et al. Immunohistochemical Characterization of Histone Deacetylase as a Potential Prognostic Marker and Therapeutic Target in Endometrial Stromal Sarcoma. Anticancer research. 2016;36:2527–34. [PubMed] [Google Scholar]
- 10.Pacheco M, Nielsen TO. Histone deacetylase 1 and 2 in mesenchymal tumors. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:222–30. doi: 10.1038/modpathol.2011.157. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Wei L, Chen Q, Terek RM. HDAC4 represses vascular endothelial growth factor expression in chondrosarcoma by modulating RUNX2 activity. The Journal of biological chemistry. 2009;284:21881–90. doi: 10.1074/jbc.M109.019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Z, Choy E, Nielsen GP, Rosenberg A, Iafrate J, Yang C, et al. Differential expression of microRNA (miRNA) in chordoma reveals a role for miRNA-1 in Met expression. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28:746–52. doi: 10.1002/jor.21055. [DOI] [PubMed] [Google Scholar]
- 13.Scheipl S, Lohberger B, Rinner B, Froehlich EV, Beham A, Quehenberger F, et al. Histone deacetylase inhibitors as potential therapeutic approaches for chordoma: an immunohistochemical and functional analysis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31:1999–2005. doi: 10.1002/jor.22447. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Xia J, Yu YL, Wang SQ, Wei YB, Chen FY, et al. HDAC5 promotes osteosarcoma progression by upregulation of Twist 1 expression. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:1383–7. doi: 10.1007/s13277-013-1189-x. [DOI] [PubMed] [Google Scholar]
- 15.Schofield AV, Gamell C, Bernard O. Tubulin polymerization promoting protein 1 (TPPP1) increases beta-catenin expression through inhibition of HDAC6 activity in U2OS osteosarcoma cells. Biochemical and biophysical research communications. 2013;436:571–7. doi: 10.1016/j.bbrc.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Xie T, Xian M, Wang YJ, Li HY, Ying MD, et al. SIRT1 promotes metastasis of human osteosarcoma cells. Oncotarget. 2016;7:79654–69. doi: 10.18632/oncotarget.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ban J, Aryee DN, Fourtouna A, van der Ent W, Kauer M, Niedan S, et al. Suppression of deacetylase SIRT1 mediates tumor-suppressive NOTCH response and offers a novel treatment option in metastatic Ewing sarcoma. Cancer research. 2014;74:6578–88. doi: 10.1158/0008-5472.CAN-14-1736. [DOI] [PubMed] [Google Scholar]
- 18.Dickson BC, Riddle ND, Brooks JS, Pasha TL, Zhang PJ. Sirtuin 1 (SIRT1): a potential immunohistochemical marker and therapeutic target in soft tissue neoplasms with myoid differentiation. Human pathology. 2013;44:1125–30. doi: 10.1016/j.humpath.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Yu TK, et al. Expression of SIRT1 and DBC1 is associated with poor prognosis of soft tissue sarcomas. PloS one. 2013;8:e74738. doi: 10.1371/journal.pone.0074738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu JQ, Liu P, Si MJ, Ding XY. MicroRNA-126 inhibits osteosarcoma cells proliferation by targeting Sirt1. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:3871–7. doi: 10.1007/s13277-013-0974-x. [DOI] [PubMed] [Google Scholar]
- 21.Ying S, Jianjun H, Xue Y, Shuwei Y, Liyuan Z, Jie W, et al. MicroRNA-133b inhibits cell proliferation and invasion in osteosarcoma by targeting to Sirt1. Oncology research. 2017 doi: 10.3727/096504016X14826089198805. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Shi Y, Huang J, Zhou J, Liu Y, Fu X, Li Y, et al. MicroRNA-204 inhibits proliferation, migration, invasion and epithelial-mesenchymal transition in osteosarcoma cells via targeting Sirtuin 1. Oncology reports. 2015;34:399–406. doi: 10.3892/or.2015.3986. [DOI] [PubMed] [Google Scholar]
- 23.Foss F, Horwitz S, Pro B, Prince HM, Sokol L, Balser B, et al. Romidepsin for the treatment of relapsed/refractory peripheral T cell lymphoma: prolonged stable disease provides clinical benefits for patients in the pivotal trial. Journal of hematology & oncology. 2016;9:22. doi: 10.1186/s13045-016-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie C, Wu B, Chen B, Shi Q, Guo J, Fan Z, et al. Histone deacetylase inhibitor sodium butyrate suppresses proliferation and promotes apoptosis in osteosarcoma cells by regulation of the MDM2–p53 signaling. OncoTargets and therapy. 2016;9:4005–13. doi: 10.2147/OTT.S105418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou WB, Zhu J, Eilers G, Li X, Kuang Y, Liu L, et al. HDACi inhibits liposarcoma via targeting of the MDM2-p53 signaling axis and PTEN, irrespective of p53 mutational status. Oncotarget. 2015;6:10510–20. doi: 10.18632/oncotarget.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Li X, Fan G, Fukushi J, Matsumoto Y, Iwamoto Y, et al. Impairment of p53 acetylation by EWS-Fli1 chimeric protein in Ewing family tumors. Cancer letters. 2012;320:14–22. doi: 10.1016/j.canlet.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Shen T, Li Y, Yang L, Xu X, Liang F, Liang S, et al. Upregulation of Polo-like kinase 2 gene expression by GATA-1 acetylation in human osteosarcoma MG-63 cells. The international journal of biochemistry & cell biology. 2012;44:423–9. doi: 10.1016/j.biocel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Lopez G, Song Y, Lam R, Ruder D, Creighton CJ, Bid HK, et al. HDAC Inhibition for the Treatment of Epithelioid Sarcoma: Novel Cross Talk Between Epigenetic Components. Molecular cancer research: MCR. 2016;14:35–43. doi: 10.1158/1541-7786.MCR-15-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayarthodi S, Fujimura Y, Fang J, Morsalin S, Rao VN, Reddy ES. Anti-Epileptic Drug Targets Ewing Sarcoma. Journal of pharmaceutical sciences and pharmacology. 2014;1:87–100. doi: 10.1166/jpsp.2014.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gastaldi T, Bonvini P, Sartori F, Marrone A, Iolascon A, Rosolen A. Plakoglobin is differentially expressed in alveolar and embryonal rhabdomyosarcoma and is regulated by DNA methylation and histone acetylation. Carcinogenesis. 2006;27:1758–67. doi: 10.1093/carcin/bgl008. [DOI] [PubMed] [Google Scholar]
- 31.Shim JS, Kim DH, Kwon HJ. Plakoglobin is a new target gene of histone deacetylase in human fibrosarcoma HT1080 cells. Oncogene. 2004;23:1704–11. doi: 10.1038/sj.onc.1207289. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Cheng H, Kwan W, Lubieniecka JM, Nielsen TO. Histone deacetylase inhibitors induce growth arrest, apoptosis, and differentiation in clear cell sarcoma models. Molecular cancer therapeutics. 2008;7:1751–61. doi: 10.1158/1535-7163.MCT-07-0560. [DOI] [PubMed] [Google Scholar]
- 33.Cironi L, Petricevic T, Fernandes Vieira V, Provero P, Fusco C, Cornaz S, et al. The fusion protein SS18-SSX1 employs core Wnt pathway transcription factors to induce a partial Wnt signature in synovial sarcoma. Scientific reports. 2016;6:22113. doi: 10.1038/srep22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Ouchida M, Morimoto Y, Yoshida A, Jitsumori Y, Ozaki T, et al. Significant growth suppression of synovial sarcomas by the histone deacetylase inhibitor FK228 in vitro and in vivo. Cancer letters. 2005;224:311–9. doi: 10.1016/j.canlet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Su L, Sampaio AV, Jones KB, Pacheco M, Goytain A, Lin S, et al. Deconstruction of the SS18-SSX fusion oncoprotein complex: insights into disease etiology and therapeutics. Cancer cell. 2012;21:333–47. doi: 10.1016/j.ccr.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubieniecka JM, de Bruijn DR, Su L, van Dijk AH, Subramanian S, van de Rijn M, et al. Histone deacetylase inhibitors reverse SS18-SSX-mediated polycomb silencing of the tumor suppressor early growth response 1 in synovial sarcoma. Cancer research. 2008;68:4303–10. doi: 10.1158/0008-5472.CAN-08-0092. [DOI] [PubMed] [Google Scholar]
- 37.Abraham J, Nunez-Alvarez Y, Hettmer S, Carrio E, Chen HI, Nishijo K, et al. Lineage of origin in rhabdomyosarcoma informs pharmacological response. Genes & development. 2014;28:1578–91. doi: 10.1101/gad.238733.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–6. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 39.Walczak H. Death Receptor–Ligand Systems in Cancer, Cell Death, and Inflammation. Cold Spring Harbor Perspectives in Biology. 2013;5:a008698. doi: 10.1101/cshperspect.a008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnemann J, Dreyer L, Hartwig M, Palani CD, Hong le TT, Klier U, et al. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma cells. Journal of cancer research and clinical oncology. 2007;133:847–58. doi: 10.1007/s00432-007-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koshkina NV, Rao-Bindal K, Kleinerman ES. Effect of the histone deacetylase inhibitor SNDX-275 on Fas signaling in osteosarcoma cells and the feasibility of its topical application for the treatment of osteosarcoma lung metastases. Cancer. 2011;117:3457–67. doi: 10.1002/cncr.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamanegi K, Yamane J, Hata M, Ohyama H, Yamada N, Kato-Kogoe N, et al. Sodium valproate, a histone deacetylase inhibitor, decreases the secretion of soluble Fas by human osteosarcoma cells and increases their sensitivity to Fas-mediated cell death. Journal of cancer research and clinical oncology. 2009;135:879–89. doi: 10.1007/s00432-008-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao-Bindal K, Zhou Z, Kleinerman ES. MS-275 sensitizes osteosarcoma cells to Fas ligand-induced cell death by increasing the localization of Fas in membrane lipid rafts. Cell death & disease. 2012;3:e369. doi: 10.1038/cddis.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe K, Okamoto K, Yonehara S. Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. Cell death and differentiation. 2005;12:10–8. doi: 10.1038/sj.cdd.4401507. [DOI] [PubMed] [Google Scholar]
- 45.Rao-Bindal K, Koshkina NV, Stewart J, Kleinerman ES. The histone deacetylase inhibitor, MS-275 (entinostat), downregulates c-FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Current cancer drug targets. 2013;13:411–22. doi: 10.2174/1568009611313040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croce CM, Reed JC. Finally, An Apoptosis-Targeting Therapeutic for Cancer. Cancer research. 2016;76:5914–20. doi: 10.1158/0008-5472.CAN-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang CK, Yu XD, Li Q, Xie G, Teng Y. Chloroquine and valproic acid combined treatment in vitro has enhanced cytotoxicity in an osteosarcoma cell line. Asian Pacific journal of cancer prevention: APJCP. 2013;14:4651–4. doi: 10.7314/apjcp.2013.14.8.4651. [DOI] [PubMed] [Google Scholar]
- 48.Imai T, Adachi S, Nishijo K, Ohgushi M, Okada M, Yasumi T, et al. FR901228 induces tumor regression associated with induction of Fas ligand and activation of Fas signaling in human osteosarcoma cells. Oncogene. 2003;22:9231–42. doi: 10.1038/sj.onc.1207184. [DOI] [PubMed] [Google Scholar]
- 49.Roh MS, Kim CW, Park BS, Kim GC, Jeong JH, Kwon HC, et al. Mechanism of histone deacetylase inhibitor Trichostatin A induced apoptosis in human osteosarcoma cells. Apoptosis: an international journal on programmed cell death. 2004;9:583–9. doi: 10.1023/B:APPT.0000038037.68908.6e. [DOI] [PubMed] [Google Scholar]
- 50.Frohlich LF, Mrakovcic M, Smole C, Lahiri P, Zatloukal K. Epigenetic silencing of apoptosis-inducing gene expression can be efficiently overcome by combined SAHA and TRAIL treatment in uterine sarcoma cells. PloS one. 2014;9:e91558. doi: 10.1371/journal.pone.0091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su L, Cheng H, Sampaio AV, Nielsen TO, Underhill TM. EGR1 reactivation by histone deacetylase inhibitors promotes synovial sarcoma cell death through the PTEN tumor suppressor. Oncogene. 2010;29:4352–61. doi: 10.1038/onc.2010.204. [DOI] [PubMed] [Google Scholar]
- 52.Shats I, Gatza ML, Liu B, Angus SP, You L, Nevins JR. FOXO transcription factors control E2F1 transcriptional specificity and apoptotic function. Cancer research. 2013;73:6056–67. doi: 10.1158/0008-5472.CAN-13-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng Z, Liu X, Jin J, Xu H, Gao Q, Wang Y, et al. Histone Deacetylase Inhibitor Trichostatin a Promotes the Apoptosis of Osteosarcoma Cells through p53 Signaling Pathway Activation. International journal of biological sciences. 2016;12:1298–308. doi: 10.7150/ijbs.16569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ververis K, Hiong A, Karagiannis TC, Licciardi PV. Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics: targets & therapy. 2013;7:47–60. doi: 10.2147/BTT.S29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hrzenjak A, Moinfar F, Kremser ML, Strohmeier B, Staber PB, Zatloukal K, et al. Valproate inhibition of histone deacetylase 2 affects differentiation and decreases proliferation of endometrial stromal sarcoma cells. Molecular cancer therapeutics. 2006;5:2203–10. doi: 10.1158/1535-7163.MCT-05-0480. [DOI] [PubMed] [Google Scholar]
- 56.Hrzenjak A, Moinfar F, Kremser ML, Strohmeier B, Petru E, Zatloukal K, et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Molecular cancer. 2010;9:49. doi: 10.1186/1476-4598-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kutko MC, Glick RD, Butler LM, Coffey DC, Rifkind RA, Marks PA, et al. Histone deacetylase inhibitors induce growth suppression and cell death in human rhabdomyosarcoma in vitro. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:5749–55. [PubMed] [Google Scholar]
- 58.Sowa Y, Orita T, Hiranabe-Minamikawa S, Nakano K, Mizuno T, Nomura H, et al. Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites. Annals of the New York Academy of Sciences. 1999;886:195–9. doi: 10.1111/j.1749-6632.1999.tb09415.x. [DOI] [PubMed] [Google Scholar]
- 59.Nakatani F, Tanaka K, Sakimura R, Matsumoto Y, Matsunobu T, Li X, et al. Identification of p21WAF1/CIP1 as a direct target of EWS-Fli1 oncogenic fusion protein. The Journal of biological chemistry. 2003;278:15105–15. doi: 10.1074/jbc.M211470200. [DOI] [PubMed] [Google Scholar]
- 60.Sakimura R, Tanaka K, Nakatani F, Matsunobu T, Li X, Hanada M, et al. Antitumor effects of histone deacetylase inhibitor on Ewing’s family tumors. International journal of cancer. 2005;116:784–92. doi: 10.1002/ijc.21069. [DOI] [PubMed] [Google Scholar]
- 61.Lopez G, Bill KL, Bid HK, Braggio D, Constantino D, Prudner B, et al. HDAC8, A Potential Therapeutic Target for the Treatment of Malignant Peripheral Nerve Sheath Tumors (MPNST) PloS one. 2015;10:e0133302. doi: 10.1371/journal.pone.0133302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Z, Ma C, Shan Z, Ju Y, Li S, Zhao Q. Histone deacetylase inhibitors suppress the growth of human osteosarcomas in vitro and in vivo. Journal of BUON: official journal of the Balkan Union of Oncology. 2013;18:1032–7. [PubMed] [Google Scholar]
- 63.Zhu J, Gu J, Ma J, Xu Z, Tao H. Histone deacetylase inhibitors repress chondrosarcoma cell proliferation. Journal of BUON: official journal of the Balkan Union of Oncology. 2015;20:269–74. [PubMed] [Google Scholar]
- 64.Jin S, Tong T, Fan W, Fan F, Antinore MJ, Zhu X, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21:8696–704. doi: 10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- 65.Cheng DD, Yang QC, Zhang ZC, Yang CX, Liu YW. Antitumor activity of histone deacetylase inhibitor trichostatin A in osteosarcoma cells. Asian Pacific journal of cancer prevention: APJCP. 2012;13:1395–9. doi: 10.7314/apjcp.2012.13.4.1395. [DOI] [PubMed] [Google Scholar]
- 66.Lee DH, Zhang Y, Kassam AB, Park MJ, Gardner P, Prevedello D, et al. Combined PDGFR and HDAC Inhibition Overcomes PTEN Disruption in Chordoma. PloS one. 2015;10:e0134426. doi: 10.1371/journal.pone.0134426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arsenault D, Brochu-Gaudreau K, Charbonneau M, Dubois CM. HDAC6 deacetylase activity is required for hypoxia-induced invadopodia formation and cell invasion. PloS one. 2013;8:e55529. doi: 10.1371/journal.pone.0055529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mu X, Brynien D, Weiss KR. The HDAC inhibitor Vorinostat diminishes the in vitro metastatic behavior of Osteosarcoma cells. BioMed research international. 2015;2015:290368. doi: 10.1155/2015/290368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vleeshouwer-Neumann T, Phelps M, Bammler TK, MacDonald JW, Jenkins I, Chen EY. Histone Deacetylase Inhibitors Antagonize Distinct Pathways to Suppress Tumorigenesis of Embryonal Rhabdomyosarcoma. PloS one. 2015;10:e0144320. doi: 10.1371/journal.pone.0144320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aili A, Chen Y, Zhang H. MicroRNA10b suppresses the migration and invasion of chondrosarcoma cells by targeting brainderived neurotrophic factor. Molecular medicine reports. 2016;13:441–6. doi: 10.3892/mmr.2015.4506. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M, Zhang X. Association of MMP-2 expression and prognosis in osteosarcoma patients. International journal of clinical and experimental pathology. 2015;8:14965–70. [PMC free article] [PubMed] [Google Scholar]
- 72.Ailenberg M, Silverman M. Differential effects of trichostatin A on gelatinase A expression in 3T3 fibroblasts and HT-1080 fibrosarcoma cells: implications for use of TSA in cancer therapy. Biochemical and biophysical research communications. 2003;302:181–5. doi: 10.1016/s0006-291x(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 73.Ma L, Maruwge W, Strambi A, D’Arcy P, Pellegrini P, Kis L, et al. SIRT1 and SIRT2 inhibition impairs pediatric soft tissue sarcoma growth. Cell death & disease. 2014;5:e1483. doi: 10.1038/cddis.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hrzenjak A, Kremser ML, Strohmeier B, Moinfar F, Zatloukal K, Denk H. SAHA induces caspase-independent, autophagic cell death of endometrial stromal sarcoma cells by influencing the mTOR pathway. The Journal of pathology. 2008;216:495–504. doi: 10.1002/path.2434. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto S, Tanaka K, Sakimura R, Okada T, Nakamura T, Li Y, et al. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer research. 2008;28:1585–91. [PubMed] [Google Scholar]
- 76.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. European journal of cancer (Oxford, England: 1990) 2004;40:1934–40. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 77.Cheng Y, Cai L, Jiang P, Wang J, Gao C, Feng H, et al. SIRT1 inhibition by melatonin exerts antitumor activity in human osteosarcoma cells. European journal of pharmacology. 2013;715:219–29. doi: 10.1016/j.ejphar.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Hedrick E, Crose L, Linardic CM, Safe S. Histone Deacetylase Inhibitors Inhibit Rhabdomyosarcoma by Reactive Oxygen Species-Dependent Targeting of Specificity Protein Transcription Factors. Molecular cancer therapeutics. 2015;14:2143–53. doi: 10.1158/1535-7163.MCT-15-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laporte AN, Barrott JJ, Yao RJ, Poulin NM, Brodin BA, Jones KB, et al. HDAC and Proteasome Inhibitors Synergize to Activate Pro-Apoptotic Factors in Synovial Sarcoma. PloS one. 2017;12:e0169407. doi: 10.1371/journal.pone.0169407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawamata H, Tachibana M, Fujimori T, Imai Y. Differentiation-inducing therapy for solid tumors. Current pharmaceutical design. 2006;12:379–85. doi: 10.2174/138161206775201947. [DOI] [PubMed] [Google Scholar]
- 81.Sakimura R, Tanaka K, Yamamoto S, Matsunobu T, Li X, Hanada M, et al. The effects of histone deacetylase inhibitors on the induction of differentiation in chondrosarcoma cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:275–82. doi: 10.1158/1078-0432.CCR-06-1696. [DOI] [PubMed] [Google Scholar]
- 82.Cain JE, McCaw A, Jayasekara WS, Rossello FJ, Marini KD, Irving AT, et al. Sustained Low-Dose Treatment with the Histone Deacetylase Inhibitor LBH589 Induces Terminal Differentiation of Osteosarcoma Cells. Sarcoma. 2013;2013:608964. doi: 10.1155/2013/608964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Pompo G, Salerno M, Rotili D, Valente S, Zwergel C, Avnet S, et al. Novel histone deacetylase inhibitors induce growth arrest, apoptosis, and differentiation in sarcoma cancer stem cells. Journal of medicinal chemistry. 2015;58:4073–9. doi: 10.1021/acs.jmedchem.5b00126. [DOI] [PubMed] [Google Scholar]
- 84.Blattmann C, Thiemann M, Stenzinger A, Christmann A, Roth E, Ehemann V, et al. Radiosensitization by histone deacetylase inhibition in an osteosarcoma mouse model. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2013;189:957–66. doi: 10.1007/s00066-013-0372-8. [DOI] [PubMed] [Google Scholar]
- 85.Blattmann C, Oertel S, Ehemann V, Thiemann M, Huber PE, Bischof M, et al. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. International journal of radiation oncology, biology, physics. 2010;78:237–45. doi: 10.1016/j.ijrobp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Blattmann C, Oertel S, Thiemann M, Dittmar A, Roth E, Kulozik AE, et al. Histone deacetylase inhibition sensitizes osteosarcoma to heavy ion radiotherapy. Radiation oncology (London, England) 2015;10:146. doi: 10.1186/s13014-015-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sampson ER, Amin V, Schwarz EM, O’Keefe RJ, Rosier RN. The histone deacetylase inhibitor vorinostat selectively sensitizes fibrosarcoma cells to chemotherapy. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29:623–32. doi: 10.1002/jor.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heinicke U, Kupka J, Fulda S. JNJ-26481585 primes rhabdomyosarcoma cells for chemotherapeutics by engaging the mitochondrial pathway of apoptosis. Oncotarget. 2015;6:37836–51. doi: 10.18632/oncotarget.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heinicke U, Fulda S. Chemosensitization of rhabdomyosarcoma cells by the histone deacetylase inhibitor SAHA. Cancer letters. 2014;351:50–8. doi: 10.1016/j.canlet.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 90.Sampson VB, Vetter NS, Kamara DF, Collier AB, Gresh RC, Kolb EA. Vorinostat Enhances Cytotoxicity of SN-38 and Temozolomide in Ewing Sarcoma Cells and Activates STAT3/AKT/MAPK Pathways. PloS one. 2015;10:e0142704. doi: 10.1371/journal.pone.0142704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Unland R, Clemens D, Heinicke U, Potratz JC, Hotfilder M, Fulda S, et al. Suberoylanilide hydroxamic acid synergistically enhances the antitumor activity of etoposide in Ewing sarcoma cell lines. Anti-cancer drugs. 2015;26:843–51. doi: 10.1097/CAD.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 92.Schonn I, Hennesen J, Dartsch DC. Ku70 and Rad51 vary in their importance for the repair of doxorubicin- versus etoposide-induced DNA damage. Apoptosis: an international journal on programmed cell death. 2011;16:359–69. doi: 10.1007/s10495-010-0564-y. [DOI] [PubMed] [Google Scholar]
- 93.Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:3472–83. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 94.Becker M, Graf C, Tonak M, Radsak MP, Bopp T, Bals R, et al. Xenograft models for undifferentiated pleomorphic sarcoma not otherwise specified are essential for preclinical testing of therapeutic agents. Oncology letters. 2016;12:1257–64. doi: 10.3892/ol.2016.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X, et al. Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer chemotherapy and pharmacology. 2011;67:439–46. doi: 10.1007/s00280-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 96.Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X, et al. Histone deacetylase inhibitor PCI-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer research. 2011;31:1115–23. [PMC free article] [PubMed] [Google Scholar]
- 97.Capobianco E, Mora A, La Sala D, Roberti A, Zaki N, Badidi E, et al. Separate and combined effects of DNMT and HDAC inhibitors in treating human multi-drug resistant osteosarcoma HosDXR150 cell line. PloS one. 2014;9:e95596. doi: 10.1371/journal.pone.0095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakamura T, Matsumine A, Kawai A, Araki N, Goto T, Yonemoto T, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122:1408–16. doi: 10.1002/cncr.29961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tavallai S, Hamed HA, Grant S, Poklepovic A, Dent P. Pazopanib and HDAC inhibitors interact to kill sarcoma cells. Cancer biology & therapy. 2014;15:578–85. doi: 10.4161/cbt.28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thayanithy V, Park C, Sarver AL, Kartha RV, Korpela DM, Graef AJ, et al. Combinatorial treatment of DNA and chromatin-modifying drugs cause cell death in human and canine osteosarcoma cell lines. PloS one. 2012;7:e43720. doi: 10.1371/journal.pone.0043720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hurtubise A, Bernstein ML, Momparler RL. Preclinical evaluation of the antineoplastic action of 5-aza-2′-deoxycytidine and different histone deacetylase inhibitors on human Ewing’s sarcoma cells. Cancer cell international. 2008;8:16. doi: 10.1186/1475-2867-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ecke I, Petry F, Rosenberger A, Tauber S, Monkemeyer S, Hess I, et al. Antitumor effects of a combined 5-aza-2′deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer research. 2009;69:887–95. doi: 10.1158/0008-5472.CAN-08-0946. [DOI] [PubMed] [Google Scholar]
- 103.Zhu S, Denman CJ, Cobanoglu ZS, Kiany S, Lau CC, Gottschalk SM, et al. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharmaceutical research. 2015;32:779–92. doi: 10.1007/s11095-013-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamanegi K, Yamane J, Kobayashi K, Kato-Kogoe N, Ohyama H, Nakasho K, et al. Valproic acid cooperates with hydralazine to augment the susceptibility of human osteosarcoma cells to Fas- and NK cell-mediated cell death. International journal of oncology. 2012;41:83–91. doi: 10.3892/ijo.2012.1438. [DOI] [PubMed] [Google Scholar]
- 105.Berghuis D, Schilham MW, Vos HI, Santos SJ, Kloess S, Buddingh EP, et al. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clinical sarcoma research. 2012;2:8. doi: 10.1186/2045-3329-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quan P, Moinfar F, Kufferath I, Absenger M, Kueznik T, Denk H, et al. Effects of targeting endometrial stromal sarcoma cells via histone deacetylase and PI3K/AKT/mTOR signaling. Anticancer research. 2014;34:2883–97. [PubMed] [Google Scholar]
- 107.Morgan SS, Cranmer LD. Vorinostat synergizes with ridaforolimus and abrogates the ridaforolimus-induced activation of AKT in synovial sarcoma cells. BMC research notes. 2014;7:812. doi: 10.1186/1756-0500-7-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Maldegem AM, Bovee JV, Gelderblom H. Panobinostat-A Potential Treatment for Metastasized Ewing Sarcoma? A Case Report. Pediatric blood & cancer. 2016;63:1840–3. doi: 10.1002/pbc.26077. [DOI] [PubMed] [Google Scholar]
- 109.Lee J, McGuire C. Clinical efficacy of vorinostat in a patient with leiomyosarcoma. Clinical Medicine Insights Oncology. 2012;6:101–5. doi: 10.4137/CMO.S7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassier PA, Lefranc A, Amela EY, Chevreau C, Bui BN, Lecesne A, et al. A phase II trial of panobinostat in patients with advanced pretreated soft tissue sarcoma. A study from the French Sarcoma Group. British journal of cancer. 2013;109:909–14. doi: 10.1038/bjc.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chu QS, Nielsen TO, Alcindor T, Gupta A, Endo M, Goytain A, et al. A phase II study of SB939, a novel pan-histone deacetylase inhibitor, in patients with translocation-associated recurrent/metastatic sarcomas-NCIC-CTG IND 200dagger. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26:973–81. doi: 10.1093/annonc/mdv033. [DOI] [PubMed] [Google Scholar]
- 112.Schmitt T, Mayer-Steinacker R, Mayer F, Grunwald V, Schutte J, Hartmann JT, et al. Vorinostat in refractory soft tissue sarcomas - Results of a multi-centre phase II trial of the German Soft Tissue Sarcoma and Bone Tumour Working Group (AIO) European journal of cancer (Oxford, England: 1990) 2016;64:74–82. doi: 10.1016/j.ejca.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 113.Choy E, Flamand Y, Balasubramanian S, Butrynski JE, Harmon DC, George S, et al. Phase 1 study of oral abexinostat, a histone deacetylase inhibitor, in combination with doxorubicin in patients with metastatic sarcoma. Cancer. 2015;121:1223–30. doi: 10.1002/cncr.29175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vitfell-Rasmussen J, Judson I, Safwat A, Jones RL, Rossen PB, Lind-Hansen M, et al. A Phase I/II Clinical Trial of Belinostat (PXD101) in Combination with Doxorubicin in Patients with Soft Tissue Sarcomas. Sarcoma. 2016;2016:2090271. doi: 10.1155/2016/2090271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas S, Aggarwal R, Jahan T, Ryan C, Troung T, Cripps AM, et al. A phase I trial of panobinostat and epirubicin in solid tumors with a dose expansion in patients with sarcoma. Annals of oncology: official journal of the European Society for Medical Oncology. 2016;27:947–52. doi: 10.1093/annonc/mdw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aggarwal R, Thomas S, Pawlowska N, Bartelink I, Grabowsky J, Jahan T, et al. Inhibiting Histone Deacetylase as a Means to Reverse Resistance to Angiogenesis Inhibitors: Phase I Study of Abexinostat Plus Pazopanib in Advanced Solid Tumor Malignancies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:1231–9. doi: 10.1200/JCO.2016.70.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park H, Garrido-Laguna I, Naing A, Fu S, Falchook GS, Piha-Paul SA, et al. Phase I dose-escalation study of the mTOR inhibitor sirolimus and the HDAC inhibitor vorinostat in patients with advanced malignancy. Oncotarget. 2016;7:67521–31. doi: 10.18632/oncotarget.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dickson MA, Rathkopf DE, Carvajal RD, Grant S, Roberts JD, Reid JM, et al. A phase I pharmacokinetic study of pulse-dose vorinostat with flavopiridol in solid tumors. Investigational new drugs. 2011;29:1004–12. doi: 10.1007/s10637-010-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schelman WR, Traynor AM, Holen KD, Kolesar JM, Attia S, Hoang T, et al. A phase I study of vorinostat in combination with bortezomib in patients with advanced malignancies. Investigational new drugs. 2013;31:1539–46. doi: 10.1007/s10637-013-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deming DA, Ninan J, Bailey HH, Kolesar JM, Eickhoff J, Reid JM, et al. A Phase I study of intermittently dosed vorinostat in combination with bortezomib in patients with advanced solid tumors. Investigational new drugs. 2014;32:323–9. doi: 10.1007/s10637-013-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsubara H, Watanabe M, Imai T, Yui Y, Mizushima Y, Hiraumi Y, et al. Involvement of extracellular signal-regulated kinase activation in human osteosarcoma cell resistance to the histone deacetylase inhibitor FK228 [(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone] The Journal of pharmacology and experimental therapeutics. 2009;328:839–48. doi: 10.1124/jpet.108.147462. [DOI] [PubMed] [Google Scholar]