Abstract
MnO2@PPy core-shell micromaterials are prepared by chemical polymerization of pyrrole on the MnO2 surface. The polypyrrole (PPy) is formed as a homogeneous organic shell on the MnO2 surface. The thickness of PPy shell can be adjusted by the usage of pyrrole. The analysis of SEM, FT-IR, X-ray photoelectron spectroscopy (XPS), thermo-gravimetric analysis (TGA), and XRD are used to confirm the formation of PPy shell. Galvanostatic cell cycling and electrochemical impedance spectroscopy (EIS) are used to evaluate the electrochemical performance as anode for lithium-ion batteries. The results show that after formation of MnO2@PPy core-shell micromaterials, the cyclic performance as anode for lithium-ion batteries is improved. Fifty microliters of PPy-coated caddice-clew-like MnO2 has the best cyclic performances as has 620 mAh g−1 discharge specific capacities after 300 cycles. As a comparison, the discharge specific capacity of bare MnO2 materials falls to below 200 mAh g−1 after 10 cycles. The improved lithium-storage cyclic stability of the MnO2@PPy samples attributes to the core-shell hybrid structure which can buffer the structural expansion and contraction of MnO2 caused by the repeated embedding and disengagement of Li ions and can prevent the pulverization of MnO2. This experiment provides an effective way to mitigate the problem of capacity fading of the transition metal oxide materials as anode materials for (lithium-ion batteries) LIBs.
Electronic supplementary material
The online version of this article (10.1186/s11671-017-2286-3) contains supplementary material, which is available to authorized users.
Keywords: Manganese dioxide, PPy, Lithium-ion battery, Anode material
Background
Since 3d transition metal oxides (MO; where M is Fe, Co, Ni, and Cu) were proposed to serve as high theoretic capacity anodes for lithium-ion batteries by Tarascon et al. [1], many efforts have been made in preparing micro/nano-metal oxides with various morphologies and researching their electrochemical performance as anode for lithium-ion batteries [2–6]. For examples, Zhu’s research group had made monodisperse Fe3O4 and γ-Fe2O3 microspheres via a surfactant-free solvothermal method [3]. They had a high initial discharge capacity of 1307 and 1453 mAh g−1, respectively. After 110 cycles, the discharge capacity remained at 450 mAh g−1 for Fe3O4 and 697 mAh g−1 for γ-Fe2O3. Hongjing Wu et al. had prepared uniform multi-shelled especially quintuple-shelled NiO hollow spheres by a simple shell-by-shell self-assembly hydrothermal treatment. The merit of this research made a significant contribution to the synthetic methodology of multi-shelled hollow structures. But the lithium storage performances of the NiO hollow spheres were not very excellent [4]. MnO2 possess high theoretically gravimetric lithium storage capacity of about 1230 mAh g−1; therefore, many researches are made to the design, synthesis, and applications of MnO2 anodes for lithium-ion battery [7–10]. For instance, Chen’s research group had made γ-MnO2 with hollow microspherical shape and nanocubic shape [11]. After 20 cycles, the discharge capacities of the nanocubes and microspheres were 656.5 and 602.1 mAh g−1. In addition, they had made many researches on MnO2 materials for lithium-ion battery from the year 2000 to now [12, 13]. We also studied the applications of MnO2 anodes for lithium-ion battery, but the discharge specific capacity of bare MnO2 materials felled so fast to below 200 mAh g−1 after 10 cycles [14].
Although transition metal oxides materials have large theoretical specific capacities, all these materials including MnO2 anodes are generally plagued by rapid capacity fading. The reasons for the poor cycling stability are as follows: (1) the electronic conductivity of transition metal oxides materials are usually low, and the electron or ion have difficulties in the diffusion process, resulting in irreversible electrode reaction and fast capacity decay. (2) After charge/discharge cycles, transition metal oxides suffer from enormous mechanical stress and pulverize, leading to electrical contact loss between active particles and current collector. The transition metal oxide particles without electrical contact can no longer participate in the charge/discharge cycles, resulting in capacity fading [15, 16].
Shell coating is an effective strategy to improve the cycling stability. In this structure, to a certain extent, the shell can buffer the structural expansion and contraction of metal oxide materials caused by the repeated embedding and disengagement of the Li ions. For the moment, carbon coating, organic conducting polymer coating, graphene hybrid, and other inorganic compound coating have been used [17, 18]. For instance, Yin et al. prepared polypyrrole (PPy)-coated CuO nanocomposites. The core-shell sample had a high reversible capacity of 760 mAh g−1 which was much better than those of the bare CuO sample [19]. Li et al. prepared graphene-wrapped MnO2 nanoribbons. The reversible specific discharge capacity reached 890 mAh g−1 at 0.1 A g−1 after 180 cycles. Therefore, it is necessary and urgent to make PPy shell coating on MnO2 materials to improve the cyclic stability as anode for lithium-ion batteries [20].
In the present work, to improve the cyclic performance of MnO2 materials as anode for lithium-ion batteries, polypyrrole (an organic conducting polymer) coating had been prepared by chemical polymerization. As a result, the cyclic performance was improved after formation of MnO2@PPy core-shell micromaterials. This experiment provides an effective way to mitigate the problem of capacity fading of the transition metal oxides materials as anode materials for (lithium-ion batteries) LIBs.
Methods
Preparation of Samples
All reagents were of analytical grade and purchased from the Shanghai Chemical Company. The pyrrole was purified by decompressional distillation prior to use and stored at 0–5 °C and guarded against exposure to light to prevent residual polymerization. Other reagents were used without further purification.
The MnO2 micromaterials were prepared using the similar method described by Yu et al. [14, 21] as some modification. To prepare caddice-clew-like MnO2 micromaterial, 1.70 g MnSO4·H2O was dissolved in 15 mL distilled water with vigorous stirring. When the solution was clear, 20 mL aqueous solution containing 2.72 g K2S2O8 were added to the above solution under continuous stirring. Then, the resulting transparent solution was transferred into a Teflon-lined stainless steel autoclave (50 mL) of 80% capacity of the total volume. The autoclave was sealed and maintained at 110 °C for 6 h. After the reaction was completed, the autoclave was allowed to cool to room temperature naturally. The solid black precipitate was filtered, washed several times with distilled water to remove impurities, and then dried at 80 °C in air for 3 h. The obtained caddice-clew-like MnO2 micromaterial was collected for the fabrication of PPy-coated MnO2 materials. Urchin-like MnO2 micromaterial was prepared by the similar method; after adding 1.70 g MnSO4·H2O and 2.72 g K2S2O8 into 35 mL distilled water, 2 mL H2SO4 was then added.
The MnO2@PPy hybrid micromaterials were prepared by chemical polymerization of pyrrole on the MnO2 surface using sodium benzenesulfonate (BSNa) as surfactant and FeCl3 as oxidant. The molar ratio of monomer pyrrole to BSNa was 3:1. First, 0.2 g MnO2 was dispersed into a beaker containing 50 mL of 0.01 mol L−1 BSNa aqueous solution and stirred for 0.5 h. The mixture was put in an ice/water bath (0–5 °C) under stirring. Then, a certain amount of pyrrole was added to the mixture. After stirring for 0.5 h, a small amount of FeCl3 solution was dropwise added into the aqueous solution to start the polymerization process. The gradual change of color from light black to deep black indicated the formation of PPy. The mixture was kept at 0–5 °C under stirring for 12 h to form MnO2@PPy core-shell micromaterials. The thickness of PPy was controlled by pyrrole usage. Finally, the obtained composite was filtered, washed with water and ethanol, and then dried under vacuum at 60 °C for 4 h.
Characterization of Samples
The morphological investigations of SEM images and energy dispersive spectroscopy (EDS) were taken on a scanning electron microscope (QUANTA-200 America FEI Company). The crystallographic structures of the products were determined with XRD which were recorded on a Rigaku D/max-2200/PC with Cu target at a scanning rate of 7°/min with 2θ ranging from 10° to 70°. Fourier transform infrared (FT-IR) spectra of the MnO2@PPy hybrid micromaterials palletized with KBr were performed on a Nicolet IS10 spectrometer. Thermo-gravimetric analysis (TGA) was also used to determine the weight loss of MnO2@PPy hybrid micromaterials at 10 °C/min from 25 to 800 °C in air (MELER/1600H Thermogravimetric Analyzer). X-ray photoelectron spectroscopy (XPS) measurements were recorded on a Ulvac-PHI, PHI5000 Versaprobe-II X-ray photoelectron spectroscope, using Al Kα X-rays as the excitation source. The binding energy obtained in the XPS analysis was calibrated against the C1s peak at 284.8 eV.
Cell Assemply and Electrochemical Studies
Electrochemical lithium-storage properties of the synthesized products were measured by using CR2025 coin-type test cells assembled in a dry argon-filled glove box. To fabricate the working electrode, a slurry consisting of 60 wt.% active materials, 10 wt.% acetylene black, and 30 wt.% poly-vinylidene fluoride (PVDF) dissolved in N-methyl pyrrolidinone was casted on a copper foil, dried at 80 °C under vacuum for 5 h. A lithium sheet was served as counter and reference electrode, while a Celgard 2320 membrane was employed as a separator. The electrolyte was a solution of 1 M LiPF6 in ethylene carbonate (EC)-1,2-dimethyl carbonate (DMC) (1:1 in volume). Galvanostatical charge-discharge experiments were performed by Land electric test system CT2001A (Wuhan Land Electronics Co., Ltd.) at a current density of 0.2 C between 0.01 and 3.00 V (versus Li/Li+). When calculating the specific capacity of MnO2@PPy core-shell micromaterials, the mass of PPy was included. Electrochemical impedance spectroscopy (EIS) measurements were performed on an electrochemical workstation (CHI604D, Chenhua, Shanghai), and the frequency ranged from 0.1 Hz to 100 KHz with an applied AC signal amplitude of 5 mV.
Results and Discussion
Morphological Features of Samples
The morphologies of pure PPy sample, sea-urchin-like MnO2 sample, and the MnO2@PPy hybrid micromaterials with different pyrrole polymerization amount are characterized by SEM measurements. As shown in Fig. 1, the pure PPy sample has a sphere shape with about 800 nm in diameter and tends to agglomerate together as layered rocks. The urchin-like MnO2 sample is shown in Fig. 1a. The MnO2 micromaterial is a uniform sea-urchin-like shape with a diameter of approximately 3 μm, which consists of several straight and radially grown nanorods with uniform length of about 1 μm. The evolution of the morphologies of MnO2@PPy hybrid micromaterials is shown in Fig.1b–e. When the amount of pyrrole is small, the PPy first nucleates and then embeds into the gap of needle-like nanorods of MnO2 samples. The needle-like nanorods in Fig. 1b are obviously wider than those showed in Fig. 1a. When the amount of pyrrole increases to 20 μL, the nanorod structure still exists but not obvious. As pyrrole quantity increases to 30 μL, the needle-like nanorod structure of MnO2 micromaterials disappears completely and become spherical in shape. When pyrrole quantity further increases (Fig. 1e), the PPy shell becomes very thick. Scheme 1 illustrates the possible formation processes for the MnO2@PPy hybrid micromaterials. In the first stage, a tiny crystal nucleus of PPy generates from monomer pyrrole by the oxidation of FeCl3. Then, the crystal nucleus deposits into the gap between the thorns on the surface of the “urchin.” With the continuous polymerization of PPy, the gap between the thorns is gradually filled up. At the end, the whole “urchin” is uniformly coated by PPy. The low magnification SEM images of MnO2@PPy hybrid micromaterials in Additional file 1 confirm that PPy shell is formed uniformly on MnO2@PPy sample.
Fig. 1.
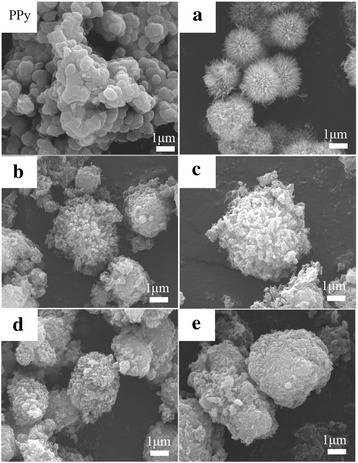
SEM images of PPy coated urchin-like MnO2 sample. In the top left-hand corner is pure PPy, a urchin-like MnO2 sample, b 10 μL, c 20 μL, d 30 μL, and e 50 μL pyrrole-coated urchin-like MnO2 sample. The scale bar is 1 μm
Scheme 1.
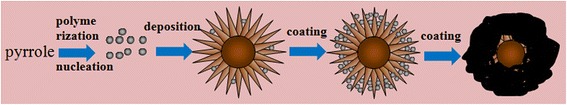
Schematic illustration of the formation mechanism proposed for MnO2@PPy material
In this work, caddice-clew-like MnO2 micromaterial is also coated by PPy using the similar method. The SEM morphologies are shown in Additional file 1: Supporting information 1. The caddice-clew-like MnO2 micromaterial is nanowire shaped and aggregates into 2–4 μm diameter spheres which look like a caddice-clew. When the amount of pyrrole is small, the PPy first forms as small particles and adheres on the surface of the MnO2 samples. With the amount of pyrrole increasing, PPy gradually cover the caddice-clew-like MnO2 completely to form a large block structure which looks like rocks.
The uniform coating of PPy is further verified by energy-dispersive X-ray (EDX) spectroscopy analysis (shown in Table 1). No carbon and nitrogen signals are detected on pure MnO2 sample. Significant amount of carbon and nitrogen signals are detected on PPy and MnO2@PPy samples due to the formation of the PPy shell. With increasing pyrrole usage, the content of carbon and nitrogen are also increased. The EDX data of caddice-clew-like MnO2@PPy samples are shown in Additional file 1: Supporting Information 4.
Table 1.
EDX data for PPy, MnO2, and PPy-coated urchin-like MnO2 sample
| Element | PPy At% | MnO2 At% | MnO2@PPy(10 μL) At% | MnO2@PPy(20 μL) At% | MnO2@PPy(30 μL) At% | MnO2@PPy(50 μL) At% |
|---|---|---|---|---|---|---|
| C | 62.99 | – | 13.78 | 06.51 | 35.11 | 21.50 |
| N | 19.03 | – | 04.94 | 04.62 | 09.64 | 07.03 |
| O | 10.13 | 34.02 | 36.57 | 36.53 | 22.27 | 30.06 |
| Mn | – | 65.98 | 44.71 | 38.84 | 18.23 | 28.35 |
FT−IR Analysis of Samples
The structure features and compositions of the synthesized PPy and MnO2@PPy samples are further characterized by FT-IR spectroscopy (shown in Fig. 2). For all the MnO2@ PPy samples and PPy sample, the bands at 1550, 1448, 1283, and 1130 cm−1 are the characteristic peaks of the PPy rings. Among them, the peak at about 1550 cm−1 is due to C-C and C=C stretching, and the peak at about 1448 cm−1 is from C-N stretching of PPy. The peak at about 1130 cm−1 is due to the S=O stretching vibration peak that belongs to the BSNa, which indicates that the sulfonate ion is doped into the pyrrole ring. The ratio of I1550 and I1448 is usually ascribed to the conjugate and the doping degree of the PPy [22]. The higher the I1550/I1448 is the higher conjugate and doping degree of PPy is. That is, if I1550/I1448 is high, the conductivity of PPy should be better. The bands at 1550, 917, and 778 cm−1 of 30 μL PPy-coated urchin-like MnO2 sample are weaker than those of 50 μL PPy-coated caddice-clew-like MnO2 sample. So, the conductivity of PPy-coated caddice-clew-like MnO2 sample should be better, and the 50 μL PPy-coated caddice-clew-like MnO2 sample should have better lithium-storage performance. Bands at 1040 and 778 cm−1 are the in-plane and out-of-plane vibrations of C-H deformation of Cβ-H absorption band. No Cα-H absorption band is observed in the spectrum, which indicates that the pyrrole ring is predominantly linked by α-α in PPy. The absorption band at 1657 cm−1 is due to the existence of water molecules in the products. Therefore, the FT-IR results prove that PPy shell is formed on MnO2@PPy sample.
Fig. 2.
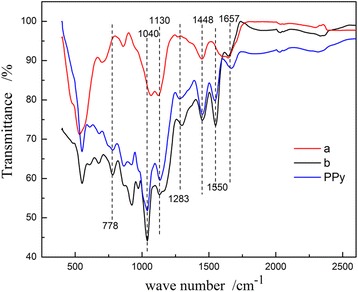
FT-IR spectra of (a) 30 μL PPy-coated urchin-like MnO2 sample and (b) 50 μL PPy-coated caddice-clew-like MnO2 sample and pure PPy
XPS Results
Usually, core-shell structure should be verified by TEM. However, the pure MnO2 sample here is too thick to take good TEM images. So, to verify the core-shell structure, we did XPS test and EDS test to verify the different components in the surface and the whole sample. For clarity, only the spectroscopy of 30 μL PPy-coated urchin-like MnO2 sample and 50 μL PPy-coated caddice-clew-like MnO2 sample are shown in Fig. 3. Others are in the Additional file 1: Supporting Information 5. The final results are listed in Table 2. The main binding energies (BE) of O1s, N1s, C1s, and Mn(2p1/2, 2p3/2) are determined to be 531.2, 398.9, 284.8 and 651.4 and 640.3 eV, respectively. The peaks at 973 and 901.6, and 848.9 eV are O KLL peaks (Auger peaks from oxygen atoms) and Mn LMM peaks (Auger peaks from Mn atoms). There is a few Fe or Cl detected by XPS, shown in Fig. 3. Here, the appearance of Fe or Cl signals is due to the use of FeCl3 as polymerization oxidant in preparing PPy shell. As can be seen in Table 2, the differences of EDS analysis and XPS analysis are distinct. In XPS analysis, the content of O, N, and C are much higher; the content of Mn is lower. The max analysis depth of XPS is about 5–10 nm. The strong O, N, and C peaks confirm that the MnO2 samples are covered by the PPy organic film (as described in the SEM paragraph).
Fig. 3.
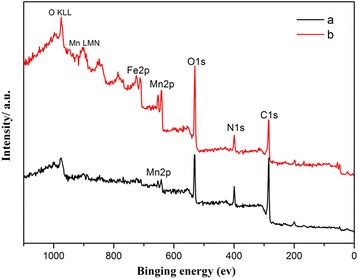
XPS spectra of (a) 30 μL PPy-coated urchin-like MnO2 sample and (b) 50 μL PPy-coated caddice-clew-like MnO2 sample
Table 2.
XPS data for urchin-like MnO2@PPy sample
| Element | MnO2@PPy(10 μL) At% | MnO2@PPy(30 μL)At% | MnO2@PPy(50 μL)At% | |||
|---|---|---|---|---|---|---|
| EDX | XPS | EDX | XPS | EDX | XPS | |
| C | 07.39 | 49.1 | 35.11 | 65.8 | 21.50 | 69.4 |
| N | – | 1.30 | 09.64 | 11.0 | 07.03 | 12.8 |
| O | 16.24 | 46.8 | 22.27 | 22.0 | 30.06 | 16.4 |
| Mn | 13.61 | 2.90 | 18.23 | 0.50 | 28.35 | 1.10 |
TGA Results
To prove the PPy shell on the synthesized MnO2@PPy samples, TGA of bare MnO2 sample, bare PPy, and MnO2@PPy samples are carried out in air. Figure 4 is the TGA results. As can be seen from Fig. 4, the bare PPy powder displays two weight loss regions. The first weight loss about 12% in the temperature range of 60–260 °C can be attributed to the desorption of physisorbed water and removal of surface-absorbed solvents as mentioned in the previous literatures [19, 23, 24]. While the second weight loss about 88% in the range of 260–600 °C is ascribed to the oxidation of PPy. As a result, bare PPy powder is thoroughly burn off at 600 °C. After TGA test, the bare urchin-like MnO2 sample and caddice-clew-like MnO2 sample remain 88.7wt.% and 91.6% at 800 °C. The most weight loss is in the temperature range of 60–300 °C, so it can be ascribed to the removal of surface absorbed solvents, although both samples looked very dry. For 30 μL PPy-coated urchin-like MnO2 sample, the weight loss in the range of 60–260 °C is 10%, and the whole weight loss in the range of 0–800 °C is 32.3%. The change in weight before and after the oxidation of PPy can be directly translated into the amount of PPy in the MnO2@PPy sample [25]. Using this method, the amounts of PPy in the 30 μL PPy-coated urchin-like MnO2 sample is about 22%. This value is close to the theoretical amounts of PPy. For 50 μL PPy-coated caddice-clew-like MnO2 sample, the whole weight loss in the range of 0–800 °C is 43.9% and the weight loss in the range of 60–260 °C is 14%. So, the actual amounts of PPy in 50 μL PPy-coated caddice-clew-like MnO2 sample is about 30% which is much near to the theoretical value. Therefore, the results confirm that the MnO2 particles are covered by the PPy organic film.
Fig. 4.
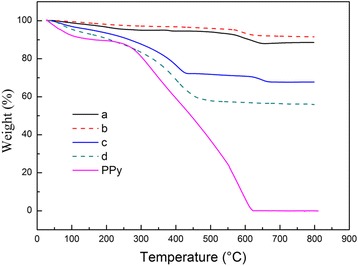
TGA curves of PPy and MnO2 samples. (a) urchin-like MnO2 sample, (b) caddice-clew-like MnO2 sample, (c) 30 μL PPy-coated urchin-like MnO2 sample, and (d) 50 μL PPy-coated caddice-clew-like MnO2 sample
XRD Characterization of Samples
The crystalline structures of MnO2@PPy samples are examined by XRD (Fig. 5). As shown, PPy is an amorphous structure. When coated by PPy, the urchin-like MnO2@PPy samples retain the α-MnO2 structure. The diffraction peaks appear at 2θ = 12.7°, 18.1°, 28.8°, 37.5°, 42.1°, 49.9°, 56.2°, and 60.3° match well with the diffraction peaks of (110),(200),(310),(211),(301),(411),(600), and (521) crystal planes of α-MnO2 standard data (JCPDS card PDF file No. 44-0141). With the increasing of the amount of PPy, the intensity of XRD peaks decrease gradually due to the formation of amorphous PPy. As shown in the PPy-coated caddice-clew-like MnO2 samples, there are obvious amorphous peaks from 15° to 30° in 75 and 100uL samples. When coated by PPy, the caddice-clew-like MnO2@PPy samples retain α-MnO2 structure too. With the increasing of the amount of PPy, the materials obviously change from crystalline to amorphous. These results further prove that the PPy organic film have successfully coated on MnO2 particles.
Fig. 5.
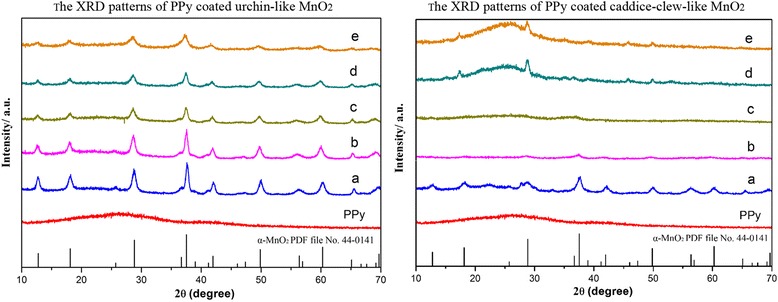
The XRD patterns of PPy-coated MnO2 samples. The left is (a) urchin-like MnO2 sample and (b) 10 μL, (c) 20 μL, (d) 30 μL, and (e) 50 μL PPy-coated. The right is (a) caddice-clew-like MnO2 sample and (b) 30 μL, (c) 50 μL, (d) 75 μL(e), and 100 μL PPy-coated
Electrochemical Performance
The electrochemical performances of these MnO2@PPy samples as anode materials for LIBs are investigated. Figure 6a, b present the typical charge-discharge curves of the anodes (compared to the full battery) constructed from the bare MnO2 sample and MnO2@PPy samples at 0.2 C rate in the voltage range of 0.01–3.00 V (vs. Li/Li+). For clarity, only the bare MnO2 sample and the MnO2@PPy with the best charge-discharge performances are shown. As can be seen, the discharge-charge profiles of MnO2@PPy samples are similar to those of bare MnO2, which indicates that the hybrid products coated by organic PPy shells do not change the electrochemical nature of MnO2 LIBs anodes. However, the lithium storage performance of PPy-coated MnO2 sample has been improved greatly. The bare urchin-like MnO2 sample and PPy-coated urchin-like MnO2 sample both have high initial discharge specific capacity as approximate 1200–1400 mAh g−1, while the theoretical discharge specific capacity is 1232 mAh g−1. The extra discharge specific capacities may result from the formation of SEI layer [14]. After 10 cycles, the discharge specific capacity of bare urchin-like MnO2 sample decreases to below 200 mAh g−1. As a comparison, the discharge specific capacity of PPy-coated urchin-like MnO2 sample remains at about 500 mAh g−1 even after 300 cycles. The caddice-clew-like MnO2 and the PPy-coated caddice-clew-like MnO2 are much similar. After 10 cycles, the discharge specific capacity of bare caddice-clew-like MnO2 decreases to below 200 mAh g−1. The PPy-coated caddice-clew-like MnO2 sample maintains at 500–600 mAh g−1 after 300 cycles.
Fig. 6.
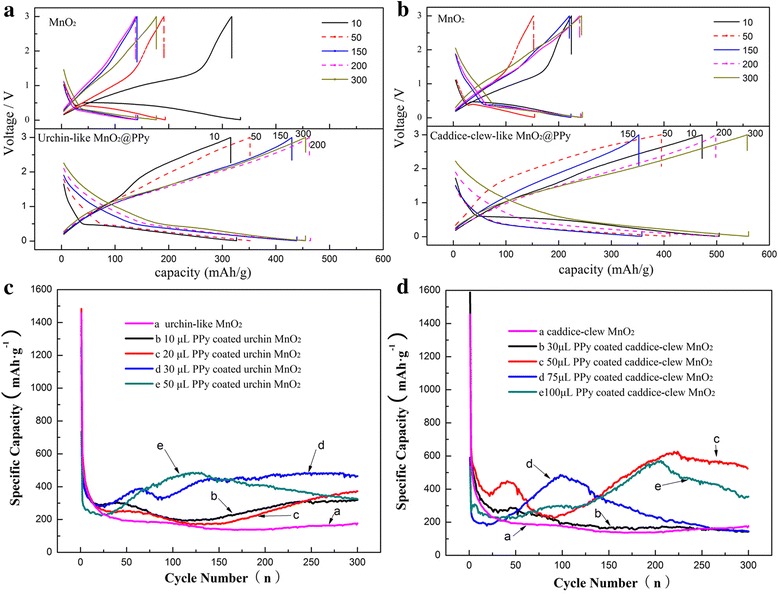
a, b Charge-discharge curves for selected cycles of 30 μL PPy-coated MnO2 sample and 50 μL PPy-coated caddice-clew-like MnO2 sample. c, d The cycling performance of the MnO2 sample and PPy-coated MnO2 samples
To evaluate their lithium-storage cyclic stability, discharge/charge measurements are performed for 300 cycles on MnO2@PPy samples with different pyrroles coated. The thickness of PPy is controlled by the amount of pyrrole. As shown in Fig. 6c, d, when the amount of pyrrole is small (such as 30 uL for caddice-clew-like MnO2 and 10 uL for urchin-like MnO2), the lithium-storage capacity of this hybrid MnO2@PPy sample improves not clearly. This indicates that the PPy film is too thin to prevent MnO2 materials suffering from pulverization. However, when the amount of pyrrole increases, the discharge specific capacities of hybrid MnO2@PPy samples are remarkably enhanced. For caddice-clew-like MnO2, when the amount of pyrrole increases to 50 uL, the hybrid MnO2@PPy sample has the biggest discharge specific capacities as 620 mAh g−1 after 300 cycles. For urchin-like MnO2, the biggest discharge specific capacity appears when 30 uL pyrrole is used. The discharge specific capacity at the 300th cycle is 480 mAh g−1. Furthermore, as can be seen from Fig. 6c, d, all the hybrid MnO2@PPy samples have improved cyclic stabilities. The improved lithium-storage cyclic stabilities of the hybrid MnO2@PPy samples can attribute to the unique structure of the metal oxide/conducting polymer core-shell hybrid products. In this structure, the flexible PPy shell can effectively buffer the structural expansion and contraction of MnO2 caused by the repeated embedding and disengagement of the Li ions. In addition, the PPy shell can prevent the pulverization of MnO2, as well as protect the loss of electrical contact between the MnO2 material and the current collector (copper foil). Whereas, the low capacity and fast capacity fading of bare MnO2 can attribute to the pulverization and loss of inter-particle contact of MnO2 or the contact of MnO2 with copper foil collector due to large volume expansion/contraction during repeated charging-discharging processes. Therefore, this experiment of PPy coating provides an effective way to mitigate the problem of capacity fading of all the transition metal oxide materials as anode materials for LIBs.
The rate performance of MnO2@PPy samples are shown in Fig. 7. To test the rate capability, charge/discharge cycles are performed at the voltage range of 0.01–3.0 V and the discharge rate as 0.2C → 0.5C → 1.0C → 2.0C → 5.0C → 2.0 C → 1.0 C → 0.5C → 0.2C. Figure 7a is the rate capability in the stage from 5.0 to 0.2 C. As shown, the discharge specific capacity of all the MnO2 samples in the 5.0 to 0.2 C stage is much similar to that in the stage from 0.2 to 5 C, which proofs that the MnO2 samples have relatively high reversibility. However, the discharge specific capacities of all the MnO2 samples are poor above the 1 C rate. The merit of the hybrid MnO2@PPy samples in the rate performance can be seen at the low rates (0.2, 0.5, and 1 C). After the discharge at 5 C, the discharge capacity of the PPy coated caddice-clew-like MnO2 sample is 508 mAh g−1 at 0.2 C, while much smaller discharge capacity is obtained as only 160 mAh g−1 at 0.2 C of the bare caddice-clew-like MnO2 sample. So, the PPy-coated caddice-clew-like MnO2 sample has improved rate performance. The situation of the PPy-coated urchin-like MnO2 sample is much similar; nevertheless, the discharge capacity is a little lower than that of the PPy-coated caddice-clew-like MnO2 sample.
Fig. 7.
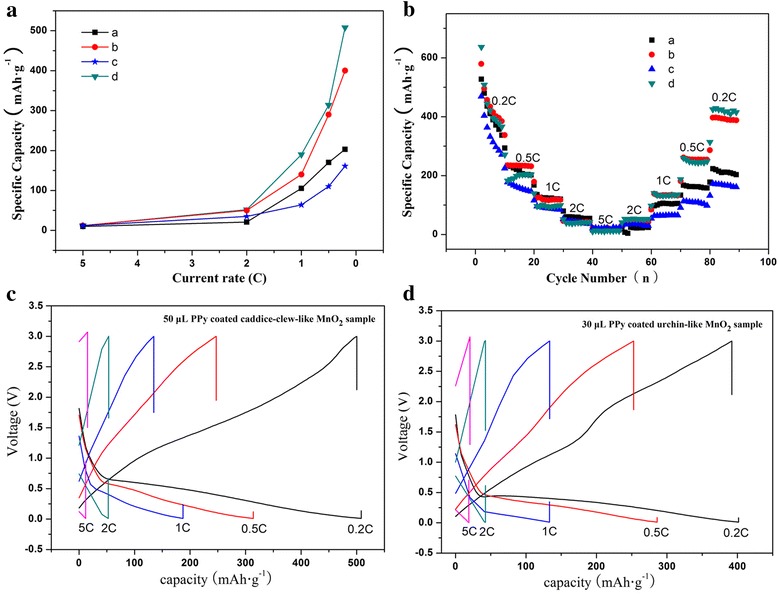
a Rate capability, b rate performance, and c, d charge-discharge curves of the MnO2@PPy samples. (a, b) Urchin-like MnO2 sample and 30 μL PPy-coated sample. (c, d) Caddice-clew-like MnO2 sample and 50 μL PPy-coated sample
As shown in the rate performance, the urchin-like MnO2 micromaterial has relatively higher discharge specific capacity than caddice-clew-like MnO2 micromaterial, which is consistent with previous reports [14]. However, after PPy coating, the caddice-clew-like MnO2@PPy sample has better lithium-storage cyclic stability. Here, the conjugate degree of the PPy may be one reason. The FT-IR analysis indicates that the PPy conjugate degree of the caddice-clew-like MnO2@PPy sample is higher. So, the caddice-clew-like MnO2@PPy sample should have better conductivity and better electrochemical performance. To confirm it, the EIS tests are carried out.
Figure 8 presents the EIS results for lithium cells after the fifth cycle at an ope-circuit voltage. As shown in Fig. 8a, the impedance spectra of caddice-clew-like MnO2 obviously consists of two oblate semicircles in the high-to-medium-frequency region and an inclined line in the low-frequency region. However, the two semicircles of the other three samples are not easily distinguishable. An intercept at the Z real axis in the high-frequency region corresponds to the ohmic electrolyte resistance (R s). The first semicircle in the high frequency ascribes to the Li-ion migration resistance (R sf) through the SEI films. The second semicircle in the high-to-medium frequency ascribes to the charge transfer resistance (R ct). The inclined line at low-frequency region represents the Warburg impedance (W s), which is associated with lithium-ion diffusion in the active material. The semicircular parts of both the hybrid MnO2@PPy samples are much smaller than that of the uncoated MnO2 sample. This indicates that the conductivities of the hybrid MnO2@PPy samples are better and the charge transfer resistance of Li ion decreases after PPy coating. The semicircle resistance of caddice-clew-like MnO2@PPy sample is only 77 Ω. The semicircle resistance of urchin-like MnO2@PPy sample is only 95 Ω. Here, after PPy coating, the lower resistance of caddice-clew-like MnO2 micromaterial can explain the better lithium-storage cyclic stability.
Fig. 8.
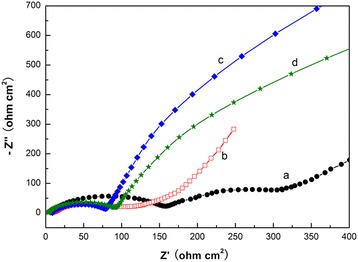
Nyquist plot of Li/MnO2 cells at open-circuit voltage. (a) caddice-clew-like MnO2 sample. (b) Urchin-like MnO2 sample. (c) 50 μL PPy-coated caddice-clew-like MnO2 sample. (d) 30 μL PPy-coated urchin-like MnO2 sample
Conclusions
In summary, MnO2@PPy core-shell micromaterials are successfully prepared by chemical polymerization of pyrrole on the MnO2 surface. The thickness of the PPy shell can be adjusted by the usage of pyrrole. After formation of MnO2@PPy core-shell micromaterials, the cyclic performances as an anode for lithium-ion batteries are improved. Fifty microliters of PPy-coated caddice-clew-like MnO2 has the best cyclic performances and has 620 mAh g−1 discharge specific capacities after 300 cycles. As a comparison, the discharge specific capacity of bare MnO2 materials falls below 200 mAh g−1 after 10 cycles. The improved lithium-storage cyclic stability of the MnO2@PPy samples can attribute to the core-shell hybrid structure. In this structure, the flexible PPy shell can effectively buffer the structural expansion and contraction of MnO2 caused by the repeated embedding and disengagement of Li ions and can prevent the pulverization of MnO2. Therefore, this experiment of PPy coating provides us an effective way to mitigate the problem of capacity fading of the transition metal oxide materials as anode materials for LIBs.
Acknowledgements
This work was financially supported by the Program for National Natural Scientific Fund (Nos. 21463028, 21761035, 21565031, and 21665027), YMU-DEAKIN International Associated Laboratory on Functional Materials, Key Laboratory of Resource Clean Conversion in Ethnic Region, Education Department of Yunnan Province, the general program of the application and basic research foundation of Yunnan province (2013FZ080, 2016FD059); the key project of scientific research foundation of educational bureau of Yunnan province (2015Z118); and the Innovative Training Program of Yunnan Minzu University for Undergraduates and Open Laboratory Project of Yunnan Minzu University for Undergraduates.
Additional file
Supporting information. (DOC 3297 kb)
Authors’ Contributions
The experiments and characterization presented in this work were carried out by LF, ZX, YZ, and RW. The experiments were designed by LF. The experiments were discussed in the results by LF, Y Z, WB, SJ JY, ZZ, and HG. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s11671-017-2286-3) contains supplementary material, which is available to authorized users.
References
- 1.Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM. Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature. 2000;407:496–499. doi: 10.1038/35035045. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Yu A. Nanostructured transition metal oxides as advanced anodes for lithium-ion batteries. Sci Bull. 2016;60:823–838. doi: 10.1007/s11434-015-0771-6. [DOI] [Google Scholar]
- 3.Xu JS, Zhu YJ. Monodisperse Fe3O4 and γ-Fe2O3 magnetic mesoporous microspheres as anode materials for lithium-ion batteries. ACS Appl Mater Interfaces. 2012;4:4752–4757. doi: 10.1021/am301123f. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Wang Y, Zheng C, Zhu J, Wu G, Li X. Multi-shelled NiO hollow spheres: easy hydrothermal synthesis and lithium storage performances. J Alloys Compd. 2016;685:8–14. doi: 10.1016/j.jallcom.2016.05.264. [DOI] [Google Scholar]
- 5.Wang Z, Qu S, Cheng Y, Zheng C, Chen S, Wu H. Facile synthesis of Co3O4 spheres and their unexpected high specific discharge capacity for Lithium-ion batteries. Appl Surf Sci. 2017;416:338–343. doi: 10.1016/j.apsusc.2017.04.194. [DOI] [Google Scholar]
- 6.Wu G, Wu H, Wang K, Zheng C, Wang Y, Feng A. Facile synthesis and application of multi-shelled SnO2 hollow spheres in lithium ion battery. RSC Adv. 2016;6:58069–58076. doi: 10.1039/C6RA11771F. [DOI] [Google Scholar]
- 7.Li B, Rong G, Xie Y, Huang L, Feng C. Low-temperature synthesis of γ-MnO2 hollow urchins and their application in rechargeable Li+ batteries. Inorg Chem. 2006;45:6404–6410. doi: 10.1021/ic0606274. [DOI] [PubMed] [Google Scholar]
- 8.Gu X, Yue J, Li L, Xue H, Yang J, Zhao X. General synthesis of MnOx (MnO2, Mn2O3, Mn3O4, MnO) hierarchical microspheres as lithium-ion battery anodes. Electrochim Acta. 2015;184:250–256. doi: 10.1016/j.electacta.2015.10.037. [DOI] [Google Scholar]
- 9.Liu X, Piao J, Bin D, Zhang T, Duan S, Wu Z, Cao A, Wan L. Controlled formation of uniform nanoshells of manganese oxide and their potential in lithium ion batteries. Chem Commun. 2017;53:2846–2849. doi: 10.1039/C7CC00284J. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Hu Z, Ruan H, Hu R, Su Y, Zhang L, Zhang J. Nanostructured MnO2 anode materials for advanced lithium ion batteries. J Mater Sci Mater Electron. 2016;27:11541–11547. doi: 10.1007/s10854-016-5284-9. [DOI] [Google Scholar]
- 11.Zhao J, Tao Z, Liang J, Chen J. Facile synthesis of nanoporous γ-MnO2 structures and their application in rechargeable Li-ion batteries. Cryst Growth Des. 2008;8:2799–2805. doi: 10.1021/cg701044b. [DOI] [Google Scholar]
- 12.Cheng F, Zhao J, Song W, Li C, Ma H, Chen J, Shen P. Facile controlled synthesis of MnO2 nanostructures of novel shapes and their application in batteries. Inorg Chem. 2006;45:2038–2044. doi: 10.1021/ic051715b. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Han X, Hu Z, Zhang X, Tao Z, Chen J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem Soc Rev. 2015;44:699–728. doi: 10.1039/C4CS00218K. [DOI] [PubMed] [Google Scholar]
- 14.Feng L, Xuan Z, Zhao H, Bai Y, Guo J, Su C. MnO2 prepared by hydrothermal method and electrochemical performance as anode for lithium-ion battery. Nanoscale Res Lett. 2014;9:290. doi: 10.1186/1556-276X-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zhang N, Sun K. 3d transition-metal oxides as anode micro/nano-materials for lithium ion batteries. Process Chem (Chin) 2011;23:2045–2054. [Google Scholar]
- 16.Feng L, Su C, Xuan Z, Guo J, Zhang Y. Recent progress in metal oxide based materials as anode materials for lithium- ion batteries. Nano Biomed Eng. 2013;5:57–64. doi: 10.5101/nbe.v5i1.p57-64. [DOI] [Google Scholar]
- 17.Mao W, Ai G, Dai Y, Fu Y, Ma Y, Shi S, Soe R, Zhang X, Qu D, Tang Z, Battaglia VS. In-situ synthesis of MnO2@CNT microsphere composites with enhanced electrochemical performances for lithium-ion batteries. J Power Sources. 2016;310:54–60. doi: 10.1016/j.jpowsour.2016.02.002. [DOI] [Google Scholar]
- 18.Zou BK, Zhang YY, Wang JY, Liang X, Ma XH, Chen CH. Hydrothermally enhanced MnO/reduced graphite oxide composite anode materials for high performance lithium-ion batteries. Electrochim Acta. 2015;167:25–31. doi: 10.1016/j.electacta.2015.03.108. [DOI] [Google Scholar]
- 19.Yin Z, Fan W, Ding Y, Li J, Guan L, Zheng Q. Shell structure control of PPy-modified CuO composite nanoleaves for lithium batteries with improved cyclic performance. ACS Sustain Chem Eng. 2015;3:507–517. doi: 10.1021/sc500755d. [DOI] [Google Scholar]
- 20.Li L, Raji ARO, Tour JM. Graphene-wrapped MnO2—graphene nanoribbons as anode materials for high-performance lithium ion batteries. Adv Mater. 2013;25:6298–6302. doi: 10.1002/adma.201302915. [DOI] [PubMed] [Google Scholar]
- 21.Yu P, Zhang X, Wang D, Wang L, Ma Y. Shape-controlled synthesis of 3D hierarchical MnO2 nanostructures for electrochemical supercapacitors. Cryst Growth Des. 2009;9:528–533. doi: 10.1021/cg800834g. [DOI] [Google Scholar]
- 22.Gangopadhyay R, De A. Polypyrrole-ferric oxide conducting nanocomposites, 1. Synthesis and characterization. Eur Polym J. 1999;35:1985–1992. doi: 10.1016/S0014-3057(99)00008-7. [DOI] [Google Scholar]
- 23.Cui L, Shen J, Cheng F, Tao Z, Chen J. SnO2 nanoparticles@polypyrrole nanowires composite as anode materials for rechargeable lithium-ion batteries. J Power Sources. 2011;196:2195–2201. doi: 10.1016/j.jpowsour.2010.09.075. [DOI] [Google Scholar]
- 24.Idris NH, Wang J, Chou S, Zhong C, Rahman MM, Liu H. Effects of polypyrrole on the performance of nickel oxide anode materials for rechargeable lithium-ion batteries. J Mater Res. 2011;26:860–866. doi: 10.1557/jmr.2011.12. [DOI] [Google Scholar]
- 25.Yuan L, Wang J, Chew SY, Chen J, Guo ZP, Zhao L, Konstantinov K, Liu HK. Synthesis and characterization of SnO2-polypyrrole composite for lithium-ion battery. J Power Sources. 2007;174:1183–1187. doi: 10.1016/j.jpowsour.2007.06.179. [DOI] [Google Scholar]


