Abstract
Iatrogenic laryngotracheal injury is the most serious complication of endotracheal intubation since this method of establishing airway was first described by Eugene Bouchut in 1858. Even today, subglottic stenosis is the most dreaded complication of intubation. This animal study is focused on the host tissue response to intubation induced injury resulting in subglottic stenosis and methods to prevent this complication. To assess the role of topically applied Mitomycin-C and Triamcinolone Acetonide in wound healing process following post-extubation subglottic injury. Prospective Randomized block, single-blinded, experimental study. Forty New-Zealand white rabbits where block randomized and allocated into 4 groups based on the type of topical medication that was applied post-extubation. Further these groups where subdivided into 3 subgroups based on the time of sacrifice (4, 6 and 12 weeks) to study the histopathological changes that occurred in a temporal sequence at the subglottis. It was observed that the rabbits in the control group and those that received Mitomycin-C only had more respiratory distress compared to those treated with Triamcinolone Acetonide. Statistically significant histopathological changes were observed in all the 4 groups. Mitomycin-C applied topically did not alter the wound healing process following post-extubation injury in the subglottis. Triamcinolone Acetonide significantly altered wound healing in the subglottis and prevented occurrence of respiratory distress.
Keywords: Post-extubation laryngotracheal injury, Subglottic stenosis, Mitomycin-C, Steroids, Triamcinolone
Introduction
Laryngotracheal injuries (LTI) has been the main concern since Eugene Bouchut (1818–1891) described his technique of establishing and maintaining airway by endotracheal intubation (ETI) at the French Academy of medical sciences in 1858 [1]. He was so highly criticized by the members of the academy that he abandoned this procedure. William MaCewen (1880) revived this technique that was later popularized by Joseph P O’Dwyer (1890) [1–3]. Technological advances that aided better visualization of laryngotracheal airway, better quality and design of the endotracheal tube along with the training in performing ETI has revolutionized the way airway intubation is done and made it a fairly safe procedure.
Today, endotracheal intubation is the preferred method for establishing and maintaining airway. There are many complications that have been reported due to this procedure. The most challenging of them, especially in children and in neonates is subglottic stenosis. Schweiger et al. [4] reported an incidence post-intubation subglottic stenosis (PISGS) in children to be around 11.38%. This incidence of subglottic stenosis (SGS) is definitely high and the authors have emphasized the need to identify the risk factors that could alter the wound healing process and prevent SGS [4]. Many drugs have been evaluated and have been described to modulate wound healing in the upper airway [5, 6]. Thus there is a need to identify drugs that could modify the wound healing process in post-extubation laryngotracheal injury (PELTI) and prevent the formation of laryngotracheal stenosis (LTS).
Aim of the study
To assess the role of topical applied Mitomycin-C (MC) and Triamcinolone Acetonide (TA) in wound healing process following post-extubation subglottic injury.
Materials and methods
This study was done at the Central Animal Facility for Toxicology and Developmental Research at Sri Ramachandra University, Chennai, India. Institutional animal ethics committee approval was obtained.
In an earlier study, a rabbit model for inducing laryngotracheal injuries following endotracheal intubation was established. In this study it was observed that New-Zealand white rabbits weighing 2.0–2.5 kg, when intubated by direct laryngoscopic method under sedation with a 4.0 mm cuffed Portex endotracheal tube (Smiths Medicals, Dublin, OH 43017, USA) for 4 h had significant subglottic injury which later had histopathological evidence of subglottic stenosis. All rabbits thus intubated had survived for 12 weeks post-extubation. The group B animal model of laryngotracheal intubation induced injuries that was established and standardized as described in our earlier article was used in the present study [7]. Forty New-Zealand white rabbits were intubated with a no 4.0 cuffed endotracheal tube for 4 h with hourly movement of the tube.
Further, these 40 rabbits were block randomized and allocated into 4 groups based on the topical medication that was applied as shown in Table 1. The medication was locally sprayed using a 23-gauge spinal needle under direct visualization using a laryngoscope on Day 0, end of 1st, 2nd and 3rd week post-extubation. There were 12 rabbits each in group A, B and C and 4 rabbits in group D (control group). The rabbits in the group A, B and C were further randomly divided into 3 subgroups based on the time of at the end of 4th, 6th and 12th week post-extubation when they were humanly sacrificed. This was done to study and compare the temporal sequence of wound healing in all these 3 groups. Post-extubation the rabbits were monitored for the onset and progress of respiratory distress (increased work of breathing/stridor). Post sacrifice the larynx and trachea was harvested and serial sections were done at the level of the subglottis that was histopathologically (HPE) evaluated by a senior pathologist who was blinded to the type of topical medication received by the rabbit’s post-extubation. The HPE features examined in each section were.
Table 1.
Block randomized groups based on medication applied topically
| Group A | Group B | Group C | Group D | |
|---|---|---|---|---|
| Number of rabbits | 12 | 12 | 12 | 4 |
| Type of medication | Mitomycin-C (MC) (0.4 mg/ml) | Triamcinolone acetonide (TA) (1 mg/ml) + MC (0.4 mg/ml) | TA (1 mg/ml) | Isotonic Saline Control |
| Amount of medication | 1 ml | (0.5 ml + 0.5 ml) = 1 ml | 1 ml | 1 ml |
Mucosal changes
Mucosal ulcerations, mucosal scabs, mucosal metaplasia.
Sub-mucosal changes
Sub-mucosal thickening, sub-mucosal vascular thickness, sub-mucosal fibrosis.
Cartilage changes
Perichondritis, cartilage deformity.
The scoring system was standardized. The HPE sections were examined at 12°, 3°, 6° and 9° clock position per continuous high power field for mucosal, sub-mucosal and cartilage pathology i.e. mucosal ulceration, mucosal scabs, mucosal metaplasia, sub-mucosal vascular thickness, sub-mucosal fibrosis, perichondritis and cartilage deformity. This scoring grade was done per cross-section recorded on a grade of 0–3 by a single senior pathologist who was blinded to the nature of injury and medication received by the rabbit whose histopathological slide is being examined. (Grade-0 was no significant pathology, grade-1 was 1–25%, grade-2 was 26–50%, and grade-3 was more than 51%). All these scores and measurements were statistically analyzed using IBM SSPS, Statistics for Windows, Version 20, Armonk, New York. Nonparametric test i.e. K-independent sample test using Kruskal–Wallis test was applied to identify any statistically significant difference in the scores and measurements when comparing the sub-groups i.e. comparing the statistical difference between subgroups in A, B, C, D sacrificed at 4th week. Similar statistical analysis was done between groups at 6th and 12th week of sacrifice. If there were any statistically significant results that were obtained in the above test, then 2-Independent sample test using Mann–Whitney U test was applied to compare the statistical difference between the two sub-groups.
Results
In this study, all the forty rabbits that were intubated and treated with topical medication, survived the intended period of observation according to the group and sub-group they belonged to. These rabbits were humanely sacrificed at the end of the period of observation. During this period, preclinical observations on the sign and symptoms for respiratory distress are shown in Table 2.
Table 2.
Preclinical observation across all groups
| Group A | Group B | Group C | Group D | |
|---|---|---|---|---|
| Total number of rabbits | 12 | 12 | 12 | 4 |
| Signs of airway obstructiona | 6 | 2 | 1 | 3 |
| Onset of respiratory distress | 3rd week—3, 4th week—3 | 3rd week—2 | 4th week—1 | 3rd week—3 |
| Number of week at sacrifice (sub-groups) | ||||
| 4 weeks | 4 | 4 | 4 | – |
| 6 weeks | 4 | 4 | 4 | – |
| 12 weeks | 4 | 4 | 4 | 4 |
aAll rabbits survived the intended period of time before sacrifice
Out of the twelve rabbits in group A (MC), six rabbits had signs and symptoms of airway obstruction in the acute as well as the chronic phase of wound healing. Three out of four rabbits in group D (Control) had signs and symptoms of airway obstruction.
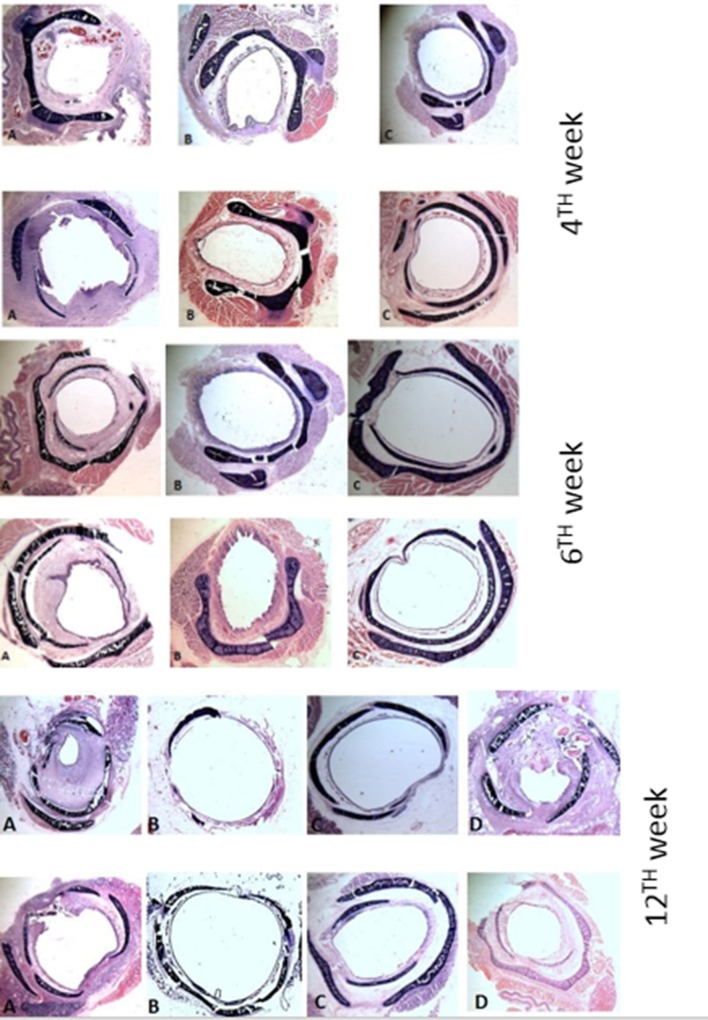
Statistical analysis of the histopathological scores of the 3 sub-groups at the level of subglottis in group A, B and C at 4th week, 6th week and group A, B, C and D at 12th week are shown in Tables 3, 4 and 5 respectively. The histopathological pictures of the cross-section at the level of subglottis at 4th, 6th and 12th week are shown in Figs. 1 and 2.
Table 3.
Histopathological scores at the level of subglottis at 4th week
| Group A (MC) Mean ± SD |
Group B.(MC + TA) Mean ± SD |
Group C (TA) Mean ± SD |
Kruskal–Wallis Test (p value) | Mann–Whitney U test | |||
|---|---|---|---|---|---|---|---|
| Group A and group B p value |
Group A and group C p value |
Group B and group C p value |
|||||
| Mucosal ulceration | 1.75 ± 0.82 | 0 | 0 | 0.005 | 0.013 | 0.013 | 1.000 |
| Mucosal scab | 1 ± 0 | 0 | 0 | 0.004 | 0.008 | 0.008 | 1.000 |
| Sub-mucosal thickening (μm) | 56.36 ± 17.19 | 23.45 ± 4.57 | 15.8 ± 5.01 | 0.012 | 0.021 | 0.021 | 0.083 |
| Sub-mucosal fibrosis | 1.5 ± 0.5 | 1.25 ± 0.5 | 0 | 0.014 | 0.495 | 0.013 | 0.011 |
p value of less than or equal to 0.05 was considered significant
Table 4.
Histopathological scores at the level of subglottis at 6th week
| Group A (MC) Mean ± SD | Group B (MC + TA) Mean ± SD | Group C (TA) Mean ± SD | Kruskal–Wallis test | Mann–Whitney U test | |||
|---|---|---|---|---|---|---|---|
| Group A and group B p value |
Group A and group C p value |
Group B and group C p value |
|||||
| Mucosal metaplasia | 1 ± 0 | 0.25 ± 0.5 | 0 | 0.017 | 0.040 | 0.008 | 0.317 |
| Sub-mucosal thickening (μm) | 90.43 ± 46.98 | 39.71 ± 25.20 | 18.55 ± 5.87 | 0.023 | 0.083 | 0.021 | 0.083 |
| Sub-mucosal fibrosis | 2 ± 0.81 | 0.25 ± 0.5 | 0 | 0.011 | 0.025 | 0.013 | 0.317 |
p value of less than or equal to 0.05 was considered significant
Table 5.
Histopathological scores at the level of subglottis at 12th week
| Group A Mean ± SD |
Group B Mean ± SD |
Group C Mean ± SD |
Group D Mean ± SD |
Kruskal–Wallis test (p value) | Mann–Whitney U test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A and group B p value |
Group A and group C p value |
Group A and group D p value |
Group B and group C p value |
Group B and group D p value |
Group C and group D p value |
||||||
| Sub-mucosal thickening | 86.16 ± 56.82 | 15.77 ± 4.17 | 17.5 ± 2.66 | 84.22 ± 42.40 | 0.01 | 0.021 | 0.021 | 0.773 | 0.773 | 0.021 | 0.021 |
| Sub-mucosal fibrosis | 2.75 ± 0.5 | 0.75 ± 0.5 | 0 | 2.25 ± 0.95 | 0.006 | 0.015 | 0.011 | 0.405 | 0.040 | 0.044 | 0.013 |
p value of less than or equal to 0.05 was considered significant
Fig. 1.
Picture of Hematoxylin and eosinophil stains at the level of subglottis, at 4th, 6th and 12th week, the figures are marked A for group A (MC), B for group B (MC + TA), C for group C (TA) and D for control group
Fig. 2.
Picture of Masson Trichrome stains at the level of subglottis, at 4th, 6th and 12th week, the figures are marked A for group A (MC), B for group B (MC + TA), C for group C (TA) and group D for controls
Discussion
It has been reported that temporary LTI occurred in 62–94% of intubations [8]. A number of factor influence the severity of injury and the progress to formation of SGS. The most critical risk factor for PESGS in neonates and children is the size/diameter of the tube [9, 10]. In a multicentric study that was conducted across seven neonatal intensive care units, Contencin et al. [10] have reported that 5 out of 247 post-intubated neonates showed evidence of respiratory distress that recovered with medical therapy. These authors identified that the size of the endotracheal tube that was used for intubation as the major risk factor for formation of SGS [9, 10].
Identification and prevention of PESGS is the need of the hour. One must be familiar not only with the risk factors but also the use of drugs that could modulate wound healing so as to prevent the occurrence of SGS. Hirshoren and Eliashar [5] have comprehensively reviewed the literature and summarized the role of drugs that modulate wound healing. Of the many drugs used for modulating wound healing, corticosteroids and MC are the commonly used drugs in clinical practice. This study has compared the role of these two drugs individually as well as in combination on the wound healing process in the PESGS with special reference to the subglottis.
Corticosteroids act on all the three phases of wound healing and hence have a definitive role in modulation of wound healing [11, 12]. Human studies on the efficacy of using corticosteroids in SGS are largely limited by case reports. Ratna et al. [13] in a prospective study in 150 patients observed that corticosteroids did not have any beneficial effects in protection of the airway, but, rather resulted in doubling the pulmonary complications.
Initial prospective experimental studies in animal using the combination of steroids along with antibiotics was found to be beneficial for preventing SGS [14, 15]. Aerosolised Dexamethasone was found to be effective in reducing oedema after acute subglottic injury in a ferret animal model when given in the immediate post-injury period at 2, 4 and 6 h [16].
MC is an antimicrobial drug which has both antimetabolic as well as antiproliferative properties. It inhibits fibroblast proliferation and hence modulates wound healing. It is also suggested that MC inhibits wound healing by down regulating the gene expression for extracellular matrix proteins [17]. The biological effect of topical MC is influenced by the concentration and exposure time [18–20]. The role of MC in LTS has been studied in various experimental animal models as shown in Table 6. As can be seen in Table 6, earlier studies have shown that MC has a beneficial effect in reducing fibrosis and stenosis but later studies have shown that MC does not have any beneficial effect in preventing or reducing LTS.
Table 6.
Animal studies for efficacy of Mitomycin-C in LTS
| Sl. no. | References | Animal; number | Type of injury | Dose of MC | Results |
|---|---|---|---|---|---|
| 1. | Eliashar et al. [21] | Canine, 60 | Direct trauma | 0.2 mg/ml | Beneficial |
| 2. | Correa et al. [22] | Canine, 10 | CO2 laser injury | 1% | Reduced subglottic collagen formation |
| 3. | George et al. [23] | Pigs, 26 | Single stage laryngotracheal reconstruction | 0.5 mg/ml | Prevent the liquefactive necrosis Promote neo-chondrification, Improved graft incorporation |
| 4. | Eliashar et al. [24] | Canine, 16 | Induced LTS | 0.5 mg/ml | Does not prevent stenosis |
| 5. | Cincik et al. [25] | Rabbits, 4 in each sub-group | Standardised trauma | 0.4 mg/ml | Reduce fibrosis |
| 6. | Roh et al. [26] | Rabbits, 26 | Laser injury | 0.4 mg/ml | Prevents progression of posterior glottis stenosis |
| 7. | Roh et al. [27] | Canine, 12 | Stripping of vocal cords | 1.0 mg/ml | Minimise anterior glottic stenosis |
| 8. | Roh et al. [28] | Rabbits, 60 | Diode laser | 0.4 mg/ml 1.0 mg/ml | Significant risk of airway obstruction |
| 9. | Roh et al. [29] | Rabbits, 60 | Laser injury | 1.0 mg/ml | No benefit to prevent stenosis |
| 11. | Shvidler, et al. [30] | Ferrets, 20 | Simulated intubation injury | – | No benefit to prevent stenosis |
| 12. | Iñiguez-Cuadra et al. [31] | Rabbits, 18 | End to end anastomosis | 0.2 mg/ml, 0.5 mg/ml | Not effective, can provoke opposite effect |
| 13 | Present study | Rabbits, 40 | Post intubation injury | 0.4 mg/ml | Does not alter the wound heling process |
In this study there was significant reduction in preclinical symptoms of respiratory distress and histopathological evidence of subglottic stenosis in the group treated with TA as compared to the group treated with TA and MC and the group treated with MC only. It was observed that there was no statistically significant modulation of wound healing in the group treated with MC as compared with the control group. Thus it was inferred that MC sprayed topically in the dose of 0.4 mg/dl did not alter the wound healing process as compared to TA. TA when sprayed topically in the immediate post extubation period in multiple doses at weekly interval modulated wound healing in such a way as to prevent the formation subglottic stenosis. Thus, extrapolating to clinical scenario it is suggested that further studies using TA in the form of topical inhalations in high risk children in the immediate post extubation period will be useful.
Conclusion
The best way to manage acquired/iatrogenic laryngotracheal stenosis is to prevent its occurrence. Identifying the high risk patients and using drugs that can modulate wound healing in the immediate post-extubation period to prevent stenosis is the need of the hour. Mitomycin-C in a dosage of 0.4 mg/ml, applied topically in 4 or more sittings does not seem to alter the progress of the healing process and does not prevent post-extubation SGS in rabbits as evidenced in this study. Triamcinolone Acetonide applied topically has shown to be a better modulator of wound healing in the laryngotracheal airway following intubation induced injury compared to Mitomycin-C.
Compliance with ethical standards
Conflict of interest
None.
Ethical approval
Institutional animal ethics committee approval was obtained prior to the start of the study. “All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.” “All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.”
References
- 1.Sperati G, Felisati D. Bouchut, O’Dwyer and laryngeal intubation in patients with croup. Acta Otorhinolaryngol Ital. 2007;27(6):320–333. [PMC free article] [PubMed] [Google Scholar]
- 2.O’Dwyer JP. Fifty cases of croup in private practice treated by intubation of the larynx, with a description for the method and the dangers incident thereto. Med Rec. 1887;32:557–561. [Google Scholar]
- 3.O’Dwyer JP. Two cases of croup treated by tubage of the glottis. NY Med J. 1885;421:146–151. [Google Scholar]
- 4.Schweiger C, Marostica PJ, Smith MM, Manica D, Carvalho PR, Kuhl G. Incidence of post-intubation subglottic stenosis in children: prospective study. J Laryngol Otol. 2013;127(4):399–403. doi: 10.1017/S002221511300025X. [DOI] [PubMed] [Google Scholar]
- 5.Hirshoren N, Eliashar R. Wound-healing modulation in upper airway stenosis-Myths and facts. Head Neck. 2009;31(1):111–126. doi: 10.1002/hed.20925. [DOI] [PubMed] [Google Scholar]
- 6.Hirshoren N, Eliashar R, Weinberger JM. Hydroxychloroquine for subglottic stenosis: a novel therapy in the battle for air. Laryngoscope. 2010;120(4):743–744. doi: 10.1002/lary.20848. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SP, Ravikumar A, Thanka J. An animal model for laryngotracheal injuries: an experimental study. Laryngoscope. 2015;125:E23–E27. doi: 10.1002/lary.24867. [DOI] [PubMed] [Google Scholar]
- 8.Rangachari V, Sundararajan I, Sumathi V, Krishna KK. Laryngeal sequelae following prolonged intubation: a prospective study. Indian J Crit Care Med. 2006;10(3):171–175. doi: 10.4103/0972-5229.27858. [DOI] [Google Scholar]
- 9.Pashley NRT. Risk factors and the prediction of outcome in acquired subglottic stenosis in children. Int J Pediatr Otorhinolaryngol. 1982;4(1):1–6. doi: 10.1016/0165-5876(82)90071-4. [DOI] [PubMed] [Google Scholar]
- 10.Contencin P, Narcy P, Holinger LD. Size of endotracheal tube and neonatal acquired subglottic stenosis. Arch Otolaryngol Head Neck Surg. 1993;119(8):815–819. doi: 10.1001/archotol.1993.01880200015002. [DOI] [PubMed] [Google Scholar]
- 11.Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998;11:277–285. [PubMed] [Google Scholar]
- 12.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/S0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 13.Ratna TE, Vijaya K, Mohan K, Ahmed S, Saeed Al G, Anil PM, Oka BC. Post intubation laryngeal sequelae in an intensive care unit. J Laryngol Otol. 1995;109:313–316. doi: 10.1017/s0022215100130002. [DOI] [PubMed] [Google Scholar]
- 14.Croft CB, Zub K, Borowiecki B. Therapy of iatrogenic subglottic stenosis: a steroid/antibiotic regimen. Laryngoscope. 1979;89(3):482–489. doi: 10.1288/00005537-197903000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Supance JS. Antibiotics and steroids in the treatment of acquired subglottic stenosis: a canine model study. Ann Otol Rhinol Laryngol. 1983;92(4 Pt 1):377–382. doi: 10.1177/000348948309200417. [DOI] [PubMed] [Google Scholar]
- 16.Kryzer TC, Jr, Gonzalez C, Burgess LP. Effects of aerosolized dexamethasone on acute subglottic injury. Ann Otol Rhinol Laryngol. 1992;101(1):95–99. doi: 10.1177/000348949210100121. [DOI] [PubMed] [Google Scholar]
- 17.Gray SD, Tritle N, Li W. The effect of mitomycin on extracellular matrix proteins in a rat wound model. Laryngoscope. 2003;113(2):237–242. doi: 10.1097/00005537-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Cheung JC, Wright MM, Shobana M, Pederson JE. Intermediate-term outcome variable dose mitomycin-C filtering surgery. Ophthalmology. 1997;104:143–149. doi: 10.1016/S0161-6420(97)30347-9. [DOI] [PubMed] [Google Scholar]
- 19.Hu D, Sires BS, Tong DC, Royak GA, Oda D. Effect of brief exposure to mitomycin C on cultured human nasal mucosa fibroblast. Ophthalmic Plast Recontr Surg. 2000;16:119–125. doi: 10.1097/00002341-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: Are two applications better than one? Laryngoscope. 2009;119:272–283. doi: 10.1002/lary.20056. [DOI] [PubMed] [Google Scholar]
- 21.Eliashar R, Eliachar I, Esclamado R, Gramlich T, Strome M. Can topical mitomycin prevent laryngotracheal stenosis? Laryngoscope. 1999;109:1594–1600. doi: 10.1097/00005537-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Correa AJ, Reinisch L, Sanders DL, Huang S, Deriso W, Duncavage JA, Garrett CG. Inhibition of subglottic stenosis with mitomycin-C in the canine model. Ann Otol Rhinol Laryngol. 1999;108(11 Pt 1):1053–1060. doi: 10.1177/000348949910801106. [DOI] [PubMed] [Google Scholar]
- 23.Coppit G, Perkins J, Munaretto J, Nielsen R, McKinney L, Ulnick K. The effects of mitomycin-C and stenting on airway wound healing after laryngotracheal reconstruction in a pig model. Int J Pediatr Otorhinolaryngol. 2000;53(2):125–135. doi: 10.1016/S0165-5876(00)00322-0. [DOI] [PubMed] [Google Scholar]
- 24.Eliashar R, Gross M, Maly B, Sichel JY. Mitomycin does not prevent laryngotracheal repeat stenosis after endoscopic dilation surgery: an animal study. Laryngoscope. 2004;114:743–746. doi: 10.1097/00005537-200404000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Cincik H, Gungor A, Cakmak A, Omeroglu A, Poyrazoglu E, Yildirim S, Cekin E, Candan H. The effects of mitomycin C and 5-fluorouracil/triamcinolone on fibrosis/scar tissue formation secondary to subglottic trauma (experimental study) Am J Otolaryngol. 2005;26(1):45–50. doi: 10.1016/j.amjoto.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Roh JL. Prevention of posterior glottic stenosis by mitomycin C. Ann Otol Rhinol Laryngol. 2005;114(7):558–562. doi: 10.1177/000348940511400712. [DOI] [PubMed] [Google Scholar]
- 27.Roh JL, Yoon YH. Prevention of anterior glottic stenosis after bilateral vocal fold stripping with mitomycin C. Arch Otolaryngol Head Neck Surg. 2005;131(8):690–695. doi: 10.1001/archotol.131.8.690. [DOI] [PubMed] [Google Scholar]
- 28.Roh JL, Yoon YH. Effect of acid and pepsin on glottic wound healing: a simulated reflux model. Arch Otolaryngol Head Neck Surg. 2006;132(9):995–1000. doi: 10.1001/archotol.132.9.995. [DOI] [PubMed] [Google Scholar]
- 29.Roh JL, Kim D, Rha K, Sung M, Kim K, Park C. Benefits and risks of mitomycin use in the traumatized tracheal mucosa. Otolaryngol Head Neck Surg. 2007;136(3):459–463. doi: 10.1016/j.otohns.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Shvidler J, Bothwell NE, Cable B. Refining indications for the use of mitomycin C using a randomized controlled trial with an animal model. Otolaryngol Head Neck Surg. 2007;136(4):653–657. doi: 10.1016/j.otohns.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Iñiguez-Cuadra R, San Martín Prieto J, Iñiguez-Cuadra M, Zúñiga Erranz S, Jofré Pavez D, González Bombardiere S, Guilemany Toste JM, Iñiguez-Sasso R. Effect of mitomycin in the surgical treatment of tracheal stenosis. Arch Otolaryngol Head Neck Surg. 2008;134(7):709–714. doi: 10.1001/archotol.134.7.709. [DOI] [PubMed] [Google Scholar]