Abstract
Purpose
This study aims to report a case of ovarian hyperstimulation syndrome (OHSS) following GnRH agonist trigger for final follicular maturation.
Methods
This study is a retrospective chart review.
Results
We report the first case of OHSS following GnRH agonist trigger for final follicular maturation and freeze-all, masking extrauterine pregnancy (EUP). The present case report elucidates the feasibility of stimulating and recruiting ovarian follicles yielding mature oocytes during early pregnancy and the ability of GnRH agonist to trigger final follicular maturation during pregnancy, in the presence of high progesterone and hCG levels.
Conclusions
Since OHSS almost always develops after hCG administration or in early pregnancy, its occurrence following GnRH agonist trigger should alert physician to search for either an inadvertent administration of exogenous hCG, or the endogenous secretion of hCG by pregnancy, e.g. EUP, or as part of a paraneoplastic syndrome.
Keywords: GnRH agonist, Trigger, OHSS, EUP
Introduction
Ovarian hyperstimulation syndrome is a serious complication of controlled ovarian hyperstimulation (COH), usually presents either after hCG administration in susceptible patients or during early pregnancy [1]. Despite many years of clinical experience, the pathophysiology of ovarian hyperstimulation syndrome (OHSS) is poorly understood and there is no reliable test to predict patients who will subsequently develop severe OHSS [2].
Controlled ovarian hyperstimulation, which combines GnRH antagonist (GnRHant) co-treatment and GnRH agonist (GnRHa) trigger, has recently become a common tool aiming to eliminate severe early OHSS and to support the concept of an OHSS-free clinic [3–5]. However, due to the reported significantly reduced clinical pregnancy and increased first trimester pregnancy loss [6, 7], freeze-all policy is offered, as one option, in an attempt to ensure OHSS-free clinic and maintain a reasonable cumulative pregnancy rate [5].
Recently, three cases of OHSS following GnRHa trigger for final oocyte maturation and a freeze-all approach were reported [8, 9]. These cases are debatable, since the presence of hCG (inadvertently injected or as a result of early pregnancy), a mandatory culprit in the etiology of OHSS, was not utterly excluded.
Herewith, we report the case of OHSS following GnRHa trigger for final follicular maturation, masking EUP.
Case report
A 36-year-old woman, gravida 2, para 1, was referred to our Infertility and IVF Unit for IVF treatment, following two failed timed intrauterine inseminations (IUI) and three COH with IUI. Her gynecological and medical histories were unremarkable, except for a history of artificial abortion and a previous cesarean section due to no progress of labor. She reported on normal PAP smear, previous use of combined oral contraception with no history of pelvic inflammatory disease. On infertility evaluation, the day 3 hormonal profile was within normal range and her hyterosalpingography revealed normal uterine cavity with two patent tubes. Her husband was healthy with normal spermogram. With the diagnosis of secondary unexplained infertility, she was offered an IVF cycle using the multiple GnRHant protocol, according to our unit policy [3].
Her COH chart is presented in Fig. 1. Stimulation was started after hormonal and ultrasonographic confirmation of an early follicular phase (day 3 estradiol (E2) and progesterone (P) levels were 146 pmol/L and 0.8 nmol/L, respectively, and transvaginal sonography (TVS) revealed an endometrial thickness of 4 mm, with no free pelvic fluid and no ovarian activity follicles <10 mm in diameter). Following 4 days of 150 unit/day of recFSH (Gonal-F, Merck Serono Ltd. Herzliya Pituach, Israel), she demonstrated high E2 (4063 pmol/L) and P (9.8 nmol/L) levels, dictating the addition of GnRHant (Cetrorelix 0.25 mg/day. Cetrotide, Merck Serono Ltd. Herzliya Pituach, Israel) and the substitution of recFSH with HP-hMG (Menopur, Ferring Pharmaceuticals Ltd., Caesarea, Israel). In our practice, we routinely substitute FSH to preparations containing LH activity, with the initiation of GnRHant. During the following days, her E2 and P levels continued to rise despite the reduction in the daily gonadotropin dose, reaching a peak E2 level of 14,533 pmol/L and a peak P level of 12.8 nmol/L. Due to the high E2 and P levels, she was triggered with GnRHa (Triptorelin 0.3 mg, Decapeptyl, Ferring Pharmaceuticals Ltd., Caesarea, Israel) for final follicular maturation. The patient underwent ovum pick-up (OPU) 36 h following GnRHa trigger, yielding six oocytes that were vitrified (freeze-all policy).
Fig. 1.
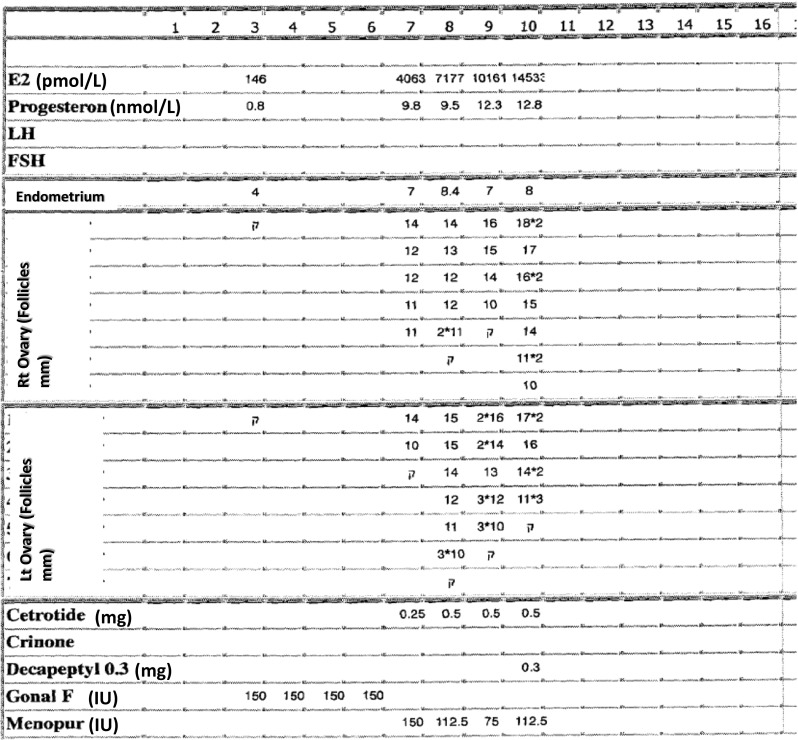
Patient’s COH chart
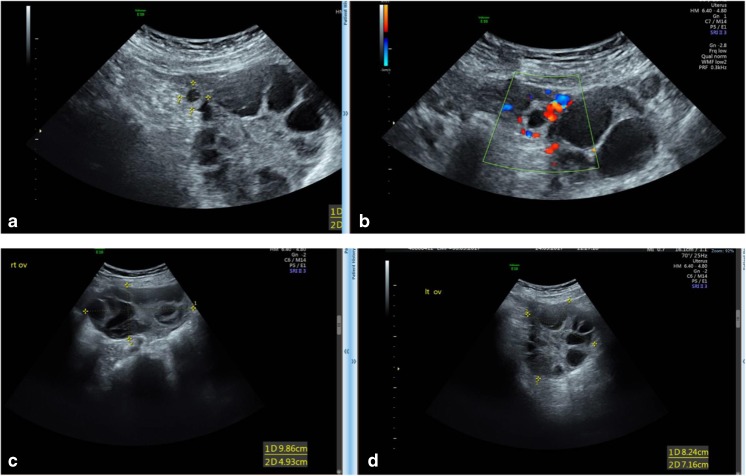
Six days following OPU, the patient presented to our unit with abdominal discomfort, blotting, with no nausea or vomiting and no respiratory distress. Upon examination, her abdomen was tender, especially on the right lower abdominal quadrant, with no signs of peritoneal irritation. Her transabdominal ultrasonography revealed a normal uterus with trilinear 13.8 mm endometrium and two enlarged ovaries. The right ovary was located outside the pelvis and above the uterus, 9.86*4.9 cm in diameter and the left ovary was positioned 8.24*7.16 cm in diameters, with sonographically detected free clear fluid in the pouch of Douglas. The patient denied any inadvertent injection of exogenous hCG. With a diagnosis of suspected sub-torsion of the right hyperstimulated ovary, she was admitted to our gynecological ward for further evaluation and treatment.
Upon admission, blood was drawn for complete blood count, chemistry, and quantitative HCG, revealing hematocrit of 42%, WBC 13200, platelets 308,000, normal kidney, liver function tests, and hCG of 2881 IU. Since the patient denied the inadvertent injection of hCG and due to the high hCG level, the working differential diagnosis was either COH during early abnormal/extrauterine pregnancy (EUP) or an ectopic hCG secretion (paraneoplastic syndrome). The patient was therefore referred to a second TVS by an expert ultrasonographer, who could demonstrate a right tubal EUP (Fig. 2), with no fetal pole or fetal cardiac activity. Due to the aforementioned ultrasonographic picture and the observed rise in hCG level (to 4144 IU, in the following day), the patient was offered intramuscular methotrexate injection, as a medical treatment of her EUP. During her hospitalization, her hCG levels decline and her symptoms improved and she was discharged after a week for further outpatient surveillance, during which she demonstrated complete resolution of her EUP and her OHSS, as noted by the decline in hCG levels and normalization of her pelvic ultasonographic findings.
Fig. 2.
Transvaginal ultrasound demonstrating the Rt EUP (a, b) and the patient’s hyperstimulated ovaries (c, d)
Discussion
In the present case, we report for the first time, on the occurrence of OHSS following GnRHa trigger for final follicular maturation masking EUP. Since OHSS almost always develops after hCG administration or in early pregnancy, its occurrence following GnRHa trigger should alert physician to search for either an inadvertent administration of exogenous hCG, or the endogenous secretion of hCG by early pregnancy, e.g., EUP, or as part of a paraneoplastic syndrome [10]. Moreover, this case may suggest the necessity to examine hCG level during COH, in the case where early follicular hormonal (E2\P) or TVS are suspicious (for pregnancy) or whenever E2 and P levels are high during the mid-follicular phase or P is extremely high at the day of triggering final follicular maturation.
Furthermore, the present case report elucidates the feasibility of stimulating and recruiting ovarian follicles yielding mature oocytes not only in the luteal phase but also during early EUP. Moreover, we could demonstrate that GnRHa can trigger final follicular maturation during pregnancy, in the presence of high P level and probably also high hCG level. These observations are in agreement with Goeckenjan et al. [11], who reported on a patient undergoing fertility preservation, with elevated hCG levels after first trimester abortion. In this case, the low hCG levels during COH supported, rather than inhibited follicular development with 28 oocytes retrieved following GnRHa trigger, as was already suggested by Filicori et al. [12].
Of emphasize, Gurbuz et al. [13] recently described four cases of failed complete luteolysis post-GnRHa trigger, which was sufficient to allow embryo implantation and development, where one of these patients developed OHSS, obviously, a consequence of the endogenous hCG secretion. This case emphasize that unprotected intercourse should be advised against, following GnRHa trigger in order to eliminate the occurrence of OHSS.
Recently, three cases of OHSS following GnRHa trigger for final oocyte maturation and a freeze-all approach were reported [8, 9]. A closer look at these cases cannot exclude an inadvertent injection of exogenous hCG or early pregnancy—which are crucial in the etiology of OHSS. In the Abu Dhabi/United Arab Emirates case [8], a 29-year-old patient with unexplained infertility and polycystic ovary-like ovaries was triggered by GnRHa. Her peak E2 and P levels were 4300 pg/mL and 2.0 ng/mL, respectively. A total of 30 oocytes were retrieved, resulting in the vitrification of 28 metaphase II oocytes. Day 1 following OPU, the patient was hospitalized with signs and symptoms of severe OHSS. To exclude natural conception, serum hCG was measured, with a negative result 3 days following OPU (5 days after triggering final follicular maturation). Menses occurred as late as 14 days after the oocyte retrieval. In the India case [8], a 27-year-old egg donor underwent OPU with a yield of 30 oocytes. Six days after oocyte retrieval, the patient was admitted with signs and symptoms of moderate–severe OHSS. Serum hCG was not measured, and similar to the Abu Dhabi/United Arab Emirates case, the GnRHa trigger did not cause a “clinical luteal-phase defect”/luteolysis and the patient menstruated 12 days after OPU. Since after GnRHa triggering progesterone serum levels return to baseline 5 days following OPU [14], the unexpected delay in menstruation in the aforementioned cases raises doubts and cannot exclude the “presence” of hCG in the circulation, either from spontaneous pregnancy or an inadvertent injection of hCG. Of notice, even the negative serum hCG measured 5 days after triggering final follicular maturation does not exclude an inadvertent injection of 250 μg of rechCG [15].
In the Singapore case [9], a PCOS patient, in whom 41 oocytes were retrieved, was hospitalized 12 h following OPU with the diagnosis of OHSS. She presented with shortness of breath and abdominal distension owing to tense ascites. Of notice, during OPU, “some” ascitic fluid was already observed, while 12 h later, ultrasound examination revealed massive ascites. Therefore, intra-abdominal bleeding could not be excluded. Moreover, there is no mention of her hormonal profile (specifically progesterone levels) during COH nor serum hCG levels (to exclude previous spontaneous pregnancy) or the time of menstruation following treatment.
To conclude, since OHSS almost always develops after hCG administration or in early pregnancy, its occurrence following GnRHa trigger should alert physician to search for check for serum hCG levels, aiming to exclude, either an inadvertent administration of exogenous hCG, or the endogenous secretion of hCG by early pregnancy. Moreover, we also recommend to check for serum hCG during COH in cases where early follicular hormonal (E2\P) or TVS are suspicious (for pregnancy) or whenever E2 and P levels are high during the mid-follicular phase or P is extremely high at the day of triggering final follicular maturation.
Acknowledgments
The authors would like to thank the “Memorial Fund Griffini Miglierina” within the “Fondazione Comunitaria del Varesotto Onlus” for non-restricted financial support to Dr. VSV during the completion of the study.
References
- 1.Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril. 1992;58:249–261. doi: 10.1016/S0015-0282(16)55188-7. [DOI] [PubMed] [Google Scholar]
- 2.Orvieto R, Ben-Rafael Z. Ovarian hyperstimulation syndrome: a new insight into an old enigma. J Soc Gynecol Invest. 1998;5:110–113. doi: 10.1177/107155769800500301. [DOI] [PubMed] [Google Scholar]
- 3.Orvieto R. Ovarian hyperstimulation syndrome—an optimal solution for an unresolved enigma. J Ovarian Res. 2013;6:77. doi: 10.1186/1757-2215-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orvieto R. Can we eliminate severe ovarian hyperstimulation syndrome? Hum Reprod. 2005;20:320–322. doi: 10.1093/humrep/deh613. [DOI] [PubMed] [Google Scholar]
- 5.Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. 2011;6:2593–2597. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 6.Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–168. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- 7.Orvieto R, Rabinson J, Meltzer S, Zohav E, Anteby E, Homburg R. Substituting HCG with GnRH agonist to trigger final follicular maturation—a retrospective comparison of three different ovarian stimulation protocols. Reprod BioMed Online. 2006;13:198–201. doi: 10.1016/S1472-6483(10)60615-3. [DOI] [PubMed] [Google Scholar]
- 8.Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. 2014;101(4):1008–1011. doi: 10.1016/j.fertnstert.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Ling LP, Phoon JW, Lau MS, Chan JK, Viardot-Foucault V, Tan TY, et al. GnRH agonist trigger and ovarian hyperstimulation syndrome: relook at “freeze-all strategy”. Reprod BioMed Online. 2014;29(3):392–394. doi: 10.1016/j.rbmo.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein J, Pandey P, Fleming N, Westin S, Piha-Paul S. A non-pregnant woman with elevated beta-HCG: a case of para-neoplastic syndrome in ovarian cancer. Gynecol Oncol Rep. 2016;17:49–52. doi: 10.1016/j.gore.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goeckenjan M, Rösner S, Toth B, Strowitzki T, Germeyer A. Successful controlled ovarian stimulation despite elevated hCG levels after first-trimester abortion in the context of fertility preservation. Gynecol Endocrinol. 2013;29(11):960–962. doi: 10.3109/09513590.2013.824961. [DOI] [PubMed] [Google Scholar]
- 12.Filicori M, Cognigni GE, Taraborrelli S, et al. Luteinizing hormone activity supplementation enhances follicle-stimulating hormone efficacy and improves ovulation induction outcome. J Clin Endocrinol Metab. 1999;84:2659–2663. doi: 10.1210/jcem.84.8.5884. [DOI] [PubMed] [Google Scholar]
- 13.Gurbuz AS, Deveer R, Ozcimen N, Ozcimen EE, Lawrenz B, Banker M, et al. Absence of luteal phase defect and spontaneous pregnancy in IVF patients despite GnRH-agonist trigger and “freeze all policy” without luteal phase support: a report of four cases. Gynecol Endocrinol. 2016;32(1):18–20. doi: 10.3109/09513590.2015.1110694. [DOI] [PubMed] [Google Scholar]
- 14.Fatemi HM, Polyzos NP, van Vaerenbergh I, et al. Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle stimulating hormone-gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2013;100:742–747. doi: 10.1016/j.fertnstert.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Weissman A, Lurie S, Zalel Y, Goldchmit R, Shoham Z. Human chorionic gonadotropin: pharmacokinetics of subcutaneous administration. Gynecol Endocrinol. 1996;10(4):273–276. doi: 10.3109/09513599609012319. [DOI] [PubMed] [Google Scholar]