Abstract
Purpose
The purpose of this study was to investigate the cause of repeated multipronucleus (MPN) formation in zygotes in a patient after both in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
Method
This is a case study. A patient had unexplained primary infertility with recurring total MPN zygotes after IVF and ICSI cycles. Time-lapse monitoring of pronucleus formation was carried out. Embryos developed from MPN zygotes were analyzed by fluorescence in situ hybridization (FISH). Single-cell RNA-seq analysis was used to identify gene expression profiles of the patient’s oocyte and zygote, and these were compared to the data from oocytes and zygotes from donors with normal fertilization (patient, n = 1; donors, n = 4). Oocyte-specific genes with differential expression were selected by the Amazonia! database.
Results
From time-lapse analysis, we observed the formation of multiple micronuclei near the site of the second polar body extrusion. These micronuclei migrated, expanded, and juxtaposed with the male pronucleus leading to a multipronucleus. None of these MPN zygotes could develop to the blastocyst stage, and FISH analysis revealed a chaotic chromosomal complement in the arrested embryos. RNA-seq analysis showed 113 differentially expressed genes (DEGs) between the patient and the donor oocytes and zygotes. Moreover, 25 of the 113 DEGs were unique or highly expressed in oocytes and early embryos. From 25 DEGs, three genes, DYNC2LI1, NEK2, and CCNH, which are involved in meiosis and the chromosome separation process, were further validated by real-time PCR.
Conclusion
We identified several candidate genes affecting pronucleus formation as a new cause of infertility.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0972-9) contains supplementary material, which is available to authorized users.
Keywords: Multipronuclear, Time-lapse, Oocyte, Single-cell transcriptome
Introduction
Pronuclear (PN) formation and development is a critical process during fertilization by which the parental chromosome complements merge and give rise to the zygotic genome. The entire process has been observed in detail in humans, and includes four major steps: formation of membrane vesicles [1]; assembly of the nuclear pore complex [2]; assembly of the nuclear lamina scaffold [3]; and aggregation of the parental pronucleus [4]. During the formation of the maternal pronucleus, membrane vesicles near the second polar body (PB) first appear approximately 6–7 h after sperm penetration [5]. Then, the membrane vesicles become progressively incorporated into the maternal pronucleus either individually or in groups. At the same time, a continuous nuclear envelope (NE) is formed around the sperm components after remodeling [6, 7]. The integrity of the NE is regulated by the activity of oocyte-derived protein kinase and phosphatase interacting with lamin A, B, and C [8]. Once pronucleus formation occurs, the envelopes of the pronuclei make contact with each other. The maternal pronucleus is drawn toward the male counterpart, and the two pronuclei are juxtaposed and positioned in the central or paracentral region of the cytoplasm [9].
Occasionally, more than two pronuclei can be observed in zygotes derived from in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) [10–13]. It is generally assumed that the formation of multiple pronuclei is due to polyspermic penetration or the failure of a second PB extrusion, especially in the case of 3PN [13]. However, oocytes with more than 3PN have been reported on rare occasions. One study has reported the case of a normal birth after a 2PN-derived embryo transfer in a patient with excessive multipronucleus (MPN) zygote formation following IVF and ICSI [14]. Nevertheless, the reason for the occurrence of MPN in these zygotes remains unclear.
In this study, we used time-lapse observation and single-cell transcriptional analysis to investigate a case with repeated MPN formation after IVF and ICSI in order to elucidate the mechanism underlying MPN formation in human oocytes after fertilization and to determine the genes involved in abnormal pronucleus formation.
Materials and methods
Resources and ethical approval
This is a case study of a patient with repeated MPN formation after IVF and ICSI. One mature oocyte, one MPN zygote, and six arrested embryos from the patient were collected with informed consent for further analysis. The study protocol was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (reference LL-SC-SG-2013-012).
Case report
A 42-year-old woman with unexplained primary infertility for 16 years was admitted to the Reproductive and Genetic Hospital of CITIC-Xiangya. The woman’s body mass index was 22.64, and her average menstrual cycle was 29–37 days. In 1999, she underwent a partial oophorectomy because of a right ovarian teratoma and had no radio- or chemotherapy thereafter. She had lipiodol angiography conducted in 2008 that revealed hydrosalpinx in the left fallopian tube. Her husband had normal semen characteristics. Normal karyotypes were revealed for both husband and wife. She had two sisters and a brother in her family, all of whom had biological offspring. There was no family history of infertility.
The first treatment started in November 2008 when the patient was 36 years old. Ultra-sound examination revealed an antral follicle count of 12 in the left ovary and a basal follicle-stimulating hormone (FSH) level of 5.27 mIU/mL. The long agonist protocol followed by ovarian stimulation with a combination of recombinant follicle-stimulating hormone (Gonal-F, Merck Serono, Darmstadt, Germany) and human menopausal gonadotropin (HMG, Livzon, Zhuhai, China) was used. Ovulation was triggered with 10,000 IU human chorionic gonadotropin (HCG, Merck Serono, Darmstadt, Germany). Four mature oocytes and one immature oocyte were retrieved. The four mature oocytes were split for both ICSI and IVF. At fertilization check, 18 h post IVF/ICSI, two MPN zygotes were obtained from IVF and one obtained from ICSI. Another oocyte obtained from the ICSI group had no pronucleus formation. The three MPN zygotes further cleaved to eight-cell, six-cell, and four-cell embryos on day 3. All embryos were discarded and not used for research.
The second treatment was initiated in November 2013 when the patient was 41 years old. The patient had an antral follicle count of five in the left ovary and basal FSH levels of 6.13 mIU/mL. The patient received the ultra-short agonist protocol followed by a combination of triptorelin (Ipsen, Paris, France), HMG (Livzon), and urofollitropin (Livzon, Zhuhai, China) for ovarian stimulation. Ovulation was triggered with 6500 IU HCG (Merck Serono). Seven oocytes were retrieved. Of these, six mature oocytes were subjected to ICSI. After 18 h, we observed one 5PN zygote, one 7PN zygote, one 8PN zygotes, one NPN oocyte, and one degenerated oocyte. The four MPN zygotes cleaved to three five-cell and one three-cell embryo on day 3 and arrested in further blastocyst culture. The four arrested embryos were used for fluorescence in situ hybridization (FISH).
The third ICSI treatment was initiated in July 2014 when the patient was 42 years old. The patient had an antral follicle count of five. The basal FSH level was 6.13 mIU/mL. We conducted the GnRH antagonist protocol followed by a combination of GnRH-a (Merck Serono), HMG (Livzon), and urofollitropin (Livzon) for ovarian stimulation. Ovulation was triggered with 6500 IU HCG (Merck Serono). A total of nine oocytes were retrieved, of which four were mature. Among the four mature oocytes, three were subjected to ICSI and one oocyte was donated for single-cell transcriptional analysis. The three mature oocytes fertilized after ICSI were placed individually and cultured in G1 plus medium with time-lapse imaging by the Primo Vision system (Vitrolife, Goteborg, Sweden). Images of each embryo were recorded every 5 min. The three mature oocytes fertilized by ICSI became MPN zygotes (one 6PN, one 7PN, and one 8PN). The 8PN zygote was donated for single-cell transcriptional analysis. The other 6PN and 7PN zygotes cleaved to two five-cell embryos on day 3 and were donated for FISH analysis.
In the fourth treatment cycle in January 2015, the patient received eight donated oocytes for ICSI, which resulted in six normal fertilized 2PN zygotes and one abnormal fertilized (1PN) zygote. The six 2PN zygotes developed into one nine-cell, two eight-cell, two seven-cell, and one five-cell embryos on day 3. All embryos were cryopreserved for frozen embryo transfer. In the FET cycle, one nine-cell and one eight-cell embryo were thawed and transferred. A clinical pregnancy with a gestational sac and fetal heartbeat was achieved. Table 1 summarizes the four treatments with detailed information.
Table 1.
Summarized information of the four treatment cycles
| IVF and ICSI attempts | COH protocol | Total oocytes | MII oocytes | Fertilized oocytes | Abnormal fertilized oocytes | Pronuclear type | Outcomes |
|---|---|---|---|---|---|---|---|
| First IVF + ICSI | Long | 5 | 4 | 3 | 3 | 2-MPNa (IVF); 1-MPN (ICSI) |
Embryos cleaved to 8-cell, 6-cell, and 4-cell embryos on day 3. |
| Second ICSI | Ultra-short | 7 | 6 | 4 | 4 | 2-8PN, 1-7PN, 1-5PN | Embryos cleaved to 3 5-cell and 1 3-cell embryos on day 3 and arrested in further blastocyst culture. |
| Third ICSI with time-lapse | GnRH-a | 9 | 4b | 3 | 3 | 1-8PNc, 1-7PN, 1-6PN | The 6PN and 7PN zygotes cleaved to 2 5-cell embryos on day 3. |
| Fourth ICSI with donor oocyte | – | – | 8 | 7 | 1 | 6-2PN, 1-1PN | The 6 2PN zygotes were cleaved to 1 9-cell, 2 8-cell, 2 7-cell, and 1 5-cell embryos in day 3. 1PN zygote cleaved to 5-cell embryo. |
aMPN denotes multipronuclear zygote
bOne mature oocyte was donated for RNA-seq analysis
cAn 8PN zygote was donated for RNA-seq analysis
FISH of embryos exhibiting arrested development
The four embryos from the second cycle and two embryos from the third cycle were donated and collected for FISH assays as previously described [15]. The blastomeres were separately aspirated and fixed with 0.01 N HCL + 0.01% Tween. FISH was performed in the first round of hybridization using a DNA probe panel (Vysis, Abbott Molecular Inc., Des Plaines, USA) for chromosomes 1 (chromosome enumerating probe (CEP) 1, red), 11 (CEP 11, aqua), and 12 (CEP 12, green). Then, the slides were rinsed in 75% ethanol, 90% ethanol, and absolute ethanol for 2 min each. After air-drying, the second hybridization round used the DNA Probe Panel for chromosomes 18 (CEP 18, aqua), X (CEP X, green), and Y (CEP Y, red). Other procedures were the same as those used in the first round.
Single-cell transcriptional analysis
RNA was isolated from single cells and amplified by a modified SMART-seq2 protocol [16]. Briefly, the zona pellucida of the one oocyte and one 8PN zygote from the third treatment cycle was removed by acidic Tyrode’s solution. Individual cells were transferred into lysis buffer, and the samples were incubated at 72 °C for 3 min, and immediately placed on ice. After cell lysis, reverse transcription was carried out on a thermal cycler using the following cycle parameters: 42 °C for 90 min, 10 cycles of 50 °C for 2 min and 42 °C for 2 min, followed by incubation at 70 °C for 15 min. Then, complementary DNA (cDNAs) were amplified by PCR according to the following protocol: 3 min at 98 °C; 18 cycles of 15 s at 98 °C, 20 s at 67 °C, and 6 min at 72 °C, as well as an extension step of 5 min at 72 °C. The amplicons were purified with Ampure XP beads, and cDNA was eluted in 20 μL of EB solution (10 mM Tris-HCL, pH 8.5, Qiagen, Duesseldorf, Germany). Equal amounts of cDNA from different time periods were mixed and fragmented into 300–500 bp sequences; cDNA libraries were eventually identified by detecting the size distribution on an Agilent high-sensitivity DNA chip, and amplified using TruSeq PE Cluster Kits (Illumina, CA, USA) after purification with Ampure beads (Agencourt, Beverly, MA, USA). Sequencing was carried out on an Illumina Hiseq 2000 (Illumina). The original image data generated by the sequencing machine were converted into sequence data via base calling (Illumina pipeline CASAVA v1.8.0) and then subjected to standard QC criteria. The distribution of gene expression in the sample was analyzed by using ggplot2.
Selection of differentially expressed genes and bioinformatics analysis
Single-cell RNA-seq analysis was performed to identify the differentially expressed gene profiles from one oocyte and one 8PN zygote from the patient in the third cycle when compared with gene profiles of oocytes and zygotes from four donors with normal fertilization (data from another unpublished project). The stimulation schemes and dose of patient and donors are listed in Supplementary Table 1. Genes with an absolute fold change values of ≥2 compared with the each donor, and with a reads per kilobase of exon model per million mapped reads (RPKM) of >0.5 for at least one sample with a chosen intersection were considered to have differential expression. All genes were annotated with Gene Ontology (GO) categories using Molecular Annotation System 3.0 (http://bioinfo.capitalbio.com/mas3/). Based on the results of the enrichment analysis by MAS 3.0 and by calculating the p and q values, the genes were assigned to various categories: cellular component, molecular function, and biological processes. The Amazonia! database (https://www.amazonia.transcriptome.eu/search.php) was used to analyze the expression of differentially expressed genes (DEGs) in oocytes and tissue samples. The oocyte-specific genes were selected because they were uniquely expressed or highly expressed in oocytes compared to other tissues.
Validation of RNA-seq results by qRT-PCR
RNA-seq data were validated by determining the expression of three oocyte-specific genes by real-time PCR. Primers were designed by PrimerBank (Supplementary Table 2). Real-time PCR reactions were carried out in a LightCycler 480 II System (Roche, Basel, Switzerland) with SYBR green (LightCycler 480 SYBR Green I Master mix, Roche) using FastStart Universal SYBR Green Master (Roche). The reaction conditions were as follows: 5 min at 95 °C followed by 45 cycles of 15 s at 95 °C, 10 s at 58 °C, and 10 s at 72 °C. Amplification specificity was measured with a melting curve by heating the sample from 65 to 97 °C.
Results
Abnormal pronucleus formation and multinucleated cells in mitosis
In the last 2 cycles, MPN zygotes were observed approximately 16–18 h post-insemination (p.i.) (Fig. 1A). In the third cycle, time-lapse monitoring was utilized to observe the cellular behavior of three oocytes after ICSI (Fig. 1D). The results revealed that the second polar body (PBII) was extruded 8.1 ± 3.4 h p.i. At approximately 10.7 ± 3.2 h p.i., five to seven vesicles, which emerged next to the site at which PBII was extruded with little fragmentation, could not coalesce to form a female pronucleus. These vesicles then migrated and juxtaposed with the male pronucleus, which had emerged at 15.2 ± 2.3 h p.i. At 24.3 ± 3.9 h p.i., both the female vesicles and male pronucleus expanded to a maximum diameter of approximately 9–12 μm and 17 μm for the female vesicles and male pronucleus, respectively (Supplementary video 1). This result suggested that the abnormality of female vesicle assembly led to the formation of multiple female pronuclei in the ooplasm. Multinucleated cells were also observed in late mitosis, especially in each blastomere at the two-cell stage. These findings indicate that disordered assembly of pronuclei might affect the nuclear assembly in early mitosis (Supplementary video 2). All embryos were arrested from the two-cell stage to the eight-cell stage. FISH analysis of arrested embryos revealed a chaotic chromosome complement or separate nuclei in some of the blastomeres (Fig. 2), further indicating that abnormalities in chromosome separation and duplication occurred in early mitosis. Three of six arrested embryos showed analyzable signals, and the other three embryos could not be analyzed because of poor embryo quality.
Fig. 1.
Morphological characteristics. A MPN zygote at 12 h p.i. captured by optical microscopy in the second cycle. B Morphology of a normal 2PN zygote. C Two transferred embryos from donor oocytes cycle. D Time-lapse imaging from the patient’s zygote to two-cell stage. a Oocyte after ICSI (0 h p.i.). b PBII extruded (about 6 h p.i.) with small fragments (arrowhead). c Site next to PBII in the cytoplasm with a few emerging vesicles (8 h p.i., arrow). d PN juxtaposition (arrowhead) in the center of the cytoplasm (12 h p.i.). e Multinucleated two-cell embryo. Multiple nuclei were located in the center of the blastomere (arrowhead). Scale bar represents 10 μm
Fig. 2.
Fluorescence in situ hybridization (FISH) sketch map and images of the three embryos. A Different embryos with signals for chromosomes 1 (red), 11 (aqua), and 12 (green) in the first round of hybridization. B Different embryos with signals for chromosomes 18(aqua), X (green), and Y (red) in the second round of hybridization. Embryos with signal abnormalities affecting multiple chromosomes and the abnormalities varying from blastomere to blastomere were defined as chaotic chromosome complement. C The images from embryo c in A and B
However, morphokinetic parameters indicated that the average cell cycle time 2 (or P2, i.e., the time between the first and second mitosis) was 10.1 ± 2.3 h, while S2 (or P3, i.e., the time between or synchrony of the second and third mitosis) was 0.7 ± 0.5 h, and t5 (or the time from fertilization to the five-cell embryo stage) was 51.6 ± 2.3 h. All results were within normal ranges [17].
In the fourth cycle, the donor oocytes were fertilized with the husband’s sperm by ICSI. Apart from one 1PN and one none-pronuclear zygote, the remaining six oocytes developed into 2PN zygotes. One nine-cell, two eight-cell, two seven-cell, and one five-cell embryos were achieved on day 3. One nine-cell and one eight-cell embryos (Fig. 1C) were transferred and implanted successfully, indicating that the husband’s sperm could fertilize oocytes from other donors, further confirming that the maternal factor led to the formation of MPN.
DEGs in oocytes and MPN zygotes
To explore the molecular mechanism underlying abnormal female pronucleus formation, one oocyte and one MPN zygote from the third treatment cycle were collected for single-cell transcriptional analysis. Data were compared with transcriptional data from four oocytes and four zygotes of four normal donors. An Illumina HiSeq2000 sequencer was used to generate an average of 7 Gb of sequencing data from single cells. We obtained an average of 94,396,674 and 86,215,348 total reads from the patient’s zygote and oocyte, and an average of 90,832,331 and 72,870,337 total reads from donors’ zygotes and oocyte. The average read length was 90 bp. The percentages of total mapped reads for the patient and control were 82.96 and 81.93%, respectively. The percentages of sequenced genes with gene coverage between 90 and 100% were 22.22 and 20.84% for the patient and control groups, respectively (Supplementary Table 3). Box and whisker plot showed that the derivation of transcription levels between different MPN zygotes and different oocytes has no significant difference, only a small subset of genes displayed difference (Supplementary Fig. 1).
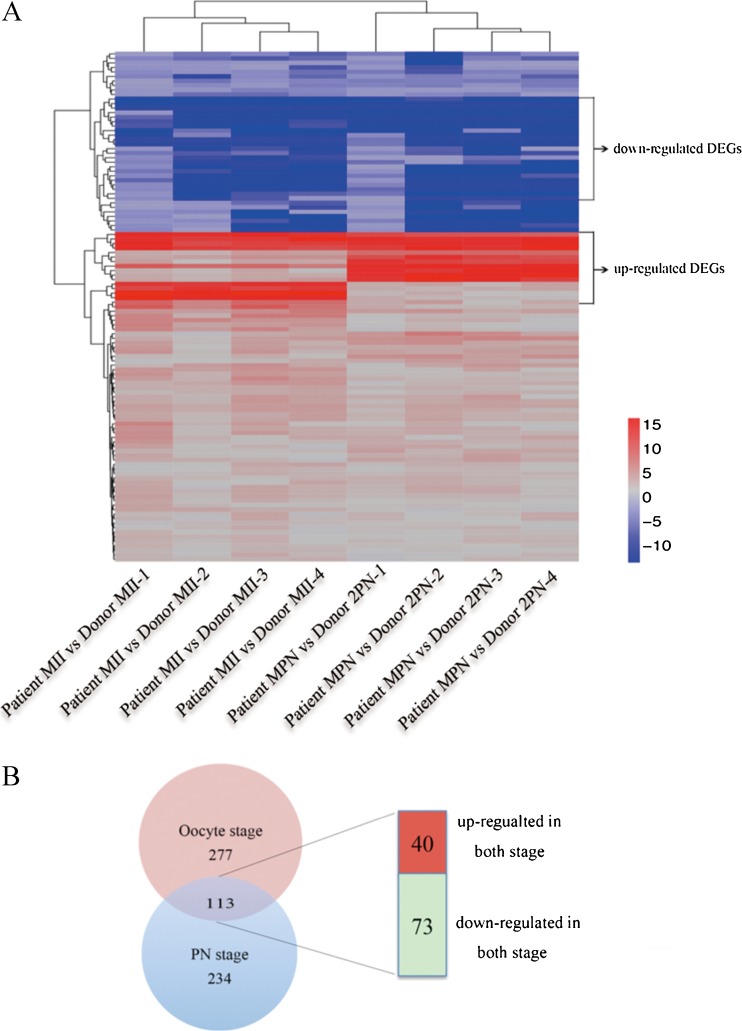
Genes with an absolute fold change values of ≥2 compared to the control and RPKM >0.5 for at least one sample were considered to be DEGs. The patient had 390 DEGs in the oocyte stage and 347 DEGs in the PN stage. Then, we identified the common DEGs that were simultaneously upregulated or downregulated in the oocyte and zygote stages. A total of 113 DEGs were identified (40 upregulated and 73 downregulated) between the patient and the controls (Fig. 3; Supplementary Table 4). The 113 DEGs were analyzed by MAS 3.0 software and divided into three major GO categories: cellular component (20.3%), biological process (48.9%), and molecular function (30.8%) (Supplementary Fig. 2).
Fig. 3.
Selection of differentially expressed genes. a Hierarchical clustering of DEGs. b Venn diagram showing the distribution of 624 differentially expressed genes between different samples, with 40 upregulated and 73 downregulated genes in both stages
Oocyte-specific genes with differential expression across tissues
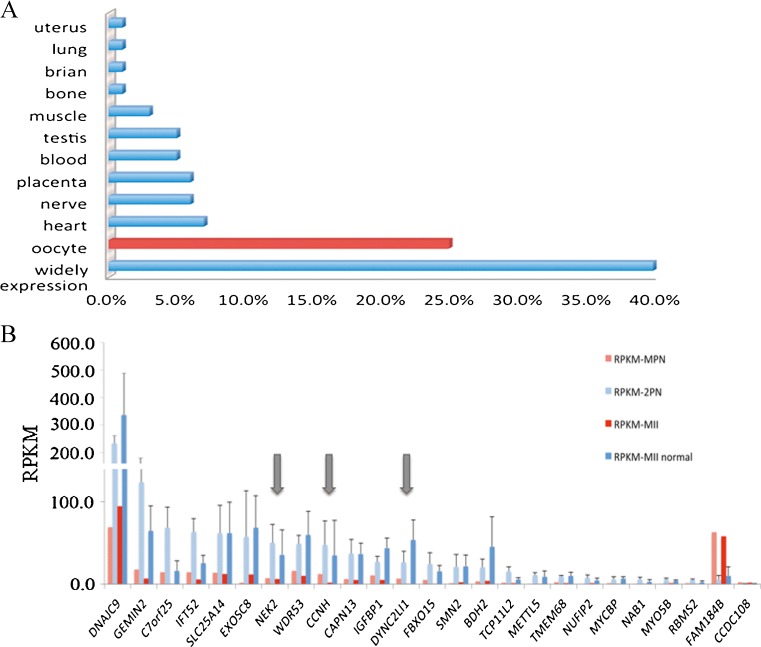
As the MPN formation might be highly related to oocyte defects, we further analyzed the relative expression levels of the 113 DEGs in different tissues by using Amazonia! database software. Most DEGs were widely expressed in tissues. Additionally, 25% of the DEGs (n = 25) were uniquely expressed or were highly expressed in oocytes compared to other tissues (Fig. 4) [18–20]. As shown in Table 2, 24 of the genes were downregulated and 1 was upregulated. These genes were listed as candidate genes by fold change, GO category, and pathway keywords. From 25 DEGs, the three genes DYNC2LI1, NEK2, and CCNH are related to the regulation of meiotic and chromosome separation, we then selected these three genes to validate the credibility of the RNA-seq analysis. The accuracy of the RNA-seq analysis was confirmed using real-time PCR to determine the differences in the mRNA expression levels between the test and control samples. The results of the fold change tendency of the three genes obtained by qRT-PCR and RNA-seq were similar, and the p values of all the genes were less than 0.05 (Fig. 5). The qRT-PCR results corroborated the RNA-seq analysis.
Fig. 4.
The percentage and expression of oocyte-specific gene. a Twenty-five percent of DEGs were specially expressed in oocytes. Others genes were tissue-specific or highly expressed in particular tissues. b Transcriptome analysis of patient and controls of the RPKM genes specifically expressed in oocytes
Table 2.
GO categories and pathways of genes specifically expressed in oocytes
| Fold change | GO categories | Pathway | |||||
|---|---|---|---|---|---|---|---|
| PN stage | MII stage | Biology process | Cell component | Molecular function | |||
| METTL5 | −1.10E4 | −273.59 | Methylation | – | Nucleic acid binding | – | |
| EXOSC8 | −39.04 | −5.95 | RNA processing | Nucleus | Exonuclease activity, binding | – | |
| MYO5B | −24.21 | −4.18 | Myosin | – | Nucleotide binding | Motor activity | |
| SMN2 | −18.31 | −9.42 | Spliceosome assembly | Nucleus | RNA binding | – | |
| NAB1 | −14.73 | −38.53 | Regulation of transcription | Nucleus | Transcriptional repressor activity | Negative regulation of transcription | |
| NUFIP2 | −10.39 | −3.94 | – | Nucleus | RNA binding | – | |
| NEK2 | −7.22 | −5.81 | Regulation of mitosis, meiosis | Nucleus | Protein serine/threonine kinase activity | Cytokinesis | |
| GEMIN2 | −7.06 | −9.45 | Spliceosome assembly | Nucleus | Protein binding | RNA splicing | |
| MYCBP | −6.23 | −11.92 | Regulation of transcription | Nucleus | Transcription coactivator activity | – | |
| CAPN13 | −6.09 | −7.44 | Proteolysis | Intracellular | Calpain activity, binding | – | |
| BDH2 | −6.00 | −11.02 | Oxidation reduction | Mitochondrion | Binding | Butanoate metabolism | |
| RBMS2 | −4.69 | −21.11 | RNA processing | Nucleus | Nucleotide binding | Translation | |
| FBXO15 | −4.65 | −36.41 | Modification-dependent protein catabolism | SCF ubiquitin ligase complex | Protein binding | Ubiquitin cycle | |
| IFT52 | −4.49 | −4.41 | – | – | Embryonic digit morphogenesis | – | |
| SLC25A14 | −4.47 | −4.99 | Transport | Mitochondrion | Binding | Mitochondrial membrane | |
| TMEM68 | −4.28 | −8.04 | Metabolism | Membrane | Acyltransferase activity | Acyltransferase activity | |
| DYNC2LI1 | −4.13 | −5.36E4 | Development | Microtubule | Motor activity | – | |
| CCNH | −3.91 | −19.15 | Cell cycle | Nucleus | Protein binding | Cell cycle | |
| DNAJC9 | −3.37 | −3.56 | – | – | Heat shock protein binding | – | |
| IGFBP1 | −2.63 | −8.31 | Regulation of cell growth | Extracellular region | Insulin-like growth factor Binding | Cell growth | |
| CCDC108 | 14.6 | 6.23 | – | Membrane | Structural molecule activity | – | |
Fig. 5.
Validation of RNA-seq results by RT-PCR. The graph shows the relative mRNA expression level of three selected genes in MPN zygote and oocytes from patient are presented as the fold of oocytes and 2PN zygotes from donors
Discussion
In this study, we evaluated a case with unexplained primary infertility caused by recurring MPN zygotes after IVF and ICSI cycles. In routine IVF practice, zygotes with more than 3PN in humans have rarely been reported. It is difficult to analyze MPN formation by traditional morphologic evaluation. Time-lapse analysis was used in the third treatment cycle, allowing us to observe the phenomenon of abnormal female PN formation, which could not be observed in the first 2 cycles. Thanks to this observation, we found for the first time that assembly error of the female pronucleus can be the cause of infertility.
The patient was treated with IVF at 36-year-old and underwent four treatment cycles during 7 years. Regardless of the fertilization method, stimulation protocol, and stimulation dose, the phenomenon of MPN still existed in all zygotes. However, normal 2PN could form with donor oocytes. So we suspect that it is not age or stimulation factors, but more likely female genetic factors that caused this phenomenon.
The mechanism of MPN formation has been described in animal models. In zebrafish, brambleberry (Bmb) is required for pronuclear membrane fusion following fertilization. Mutation of Bmb disrupts karyomere fusion, resulting in the formation of multiple pronuclei [21]. However, we did not identify a homolog in humans despite the high level of genome annotation. In mice, knockout of Kid/kinesin-10 leads to a failure of chromosomes to form a whole complement, causing in multipronuclei emerging in the center of the cytoplasm [22]. However, we did not detect expression dysregulation of its homolog gene KIF22 in the patient’s oocyte and MPN zygote. To further investigate the molecular mechanism of MPN formation in this case, we performed single-cell transcriptional analysis of the patient’s oocyte and MPN zygote, and compared the data to control samples with normal fertilization. Twenty-five candidate genes that were uniquely expressed or highly expressed in oocytes were selected, and DYNC2LI1, NEK2, and CCNH, which participate in the regulation of meiosis and chromosome separation, were further validated by qRT-PCR, and the results were consistent with those of RNA-seq. However, the mechanism of MPN formation is still unclear. This may be partially due to the limited sample size or the impact of different stimulation protocols on the oocyte transcriptome [23–27]. As such, transcriptional data from one mature oocyte and one MPN zygote only provide clues for further investigation. Future studies, including whole exon sequencing of the family or loss-of-function analysis of candidate genes, might help identify the exact mechanism of this phenomenon.
Electronic supplementary material
(DOCX 15 kb)
(DOCX 14 kb)
(DOCX 14 kb)
(DOCX 26 kb)
(DOCX 12337 kb)
(DOCX 2398 kb)
Formation of multiple pronuclei (MP4 10320 kb)
Mitosis in embryos containing multiple pronuclei (MP4 4597 kb)
Acknowledgments
We thank to the patients and staff at the Reproductive and Genetic Hospital of CITIC-Xiangya and LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81222007 to L.G. and 81471510 to L.G.), the Program for New Century Excellent Talents in University (No. 907010003), and the Fundamental Research Funds for the Central Universities of Central South University (No. 2015zzts101).
Compliance with ethical standards
This study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (reference LL-SC-SG-2013-012).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
J. Dai and L. Z. Leng contributed equally to the work.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0972-9) contains supplementary material, which is available to authorized users.
References
- 1.Collas P, Poccia D. Lipophilic organizing structures of sperm nuclei target membrane vesicle binding and are incorporated into the nuclear envelope. Dev Biol. 1995;169(1):123–135. doi: 10.1006/dbio.1995.1132. [DOI] [PubMed] [Google Scholar]
- 2.Inoue A, Zhang Y. Nucleosome assembly is required for nuclear pore complex assembly in mouse zygotes. Nat Struct Mol Biol. 2014;21(7):609–616. doi: 10.1038/nsmb.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King MC, Lusk CP. A model for coordinating nuclear mechanics and membrane remodeling to support nuclear integrity. Curr Opin Cell Biol. 2016;41:9–17. doi: 10.1016/j.ceb.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang YX, Munne S, Reing A, Schattman G, Grifo J, Cohen J. The parental origin of the distal pronucleus in dispermic human zygotes. Zygote. 1994;2(1):79–85. doi: 10.1017/S0967199400001799. [DOI] [PubMed] [Google Scholar]
- 5.Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod. 1997;12(3):532–541. doi: 10.1093/humrep/12.3.532. [DOI] [PubMed] [Google Scholar]
- 6.Jones EL, Mudrak O, Zalensky AO. Kinetics of human male pronuclear development in a heterologous ICSI model. J Assist Reprod Genet. 2010;27(6):277–283. doi: 10.1007/s10815-010-9402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collas P, Poccia D. Remodeling the sperm nucleus into a male pronucleus at fertilization. Theriogenology. 1998;49(1):67–81. doi: 10.1016/S0093-691X(97)00403-2. [DOI] [PubMed] [Google Scholar]
- 8.Isaji M, Iwata H, Harayama H, Miyake M. The localization of LAP2 beta during pronuclear formation in bovine oocytes after fertilization or activation. Zygote. 2006;14(2):157–167. doi: 10.1017/S0967199406003613. [DOI] [PubMed] [Google Scholar]
- 9.Terada Y, Schatten G, Hasegawa H, Yaegashi N. Essential roles of the sperm centrosome in human fertilization: developing the therapy for fertilization failure due to sperm centrosomal dysfunction. Tohoku J Exp Med. 2010;220(4):247–258. doi: 10.1620/tjem.220.247. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Zhao W, Xue X, Zhang S, Shi W, Shi J. Three pro-nuclei (3PN) incidence factors and clinical outcomes: a retrospective study from the fresh embryo transfer of in vitro fertilization with donor sperm (IVF-D) Int J Clin Exp Med. 2015;8(8):13997–14003. [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Xue X, Zhao W, Li W, Shi J. Effects of high three pro-nuclei (3PN) proportion incidence on clinical outcomes in the fresh cleavage-stage and blastocyst-stage embryo transfer (ET) cycles. Gynecol Endocrinol. 2016:1–4. [DOI] [PubMed]
- 12.Rosenbusch B, Schneider M, Sterzik K. The chromosomal constitution of multipronuclear zygotes resulting from in-vitro fertilization. Hum Reprod. 1997;12(10):2257–2262. doi: 10.1093/humrep/12.10.2257. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbusch BE. A preliminary concept, deduced from cytogenetic analyses, for explaining different types of multipronuclear oocytes obtained after intracytoplasmic sperm injection. Fertil Steril. 2010;94(6):2479–2481. doi: 10.1016/j.fertnstert.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 14.van der Westerlaken L, Helmerhorst F, Verburg H, Naaktgeboren N. Successful intracytoplasmic sperm injection after failed in vitro fertilization due to multipronuclear oocytes. Fertil Steril. 2003;80(3):639–640. doi: 10.1016/S0015-0282(03)00752-0. [DOI] [PubMed] [Google Scholar]
- 15.Liao HQ, OuYang Q, Zhang SP, Cheng DH, Lu GX, Lin G. Pronuclear removal of tripronuclear zygotes can establish heteroparental normal karyotypic human embryonic stem cells. J Assist Reprod Genet. 2016;33(2):255–263. doi: 10.1007/s10815-015-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc. 2014;9(1):171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 17.Freour T, Basile N, Barriere P, Meseguer M. Systematic review on clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring. Hum Reprod Update. 2015;21(1):153–154. doi: 10.1093/humupd/dmu054. [DOI] [PubMed] [Google Scholar]
- 18.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101(16):6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM, et al. Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics. 2005;86(2):127–141. doi: 10.1016/j.ygeno.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Wood JR, Dumesic DA, Abbott DH, Strauss JF., 3rd Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92(2):705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 21.Abrams EW, Zhang H, Marlow FL, Kapp L, Lu S, Mullins MC. Dynamic assembly of brambleberry mediates nuclear envelope fusion during early development. Cell. 2012;150(3):521–532. doi: 10.1016/j.cell.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohsugi M, Adachi K, Horai R, Kakuta S, Sudo K, Kotaki H, et al. Kid-mediated chromosome compaction ensures proper nuclear envelope formation. Cell. 2008;132(5):771–782. doi: 10.1016/j.cell.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Devjak R, Fon Tacer K, Juvan P, Virant Klun I, Rozman D, Vrtacnik Bokal E. Cumulus cells gene expression profiling in terms of oocyte maturity in controlled ovarian hyperstimulation using GnRH agonist or GnRH antagonist. PLoS One. 2012;7(10):e47106. doi: 10.1371/journal.pone.0047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papler TB, Bokal EV, Tacer KF, Juvan P, Virant Klun I, Devjak R. Differences in cumulus cells gene expression between modified natural and stimulated in vitro fertilization cycles. J Assist Reprod Genet. 2014;31(1):79–88. doi: 10.1007/s10815-013-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24(6):1436–1445. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, Van der Elst J, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25(5):1259–1270. doi: 10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- 27.Brannian J, Eyster K, Mueller BA, Bietz MG, Hansen K. Differential gene expression in human granulosa cells from recombinant FSH versus human menopausal gonadotropin ovarian stimulation protocols. Reprod Biol Endocrinol. 2010;8:25. doi: 10.1186/1477-7827-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)
(DOCX 14 kb)
(DOCX 14 kb)
(DOCX 26 kb)
(DOCX 12337 kb)
(DOCX 2398 kb)
Formation of multiple pronuclei (MP4 10320 kb)
Mitosis in embryos containing multiple pronuclei (MP4 4597 kb)