Abstract
Background
Obesity is associated with several fertility disorders. This prospective cohort study was designed to evaluate the effect of body mass index (BMI) (kg/m2) on oocyte diameter and treatment.
Methods
Women undergoing in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI) were enrolled in the study. They were divided into two groups according to BMI: obese (BMI > 30) and normal weight (BMI < 25). Mature oocytes were evaluated according to total diameter, zona pellucida, and oolema diameters.
Results
A total of 387 oocytes were obtained from the 46 women who participated. Significantly more mature oocytes (M2) were retrieved from normal weight patients compare to obese women (15.1 ± 6.8 vs. 9.7 ± 3.9, respectively, P < 0.001). Oocytes from women in the obese group were significantly smaller than those in the normal weight group, including oocyte diameter (157.9 ± 7.9 vs. 164.3 ± 5.1 μm, P < 0.0001), oolema diameter (110.3 ± 4.5 vs. 113.5 ± 3.5 μm, P < 0.0001), and zona pellucida thickness (17.9 ± 2.6 vs. 19.0 ± 2.4 μm, P < 0.000), respectively. Multivariate logistic regression analysis, including oolema diameter, female age, BMI, number of M2 oocytes, and zona pellucida, was conducted to predict pregnancy. Small oolema diameter in obese patient adversely correlated with pregnancy. Larger oolema diameter was positively associated with the probability of pregnancy in the obese group as well as thinner zona pellucida.
Conclusion
Obesity is associated with smaller oocytes, which adversely affect fertility outcomes.
Trial registration
NIH number NCT01672931
Keywords: Obesity, Oocyte, Oolema diameter, Zona pellucida, IVF
Background
Obesity is an important health issue worldwide. It is common among women of reproductive age, and its rates are increasing according to the World Health Organization (WHO). Overweight is defined as a body mass index (BMI) of 25 kg/m2 or above, and obesity is defined as a BMI of 30 or more [1, 2].
Obesity is associated with anovulation, menstrual disorders, and infertility, as well as lower success rates in assisted reproduction. This is manifested by lower implantation and pregnancy rates, miscarriage, adverse pregnancy outcomes, increased maternal and fetal complications during pregnancy, and congenital anomalies [3–5]. The specific factors affected by obesity are unknown. Some studies focused on oocyte quality [6, 7], and others, on the endometrium [8]. However, their results conflict [9–11].
Luke et al. found that the likelihood of conception through assisted reproductive technology (ART) decreased with increasing BMI. Outcomes improved when donor oocytes from normal weight women were used. These data support the concept that obesity has a detrimental effect on oocytes [12].
Recent studies reported a correlation between obesity and oocyte size. Among women with polycystic ovarian syndrome (PCOS), those who were obese had smaller oocytes than those who were not [13, 14]. Moreover, several studies using animal models found a relation between environmental influences, such as obesity and insulin resistance, and oocyte quality and subfertility. Oocytes from obese mouse demonstrated delayed oocyte maturation, poorer mitochondrial quality and function, and more aneuploidty [7].
This study evaluated the effects of BMI on oocyte diameter in terms of treatment outcomes.
Methods
Study design
Patients were prospectively enrolled from 1 January 2013 through 31 December 2014. This case-control study compared treatment outcomes between obese and normal weight control subjects who were undergoing in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI) at the Hillel Yaffe Medical Center.
Study participants
Inclusion criteria were women undergoing IVF-ICSI cycles, age≤39 years, FSH < 12 IU/l, and more than four oocytes collected per patient. In order to demonstrate a substantial difference, women with BMI 25–29 were also excluded.
Only patients undergoing ICSI were included, because oocyte measurements were obtainable only after the oocytes were denuded by exposure to hyaluronidase, which is part of the ICSI protocol. Women with BMI ≥ 30 kg/m2 were enrolled into the obese group and BMI < 25 kg/m2 were enrolled into the normal weight group [1, 2]. Exclusion criteria were age≥40, PCOS, poor ovarian reserve documented in previous cycles, and any adjuvant steroid treatment to improve ovarian response (DHEA, steroids, etc.).
Treatment protocol
Patients underwent controlled ovarian stimulation according to their physician’s recommendation. All protocols [15] and ICSI were performed as previously described [16]. Estrogen and progesterone (P) levels were evaluated at every follow-up visit, including on the day of hCG (Ovitrelle Merck-Serono) administration before oocyte retrieval.
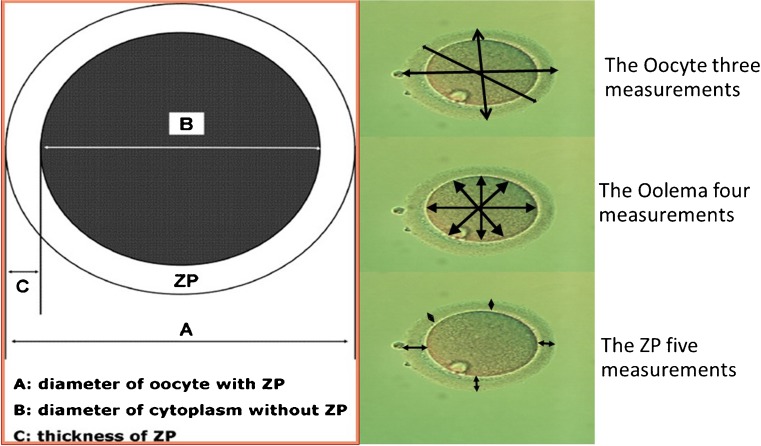
Only mature oocytes were analyzed after retrieval and before ICSI. After denudation of the granulosa cells, all oocytes were captured by an Olympus camera in an inverted microscope. A Zilos laser was used to measure computer-captured digital images using software that automatically calculated the mean and standard deviation of each measurement. Due to its spherical shape, we used the mean of three measurements of the oocyte. We took four measurements of the oolema and five of the zona pellucida (Fig. 1). To avoid intra-observer error, all measurements were taken by the same embryologist, who was blinded to the patients’ clinical information including BMI.
Fig. 1.
Representative measurements of different sections of oocyte. Three measurements for the full oocyte, four measurements of the oolema, and five measurements of the zona pellucida were taken for each oocyte in the study
Embryo quality was evaluated on the day of transfer, according to number of cells, symmetry, granularity, type, percentage of fragmentation, presence of multinucleate blastomeres, and degree of compaction, as previously described [17, 18]. A top quality embryo was described as having 4–5 cells on day 2 or >6 equal-size blastomeres on day 3, ≤10% fragmentation and no multinucleate cells [19]. A maximum of three embryos were transferred on day 2 or 3 of development. The association between different BMI in the different study group and the number of oocytes retrieved, number of mature oocytes (M2), fertilization and cleavage rates, mean oocyte diameter, mean oolema diameter, mean zona pellucida (ZP) thickness, number of top quality embryos, and pregnancy rate were compared.
Pregnancy determination
Beta hCG was measured 14 days after embryo transfer. Clinical pregnancy and implantation rates were confirmed when a gestational sac with fetal heart beat was visible by ultrasound examination 7 weeks later. Demographic data, treatment information and results, and pregnancy outcomes were recorded and followed until delivery.
Power analysis
Power analysis was conducted to answer the main study question of the difference in oocyte diameter between two distinctly different BMI groups. Significance level (alpha) was set at 0.05. Sample size was calculated using “Power and sample size” software and was based on previous results from Marquard et al. [13], assuming 80% power, 95% confidence interval and mean differences, and a difference in oocyte diameter between normal weight and overweight women of 1.6 μm. At least 136 oocytes per group were needed for analysis.
Statistical analysis
Statistical analysis was performed using the SPSS software package version 21 (SPSS Inc., Chicago, IL). Shapiro-Wilk test was used to evaluate the distribution of quantitative data. Comparisons between groups were analyzed using Student’s t test or Mann-Whitney U test. For repeated measures, we used linear mixed models to verify that no difference exist compared with other tests. Proportions were compared using chi-square test or Fisher’s exact test. A P value less than 0.05 was considered significant.
A multivariate logistic regression analysis model was used to rule out any other confounders that might influence the clinical results. The multivariate logistic regression model used the mean per patient of several independent parameters to predict pregnancy. To avoid multicollinearity, Pearson’s correlations were performed and only non-correlated parameters were included in the multivariate model.
The study was conducted at the IVF Unit at the Hillel Yaffe Medical Center. It was approved by the Institutional Review Board (NIH number NCT01672931). All participants signed an informed consent.
Results
A total of 387 oocytes were obtained from 46 women who participated in the study. Demographic characteristics are shown in Table 1. There were no differences in the indications for fertility treatment. The normal weight group were significantly younger compared to the obese group; however, both group are considered young for IVF treatments.
Table 1.
Demographic characteristics of the study group patients
| Characteristic | Normal weight | Obese | P value |
|---|---|---|---|
| Number of patients | 24 | 22 | |
| Number of oocytes tested | 215 | 172 | |
| Age | 29.0 ± 4.6 | 34.0 ± 4.5 | <0.0001 |
| Estradiol (pmol/l) (median 25–75%) | 182 (94–286) | 80 (38–151) | 0.001 |
| FSH (IU) | 6.5 ± 1.5 | 6.1 ± 1.6 | 0.41 |
| LH (IU) | 6.6 ± 2.3 | 5.7 ± 3.3 | 0.33 |
| BMI (kg/m2) | 21.2 ± 1.9 | 34.5 ± 3.8 | <0.0001 |
| Type of gonadotrophins used | |||
| Recombinant | 21 (88%) | 17 (77%) | 0.45 |
| Urinary | 2 (8%) | 5 (23%) | 0.23 |
| Combined | 1 (4%) | 0 | 1.00 |
| Total dose of gonadotrophins (IU) (median 25–75%) | 1200 (906.5–1612) | 1900 (1425–2400) | 0.003 |
| Protocol | |||
| Long agonist | 7 (29%) | 10 (45%) | 0.36 |
| Short agonist | 0 | 2 (10%) | 0.22 |
| Antagonist | 17 (71%) | 10 (45%) | 0.13 |
Mean ± SD
More mature oocytes (M2) were retrieved from normal weight than from obese women (15.1 ± 6.8 vs. 9.7 ± 3.9, respectively, P < 0.001) (Table 2). Mature oocytes from women in the obese group were significantly smaller than those in the normal weight group were, including diameter (157.9 ± 7.9 vs. 164.3 ± 5.1 μm, P < 0.0001), oolema (110.3 ± 4.5 vs. 113.5 ± 3.5 μm, P < 0.0001), and zona pellucida (17.9 ± 2.6 vs. 19.0 ± 2.4 μm, P < 0.0001).
Table 2.
Oocyte measurements between normal weight and obese women
| Measurement | Normal weight | Obese | P value |
|---|---|---|---|
| Number of patients | 24 | 22 | |
| Number of mature oocytes tested | 215 | 172 | |
| Full oocyte diameter (μm) | 164.3 ± 5.1 | 157.9 ± 7.9 | <0.0001 |
| Oolema diameter (μm) | 113.5 ± 3.5 | 110.3 ± 4.5 | <0.0001 |
| Zona pellucida thickness (μm) | 19.0 ± 2.4 | 17.9 ± 2.6 | <0.0001 |
Mean ± SD
We used linear mixed models for repeated measure analyses to evaluate whether differences exist in ZP measurements and to examine whether age, oolema, and M2 influence pregnancy. BMI was significantly correlated to ZP thickness.
There were no statistically significant differences between the groups regarding endometrial thickness and length of stimulation protocols (Table 3). The numbers of embryos per participant, as well as the number of transferred embryos, were comparable. Fertilization rate was comparable between the obese and normal weight groups (59.2 ± 21.2 vs. 67.9 ± 21.2, P = 0.17); however, cleavage rate was significantly higher in the normal weight group (100 vs. 92.5%, P = 0.03). Both groups had a high number of top quality embryos with no significant difference. Pregnancy rate was comparable as well, however, with a trend towards higher pregnancy rate in the normal weight group (50 vs. 28.6%, P = 0.22). Interestingly, we sub-analyzed the pregnancy in both groups; we found that in the obese group, zona pellucida was significantly thinner in those who conceived compare to the non-conceived group (16.64 ± 2.7 vs. 19.5 ± 2.2, P < 0.0001).
Table 3.
Clinical outcomes
| Outcomes | Normal weight | Obese | P value |
|---|---|---|---|
| Number of patients | 24 | 22 | |
| Duration of follicular phase (days) | 10.0 ± 3.1 | 10.7 ± 3.0 | 0.48 |
| Peak estradiol level (pmol/l) (median 25–75%) | 1461 (1350–1983) | 1613 (1353–2240) | 0.91 |
| Endometrial thickness (mm) | 9.97 ± 1.99 | 10.3 ± 1.9 | 0.56 |
| Number of oocytes tested | 215 | 172 | |
| No. of mature oocytes collected | 15.1 ± 6.8 | 9.7 ± 3.9 | 0.001 |
| Fertilization rate (%) | 59.2 ± 21.2 | 67.9 ± 21.2 | 0.17 |
| Cleavage rate (%) | 100 | 92.5 ± 10.2 | 0.03 |
| Number of embryos transferred | |||
| 1 | 6 (25%) | 3 (14%) | 0.46 |
| 2 | 11 (46%) | 12 (57%) | 0.55 |
| 3 | 7 (29%) | 6 (29%) | 1.00 |
| Top quality embryos (%) | 17.4 (0–40.4) | 20 (0–46.4) | 0.58 |
| Pregnancy rate (%) | 12 (50%) | 6 (28.6%) | 0.22 |
Mean ± SD
A multivariate logistic regression analysis was conducted to predict pregnancy (Table 4). Female age, BMI, number of M2 oocytes, and mean zona pellucida thickness and oolema diameter were included. Different variables contributed in each BMI group. In the overweight patients, the smaller oolema diameter adversely predicted pregnancy. As the oolema diameter increased, so did the probability of pregnancy (95% CI 1.044–1.316, OR = 1.17, P = 0.007). The zona pellucida thickness influence pregnancy rate; as the thickness decreased, pregnancy rate increased significantly (95% CI 0.54–0.793, OR = 0.65, P < 0.0001). In a combined model, normal weight patient demonstrated odds ratio of 6.1 to achieved pregnancy compare to obese patients (P < 0.0001, 95%CI = 3.03–12.4). Age did not influence oocyte parameters nor pregnancy, probably because patients were relatively young in both group.
Table 4.
A multivariate logistic regression analysis models
| Significant parameters | Confidence interval (CI) | Odds ratio (OR) | P value | |
|---|---|---|---|---|
| Model A: all | Mean zona pellucida thickness | 0.82–0.993 | 0.9 | P = 0.036 |
| BMI group | 3.03–12.4 | 6.1 | P < 0.0001 | |
| M2 oocytes | 0.78–0.87 | 0.83 | P < 0.0001 | |
| Mean oolema diameter | 0.99–1.14 | 1.06 | P = 0.06 | |
| Age | 0.98–1.12 | 1.05 | P = 0.126 | |
| Model B: overweight | Mean zona pellucida thickness | 0.54–0.79 | 0.65 | P < 0.0001 |
| Mean oolema diameter | 1.04–1.31 | 1.17 | P = 0.007 | |
| Age | 0.84–1.03 | 0.94 | P = 0.183 |
Discussion
It is well-established that obesity has a major influence on fertility and reproductive medicine outcomes due to its relation to anovulation, hyperinsulinemia, and ovarian hyperandrogenism [12, 20–23]. Yet, the exact mechanisms of how obesity results in lower implantation and pregnancy rates and higher miscarriage rate are debated [9, 14, 24, 25].
The current study prospectively evaluated the effect of obesity on the quantity, size, and quality of oocytes. We found that oocytes were significantly smaller in obese women compared to normal weight women. Obesity had an adverse influence on the total number of mature oocytes and cleavage rate. However, fertilization rate and embryo quality did not differ between the groups. In agreement with our results, Lazzaroni-Tealdi E. et al. [26] demonstrate that oocyte size showed significant influence on embryo quality and an independent predictor of embryo quality after adjustment to age.
Obese women received significantly larger doses of gonadotropin. The normal weight group showed a trend towards better pregnancy rates, but lacked the power to establish significance.
The literature proposes various theories to explain poorer treatment outcomes among obese women. These include abnormal hormonal axis and anovulation, hostile endometrial receptivity, and endogenous oocyte factors [4, 5, 12, 21, 27, 28]. Oocyte quality has been widely investigated [6, 24, 29, 30]. Marquand et al. reported that oocytes collected from obese women with PCOS were significantly smaller than those from normal weight controls with PCOS [13]. Lazzaroni-Tealdi E. et al. found six oocyte parameters with a significant influence on embryo quality, including morphology, ooplasm, ZP structure, PVS, and morphology of the first PB and as well oocyte size [26]. In contrast, Salata et al. did not find a correlation between oocyte diameter, fertilization rate, and embryo quality [31]. Similar to Marquard et al., we found significantly smaller oocytes in obese as compared with normal weight women without PCOS. We did not detect a difference in embryo quality between the groups.
Y.P. Sun et al. demonstrated that ZP thickness variation influenced pregnancy rate more than the mean thickness of ZP [32]. Balakier H. et al. found that embryos with good morphology exhibited considerably thinner ZP compared with those of less favorable morphology. The ZP thickness had no significant impact on the implantation and pregnancy rates [33]. In our study, we found that obese women had thicker ZP and in the obese group, it significantly influenced the pregnancy rate (Table 4). We did not find any significant results when ZP thickness variation was calculated.
Using an animal model, Jungheim et al. demonstrated the effects of maternal obesity in the pre- and peri-conceptional periods by comparing reproductive tissues from diet-induced obese female mice to those of control mice. They found fewer and smaller mature oocytes in mice on the rich fat diet as compared to the controls. Other possible mechanisms related to poor reproductive outcomes include decreased IGF-1 receptor expression in the pre-implantation blastocyst, which influences embryonic implantation and fetal growth, and increased mid-gestational placental transcription of IGF-2 receptors, a maternally expressed gene known to influence placental and fetal size [7].
Robker et al. found that obesity is associated with intrafollicular changes in multiple cellular systems including steroidogenesis, intrinsic metabolism, and inflammation in women undergoing in vitro fertilization [24]. Consistent with the above and in agreement with our results, Leary et al. showed that oocytes from overweight and obese women are significantly smaller compared to those from normal weight women and that the smaller oocytes were less likely to reach the blastocyst stage. In addition, the embryos demonstrated significant metabolic abnormalities, including decreased glucose consumption, altered amino acid metabolism profile, and increased endogenous triglyceride content [14].
Our findings suggest that obese patients have significantly fewer mature oocytes than normal weight women do, but similar fertilization rates. This agrees with Wittemer et al. who also found significantly fewer mature oocytes in obese women [34], but is in contrast to Shah et al. who found that obesity was associated with fewer normally fertilized oocytes despite similar numbers of mature oocytes [35].
According to our regression model, we found small oocyte and zona pellucida diameters in the obese patients, but not in the normal weight patients. We assume that the smaller oocyte diameters among the obese patients represent mal-synthesis of fat and cholesterol, and presumably adverse mitochondrial function, which adversely affects pregnancy outcomes.
To the best of our knowledge, this prospective study is the first to demonstrate the connection between obesity, oocyte size, and fertility outcomes among women without PCOS. The small sample size limited the ability to demonstrate statistically significant differences in the clinical outcomes, and only a trend was demonstrated. The two groups differed in age; however, since we limited the patients in the study to be younger than 40 years, we believe this difference had no clinical effect. The average age in each group was younger than 35 years (Table 1).
To expand on our findings and assess the assumption about oocyte metabolism, we plan to increase the sample size, assess our assumption of mal-synthesis of lipids, and compare the follicular fluids between the groups in a future study.
Conclusion: Obesity can result in smaller oocytes, which adversely affect fertility outcomes. Regarding our findings, we recommend lifestyle changes for patients who are above a normal weight range. Despite not having a significant effect on pregnancy rates, it is well-established that obesity predisposes women to several general and obstetrical complications such as pre-eclampsia, gestational diabetes, and birth defects, as well as higher cesarean section rates [36–38]. Therefore, it is important to discuss fertility and pregnancy complications with obese patients prior to conception, if possible [39–41].
BMI, body mass index; ICSI, intracytoplasmatic sperm injection; IVF, in vitro fertilization; PCOS, polycystic ovarian syndrome
Contributor Information
Yuval Atzmon, Email: atzmony@gmail.com.
Ester Shoshan-Karchovsky, Email: etykar@yahoo.com.
Medeia Michaeli, Email: medeia_michaeli@yahoo.com.
Nardin Aslih, Email: nardin_aslih@yahoo.com.
Guy Shrem, Email: gai.shrem@gmail.com.
Adrian Ellenbogen, Email: ellenbogen55@yahoo.com.
Einat Shalom-Paz, Email: einatshalompaz@gmail.com.
References
- 1.Losing weight, body mass index [Internet]. Available from: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm
- 2.WHO | Obesity and overweight. WHO. World Health Organization; 2016.
- 3.Practice Committee of American Society for Reproductive Medicine Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90:S21–S29. doi: 10.1016/j.fertnstert.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29:498–506. doi: 10.1016/j.bpobgyn.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod BioMed Online. 2011;23:421–439. doi: 10.1016/j.rbmo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 6.MacHtinger R, Combelles CMH, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod. 2012;27:3198–3207. doi: 10.1093/humrep/des308. [DOI] [PubMed] [Google Scholar]
- 7.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock IA, Diaz-Gimeno P, Cabanillas S, Bellver J, Sebastian-Leon P, Shah M, et al. Does an increased body mass index affect endometrial gene expression patterns in infertile patients? A functional genomics analysis. Clin. Trial Regist. Number NCT02205866.Fertil Steril Ò. 2016. [DOI] [PubMed]
- 9.Bellver J, Ayllon Y, Ferrando M, Melo M, Goyri E, Pellicer A, et al. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93:447–454. doi: 10.1016/j.fertnstert.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 10.van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015. [DOI] [PMC free article] [PubMed]
- 11.Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10:267–280. doi: 10.1093/humupd/dmh018. [DOI] [PubMed] [Google Scholar]
- 12.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26:245–252. doi: 10.1093/humrep/deq306. [DOI] [PubMed] [Google Scholar]
- 13.Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95:2146–2149.e1. doi: 10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30:122–132. doi: 10.1093/humrep/deu276. [DOI] [PubMed] [Google Scholar]
- 15.Macklon NS, Stouffer RL, Giudice LC, Fauser BCJM. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 16.Payne D, Flaherty SP, Jeffrey R, Warnes GM, Matthews CD. Successful treatment of severe male factor infertility in 100 consecutive cycles using intracytoplasmic sperm injection. Hum Reprod. 1994;9:2051–2057. doi: 10.1093/oxfordjournals.humrep.a138392. [DOI] [PubMed] [Google Scholar]
- 17.Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95:1985–1989. doi: 10.1016/j.fertnstert.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3:284–295. doi: 10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- 19.Balaban B, Brison D, Calderón G, Catt J, Conaghan J, Cowan L, et al. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed. 2011;22:632–646. doi: 10.1016/j.rbmo.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Dağ ZÖ, Dilbaz B. Impact of obesity on infertility in women. J Turkish Ger Gynecol Assoc. 2015;16:111–117. doi: 10.5152/jtgga.2015.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13:433–444. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- 22.Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. 2017. [DOI] [PubMed]
- 24.Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94:1533–1540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- 25.Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod BioMed Online. 2007;15:532–538. doi: 10.1016/S1472-6483(10)60385-9. [DOI] [PubMed] [Google Scholar]
- 26.Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, et al. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0143632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols JE, Crane MM, IV, Higdon HL, Miller PB, Boone WR. Extremes of body mass index reduce in vitro fertilization pregnancy rates. Fertil Steril. 2003;79:645–647. doi: 10.1016/S0015-0282(02)04807-0. [DOI] [PubMed] [Google Scholar]
- 28.Seif MW, Diamond K, Nickkho-Amiry M. Obesity and menstrual disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2015;29:516–527. doi: 10.1016/j.bpobgyn.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod BioMed Online. 2006;12:507–512. doi: 10.1016/S1472-6483(10)62006-8. [DOI] [PubMed] [Google Scholar]
- 31.Salata RG, Araujo MCPM, de Melo AS, de Albuquerque Salles Navarro PA, Ferriani RA, dos Reis RM. Oocyte diameter as a predictor of fertilization and embryo quality in assisted reproduction cycles. Fertil Steril. 2010;93:621–625. doi: 10.1016/j.fertnstert.2008.12.124. [DOI] [PubMed] [Google Scholar]
- 32.Sun YP, Xu Y, Cao T, Su YC, Guo YH. Zona pellucida thickness and clinical pregnancy outcome following in vitro fertilization. Int J Gynaecol Obstet. 2005;89:258–262. doi: 10.1016/j.ijgo.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Balakier H, Sojecki A, Motamedi G, Bashar S, Mandel R, Librach C. Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels? Fertil Steril. 2012;98:77–83. doi: 10.1016/j.fertnstert.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Wittemer C, Ohl J, Bailly M, Bettahar-Lebugle K, Nisand I. Does body mass index of infertile women have an impact on IVF procedure and outcome? J Assist Reprod Genet. 2000;17:547–552. doi: 10.1023/A:1026477628723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet Gynecol. 2011;118:63–70. doi: 10.1097/AOG.0b013e31821fd360. [DOI] [PubMed] [Google Scholar]
- 36.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16:621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]
- 37.Salihu HM, De La Cruz C, Rahman S, August EM. Does maternal obesity cause preeclampsia? A systematic review of the evidence. Minerva Ginecol. 2012;64:259–280. [PubMed] [Google Scholar]
- 38.Carmichael SL, Rasmussen SA, Shaw GM. Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res A Clin Mol Teratol. 2010;88:804–810. doi: 10.1002/bdra.20679. [DOI] [PubMed] [Google Scholar]
- 39.Radulescu L, Munteanu O, Popa F, Cirstoiu M. The implications and consequences of maternal obesity on fetal intrauterine growth restriction. J Med Life. 2013;6:292–298. [PMC free article] [PubMed] [Google Scholar]
- 40.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Low maternal weight, failure to thrive in pregnancy, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2003;189:1726–1730. doi: 10.1016/S0002-9378(03)00860-3. [DOI] [PubMed] [Google Scholar]
- 41.Consultation W Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Heal Organ - Tech Rep Ser. 2000;894:i–xii. [PubMed] [Google Scholar]