Introduction
Helicobacter cinaedi is an unusual cause of cellulitis in immunocompromised patients.1 The organism is fastidious, and blood cultures are often negative, making the diagnosis challenging, especially in those without systemic signs. We report a case of chronic H cinaedi cellulitis in a patient with X-linked agammaglobulinemia (XLA) diagnosed by universal microbial polymerase chain reaction (PCR).
Case report
A 48-year-old man with XLA presented to an outside clinic with chronic leg lesions. He had been treated with intravenous immunoglobulin since 1974. Painful, bruiselike lesions developed below his knees 5 to 6 months before presentation. He also reported fatigue, inguinal discomfort, joint pain, and shortness of breath concomitant with worsening of his leg lesions. The lesions were initially thought to be clinically consistent with erythema nodosum, and he was started on prednisone, 20 mg daily. After a few weeks of treatment there was limited improvement, so he was referred to the dermatology clinic for evaluation.
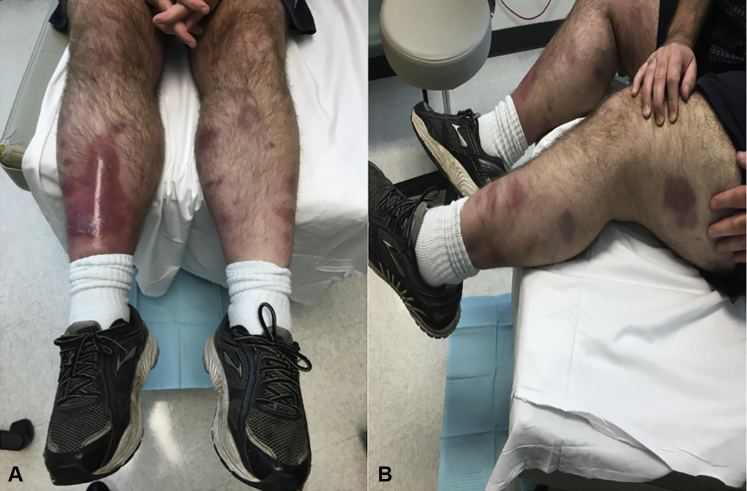
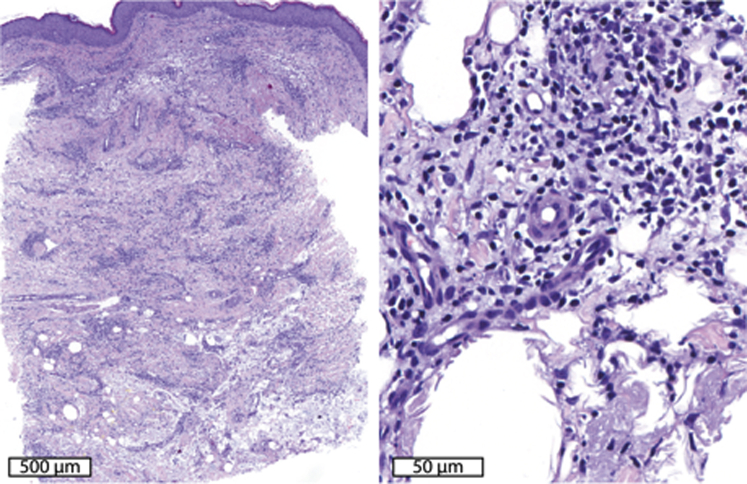
On examination, he had normal vital signs and appeared in no acute distress. Skin examination found scattered, variably erythematous, violaceous, hyperpigmented, and firm plaques on the distal legs that were several centimeters in diameter, some with overlying superficial desquamation (Fig 1). Our differential diagnoses included infection (including atypical bacterial and fungal), erythema nodosum, erythema induratum, Sweet syndrome, and primary cutaneous granulomas as a manifestation of immunodeficiency.2 Laboratory evaluation found a mild transaminitis, white blood cell count of 11.4*109/L (absolute neutrophil count of 10.7, absolute lymphocyte count of 0.3, and CD4 count of 154 cells/mm3), IgM and IgA below the measurable range, IgG in the normal range, C-reactive protein of 130 mg/L, erythrocyte sedimentation rate of 82 mm/h, procalcitonin of 0.75 ng/mL, and a negative interferon-γ release assay (QuantiFERON, QIAGEN, Hilden, Germany). During the course of his evaluation, 3 punch biopsies found granulomatous and suppurative dermatitis with lipomembranous fat necrosis (Fig 2). Periodic acid–Schiff–diastase, Brown-Brenn, and Fite stains were negative for fungi, bacteria, and acid-fast bacilli, respectively. Multiple cultures of the biopsied skin for bacteria, fungi, and mycobacteria were negative as well. During this time, he was evaluated by the urology department for orchalgia and epididymitis was diagnosed. He was started on trimethoprim-sulfamethoxazole (TMP-SMX), 160 mg to 800 mg twice a day for 6 weeks and then daily for prevention. Treatment with TMP-SMX improved his orchalgia but did not improve his rash. As his prednisone was tapered down, the rash spread further, and tender, violaceous, mobile, subcutaneous nodules developed on his trunk.
Fig 1.
Violaceous, hyperpigmented firm plaques of several centimeters on anterior shins (A) and left lateral leg (B).
Fig 2.
Histopathologic features. Left panel shows an interstitial suppurative and granulomatous infiltrate involving the dermis and superficial subcutis. Right panel shows lipomembranous change in the subcutis with a mixed infiltrate with lymphocytes, neutrophils, and plasma cells.
Although stains and cultures were negative, the patient's symptoms, immunodeficiency, and pathology suggested infection. The infectious disease department was consulted and recommended sending tissue from a skin biopsy for universal microbial PCR. This PCR used a 16S rRNA primer set to identify bacteria in addition to other primer sets for mycobacteria and fungi. An amplified PCR product from leg skin was sequenced and identified as coming from H cinaedi. To confirm the diagnosis, one of the recently appeared, subcutaneous nodules from the patient's trunk was excised and sent for pathology, culture, and repeat microbial PCR. The pathology findings showed a neutrophilic abscess with fat necrosis. Bacterial PCR was once again positive for H cinaedi. Blood cultures obtained while the patient was on TMP-SMX were incubated for 6 days but were negative. The patient was started on doxycycline, 100 mg twice a day, in addition to TMP-SMX. There was significant improvement in his rash and fatigue 3 weeks later. After 4 months, his systemic symptoms had completely resolved, and his rash had resolved to hyperpigmentation and mild atrophy so all antibiotics were stopped.
Discussion
H cinaedi is a gram-negative enteric curved (helix-shaped) bacillus that causes gastroenteritis, bacteremia, and rash, usually in immunocompromised patients. Patients typically appear acutely ill and are found to have fever and bacteremia. According to a retrospective review of 73 mostly immunocomprimised patients with H cinaedi bacteremia, skin lesions occur in 30% of patients and appear as erythematous patches and plaques on the extremities.3 Skin biopsy often finds a mixed inflammatory infiltrate in the dermis and subcutis. Diagnosis is very difficult by traditional culture techniques. The authors of the previously mentioned study examined tissue culture and bacterial stains of skin lesions of 6 patients with H cinaedi bacteremia, and all were found to be negative.3 Correct diagnosis requires a high index of suspicion and microbiologic confirmation. H cinaedi infections generally respond to extended courses of penicillins, carbapenems, aminoglycosides, or tetracyclines.3 The lack of response of our patient's rash to TMP-SMX prescribed for epididymitis is in line with documented resistance of H cinaedi to that antibiotic.4
Recognition of H cinaedi infection in our case was delayed because the bacterium was not detected by routine stains or cultures, consistent with current literature in which cultures are often negative.3 One possibility is that our patient's immunodeficiency allowed for indolent infection and bacteremia (as demonstrated by the late onset of bacteria-positive abscesses at distant sites) without fever or positive blood culture. Indeed, a case of afebrile but ulcerating H cinaedi skin infection has been reported in a patient with XLA.5 This patient had an evolving rash for 5 years before it formed a pyoderma gangrenosum–like ulcer, and H cinaedi was detected via PCR of the skin. Similarly, another case of an afebrile patient with XLA who had hyperpigmented macules, whom later was found to have positive blood cultures and PCR findings for H cinaedi, has been reported.6 PCR has also been used to detect the bacterium in the blood, urine, and stool of infected hospital patients.4, 5, 7, 8 These observations suggest that PCR may be the best test to rule out H cinaedi infection, which can mimic other illnesses and avoid detection by other means. Before PCR was available, it is conceivable that similar cases of indolent infections like this one went misdiagnosed from lack of a sufficiently sensitive microbiologic test.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Shimizu T., Choi E., Petersen C.P. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J Pathol. 2016;239(4):399–410. doi: 10.1002/path.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harp J., Coggshall K., Ruben B.S., Ramirez-Valle F., He S.Y., Berger T.G. Cutaneous granulomas in the setting of primary immunodeficiency: a report of four cases and review of the literature. Int J Dermatol. 2015;54(6):617–625. doi: 10.1111/ijd.12765. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu S., Shimizu H. Cutaneous manifestations of Helicobacter cinaedi: a review. Br J Dermatol. 2016;175(1):62–68. doi: 10.1111/bjd.14353. [DOI] [PubMed] [Google Scholar]
- 4.Uckay I., Garbino J., Dietrich P.Y., Ninet B., Rohner P., Jacomo V. Recurrent bacteremia with Helicobacter cinaedi: case report and review of the literature. BMC Infect Dis. 2006;6:86. doi: 10.1186/1471-2334-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dua J., Elliot E., Bright P. Pyoderma gangrenosum-like ulcer caused by Helicobacter cinaedi in a patient with x-linked agammaglobulinaemia. Clin Exp Dermatol. 2012;37(6):642–645. doi: 10.1111/j.1365-2230.2011.04293.x. [DOI] [PubMed] [Google Scholar]
- 6.Simons E., Spacek L.A., Lederman H.M., Winkelstein J.A. Helicobacter cinaedi bacteremia presenting as macules in an afebrile patient with X-linked agammaglobulinemia. Infection. 2004;32(6):367–368. doi: 10.1007/s15010-004-3152-7. [DOI] [PubMed] [Google Scholar]
- 7.Oyama K., Khan S., Okamoto T. Identification of and screening for human Helicobacter cinaedi infections and carriers via nested PCR. J Clin Microbiol. 2012;50(12):3893–3900. doi: 10.1128/JCM.01622-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasahara Y., Noguchi S., Orihashi T. [Three cases of bacteremia due to Helicobacter cinaedi infection and the usefulness of gene analysis of isolated bacteria] J UOEH. 2015;37(4):293–298. doi: 10.7888/juoeh.37.293. [DOI] [PubMed] [Google Scholar]