Abstract
Aim
To use plan analysis software to evaluate a class solution for prostate intensity modulated radiotherapy (IMRT) planning.
Background
Class solutions for radiotherapy planning are increasingly being considered for streamlining planning. Plan analysis software provides an objective approach to evaluating radiotherapy plans.
Materials and methods
Three iterations of a class solution for prostate IMRT planning (T1, T2 and Tfinal) were compared to the clinical plan of 74 prostate patients using radiotherapy plan analysis software (Plan IQ™, Sun Nuclear Corporation). A set of institution-specific plan quality metrics (scores) were established, based on best practice guidelines.
Results
For CTV coverage, Tfinal was not significantly different to the clinical plan. With the exception of 95% PTV coverage, Tfinal metrics were significantly better than the clinical plan for PTV coverage. In the scoring analysis, mean dose, 95% and 107% isodose coverage scores were similar for all the templates and clinical plan. 100% coverage of the CTV clinical plan was similar to Tfinal but scored higher than T1 and T2. There were no significant differences between Tfinal and the clinical plan for the metrics and scores associated with organs at risk. The total plan score was similar for Tfinal and the clinical plan, although the scores for volume receiving total dose outside the PTV were higher for Tfinal than for the clinical plan (P < 0.0001).
Conclusions
The radiotherapy plan analysis software was useful for evaluating a class solution for prostate IMRT planning and provided evidence that the class solution produced clinically acceptable plans for these patients.
Keywords: Prostate, Radiotherapy planning, Software, Solutions
1. Background
Intensity modulated radiotherapy (IMRT) is a widely used and well established technique for external beam radiotherapy. The potential for automation was identified early on1; the possibility of a class solution for prostate radiotherapy treatment planning was also considered at an early stage.2, 3 The concept of a class solution has received increasing attention as it has moved from concept to clinical reality.4, 5, 6, 7, 8, 9, 10 Some authors consider class solutions as a starting point8 for the treatment planning process and more recently it has been suggested that radiotherapy planning will become fully automated within the next decade.11
A class solution for radiotherapy planning can be defined as a set of dosimetric objectives and geometric beam arrangements that are sufficiently robust to produce a clinically acceptable dose distribution regardless of the patient anatomy, target volume or organs at risk (OAR).7 The resulting plan should also be less dependent on treatment planner experience12 and resulting in an efficient clinical workflow.
Treatment plan dose distributions are often deemed clinically acceptable with respect to department protocols (including clinician experience) and international recommendations. With the introduction of IMRT, assessment using objective dose volume histogram (DVH) criteria has increased; however, it can still often be subjective in determining whether the treatment plan is considered clinically acceptable. Current literature12, 13 suggests that clinical experience, individual knowledge and planner skill introduce a bias when determining quality of treatment plans and a more structured analysis or quantitative evaluation of treatment plans is required. Ruan et al.12 developed a set of evolving institution specific criteria as a standard by which to assess plan quality with a view to reducing or eliminating bias. Ventura et al.14 incorporated planning and clinical criteria into a plan evaluation tool (SPIDERplan) which was based on a scoring approach and included graphical representation in the form of radar plots. The quality of the dose distribution in the PTV and OARs of possible treatment plans could be easily compared.
Plan quality can be evaluated using dedicated task specific software. Software developed to analyse radiotherapy plans have tools to measure, compare and validate treatment plan quality. For example, Plan IQ™ is a treatment planning system independent, DVH analysis tool that enables the user to create plan quality algorithms.15 A plan quality algorithm may contain many plan quality metrics (PQM) with specific weightings or scores assigned to each, enabling plans to be given an overall quality score for ease of comparison and validation.13 This type of software provides effective tools for analysing and validating class solutions.
The development of a class solution for prostate IMRT has recently been described.16 In the current study, we use analytical software tools to evaluate a class solution for intact prostate radiotherapy planning.
2. Methods and materials
2.1. Patient selection
Retrospective planning data was collected for 74 prostate patients treated in late 2014 through to early 2015. All patients were treated with 81 Gy in 45 fractions in a single phase treatment approach. All patients were simulated with the same computed tomography (CT) scanning protocol. Patients were contoured in accordance with the institutional protocol which is developed from evidence-based guidelines.16 The work met the criteria for a Quality Improvement project according to the NSW Health Ethics Guideline document GL2007_020 and did not require formal ethical review (HREC reference number QA160).
2.2. Class solution for prostate IMRT
As a quality improvement initiative, an optimal template (class solution) for IMRT prostate patients has been developed within our institution.16 Using a stepwise quality improvement model and evidence-based guidelines, a template based on 10 patients underwent three stages of development, refinement and evaluation. The first evaluation involved 20 patients at two centres using sensitivity analysis; the second involved a major review of treated plans across a larger number of cases (n = 50); the review informed the development of a final template, Tfinal. In the current study, the criteria of the developmental templates (termed T1 and T2) and Tfinal were applied and calculated on each patient data set. These class solutions were compared to each other and the clinically treated plan (CP). Along with the final clinical treatment plan, this information was then exported to radiotherapy plan analysis software (Plan IQ™, Sun Nuclear Corporation, Melbourne, FL, USA) where the plan quality metrics (PQM) was applied. Monaco V5.0 (Elekta-CMS Software, MO, USA) was used to generate all treatment plans using plan templates.
2.3. Building the metrics
A prerequisite to the analysis was to establish a robust set of institution-specific metrics that would identify a high-quality treatment plan. The PQM for this study were drawn from the IMRT prostate protocol used at our institution which outlined the target planning aims and organ at risk (OAR) dose constraints. The metrics used and the dose constraints from the local clinical protocol are summarised in Table 1. To further assess plan quality, other metrics including conformity, regions of high dose outside the planning target volume (PTV) and plan global maximum location were assessed. Each metric was assigned a weighting, achieved through a maximum score, with respect to clinical importance (see Table 1). In total there were eight metrics associated with target coverage, ten metrics associated with OARs and three metrics that had a lesser weighting and were indicators of overall plan quality.
Table 1.
Plan quality metrics and assigned scores for assessing prostate plan quality: the maximum possible score is 146.
| Structure | Metric | Maximum |
Middle range |
Minimum |
|||
|---|---|---|---|---|---|---|---|
| Score | Criteria | Score | Criteria | Score | Criteria | ||
| CTV | % covered by TD | 20 | 100–99.5% | 19.9–0.1 | 99.6–99% | 0 | <98.9% |
| PTV | % covered by TD | 20 | 100–95% | 19.9–0.1 | 94.9–90% | 0 | <89.9% |
| % covered by 95%TD | 10 | 100–99% | 9.9–0.1 | 98.9–98 | 0 | <97.9% | |
| % covered by 107%TD | 10 | <1% | 9.9–0.1 | 1.1–2% | 0 | >2% | |
| % covered by 105%TD | 10 | 0% | 0 | >15% | |||
| Mean dose | 5 | 81–83 Gy | 2.9–0.1 | 84.6–85.6 Gy | 0 | >85.6 Gy | |
| Homogeneity index | 5 | 0.02 | 0 | >0.15 | |||
| Conformation number | 5 | 1 | 4.9–4 3.9–0 |
0.9–0.7 0.69–0.51 |
0 | <0.5 | |
| Volume (cc) covered by TD outside PTV | 10 | 8–10 cc | 7.9–0 | 10.1–20 cc | 0 | >20 cc | |
| Rectum | Volume (cc) covered by 102.5%TD | 0 | <2 cc | 0.1 to −5 | 2–3 cc | −5 | >3cc |
| % covered by 40 Gy | 10 | <35% | 8–10 7.9–0.1 |
35.1–40% 40.1–45% |
0 | >45% | |
| % covered by 65 Gy | 10 | <17% | 9.9–0.1 | 17.1–21% | 0 | >21% | |
| % covered by 75 Gy | 10 | <10% | 9.9–0.1 | 10.1–15% | 0 | >15% | |
| Coverage of 50%TD axial slice evaluation | 0 | Pass | −10 | Fail | |||
| Bladder | % covered by 60 Gy | 5 | <35% | 4.9–0.1 | 35.1–40% | 0 | >40% |
| % covered by 40 Gy | 5 | <50% | 4.9–0.1 | 50.1–60% | 0 | >60% | |
| Penile bulb | % covered by 50 Gy | 2 | <95% | 1.9–0.1 | 95.1–100% | 0 | >100% |
| Rt femoral head | % covered by 45 Gy | 2 | <2% | 1.9–0.1 | 2–5% | 0 | >5% |
| Lt femoral head | % covered by 45 Gy | 2 | <2% | 1.9–0.1 | 2–5% | 0 | >5% |
| Patient | Volume (cc) covered by 110%TD | 0 | <2 cc | −0.1 to −5 | 2–5 cc | −5 | >5 cc |
| Global max location | Within CTV/PTV/elsewhere/rectum | 5 | CTV | 3 0 |
PTV Elsewhere |
−5 | Rectum |
Abbreviations: CTV, clinical target volume; PTV, planning target volume; TD, total dose (Gy).
Definitions: homogeneity index = [dose covering 1% of PTV − dose covering 99% of PTV] (Gy)/prescribed dose (Gy). The prescribed dose was 81 Gy.
Conformation number = [PTV volume (cc) covered by specified dose (95% in Gy)]2/[total volume covered by 95% dose (Gy) × total volume of PTV (cc)].
2.4. Metric verification
PQM development followed a heuristic process and empirical assessment by five experienced radiation therapist/dosimetrists who determined that the PQM score functions selected were providing a suitable measure of plan quality and were adequate for the subsequent assessments. The process included a review of all low scoring plans where the PQM was <120; plans reviewed exhibited major violations of the clinical protocol requirements. While not assessed further it would appear that a score of <120 would correlate strongly with plans that do not meet required clinical objectives.
2.5. Plan assessments
Planning criteria and OAR constraints were assessed against evidence-based guidelines as previously described.16, 17 Plans that met all constraints were considered “ideal”; those with only minor violations were considered acceptable for treatment. Plans with major violations were considered unacceptable (fail).
2.6. Statistical analysis
Statistical procedures were carried out using MaxStat Pro (Jever, Germany). The score function, total score and metrics were extracted from Plan IQ and tabulated in Microsoft Excel. Since the metrics were repeated on each subject, with one clinical plan and three class solution plan data sets available, a repeated measures analysis of variance (RM-ANOVA) was considered the statistical method of choice.
Data were assessed for normality using the Anderson–Darling test; data which failed the test were analysed by the Friedman test, a non-parametric equivalent of RM-ANOVA. Where significant differences were noted between groups, multiple comparisons were carried out using Newman–Keuls test for normally distributed data and Conover's test for non-parametric data. Proportions were compared using the z-test. A probability P < 0.05 was considered statistically significant.
3. Results
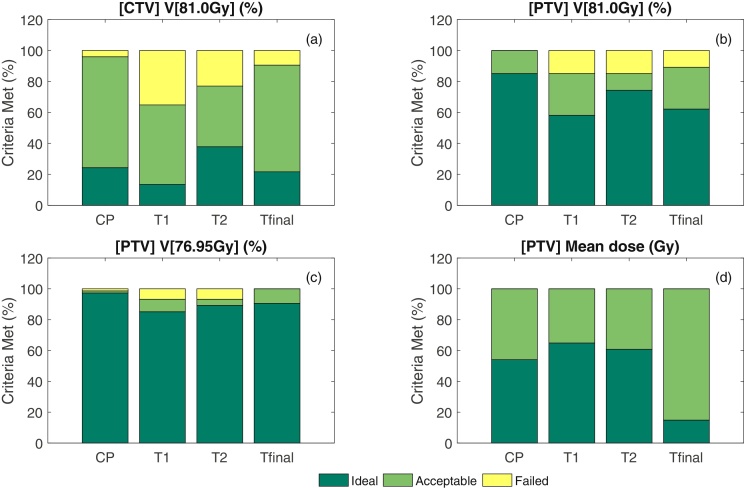
The templates and clinical plan are compared in Fig. 1. In general, successive templates showed an improvement in performance, with T2 meeting acceptable plan criteria in more cases than T1 (58/74 [78.4%] versus 63.5% [47/74]; P = 0.0229) and Tfinal (66/74 [89.2%]) meeting acceptable plan criteria in more cases than T2 (P = 0.0373).
Fig. 1.
Comparison of the clinical plan (CP) and template plans (T1, T2 and Tfinal) for (a) CTV V[81.0 Gy], (b) PTV V[81.0 Gy], (c) PTV V[76.95 Gy] and (d) the mean dose constraints.
3.1. Target coverage
The metrics associated with target coverage for the clinical plan (CP), T1, T2 and Tfinal versions of the template plans are compared in Table 2. T1 showed significant differences with respect to the clinical plan for all target metrics. With the CP as the reference, T2 performed better than T1, except for the percentage covered by 105, 107% of total dose (TD; the prescribed dose was 81 Gy.) and mean dose. Tfinal performed better than T1 and T2 for all metrics with the exception of the percentage covered by 100% TD and mean dose.
Table 2.
Evaluation of a class solution for prostate IMRT. Percentage volumes associated with target coverage (median [range] or means [SD]): comparison of the clinical plan (CP) and class solutions T1, T2 and Tfinal.
| Metric | CP | T1 | T2 | Tfinal | RM-ANOVA (P value) |
|---|---|---|---|---|---|
| [CTV] % covered by TD | 99.80 (94.50–100.0) | 99.53***,††† (92.81–100.0) | 99.81 (46.08–100.0) | 99.81 (88.50–100.0) | <0.001 |
| [PTV] % covered by TD | 97.02 (90.35–99.96) | 96.36***,† (83.19–99.86) | 97.99††† (40.43–99.98) | 96.87* (76.04–99.92) | <0.0001 |
| [PTV] % covered by 95% TD | 99.94 (95.33–100.0) | 99.93** (96.34–100.0) | 99.97†† (71.82–100.0) | 99.93 (98.28–100.0) | 0.0045 |
| [PTV] % covered by 107% TD | 0.004 (0–1.31) | 0.000*** (0.000–0.010) | 0.019*,††† (0.000–0.261) | 0.000*** (0.000–0.012) | <0.0001 |
| [PTV] % covered by 105% TD | 1.65 (0.00–15.67) | 2.20††† (1.15–3.61) | 2.79*,††† (0.000–11.24) | 0.23*** (0.02–0.70) | <0.0001 |
| [PTV] Mean dose (Gy) | 83.03 (82.44–83.82) | 83.16*,††† (82.13–83.61) | 83.15††† (79.31–83.89) | 82.80*** (81.86–83.14) | <0.0001 |
| [PTV] Homogeneity index [81.0 Gy] | 0.066 (0.038–0.251) | 0.071***,††† (0.042–0.203) | 0.065 (0.032–0.228) | 0.061* (0.035–0.110) | <0.0001 |
| [PTV] Conformation number [76.95 Gy] | 0.685 (0.578–0.782) | 0.636***,††† (0.551–0.778) | 0.689††† (0.582–0.884) | 0.697*** (0.602–1.000) | <0.0001 |
| Volume (cc) covered by TD outside PTV | 16.83 (6.30) | 18.72††† (5.41) | 16.93††† (6.51) | 13.64*** (3.71) | <0.0001 |
Compared to CP, P < 0.05.
P < 0.01.
P < 0.0001.
Compared to Tfinal, P < 0.05.
P < 0.01.
P < 0.0001.
Homogeneity index = [dose covering 1% of PTV − dose covering 99% of PTV] (Gy)/prescribed dose (Gy). The prescribed dose was 81 Gy.
Conformation number = [PTV volume (cc) covered by specified dose (95% in Gy)]2/[total volume covered by 95% dose (Gy) × total volume of PTV (cc)].
Fig. 1 illustrates the performance of the clinical and template plans against the clinical protocol for the CTV V[81.0 Gy], PTV V[81.0 Gy], PTV V[76.95 Gy] and the mean dose constraints. Tfinal clearly out performed T1 and T2 for all metrics. It is worth noting that even the original clinically treated plans used for comparison had a small percentage of “failures” where the accepted plan had minor violations and did not fully meet clinical protocol requirements.
The scores associated with the different target metrics are summarised in Table 3. Both the Tfinal and clinical plan showed significantly better scores with respect to percentage covered by 100% TD of the CTV when compared with the T1 and T2 class solution templates. There was no statistically significant difference of the mean dose, the percentage covered by 105% TD and 107% TD scores between the templates and clinical plan.
Table 3.
Evaluation of a class solution for prostate IMRT. Scores associated with target coverage (median [range]): comparison of the clinical plan (CP) and class solutions T1, T2 and Tfinal.
| Metric | CP | T1 | T2 | Tfinal | RM-ANOVA (P value) |
|---|---|---|---|---|---|
| [CTV] % covered by TD | 20.00 (0.10–20.00) | 20.00***,††† (0.10–20.00) | 20.00***,†† (0.10–20.00) | 20.00 (0.10–20.00) | 0.0055 |
| [PTV] % covered by TD | 20.00 (3.29–20.00) | 20.00***,† (0.10–20.00) | 20.00** (0.10–20.00) | 20.00*** (0.10–20.00) | 0.0105 |
| [PTV] % covered by 95% TD | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 10.00 (7.75–10.00) | 0.7471 |
| [PTV] % covered by 107% TD | 10.00 (7.05–10.00) | 10.00 (10.00–10.00) | 10.00 (10.00–10.00) | 10.00 (10.00–10.00) | 0.9747 |
| [PTV] % covered by 105% TD | 8.91 (0.00–10.00) | 8.54††† (7.60–9.66) | 8.15*,††† (2.55–10.00) | 9.85*** (7.85–10.00) | <0.0001 |
| [PTV] Mean dose (Gy) | 5.00 (4.46–5.00) | 5.00 (4.75–5.00) | 5.00 (4.37–5.00) | 5.00 (5.00–5.00) | 0.0991 |
| [PTV] Homogeneity index [81.0 Gy] | 3.54 (0.00–4.70) | 3.325***,††† (0.00–4.49) | 3.565† (0.00–4.91) | 3.71* (1.69–5.00) | 0.0001 |
| [PTV] Conformation number [76.95 Gy] | 3.745 (1.62–4.29) | 2.85***,††† (1.07–4.27) | 3.85††† (1.69–4.63) | 4.00*** (2.11–5.00) | <0.001 |
Compared to CP, P < 0.05.
P < 0.01.
P < 0.0001.
Compared to Tfinal, P < 0.05.
P < 0.01.
P < 0.0001.
Homogeneity index = [dose covering 1% of PTV − dose covering 99% of PTV] (Gy)/prescribed dose (Gy). The prescribed dose was 81 Gy.
Conformation number = [PTV volume (cc) covered by specified dose (95% in Gy)]2/[total volume covered by 95% dose (Gy) × total volume of PTV (cc)].
3.2. Organs at risk
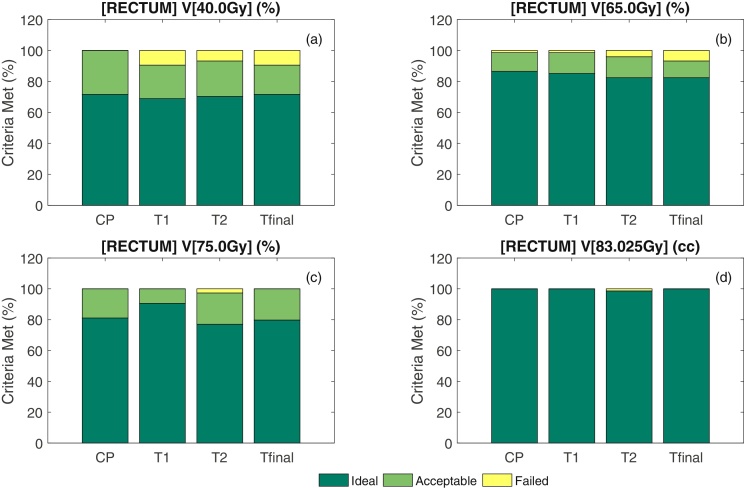
The metrics associated with OAR for the CP, T1, T2 and Tfinal versions of the class solution plans are summarised in Table 4. While T1 differed from the CP on all measures, T2 and Tfinal were not significantly different to the CP. Fig. 2 illustrates the performance of the clinical and template plans against the clinical protocol for the Rectum V[40.0 Gy], Rectum V[65.0 Gy], Rectum V[75.0 Gy] and Rectum V[83.025 Gy].
Table 4.
Evaluation of a class solution for prostate IMRT. Percentage volumes associated with organs at risk (mean [SD] or median [range]): comparison of the clinical plan (CP) and class solutions T1, T2 and Tfinal.
| Metric | CP | T1 | T2 | Tfinal | RM-ANOVA (P value) |
|---|---|---|---|---|---|
| [RECTUM] volume (cc) covered by 102.5%TD | 0.344 (0.000–1.915) | 0.115***,††† (0.000–0.439) | 0.424†† (0.000–2.238) | 0.194 (0.000–1.776) | <0.0001 |
| [RECTUM] % covered by 40 Gy | 29.26 (7.95) | 30.19 (9.50)***,††† | 29.41 (9.19) | 29.78 (9.24) | <0.0001 |
| [RECTUM] % covered by 65 Gy | 11.22 (5.21) | 10.57 (5.47)**,† | 11.02 (5.80) | 11.15 (5.69) | 0.0118 |
| [RECTUM] % covered by 75 Gy | 6.50 (3.60) | 5.65 (3.65)***,††† | 6.50 (4.22) | 6.36 (3.98) | <0.0001 |
| [BLADDER] % covered by 60 Gy | 13.23 (7.76) | 16.20***,††† (9.85) | 12.67 (7.71) | 13.07 (8.05) | <0.0001 |
| [BLADDER] % covered by 40 Gy | 22.97 (13.88) | 26.28***,††† (15.82) | 22.23 (13.60) | 22.53 (13.96) | <0.0001 |
| [BULB] % covered by 50 Gy | 0.000 (0.000–40.23) | 0.000 (0.000–45.12) | 0.000 (0.000–50.52) | 0.000 (0.000–35.98) | 0.0697 |
| [L FEMORAL HEAD] % covered by 45 Gy | 0.000 (0.000–1.328) | 0.000***,††† (0.000–3.834) | 0.000* (0.000–0.299) | 0.000** (0.000–0.300) | 0.0236 |
| [R FEMORAL HEAD] % covered by 45 Gy | 0.000 (0.000–1.861) | 0.000 ***,††† (0.000–4.438) | 0.000 (0.000–0.461) | 0.000 (0.000–0.443) | 0.0004 |
Compared to CP, P < 0.05.
P < 0.01.
P < 0.0001.
Compared to Tfinal, P < 0.05.
P < 0.01.
P < 0.0001.
Fig. 2.
Comparison of the clinical plan (CP) and template plans (T1, T2 and Tfinal) for (a) Rectum V[40.0 Gy], (b) Rectum V[65.0 Gy], (c) Rectum V[75.0 Gy] and (d) Rectum V[83.025 Gy].
The scores associated with the different target metrics for OAR are summarised in Table 5. There were no significant differences in the scores between the various groups in these analyses.
Table 5.
Evaluation of a class solution for prostate IMRT: Scores associated with organs at risk (median [range]): comparison of the clinical plan (CP) and class solutions T1, T2 and Tfinal.
| Metric | CP | T1 | T2 | Tfinal | RM-ANOVA (P value) |
|---|---|---|---|---|---|
| [RECTUM] Volume (cc) covered by 102.5%TD | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (−1.13 to 0.00) | 0.00 (0.00–0.00) | NSa |
| [RECTUM] % covered by 40 Gy | 10.00 (0.94–10.00) | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 0.239 |
| [RECTUM] % covered by 65 Gy | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 10.00 (0.10–10.00) | 0.6886 |
| [RECTUM] % covered by 75 Gy | 10.00 (3.75–10.00) | 10.00 (2.72–10.00) | 10.00 (0.100–10.00) | 10.00 (2.01–10.00) | 0.3182 |
| [BLADDER] % covered by 60 Gy | 5.00 (1.74–5.00) | 5.00 (0.00–5.00) | 5.00 (0.00–5.00) | 5.00 (1.14–5.00) | 0.9504 |
| [BLADDER] % covered by 40 Gy | 5.00 (0.00–5.00) | 5.00 (0.00–5.00) | 5.00 (0.00–5.00) | 5.00 (0.00–5.00) | 0.8846 |
| [BULB] % covered by 50 Gy | 2.00 (2.00–2.00) | 2.00 (2.00–2.00) | 2.00 (2.00–2.00) | 2.00 (2.00–2.00) | 1.0a |
| [L FEMORAL HEAD] % covered by 45 Gy | 2.00 (2.00–2.00) | 2.00 (0.85–2.00) | 2.00 (2.00–2.00) | 2.00 (2.00–2.00) | 0.9922 |
| [R FEMORAL HEAD] % covered by 45 Gy | 2.00 (2.00–2.00) | 2.00 (0.47–2.00) | 2.00 (2.00–2.00) | 2.00 (2.00–2.00) | 0.9922 |
NS, not significant.
3.3. Overall plan quality
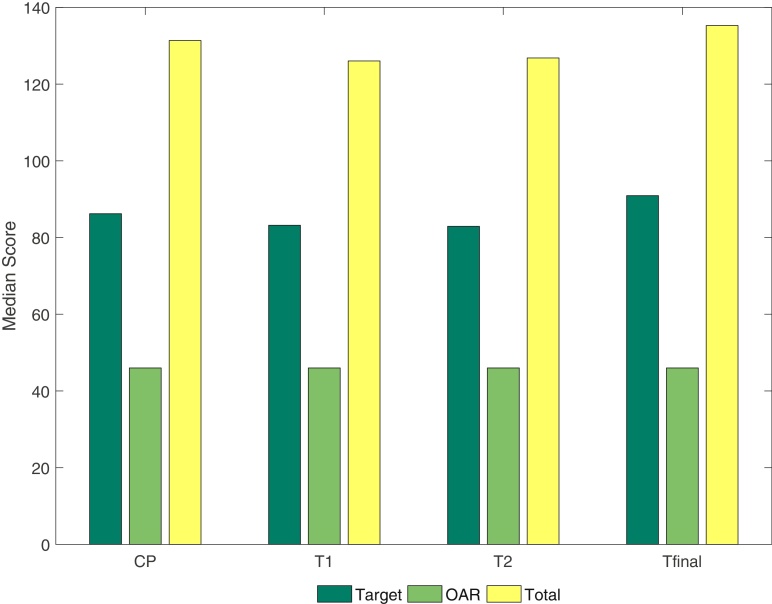
The scores associated with the overall plan quality for the CP, T1, T2 and Tfinal versions of the class solution plans are summarised in Table 6. While the median total scores for T1 and T2 were significantly lower than for the CP, there was no difference between Tfinal and the CP (Fig. 3). Interestingly, the score for volume receiving total dose outside the PTV were higher for Tfinal than for the CP (P < 0.0001).
Table 6.
Evaluation of a class solution for prostate IMRT: scores associated with overall plan quality (median [range]): comparison of the clinical plan (CP) and class solutions T1, T2 and Tfinal.
| Metric | CP | T1 | T2 | Tfinal | RM-ANOVA (P value) |
|---|---|---|---|---|---|
| Total score | 131.9 (77.8–144.1) | 126.0***,†† (68.2–141.0) | 127.3***,†† (73.7–144.0) | 135.1 (68.6–143.9) | <0.0001 |
| Volume (cc) covered by TD outside PTV | 2.775 (0.00–10.00) | 1.805**,†† (0.00–10.00) | 2.135†† (0.00–10.00) | 6.745*** (0.00–10.00) | <0.0001 |
| [Patient] Volume (cc) covered by 110%TD | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.0 |
| Global max location (ROI) | 5.00 (3.00–5.00) | 3.00**,†† (0.00–5.00) | 3.00*,† (0.00–5.00) | 5.00 (0.00–5.00) | 0.0429 |
Compared to CP, P < 0.05.
P < 0.01.
P < 0.0001.
Compared to Tfinal, P < 0.01.
P < 0.0001.
Fig. 3.
Comparison of the clinical plan (CP) and template plans (T1, T2 and Tfinal) for the median metric scores.
4. Discussion
In this study, we have demonstrated that class solution based IMRT planning (Tfinal) for prostate patients is consistent with the CP for CTV V[81 Gy], PTV V[81 Gy], and PTV V[76.95 Gy]. Interestingly, the PTV mean dose was lower in Tfinal. The reason for this is unclear but may be due to the mean dose constraint being clinically irrelevant or the constraint being restrictive in the local protocol. The PTV mean dose constraint in the clinical protocol used set the ideal dose between 83 Gy and 84.5 Gy with an acceptable dose of 84.5–85.5 Gy with no lower dose acceptable range. Plans that had mean doses less than 83 Gy still received full scoring for this metric. Despite these plans not falling within the constraints of clinical protocol, a mean dose that was closer to the prescribed dose was considered an optimal plan. When the mean dose falls outside the ideal criteria, it was always below 83 Gy. This suggests that there was a clinically acceptable lower dose (<83 Gy) in use but not documented in the protocol. Since the completion of this study, the clinical protocol has evolved and a lower mean dose is now deemed acceptable. On review of the templates, this could also be explained by a change in the first patient quadratic overdose cost function; resulting in less areas of high dose and a more homogenous dose distribution across the PTV closer to TD.
There were significant differences between the templates and the CP relating to OAR. These differences (which are evident in Fig. 2) are related to a struggle between meeting OAR tolerances and target coverage. However, when assessing plans based on the plan metric generated by the plan analysis software (Table 5) the differences are not evident. This suggests that the metrics developed for the OARs are not as sensitive as the metrics developed for the targets. As an initial validation of Plan IQ™ these metrics were considered acceptable. For future studies, it may be valuable to understand what metric functions are required to visualise subtle difference in plan quality.
The PQM algorithm used in this study places a priority on coverage of the target volumes, allocating each measure a maximum score of 20. The OAR related metrics individually have a maximum score of 10 with the majority scoring a maximum of 5. The ongoing tension between achievable OAR and target doses will no doubt continue in the clinical setting; however the ability to fine tune scoring functions and/or apply and compare quickly multiple differently weighted PQMs presents an opportunity to better understand how planning cost functions are behaving. In particular, the ability to produce a score outcome for a group of plans is a capability which allows systematic quality improvement to be more quantitatively assessed. Plans failing to meet goals are flagged by low PQM scores and would be safely detected by routine planning checks. Our assessment is that automating the planning process is safe for these cases when supported by objective quality control such as provided by the plan analysis software.
Generally, a clinician knows the desired planning objectives. However, what is less clear is to know when to compromise one objective in order to achieve another. An automated class solution could help clinicians make these decisions easier by abstracting that decision away to some extent. Moreover, a plan quality scoring capability adds a quality control measure that can identify individual patient level errors as well as systematic trend errors not otherwise easily detected. A plan quality scoring system can highlight the subtle differences in practice between different clinicians that may go undetected even if the best optimiser consistently finds the perfect solution on a case by case basis.
5. Conclusions
The present study has shown that radiotherapy plan analysis software is useful for evaluating a class solution for prostate IMRT planning. The present work has also provided evidence that the class solution developed in our institute compares favourably with clinically acceptable plans for these patients. We found that a semi-automated planning approach is capable of achieving a clinically acceptable result in 89.2% of cases. Based on this, we suggest there is scope to further explore automation of the dosimetry process.
Conflict of interest
Sun Nuclear Corporation (Melbourne, Florida, USA) provided access to a beta version of the software for evaluation purposes; no constraints were imposed on its use, application, or reporting of results, nor were any financial arrangements entered into.
Financial disclosure
None declared.
Acknowledgements
We thank Ben Nelms and Greg Robinson, Sun Nuclear Corporation, for early access to the Plan IQ™ software for evaluation purposes. We also thank the many members of the various Mid-North Coast Cancer Institute clinical work groups involved in the development of the prostate class solutions and the workgroup introducing the Plan IQ™ software. We thank Dr Thomas Shakespeare for his support and encouragement.
References
- 1.Intensity Modulated Radiation Therapy Collaborative Working Group Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 2.Khoo V.S., Bedford J.L., Webb S., Dearnaley D.P. Class solutions for conformal external beam prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:1109–1120. doi: 10.1016/s0360-3016(02)04393-6. [DOI] [PubMed] [Google Scholar]
- 3.Schreibmann E., Xing L. Feasibility study of beam orientation class-solutions for prostate IMRT. Med Phys. 2004;31:2863–2870. doi: 10.1118/1.1797571. [DOI] [PubMed] [Google Scholar]
- 4.Heijmen B., Sharfo A., Breedveld S., Heijkoop S., Mens J.W.M., Hoogeman M.S. Fully automated treatment plan generation for online adaptive radiation therapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2015;93:S216. [Google Scholar]
- 5.Xhaferllari I., Wong E., Bzdusek K., Lock M., Chen J. Automated IMRT planning with regional optimization using planning scripts. J Appl Clin Med Phys. 2013;14:176–191. doi: 10.1120/jacmp.v14i1.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breedveld S., Storchi P.R.M., Voet P.W.J., Heijmen B.J.M. iCycle: integrated, multicriterial beam angle, and profile optimization for generation of coplanar and noncoplanar IMRT plans. Med Phys. 2012;39:951–963. doi: 10.1118/1.3676689. [DOI] [PubMed] [Google Scholar]
- 7.Forde E., Bromley R., Kneebone A., Eade T. A class solution for volumetric-modulated arc therapy planning in postprostatectomy radiotherapy. Med Dosim. 2014;39:261–265. doi: 10.1016/j.meddos.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Neill C.J. Dosimetric comparison of intensity-modulated solutions for intact prostate cancer. Med Dosim. 2014;39:366–372. doi: 10.1016/j.meddos.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Voet P.W., Breedveld S., Dirkx M.L., Levendag P.C., Heijmen B.J. Integrated multicriterial optimization of beam angles and intensity profiles for coplanar and noncoplanar head and neck IMRT and implications for VMAT. Med Phys. 2012;39:4858–4865. doi: 10.1118/1.4736803. [DOI] [PubMed] [Google Scholar]
- 10.Voet P.W., Dirkx M.L., Breedveld S., Heijmen B.J. Automated generation of IMRT treatment plans for prostate cancer patients with metal hip prostheses: comparison of different planning strategies. Med Phys. 2013;40:071704. doi: 10.1118/1.4808117. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe M.B., Moore K.L., Orton C.G. Within the next ten years treatment planning will become fully automated without the need for human intervention. Med Phys. 2014;41:120601. doi: 10.1118/1.4894496. [DOI] [PubMed] [Google Scholar]
- 12.Ruan D., Shao W., DeMarco J. Evolving treatment plan quality criteria from institution-specific experience. Med Phys. 2012;39:2708–2712. doi: 10.1118/1.4704497. [DOI] [PubMed] [Google Scholar]
- 13.Nelms B.E., Robinson G., Markham J. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2:296–305. doi: 10.1016/j.prro.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Ventura T., Lopes M.dC., Ferreira B.C., Khouri L. SPIDERplan: a tool to support decision-making in radiation therapy treatment plan assessment. Rep Pract Oncol Radiother. 2016;21:508–516. doi: 10.1016/j.rpor.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Nuclear Corporation . 2013. Quality reports with PlanIQ. Available from: http://www.sunnuclear.com/medPhys/patientqa/QualityReports/ [cited 2015] [Google Scholar]
- 16.Wood M., Fonseca A., Sampson D. Prostate intensity-modulated radiotherapy planning in seven mouse clicks: development of a class solution for cancer. Rep Pract Oncol Radiother. 2016;21:567–570. doi: 10.1016/j.rpor.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Institute NSW . 2015. Radiation oncology urogenital page. Available from: https://www.eviq.org.au/Category/tabid/65/categoryid/185/Default.aspx [cited 21.12.15] [Google Scholar]