Introduction
Immunotherapy with immune checkpoint inhibitors that target the programmed cell-death receptor 1 (PD-1) is increasingly used for patients with advanced or metastatic melanoma, non–small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, and urothelial carcinoma. Cutaneous adverse events that occur during treatment with these agents include pruritus, vitiligo, lichenoid dermatitis, psoriasiform dermatitis, and, more recently, bullous pemphigoid or other autoimmune blistering disease.1, 2, 3, 4, 5, 6, 7, 8 Interestingly, radiation therapy alone has been reported as a trigger for bullous pemphigoid (BP), most often limited to the irradiated field.9, 10 We report a case of a patient who had BP localized to the field of radiation therapy during treatment with a PD-1 inhibitor. We hypothesize that concurrent treatment with radiation therapy and a PD-1 inhibitor may potentiate the risk of BP development.
Case report
A 70-year-old man with a history of metastatic acral lentiginous melanoma presented with new-onset tense bullae of the right thigh (Fig 1). Prior treatment for his melanoma included wide local excision, complete nodal dissection of the right inguinal basin, 3 cycles of ipilimumab, which was discontinued because of autoimmune hypophysitis, and right pelvic nodal basin radiation (48 Gy), which was started 8 months after discontinuation of ipilimumab. Given metastatic right popliteal nodal disease, the patient received 6 cycles of the PD-1 inhibitor pembrolizumab 7 months after completing radiation. Positron emission tomography/computed tomography found disease progression, and his treatment was switched to nivolumab, another PD-1 inhibitor (3 mg/kg every 2 weeks), and completed 13 cycles. While receiving nivolumab, he was also receiving 48 Gy of radiation to the in-transit metastases on his right thigh. He completed both nivolumab and radiation treatment 3 weeks before presentation with blister development.
Fig 1.
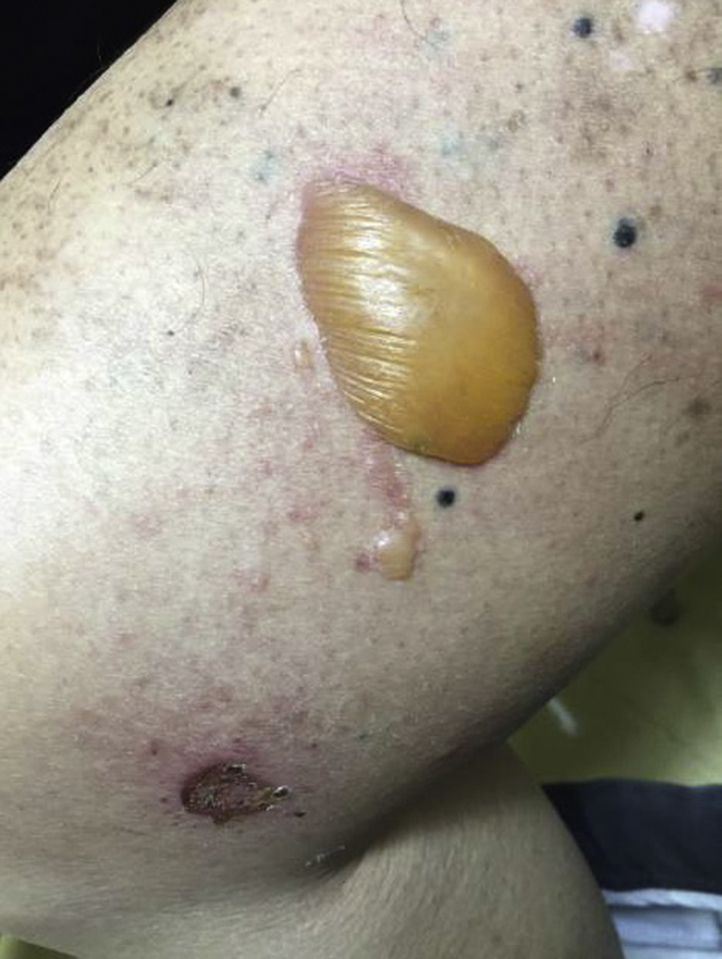
Clinical photograph of tense bullae localized to irradiated skin after anti–PD-1 therapy and radiation for in-transit cutaneous metastases to the thigh.
On skin examination, the patient had scattered tense bullae localized to the right thigh within the recent radiation treatment field. No mucosal involvement was noted, and no additional lesions were identified on total body skin examination.
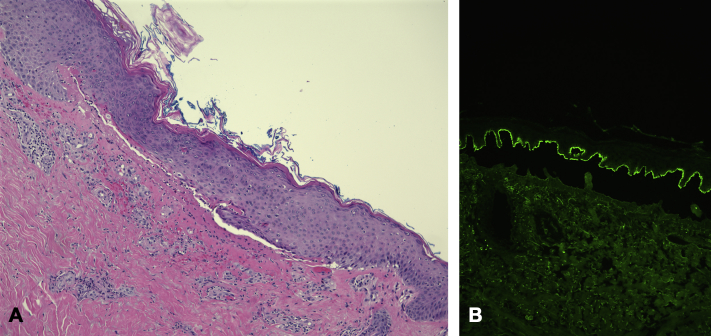
Punch biopsy revealed a subepidermal blister with numerous eosinophils. Perilesional direct immunofluorescence found 2+ linear IgG staining along the dermoepidermal junction, supportive of a diagnosis of bullous pemphigoid (Fig 2).
Fig 2.
Hematoxylin-eosin staining (A) and direct immunofluorescence on salt-split perilesional skin biopsy (B), both 100×-hematoxylin-eosin. Microscopic examination found subepidermal blister formation with numerous eosinophils in the blister cavity and a superficial dermal infiltrate consisting primarily of eosinophils. Periodic acid–Schiff stain was negative. Direct immunofluorescence studies on salt-split showed 2+ linear staining of IgG at the dermoepidermal junction.
At the time of presentation, serology for absolute eosinophil count was unremarkable, and no BP antibody levels were drawn. Given his localized and asymptomatic presentation, the patient was treated with close monitoring only. Because of disease progression of his metastatic melanoma, nivolumab was discontinued after his last dose 3 weeks before presentation. The bullae healed without scarring within 1 month of presentation without any topical or systemic treatment for BP, and no new lesions developed after resolution.
Discussion
Recognizing cutaneous adverse events associated with novel oncologic therapy is vital to the dermatologic care of the cancer patient. Our case highlights the association of both PD-1 inhibitor therapy and radiation therapy as potential triggers of BP. Further, our patient's course suggests an additive risk for BP with concurrent exposure to both therapies. Three years before presentation, he received pelvic nodal basin radiation without bullae development. Notably, he was not undergoing PD-1 therapy at that time. No lesions developed during the 8 months of treatment with pembrolizumab or nivolumab until he received concurrent radiation to the right thigh.
PD-1 inhibitor–induced BP is a relatively new and mechanistically interesting form of drug-induced BP.1, 2 Only 15 cases of anti-PD-1–associated BP exist in the literature: 7 with pembrolizumab, 7 with nivolumab, and 1 with durvalumab.1, 2, 3, 4, 5, 6, 7, 8 Most reports do not comment on the patient's radiation exposure, although in 2 cases there had been a documented history of prior radiation.4 We believe this unusual and instructive case highlights the value of assessing radiation exposure in addition to a thorough review of medications in the evaluation of the cancer patient with new-onset bullae.
Although our patient only had transient, localized disease that resolved without treatment, generalized and persistent BP necessitating systemic immunosuppression or cessation of the PD-1 inhibitor can occur.2, 4 Hwang et al1 suggest that the anti-PD1 antibody allows for autoimmune T cells to evade regulatory T cells. PD-1 blockade on B cells can also enhance antigen-specific antibody responses.1 On the other hand, radiation-induced BP is a rare and typically localized complication of radiation. Investigators suggest that radiation-induced apoptosis of epidermal cells releases BP antigen 1 and 2, which are then processed by radiation-resistant Langerhans cells.9 Radiation-induced BP typically involves breast cancer patients, and its occurrence in melanoma patients is rarely reported.9, 10 We hypothesize that the immunologic response to radiation-induced apoptosis was potentiated in our case by simultaneous autoimmune stimulation with PD-1 inhibition, thus resulting in the development of BP only with concurrent therapy.
PD-1 inhibitors are increasingly used to treat an array of cancers, many of which may also be treated with concurrent radiation. In caring for patients with dual risk factors of PD-1 inhibition and radiation exposure, it is prudent to consider the diagnosis of BP in the evaluation of urticarial or blistering dermatoses.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Hwang S.J., Carlos G., Chou S., Wakade D., Carlino M.S., Fernandez-Penas P. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26(4):413–416. doi: 10.1097/CMR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 2.Naidoo J., Schindler K., Querfeld C. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damsky W., Kole L., Tomayko M.M. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2(6):442–444. doi: 10.1016/j.jdcr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jour G., Glitza I.C., Ellis R.M. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43(8):688–696. doi: 10.1111/cup.12717. [DOI] [PubMed] [Google Scholar]
- 5.Kwon C.W., Land A.S., Smoller B.R., Scott G., Beck L.A., Mercurio M.G. Bullous pemphigoid associated with nivolumab, a programmed cell death 1 protein inhibitor. J Eur Acad Dermatol Venereol. 2017 doi: 10.1111/jdv.14143. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Mochel M.C., Ming M.E., Imadojemu S. Cutaneous autoimmune effects in the setting of therapeutic immune checkpoint inhibition for metastatic melanoma. J Cutan Pathol. 2016;43(9):787–791. doi: 10.1111/cup.12735. [DOI] [PubMed] [Google Scholar]
- 7.Rofe O., Bar-Sela G., Keidar Z., Sezin T., Sadik C.D., Bergman R. Severe bullous pemphigoid associated with pembrolizumab therapy for metastatic melanoma with complete regression. Clin Exp Dermatol. 2017;42(3):309–312. doi: 10.1111/ced.13042. [DOI] [PubMed] [Google Scholar]
- 8.Sowerby L., Dewan A.K., Granter S., Gandhi L., LeBoeuf N.R. Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatol. 2017;153(6):603–605. doi: 10.1001/jamadermatol.2017.0091. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen T., Kwan J.M., Ahmed A.R. Relationship between radiation therapy and bullous pemphigoid. Dermatology. 2014;229(2):88–96. doi: 10.1159/000362208. [DOI] [PubMed] [Google Scholar]
- 10.Mul V.E., van Geest A.J., Pijls-Johannesma M.C. Radiation-induced bullous pemphigoid: a systematic review of an unusual radiation side effect. Radiother Oncol. 2007;82(1):5–9. doi: 10.1016/j.radonc.2006.11.014. [DOI] [PubMed] [Google Scholar]