Abstract
Lesion-symptom mapping is a key tool in understanding the relationship between structure and function in neuroscience as it can provide objective evidence about which regions are crucial for a given process. Initial limitations with this approach were largely overcome by voxel-based lesion-symptom mapping (VLSM), a method introduced in the early 2000s, which allows for a whole-brain approach to study the association between damaged areas and behavioral impairment by applying an independent statistical test at every voxel. By doing so, this technique eliminated the need to predefine regions of interest or classify patients into groups based on arbitrary cutoff scores. VLSM has nonetheless its own limitations; chiefly, a bias towards recognizing cortical necrosis/gliosis but with poor sensitivity for detecting injury along long white matter tracts, thus ignoring cortical disconnection, which can per se lead to behavioral impairment. Here, we propose a complementary method that, instead, establishes a statistical relationship between the strength of connections between all brain regions of the brain (as defined by a standard brain atlas) and the array of behavioral performance seen in patients with brain injury: connectome-based lesion-symptom mapping (CLSM). Whole-brain CLSM therefore has the potential to identify key connections for behavior independently of a priori assumptions with applicability across a broad spectrum of neurological and psychiatric diseases. We propose that this approach can further our understanding of brain-structure relationships and is worth exploring in clinical and theoretical contexts.
Keywords: Voxel-based lesion-symptom mapping, Connectomics, Diffusion tensor imaging, Connectome-based lesion-symptom mapping
Highlights
-
•
Lesion-symptom mapping has been crucial to understand brain-function relations
-
•
VLSM eliminated the need to predefine regions of interest or biased patient groups.
-
•
Main limitations of VLSM relate cortical necrosis/gliosis and white matter tracts
-
•
CLSM can identify key connections for behavior independently of a priori assumptions
-
•
CLSM has applicability across several neurological and psychiatric diseases
1. Introduction
For over a century, observations of patients with brain damage have shed light on the neurobiological substrates of different brain functions. The core principle of this association is as follows: if a patient with brain injury to area X is unable to perform behavior A, one can hypothesize that area X must be crucial for the execution of A. An extension of this is the double dissociation, where those with damage to area X are impaired at task A but not task B, whereas the opposite pattern is seen for individuals with injury to area Y. These observations have proved influential in our understanding of brain function. For instance, in its origins, our understanding of the neurobiological basis of language stemmed from this approach. The classic dichotomy stipulates that while lesions to Broca's area in the left inferior frontal gyrus lead to speech production deficits (Broca, 1861), damage to Wernicke's area in the left superior temporal gyrus disrupts auditory comprehension (Wernicke, 1874). By the same principle, the interruption of fibers connecting these two regions impairs speech repetition (Lichtheim, 1885).
With the advancement of techniques that measure brain activation (e.g. functional magnetic resonance or event-related potentials), however, we have accumulated evidence to suggest a far more complex picture of brain function (Dronkers et al., 2007, Mesulam, 2005, Rudrauf et al., 2008). Following the example of language, we now recognize that substantial overlap exists between the neural systems engaged during speech production and comprehension (Silbert et al., 2014, Pickering and Garrod, 2014). Accordingly, some of the newer models stipulate the existence of a functional core that is associated with many language processes, i.e., shared domain-specific neural systems (Fedorenko and Thompson-Schill, 2014). Other authors have also suggested that specific patterns of brain activation rely on the kind of information being processed (i.e, semantic, lexical, or syntactic) rather than the modality (e.g. comprehension vs. production) (Menenti et al., 2011) and more contemporary models of speech processing propose dissociable but interacting pathways that interface sensory networks with semantic-conceptual systems (a ventral stream) with motor-articulatory systems (dorsal stream) (Hickok, 2012, Hickok and Poeppel, 2000, Hickok and Poeppel, 2004, Hickok and Poeppel, 2007, Fridriksson et al., 2016).
However, while it is undisputable that brain activation techniques have expanded our understanding of complex brain functions and functional neuroimaging, there remains a crucial problem regarding the findings from functional studies: indirect measures of neural activation detected with functional neuroimaging do not necessarily reflect a direct relationship between structure and function. Areas identified by functional studies are both the ones that are crucial for the tasks, as well as those connected to crucial areas, but not indispensable. For example, while an intra-cortical recording can identify the precise location and time-course of a neuron that predicts behavior, it is unable to reveal whether this neuron is required for the behavior. On the other hand, disruption methods such as cortical stimulation or lesions can dissociate areas that are merely involved with a task from those required by the task (though we note that these techniques are best used synergistically, e.g. a recording suggests when a neuron is involved and therefore guides the stimulation, which demonstrates that only disruption at this time influences behavior). One common example of this phenomenon is the activation of homologue language areas in the right hemisphere during speech production tasks (Bottini et al., 1994). While these right hemisphere regions may be involved in speech production, destruction of these regions due to stroke or surgery rarely leads to profound impairments of speech production (in stark contrast to the regions in the left hemisphere). At the moment, there are no discernible differences in signal quantification that can confirm which active regions are indispensable, versus those that are not.
For these reasons, there remains a gap between classic lesion-symptom mapping and functional studies. Lesion-symptom mapping is one of the few neuroscience tools that can provide objective evidence about which regions are crucial for a given process. However, it has been largely limited in its ability to elucidate the networks that extend beyond the typical lesion site. Conversely, functional studies commonly reveal broad patterns of activation, but it is unclear which networks are indeed directly associated with a specific function. A classic example is the activation of interhemispheric structures, such as the anterior cingulate or the cuneus, during task-driven language functional studies [e.g. (Geranmayeh et al., 2014)]. These areas are perfused by the anterior cerebral artery and therefore not commonly affected in individuals with language problems after an ischemic brain lesion (which are typically seen with medial cerebral artery strokes). Therefore, lesion mapping cannot determine their role in language production. Functional studies, in turn, by means of showing multiple and diverse areas of brain activity during language tasks, hinder the ability to conjecture if said areas play critical roles for specific language functions, as these assumptions are based on associations that cannot be directly tested without additional techniques or technologies. Thus, a method that could combine lesion-symptom mapping with network assessments beyond the lesion location could have a broad impact in our understanding of brain function. Here, we review available lesion-symptom mapping techniques and describe how a novel approach, entitled connectome-based lesion symptom mapping (CLSM), can help bridge this gap. This method leverages principled and modern techniques for conventional lesion-symptom mapping, combined with structural connectome data, which, in turn, provides a comprehensive measure of network damage (and residual integrity) related to, and extending beyond, the stroke lesion. There are specific aspects of this technique that require careful methodological considerations in order to derive reliable data-driven conclusions, such as how to properly measure the connectome in brains with lesions, how to combine connectome and lesion data, how to approach statistical analyses, and how to assess network measures based on link weight or more advanced graph theory approaches. These are discussed below.
2. Lesion-symptom mapping
Conventional lesion-symptom mapping, which infers that area X must be crucial for behavior A if a person can no longer perform A after damage to brain region X, has been systematically improved since its inception more than a century ago. There were initially some caveats that limited the applicability of lesion studies in a more systematic fashion. First, earlier studies lacked an objective way to quantify lesion size and location, leading to qualitative inspections and case series reporting several patients with similar lesions who exhibited comparable clinical deficits. Second, the mere overlap of lesions across patients does not necessarily imply that the area of maximal overlap is the one required for a given function (Rorden and Karnath, 2004). Third, when comparing the behavioral performance between controls and patients with injury to a specific brain region of interest (ROI), one may oversee the contribution to behavior that different subregions within that ROI may exert (for example, if the superior temporal gyrus is studied as a unique ROI, the separate contributions of the anterior and posterior aspects of this area to different language functions may go undetected). Finally, when comparing the extent of brain lesions between a group with certain behavioral impairments and another without such deficits, one must necessarily apply an often-arbitrary cut off to the outcome variable in order to classify participants into comparable groups.
In an attempt to overcome these limitations, Bates and collaborators (Bates et al., 2003) introduced voxel-based lesion symptom mapping (VLSM), which as we describe below, allowed for a whole-brain approach to study the association between damaged areas and behavioral impairment. Here, we argue that a new approach, connectome-based lesion symptom mapping (CLSM) can further complement our understanding of structure-function relationships by providing valuable information beyond lesion localization that predicts a given behavior.
3. Voxel-based lesion-symptom mapping (VLSM)
The motivation behind the development of VLSM was the analysis of how damaged tissue relates to behavioral performance on a voxel-by-voxel basis, as done by functional neuroimaging. The advantage of this approach is that it can reveal specific brain regions that contribute to behavioral performance without having to a priori define which structures may be relevant or what performance scores constitutes normal vs. impaired behavior/function. This is achieved by analyzing continuous behavioral data (for example, score on the Western Aphasia Battery) on a voxel-by-voxel basis. For instance, for each voxel, a group comparison of the behavioral score is performed (e.g. by means of a t-test or measures of effect size) between participants with and without a lesion in that specific voxel. By doing so, one is no longer required to predefine regions of interest with contentious boundaries or encompassing sub-regions with different functions. In addition, since the t-test uses the continuous behavioral scores, one does not need to classify patients into two discrete groups (those with and without an impairment) using arbitrary cutoff scores. Therefore, this method is able to capture the graded degree of impairment often seen following brain injury. This technique has been used in stroke patients to study language functions (Fridriksson et al., 2016, Mirman et al., 2015, Dronkers et al., 2004), including speech fluency (Bates et al., 2003), speech comprehension (Bates et al., 2003, Dronkers et al., 2004), and speech production (Borovsky et al., 2007, Basilakos et al., 2015, Fridriksson et al., 2010), as well as other behavioral impairments such as post-stroke swallowing difficulties (Galovic et al., 2017), somatosensory deficits (Meyer et al., 2016, Preusser et al., 2015), high level perceptual deficits (Karnath et al., 2004), and even depression (Kim et al., 2017), among others. A summary of the techniques involved in VLSM is presented in Fig. 1.
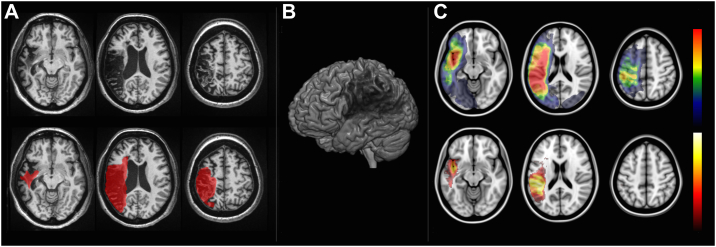
Fig. 1.
Voxel-based lesion-symptom mapping (VLSM). VLSM is performed by first defining the location of the post-stroke necrotic/gliotic tissue. Panel A demonstrates axial T1-weighted slices of one representative patient with a chronic post-stroke lesion (hypointense areas on top row slices), which is demarcated in red on the bottom row slices. Panel B is a 3D rendering that illustrates the magnitude of brain damage. Lesions from multiple individuals are then transformed into the stereotaxic MNI space and, for each voxel, a statistical analysis is performed by assessing whether there is a difference in a given behavioral measure (e.g. test score) in the group of subjects with a lesion in that voxel, versus the group of subjects without the lesion in that voxel. The results are then corrected for multiple comparisons based on the number of tested voxels. The top row in Panel C demonstrates the overlay of multiple lesions (red indicating areas with higher overlap) and the bottom row demonstrates an example of a voxel-wise statistical analyses (white-yellow voxels more strongly associated with behavioral measures).
Crucial to the interpretation of findings derived from patients with brain injury (for example, following stroke) is the fact that damage may extend beyond the area of apparent injury as seen on structural scans. Specifically, white matter damage (disconnection) may have broad ramifications outside the area of necrosis and can thus lead to remote dysfunction of apparently intact cortical lesion (Carrera and Tononi, 2014, Mukherjee, 2005, Fridriksson et al., 2007, Bonilha et al., 2014a, Bonilha et al., 2014b, Bonilha and Fridriksson, 2009, Catani and Mesulam, 2008a). This is one of the anatomical bases for diaschisis (Bonilha et al., 2014a, Catani, 2005, Catani et al., 2012) but cortical disconnection is not readily detectable by VLSM and the influence of remote cortical dysfunction on behavior is only partially examined with this approach. Furthermore, there is an inherent bias in the distribution of cortical brain lesions based on nervous system architecture/organization of vasculature which can lead to erroneously attributing crucial roles to certain areas (Mah et al., 2014, Inoue et al., 2014), specifically those naturally prone to damage (Caviness et al., 2002). In particular, based on vascular perfusion territories, there are two important phenomena that bias statistical analyses: 1) areas that are close to the stem of the vascular territory (for example, M1 segment in the middle cerebral artery) are commonly affected and are frequently co-lesioned which distal crucial areas, thus leading to the inability to statistically dissociate both areas; 2) brain regions that are outside typical perfusion areas may serve a role for behavior that goes undetected; for example, the lower aspect of the temporal pole is not perfused by the middle cerebral artery, so in VLSM studies evaluating patients with middle cerebral artery strokes, this area is not included in the extent of lesion, and thus its role remains unstudied.
In addition, as VLSM applies an independent statistical test at every voxel, this method has inherently poor sensitivity for detecting injury along long white matter tracts. While severing the tract at any location should logically lead to similar disconnection, damage at different locations generates apparent counter examples from the perspective of the mass-univariate approach. In addition, the scanning modalities typically used for VLSM are more sensitive for detecting cortical necrosis and gliosis rather than white matter disruption. As previously stated, it is well recognized that structural damage also affects white matter, and secondary white matter loss can extend to other regions beyond the post-injury (e.g. stroke) damage.
For these reasons, exploring the association between specific connectivity pathways and cognitive performance or behavior may provide complementary information to the relationship between damaged structure and function, which can be useful to better understand the role of systems integration in the neurobiology of cognition and behavior. For example, in language, the clinical characteristics of post-stroke aphasia, e.g., whether receptive or expressive, are not always predicted by the location of the necrotic cortical lesion (Fridriksson et al., 2007, Croquelois and Bogousslavsky, 2011, Dronkers, 2000). Altogether, this suggests that broader, beyond-lesion, cortical dysfunction may have a large clinical impact for at least some patients with damage to the brain. It also indicates that network pathology is crucial to shape the expression of clinical deficits, prompting the development of a complementary approach: connectome-based lesion-symptom mapping (CLSM). As we will argue below, CLSM can provide a more systematic assessment by evaluating brain damage as a combination of necrosis as well as disconnection. CLSM can also provide insight into networks located beyond the specific arterial perfusion territories (e.g., middle cerebral arteries) and thus reveal the importance of brain regions that are damaged but are not mapped as injured by VLSM.
4. Connectome-based lesion-symptom mapping (CLSM)
CLSM establishes a statistical relationship between connectome injury and behavioral performance. We employ the word ‘connectome’ to refer to the structural framework of connections across the whole brain and we discuss CLSM here in the context of structural connectivity, but the same principles could be applied to functional connectivity. Specifically, the structural connectome provides a panorama of all medium to large scale white matter connections in the brain, i.e., it is a representation of how large populations of neurons are integrated and organized. With current techniques, the structural connectome can be derived from a combination of white matter data from diffusion-weighted MRI [for review, see (Assaf et al., 2017)] and high-resolution gray matter maps. The latter permits the division of the cortex into specific regions of interest (ROIs), which can be pre-defined regions based on standard criteria (e.g. brain atlases). A whole-brain connectome approach measures the strength of the connection between all possible pairs of ROIs. This can more frequently be achieved by different methods, most commonly either by estimating fiber count (deterministic tractography) or by means of evaluating the probability of fibers between two regions (probabilistic tractography). Regardless of the tractography method employed, a two-dimensional weighted matrix can represent the connectome, where each cell is the weight (i.e., “connection strength”) between two structures. In connectomes reconstructed from deterministic tractography, the sparsity is high since many connections cannot be resolved. As such, deterministic connectome matrices are composed of a skewed distribution with many zeros and decaying prevalence of higher weight connections. Probabilistic connectome matrices, in turn, are also composed by skewed distributions with fewer links with higher weight, but the prevalence of zeros is lower. In addition, binary connectomes may be obtained from either deterministic or probabilistic tractography are also relatively sparse in that they have many connections that are either true zeros or low-weight being coded as zeros, while higher weight connections are counted as ones based on a predefined critical threshold.
Based on the above, it becomes evident that connectomes can contain information that is different in quality (continuous, binary, or skewed) depending on the choice that was used to best represent the data. This is typically a choice by the investigators depending on the quality (number of directions, number of shells) of the diffusion scan, the degree of confidence on the tractography algorithm to resolve complex anatomy, and the ability to process probabilistic tractography in parallel. As such, the statistical analysis being applied in CLSM has to take into account the type of data that are being included in the model. For example, binomial analyses or chi-square in the case of binary connections (similar to VLSM), or parametric and non-parametric continuous measures in the case of weighted connectomes.
Our standard approach has been to use probabilistic tractography, since it yields a continuous measure of probability in terms of link weight that is amenable to linear models, including applications such as support vector machine (SVM). Because link weight is a continuous measure, the general linear model (GLM) can be employed to establish the statistical relationship with the continuous behavioral variable in order to identify connections that are crucial for a given function. It is also possible to define a weight threshold to determine the presence or absence of significant connections, yielding a “binary connectome” that can also undergo GLM to compute an association with behavioral performance. A summary of the steps used to build the connectome is presented in Box 1 and in Fig. 2.
Box 1. How to build the connectome in stroke survivors?
Brain injury can lead to significant neuroanatomical distortions that pose a significant challenge for neuroimaging post-processing, particularly regarding spatial normalization and tissue segmentation. These are necessary procedures during the reconstruction of the brain connectome and this section describes a step-by-step approach to construct the connectome of individuals who survived stroke and have brain lesions secondary to post ischemic necrosis. First, the raw MR images, typically available in the DICOM format must be converted to NIfTI format popular with scientists. It is important to choose a conversion that is able to extract and transform the diffusion gradient table (Li et al., 2016). The next step is to obtain probabilistic gray and white matter maps, which will be used to define gray matter regions of interest and white matter masks for tractography. Conventional cortical segmentation programs have been designed for non-lesioned brains and they typically lead to gross errors when processing brains with tissue damage. For this reason, it is essential to employ a tool that has been optimized for brains with lesion, particularly stroke survivors. The “Clinical Toolbox” is an extension of the Statistical Parametric Mapping (SPM) software, which was developed by our group (Rorden et al., 2012) in order to optimize the segmentation and registration of brains with distorted anatomy due to large lesions. This processes requires a manually traced mask of the area of injury, which is typically drawn on T2-weighted or T1-weighted images. The lesion mask is used to minimize the impact of the lesion on the normalization estimates, either via explicit masking (Brett et al., 2001) or by substituting healthy tissue for homologous regions of the intact hemisphere (Nachev et al., 2008). This yields transformation matrices for normalization into standard stereotaxic space (MNI space) and vice versa. Normalization and tissue segmentation are performed iteratively, generating probabilistic tissue maps in native T1 and standard MNI space. Once transformations matrices are obtained, a neuroanatomical atlas, e.g., Automated Anatomical Labeling (Joliot et al., 2015, Tzourio-Mazoyer et al., 2002) can be non-linearly registered onto the probabilistic gray matter map (in native T1 space) and used to divide the gray matter into ROIs. Subsequently, the ROIs generated from the previous step, as well as the white matter map, are moved to B0 space. To achieve this, the T1-weighted image can be registered to an inverted fractional anisotropy (FA) map. Alternatively, if T2-weighted image is available, it can be linearly co-registered onto the native T1 image and then linearly transformed to the B0 image. The transformation matrices are subsequently applied to the map of segmented ROIs and to the white matter probabilistic tissue map, yielding cortical ROIs and white matter maps in DWI space. With all data in diffusion space, either a probabilistic or a deterministic DTI algorithm can now be applied to determine the strength of all pairwise connections between all ROIs. This strength is either the probability that two ROIs are connected (probabilistic approach) or the number of streamlines with a correction that accounts for distance travelled and the volumes of the ROIs involved (deterministic approach). With a matrix of link strengths, CLSM is performed by assessing link or node wise statistics. Furthermore, graph theory measures can be derived from this matrix to characterize global and regional aspects of the patient's connectome.
Alt-text: Box 1
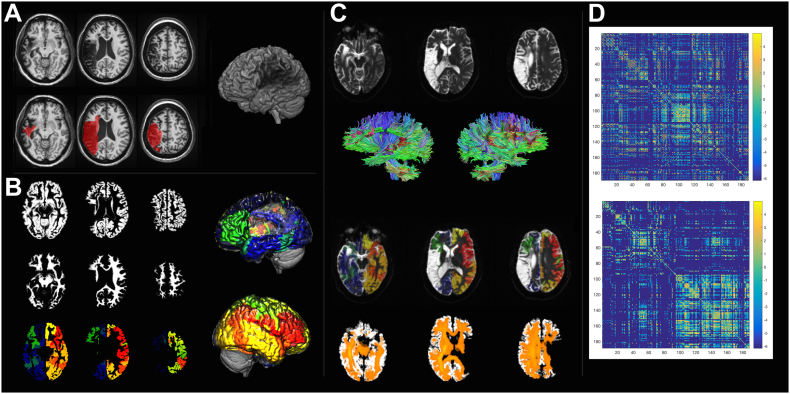
Fig. 2.
The methodological steps involved in the calculation of the connectome share similarities with VLSM. First, the necrotic/gliotic image is defined on T1 or T2 weighted images as shown in Panel A. Again, here we see a 3D render of an individual patient's brain with a lesion. Subsequently, an iterative segmentation and cost-function normalization approach is employed to define probabilistic maps of gray (Panel B, top row) and white matter (Panel B, middle row). The transformation matrix between T1 to MNI space is used to transfer an anatomical atlas to T1-weighted space and segment the probabilistic gray matter into regions of interest (Panel C, bottom row). Panel C also shows the 3D renders of segmentation into regions of interest (left and right lateral views with different colors for different regions). Tractography is performed in diffusion space, so the white matter mask and the segmented gray matter maps are transferred to B0 space (Panel C) and tractography is used to assess the number of streamlines linking each possible pairs of regions. Care is taken to ensure that tractography is performed being guided by the white matter probabilistic map, excluding the lesion site. The bottom row of Panel C shows a fiber density image in orange. Finally, a 2D matrix is generated where each entry represents the connection weight between the region in the row and column. The top matrix in Panel D shows the connectome, which is then arranged anatomically (Panel D, bottom matrix) to demonstrate the difference in the number of fibers in the left hemisphere (left upper matrix quadrant) versus the right hemisphere (right lower matrix quadrant).
The whole-brain CLSM approach has the potential to identify key connections for behavior independently of a priori assumptions. For example, we have recently (Yourganov et al., 2016) shown how CLSM can unveil connections between brain regions that contribute to specific language functions otherwise not predicted by VLSM. Specifically, we showed connections involving parietal regions that contribute to auditory comprehension and a connection between the pars orbitalis and the dorsal part of the middle frontal gyrus crucial for speech repetition. CLSM can also help explain clinical outcomes (e.g. success of epilepsy surgery or likelihood of improving post-stroke anomia with speech therapy) that may be dependent on the integrity of white matter tracts beyond cortical sparing (Gleichgerrcht et al., 2015, Bonilha et al., 2016). The flexible nature of what “behavioral data” are used for CLSM (e.g. test performance, or clinical diagnosis, or outcome, or any other quantifiable variable) gives this approach the potential to be applicable across a wide array of neurological and psychiatric diseases as well as cognitive functions [e.g. (Gleichgerrcht et al., 2016)] and behavioral domains.
What is more, complementary to CLSM, the structural connectome matrices can be modeled using frameworks such as graph theory in order to characterize their global and regional properties. For example, one can examine the network's efficiency, its organization into modules or motifs, and even determine the influence that specific regions exert on the whole network. Exploring some of these measures and how they relate to behavioral outcomes has the potential to provide valuable clinical and theoretical information. For instance, if CLSM reveals that the connection between brain regions Y and Z is critical for behavior A, understanding the influence that Y and Z exert on the network (e.g., by means of graph theory measure “betweeness-centrality”) can be important for understanding structural-functional relations as well as for clinical prognosis and development of tailored rehabilitation interventions in the future.
Alternative to CLSM, which focuses on fiber tractography between all ROIs defined by a cortical (i.e., gray matter) atlas, a number of methods have been developed that rely directly on white matter atlases (Thiebaut de Schotten et al., 2008, Thiebaut de Schotten et al., 2015). For example, white matter bundle analysis relies on a predefined tractography atlas based on a priori anatomical knowledge of white matter pathways (Catani and Mesulam, 2008b), such as the arcuate fasciculus, the superior longitudinal fasciculus, and so forth [for example, see (Catani et al., 2013, Catani, 2008, Craig et al., 2009)]. Analogous methods identify pathways based on DTI metrics (e.g. variations in functional anisotropy), either by still requiring predefined known white matter bundles [e.g. (Ivanova et al., 2016)] or by aligning DTI from multiple patients onto a single common space to infer a tract “skeleton” that represents the center of all tracts common to the pooled group (Smith et al., 2006). These types of approach require a homogeneous distribution of links across subjects, and they can both underestimate link weights (e.g. by not including fibers running in parallel) and overestimate link weights (e.g. by counting fibers that happen to be overlap with a specific predefined tract location on their way from one structure to another). This is likely prone to error in cases of damaged brains, with large variability in residual white matter connections. In favor of this approach – specifically in the context of injured brains – however, is the fact that probabilistic white matter maps can constrain tractography, thus limiting errors.
Furthermore, CLSM estimates – for each link weight – the fibers going between two specific ROIs. In contrast, these white matter bundle approaches can possibly provide information about pathways with uncertain origins (i.e., with clear approximate white matter location but without knowing exactly what cortical regions those tracts are connecting), which have been useful in several aspects of cognition, most notably language functions [e.g. (Catani and Mesulam, 2008b, Agosta et al., 2010)].
It should be emphasized that tractography in lesioned brains (i.e., with gliosis and liquefative necrosis) is not as well validated as tractography in brains without neurological disorders. In fact, lack of validation is a limitation that still applies to many other techniques, including functional MRI in the context of aging and cerebrovascular risk factors (such as hypertension, diabetes and hypercholesterolemia) due to changes in cardiac output, atherosclerosis, microangiopathic burden, and hemodynamic coupling.
Every neuroimaging method has its limitations and CLSM results should be interpreted with caution in the context of the shortcomings of tractography. We believe that special attention should be placed to maximize the sensitivity and specificity of fiber tracking, which, at the time of this review, includes avoiding tracking in areas of gliosis and liquefactive necrosis (i.e. within the stroke lesion), use of probabilistic tractography and gray matter seeding in areas of residual (non-lesioned) cortex. It is also worth noting that, in spite of methodological constraints, the confirmation of a statistical association between an imaging marker and a clinical symptom is an important confirmation of the biophysical relevance and of the utility of the tool.
Based on the aforesaid, there are objective advantages to incorporating a connectome approach to the study of behavior and function, since CLSM can identify connections crucial for behavior independently of structural presumptions. The structural connectome per se has limitations worth considering. First, directionality of fiber bundles cannot be inferred from DTI. Second, short-range fibers can be difficult to detect, and it is especially difficult to determine water diffusion directionality in areas of dense fiber crossing or complex fiber trajectories. Furthermore, because the statistical relationship between structure and function in CLSM is based on link weights (e.g. fiber count or connection probability), this approach ignores the integrity of indirect (i.e., beyond pair-wise) connections. For instance, if the white matter fibers connecting A and B are disrupted but there is sparing of the connections between A and C, and B and C, one could hypothesize that there are indirect connections between A and B that could still support function. Measuring connectome dynamics by analyzing the shortest direct and indirect pathways between regions of interest can provide valuable information about the overall integrity of the network in supporting behavior (Misic et al., 2015).
5. Conclusions
Lesion symptom mapping is a very powerful approach in furthering our understanding of the neurobiological basis of behavior and can reveal a more direct relationship between structure and function than activation techniques. The emergence of VLSM initially eliminated the need to predefine regions of interest or classify patients into groups based on arbitrary cutoff scores. It also enabled an objective and quantifiable way to statistically evaluate the relationship between brain structure and behavioral function. However, brain damage may extend well beyond the area of apparent gray matter injury, and behavioral impairment may come about from changes to the white matter tracts that provide the scaffolding for brain function. CLSM is a whole-brain approach that seeks to establish a statistical relationship between the strength of connections between all brain regions of the brain (as defined by a standard brain atlas or by discrete units as small as a voxel) and the array of behavioral performance seen in patients with brain injury. CLSM can therefore provide valuable complementary information based on lesion-symptom mapping less constrained by cortical injury.
Footnotes
Disclaimer: The authors report no conflict of interest.
Contributor Information
Ezequiel Gleichgerrcht, Email: gleichge@musc.edu.
Leonardo Bonilha, Email: bonilha@musc.edu.
References
- Agosta F., Henry R.G., Migliaccio R. Language networks in semantic dementia. Brain J. Neurol. 2010;133(Pt 1):286–299. doi: 10.1093/brain/awp233. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y., Johansen-Berg H., Thiebaut de Schotten M. The role of diffusion MRI in neuroscience. NMR Biomed. 2017 doi: 10.1002/nbm.3762. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Basilakos A., Rorden C., Bonilha L., Moser D., Fridriksson J. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke. 2015;46(6):1561–1566. doi: 10.1161/STROKEAHA.115.009211. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P. Voxel-based lesion-symptom mapping. Nat. Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Bonilha L., Fridriksson J. Subcortical damage and white matter disconnection associated with non-fluent speech. Brain J. Neurol. 2009;132(Pt 6) doi: 10.1093/brain/awn200. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L., Nesland T., Rorden C., Fillmore P., Ratnayake R.P., Fridriksson J. Mapping remote subcortical ramifications of injury after ischemic strokes. Behav. Neurol. 2014;2014:215380. doi: 10.1155/2014/215380. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L., Rorden C., Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45(4):988–993. doi: 10.1161/STROKEAHA.113.004137. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L., Gleichgerrcht E., Nesland T., Rorden C., Fridriksson J. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil. Neural Repair. 2016;30(3):266–279. doi: 10.1177/1545968315593808. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovsky A., Saygin A.P., Bates E., Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45(11):2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G., Corcoran R., Sterzi R. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain J. Neurol. 1994;117(Pt 6):1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Brett M., Leff A.P., Rorden C., Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche. Bulletin de la Societé d'Anthropologie de Paris. 1861;2:235–238. [Google Scholar]
- Carrera E., Tononi G. Diaschisis: past, present, future. Brain J. Neurol. 2014;137(Pt 9):2408–2422. doi: 10.1093/brain/awu101. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Catani M. ffytche DH. The rises and falls of disconnection syndromes. Brain J. Neurol. 2005;128(Pt 10):2224–2239. doi: 10.1093/brain/awh622. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Catani M. Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M. What is a disconnection syndrome? Cortex. 2008;44(8):911–913. doi: 10.1016/j.cortex.2008.05.001. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Dell'acqua F., Bizzi A. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012;48(10):1262–1287. doi: 10.1016/j.cortex.2012.07.001. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain J. Neurol. 2013;136(Pt 8):2619–2628. doi: 10.1093/brain/awt163. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V.S., Makris N., Montinaro E. Anatomy of stroke, part I: an MRI-based topographic and volumetric system of analysis. Stroke. 2002;33(11):2549–2556. doi: 10.1161/01.str.0000036083.90045.08. [DOI] [PubMed] [Google Scholar]
- Craig M.C., Catani M., Deeley Q. Altered connections on the road to psychopathy. Mol. Psychiatry. 2009;14(10):946–953. doi: 10.1038/mp.2009.40. http://dx.doi.org/10.1038/mp.2009.40 07. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Croquelois A., Bogousslavsky J. Stroke aphasia: 1,500 consecutive cases. Cerebrovasc. Dis. 2011;31(4):392–399. doi: 10.1159/000323217. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Dronkers N.F. The pursuit of brain-language relationships. Brain Lang. 2000;71(1):59–61. doi: 10.1006/brln.1999.2212. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Dronkers N.F., Wilkins D.P., Van Valin R.D., Jr., Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Dronkers N.F., Plaisant O., Iba-Zizen M.T., Cabanis E.A. Paul Broca's historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain J. Neurol. 2007;130(Pt 5):1432–1441. doi: 10.1093/brain/awm042. http://dx.doi.org/10.1093/brain/awm042 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Fedorenko E., Thompson-Schill S.L. Reworking the language network. Trends Cogn. Sci. 2014;18(3):120–126. doi: 10.1016/j.tics.2013.12.006. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Bonilha L., Rorden C. Severe Broca's aphasia without Broca's area damage. Behav. Neurol. 2007;18(4):237–238. doi: 10.1155/2007/785280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Kjartansson O., Morgan P.S. Impaired speech repetition and left parietal lobe damage. J. Neurosci. 2010;30(33):11057–11061. doi: 10.1523/JNEUROSCI.1120-10.2010. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Yourganov G., Bonilha L., Basilakos A., Den Ouden D.B., Rorden C. Revealing the dual streams of speech processing. Proc. Natl. Acad. Sci. U. S. A. 2016;113(52):15108–15113. doi: 10.1073/pnas.1614038114. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Pastore-Wapp M. Diverging lesion and connectivity patterns influence early and late swallowing recovery after hemispheric stroke. Hum. Brain Mapp. 2017 doi: 10.1002/hbm.23511. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F., Wise R.J., Mehta A., Leech R. Overlapping networks engaged during spoken language production and its cognitive control. J. Neurosci. 2014;34(26):8728–8740. doi: 10.1523/JNEUROSCI.0428-14.2014. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E., Kocher M., Bonilha L. Connectomics and graph theory analyses: Novel insights into network abnormalities in epilepsy. Epilepsia. 2015;56(11):1660–1668. doi: 10.1111/epi.13133. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E., Fridriksson J., Rorden C., Nesland T., Desai R., Bonilha L. Separate neural systems support representations for actions and objects during narrative speech in post-stroke aphasia. NeuroImage. Clin. 2016;10:140–145. doi: 10.1016/j.nicl.2015.11.013. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat. Rev. Neurosci. 2012;13(2):135–145. doi: 10.1038/nrn3158. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn. Sci. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Inoue K., Madhyastha T., Rudrauf D., Mehta S., Grabowski T. What affects detectability of lesion-deficit relationships in lesion studies? NeuroImage. Clin. 2014;6:388–397. doi: 10.1016/j.nicl.2014.10.002. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova M.V., Isaev D.Y., Dragoy O.V. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex. 2016;85:165–181. doi: 10.1016/j.cortex.2016.04.019. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Joliot M., Jobard G., Naveau M. AICHA: an atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods. 2015;254:46–59. doi: 10.1016/j.jneumeth.2015.07.013. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Fruhmann Berger M., Kuker W., Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb. Cortex. 2004;14(10):1164–1172. doi: 10.1093/cercor/bhh076. [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- Kim N.Y., Lee S.C., Shin J.C., Park J.E., Kim Y.W. Voxel-based lesion symptom mapping analysis of depressive mood in patients with isolated cerebellar stroke: a pilot study. NeuroImage. Clin. 2017;13:39–45. doi: 10.1016/j.nicl.2016.11.011. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Morgan P.S., Ashburner J., Smith J., Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Lichtheim L. On Aphasia. Brain J. Neurol. 1885;7:433–484. [Google Scholar]
- Mah Y.H., Husain M., Rees G., Nachev P. Human brain lesion-deficit inference remapped. Brain J. Neurol. 2014;137(Pt 9):2522–2531. doi: 10.1093/brain/awu164. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menenti L., Gierhan S.M., Segaert K., Hagoort P. Shared language: overlap and segregation of the neuronal infrastructure for speaking and listening revealed by functional MRI. Psychol. Sci. 2011;22(9):1173–1182. doi: 10.1177/0956797611418347. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Mesulam M. Imaging connectivity in the human cerebral cortex: the next frontier? Ann. Neurol. 2005;57(1):5–7. doi: 10.1002/ana.20368. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Meyer S., Kessner S.S., Cheng B. Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. NeuroImage. Clin. 2016;10:257–266. doi: 10.1016/j.nicl.2015.12.005. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D., Chen Q., Zhang Y. Neural organization of spoken language revealed by lesion-symptom mapping. Nat. Commun. 2015;6:6762. doi: 10.1038/ncomms7762. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic B., Betzel R.F., Nematzadeh A. Cooperative and competitive spreading dynamics on the human connectome. Neuron. 2015;86(6):1518–1529. doi: 10.1016/j.neuron.2015.05.035. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Mukherjee P. Diffusion tensor imaging and fiber tractography in acute stroke. Neuroimaging Clin. N. Am. 2005;15(3):655–665. doi: 10.1016/j.nic.2005.08.010. http://dx.doi.org/10.1016/j.nic.2005.08.010 xii. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Nachev P., Coulthard E., Jager H.R., Kennard C., Husain M. Enantiomorphic normalization of focally lesioned brains. NeuroImage. 2008;39(3):1215–1226. doi: 10.1016/j.neuroimage.2007.10.002. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M.J., Garrod S. Neural integration of language production and comprehension. Proc. Natl. Acad. Sci. U. S. A. 2014;111(43):15291–15292. doi: 10.1073/pnas.1417917111. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusser S., Thiel S.D., Rook C. The perception of touch and the ventral somatosensory pathway. Brain J. Neurol. 2015;138(Pt 3):540–548. doi: 10.1093/brain/awu370. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C., Karnath H.O. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat. Rev. Neurosci. 2004;5(10):813–819. doi: 10.1038/nrn1521. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Rorden C., Bonilha L., Fridriksson J., Bender B., Karnath H.O. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrauf D., Mehta S., Grabowski T.J. Disconnection's renaissance takes shape: Formal incorporation in group-level lesion studies. Cortex. 2008;44(8):1084–1096. doi: 10.1016/j.cortex.2008.05.005. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Silbert L.J., Honey C.J., Simony E., Poeppel D., Hasson U. Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc. Natl. Acad. Sci. U. S. A. 2014;111(43):E4687–96. doi: 10.1073/pnas.1323812111. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Kinkingnehun S., Delmaire C. Visualization of disconnection syndromes in humans. Cortex. 2008;44(8):1097–1103. doi: 10.1016/j.cortex.2008.02.003. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell'Acqua F., Ratiu P. From Phineas Gage and Monsieur Leborgne to H.M.: revisiting disconnection syndromes. Cereb. Cortex. 2015;25(12):4812–4827. doi: 10.1093/cercor/bhv173. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Wernicke C. 1874. Der aphasische Symptomencomplex. Breslau. [Google Scholar]
- Yourganov G., Fridriksson J., Rorden C., Gleichgerrcht E., Bonilha L. Multivariate connectome-based symptom mapping in post-stroke patients: networks supporting language and speech. J. Neurosci. 2016;36(25):6668–6679. doi: 10.1523/JNEUROSCI.4396-15.2016. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]