Abstract
Aim
To fabricate and evaluate the efficacy of individualized intraoral stents to minimize the potential side effects of radiation on oral tissues in patients with early stages of lip cancer.
Background
Lower lip cancer is a common tumor found almost exclusively in middle-aged and elderly males. Surgery is the most common treatment of choice, although for less extensive lesions, exclusive radiotherapy may be preferred. Some studies have found that the use of intraoral stents in patients with intraoral cancer (e.g., of the tongue or floor of the mouth) obtained favorable results in preventing unnecessary radiation doses to adjacent normal tissue and reducing oral complications. However, studies investigating the efficacy of individualized intraoral stents in patients with lip cancer have not been reported in the literature.
Materials and methods
Six patients with early stage lip cancer were eligible for curative radiotherapy and personalized intraoral stents. The stents were fabricated and all participants were evaluated for the occurrence of oral complications.
Results
The regions of the oral mucosa protected from radiation by intraoral stents showed no mucositis. One patient complained of mild oral dryness but without interference in habits. At follow-up, none of the patients had late xerostomia or signs of dental caries by radiation.
Conclusions
The use of individualized intraoral stents was shown to be promising in reducing the adverse effects of radiation therapy in lip cancer patients. These findings highlight the importance of a multidisciplinary team during oncological therapy.
Keywords: Head and neck cancer, Lip cancer, Radiotherapy, Stents, Side effects
1. Background
Lip cancer (LC) is a type of oral cancer that develops at the junction of the oral cavity and the skin; it is most common on the lower lip (80%), while the upper lip and commissures are involved less often (5–8% and 7–15% of cases, respectively).1, 2 Moreover, LC is the most frequent tumor of the oral and maxillofacial region, comprising 25–30% of all oral cancers.2 It has a variable incidence around the world, with the highest rates being reported in southern Australia and some regions of Canada and Spain; incidence of LC is also considerable in tropical countries.3 It is commonly developed on the vermilion border of the lip and is diagnosed as squamous cell carcinoma (SCC) in 95% of cases; basal cell carcinomas and adenocarcinomas occur more rarely.1 The worldwide available data reveal that this type of cancer occurs mostly in white men (with a male-to-female ratio of 28.5:4.3), with peak incidence in the sixth and seventh decades, and among people working under conditions of prolonged sun exposure, such as agricultural workers and those with other outdoor occupations.1, 2, 4 The etiology of lower lip SCC is highly related to chronic sunlight (especially UVB) exposure and other factors, such as low sociodemographic conditions, genetic susceptibility, and immunosuppression, which might produce a synergistic effect.2, 4, 5 However, the definitive pathogenic pathway remains unclear.
It is very important to note that LC may evolve from precancerous lesions of the lip, such as actinic cheilitis, or from healthy lips.5 Because the disease is asymptomatic, the diagnosis may be delayed, even when it occurs on an easily visible site on the lip.3, 4, 5 In general, LC shows a survival rate higher than 5 years and a mortality rate of 10–15%, exhibiting a better prognosis compared with other head and neck tumors. The accessibility of the lesion site often allows for full-thickness resection of the neoplasm.2, 3 The prognosis depends mainly on the clinical staging of the tumor, especially with regard to its size and to lymph node status.4
Surgery is the treatment of choice, despite the fact that, for less extensive lesions in the early stages (T1/T2N0), the results obtained with radiotherapy (RT) may compare favorably.6, 7 Head and neck RT may offer better functional and cosmetic results, but can lead to complications, such as oral mucositis, xerostomia, and osteoradionecrosis, with significant impairment of patient's quality of life.8, 9 Several strategies have been used to prevent oral complications, because the treatment of these conditions is still considered difficult.8, 9, 10 Individualized intraoral stent can decrease these effects by reducing unnecessary radiation doses to healthy oral tissue and thereby minimizing the adverse effects of radiation.9, 10, 11, 12, 13, 14 Some studies have evaluated the use of intraoral stents in patients with intraoral cancer (e.g., cancer of the tongue or floor of the mouth) and obtained favorable results in preventing unnecessary radiation doses to adjacent normal tissue and reducing oral complications. However, studies investigating the efficacy of individualized intraoral stents in patients with LC have not been reported in the literature.
2. Aim
The present study evaluated the efficacy of individualized intraoral stents to minimize the potential side effects of radiation on oral tissues in 6 patients with early stages (T1/T2N0) of LC.
3. Materials and methods
For this study, only patients diagnosed with LC in early stages (T1/T2N0), and following exclusively RT treatment programs with curative purpose were selected (Fig. 1 and Table 1). The staging of the patients was obtained through extra- and intraoral physical examination. Six patients meeting these study criteria were selected and referred to the Clinical Oncological Dentistry of Santa Casa Hospital of Montes Claros (Minas Gerais, Brazil) for the construction of individualized intraoral stents. All devices were built by an experienced clinician (BAR).
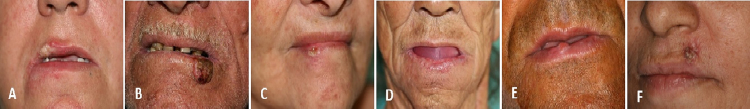
Fig. 1.
(A–F) Case 1, 2, 3, 4, 5 and 6, respectively. Clinical presentation of the lip cancer involving the lip and region around of the lip before radiotherapy.
Table 1.
Clinical features of six lip cancer patients.
| Cases | Age | Gender/occupation | Risk factors | Diagnoses | TNMc/clinical staging | Energy/radiotherapy total dose | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| 1 | 47 | Female/teacher | Smoking and sun exposure | SCCa grade Ib | T2N0M0/II | 8 MeVe/60 Gyf | 60 |
| 2 | 72 | Male/rural worker | Smoking and sun exposure | SCC grade II | T2N0M0/II | 7 MeV/60 Gy | 60 |
| 3 | 67 | Female/domestic | Sun exposure | SCC grade II | T1N0M0/I | 7 MeV/66 Gy | 56 |
| 4 | 69 | Female/rural worker | Sun exposure | SCC grade I | T1N0M0/I | 7 MeV/60 Gy | 50 |
| 5 | 47 | Male/rural worker | Alcohol consumption, smoking and sun exposure | SCC grade II | T1N0M0/I | 5 MeV/66 Gy | 39 |
| 6 | 51 | Female/at home | Sun exposure | BCCd | T1N0M0/I | 4 MeV/66 Gy | 2 |
SCC: squamous cell carcinoma.
Grade according to World Health Organization.
TNM: tumor-node-metastasis (TNM) staging system.
BCC: basal cell carcinoma.
MeV: mega electron volts.
Gy: gray, radiation dose absorbed by human tissue.
All patients underwent removal of the intraoral foci of infection prior to the fabrication of stents. Then, the dental and/or alveolar ridge arcades were molded, obtaining of the casts and assembly in articulator were held (Fig. 2A). Cerrobend alloy or lead with thickness of 5 mm was used as shielding (Fig. 2B). The stent was made of acrylic resin, adapted to the metal plate and wrapped in vinyl polychloride film and wax to avoid contact with the metal and reduce backscatter (Fig. 2C). According to the proposed treatment, the 6 patients received RT exclusively, with a total dose of 60–66 Gy fractionated at 2 Gy per day (for 5 consecutive days) for 6–7 weeks, using an electron beam with energy ranging from 4 to 8 mega electron volts (MeV) of linear accelerator (Siemens Mevatron Primus MXE-2® and Elekta Synergy Full®), with protection blocks and radioprotective stents placed in the retrolabial region (Fig. 2D). A bolus made of wax (5 mm thick) was superimposed on the immobilizing thermoplastic mask in the region corresponding to the tumor, with the patient in the supine position using anatomical head and neck support. An area with a radial margin of 2 cm around the primary lesion was included in the irradiation field. The radiation field was defined by examining the topography, dimensions, and risk of microscopic disease in adjacent tissues. For the dose prescription, we used a focus-skin distance technique with a source surface distance (SSD) of 100 cm and 80% isodose in 2 patients and 85% in 4 patients. For the electron energy selection, the lowest energy was used such that the lesion was covered in the 80% or 85% curve. The dosimetric plans of the two-dimensional treatments used in this study were based on the determination of energy, isodose, total dose and dose per fraction, bolus, and lesion anatomy.
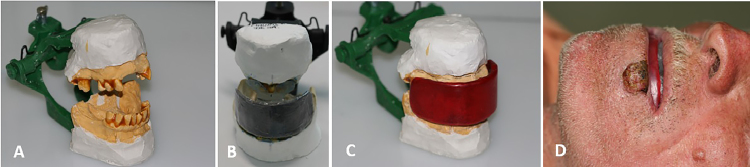
Fig. 2.
(A) Model mounted on articulator. (B) Screening plate adapted to the acrylic resin. (C) Wax-coated board. (D) The patient wearing the intraoral stent (case 2).
All participants were analyzed before, during, and after radiation for the occurrence of oral complications such as xerostomia, oral mucositis, dysphagia, and dysgeusia. To grade the intensity of signs and symptoms related to xerostomia, we used the grading system proposed by Eisbruch et al. (symptomatic assessment)14; to evaluate oral mucositis, we used the grading system proposed by the World Health Organization (WHO).15 All 6 patients signed informed consent to participate, and this study was carried out with approval from the Human Research Ethics Committee of the University of Montes Claros.
4. Results
In all cases, the regions of the oral cavity protected by the intraoral devices showed no oral mucositis but oral mucositis was reported in unprotected areas surrounding the tumor (Fig. 3). Cases 1 and 4 reached grade III oral mucositis in unprotected areas, leading to temporary interruption of RT; the other cases showed grade I or II oral mucositis without interruption of the therapy. None of the patients had dysgeusia or dysphagia, and only case 4 complained of mild oral dryness, which occurred after 13 days of RT without interference in habits (xerostomia grade 1). The others showed no xerostomia during or after RT. As part of our oral care protocol, all patients who developed oral mucositis began curative low-level laser therapy (LLLT). Applications were administered 5 days per week until the complete remission of the lesions, according to the protocol of Lalla et al.15 The laser (gallium aluminum arsenate diode laser, Twin laser, MM Optics, São Paulo, Brazil) illumination consisted of a continuous 660-nm wavelength; power of 40 mW; spot size of 4 mm2; and energy density delivered in each ulcerated area of oral mucosa of 6.2 J/cm2. The tumor area (or previous tumor area) was excluded from the field of laser illumination. The fabricated devices had good retention, stability, and patient acceptance, and were easy for the patients to use. All cancer lesions had a complete remission and great esthetic and functional results were obtained (Fig. 4). In follow-up between 2 and 60 months, there were no signs of recurrence of the cancer lesions or metastases.
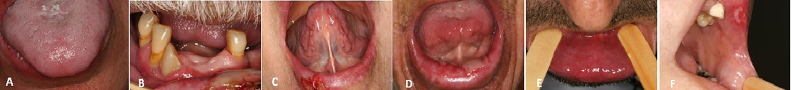
Fig. 3.
(A) Case 1 at the 12th session of the RT (24 Gy). (B, C, D, E and F) Oral mucositis in the topography of tumor and lip mucosa, but no changes induced by radiation on protected regions (cases 2, 3, 4, 5, and 6 at doses of 34 Gy, 32 Gy, 58 Gy, 48 Gy and 38 Gy, respectively).
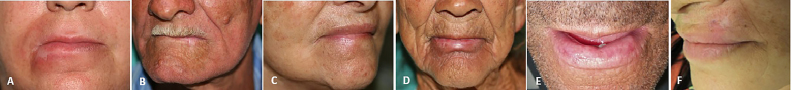
Fig. 4.
(A) Case 1: 30 days after the last session of radiotherapy. (B) Case 2: 12 months after radiotherapy. (C) Case 3: 55 months after radiotherapy. (D) Case 4: in the last session of radiotherapy. (E) Case 5: 18 months post-RT. (E) Case 6: 60 days post-RT.
Interestingly, cases 2 and 3 have undergone tooth extractions in our service in the post-RT period and showed no signs of osteoradionecrosis in follow-up visits. Case 2 is in oral prosthetic rehabilitation and will be fitted with dentures. Case 5 is in oral rehabilitation with dental implants, with no osteoradionecrosis signals observed in a follow-up of 39 months. At follow up, none of the patients had late xerostomia or signs of dental caries by radiation.
5. Discussion
Early stages of LC (T1/T2N0) can be properly treated by RT alone, as this method can reduce morbidity and achieve better esthetic and functional results. In our study, all these factors were decisive in the choice of exclusive RT for the treatment of the 5 patients with squamous cell carcinoma of the lip and one with basal cell carcinoma. However, head and neck RT is associated with a number of adverse effects—mucositis and xerostomia being two of the most serious and debilitating—that directly interfere with quality of life.8, 15 So, focusing on the modalities that prevent/reduce oral complications associated with RT, individualized intraoral stents can and should be used.9, 10, 11, 13 The available literature is not abundant as to the effectiveness of such stents, but our study demonstrated the efficacy of these individualized intraoral stents in minimizing the potential side effects of radiation on oral tissues in the patient with LC. To the best of our knowledge, this study was the first to evaluate individualized intraoral stents in patients with early stages of LC in exclusive RT treatment programs with a curative purpose.
The intraoral stents are designed to open the oral cavity and exclude healthy structures from the radiation field, protecting normal tissues adjacent to cancer. Moreover, they are able to immobilize the jaw and tongue, effectively recreating the same position during planning and all subsequent treatment sessions of RT.9, 10, 11, 12, 13 Several types of individualized intraoral stents have been fabricated using different materials such as thermal plastic, self-curing resin, polyvinyl siloxane-metal composite, polymethylmethacrylate resin, autopolymerizing acrylic resin, and wax.9, 10, 11, 12, 13 However, acrylic resin is considered an excellent material option because it is nontoxic, nonirritating to oral tissue, low cost, practical to manipulate, durable, and hygienic, and it does not interfere with radiation.9, 10, 11, 12, 13 Thus, acrylic resin was used in this study, along with wax used to protect the unaffected oral tissues from adverse effects during radiotherapy. Cerrobend alloy was used to fabricate the stents, as it is a low fusing alloy that can be easily cast into a desired form.13 As previous studies have noted, the production of individualized stents is easy, safe, and rapid; normally two or three sessions are needed.9, 13 Moreover, stents do not adversely affect comfort and allow for repeated placement.10, 13 Other studies have shown that the use of intraoral stents is beneficial in radiation therapy for the tongue, floor of the mouth, and nasopharyngeal cancer.10, 11, 12 In the present study, patients used the intraoral stents during all RT sessions without any difficulties. We verified that the oral complications associated with RT decreased with the use of intraoral devices in radiation therapy for LC. None of the 6 patients had dysgeusia or dysphagia, and only one had xerostomia grade 1. In all cases, the regions of the oral cavity protected by the intraoral devices showed no oral mucositis. Predictably, oral mucositis was reported in unprotected areas surrounding the tumor. The reduction of adverse effects of radiation has a direct impact on the clinical course of the disease, as oral mucositis may affect 80% of patients who undergo head and neck irradiation; 42% are at risk of severe mucositis (grades 3–4), which usually compromises patients’ adherence to treatment and quality of life.15 The successful use of intraoral stents during radiotherapy for LC underscores the importance of cooperation between the radiotherapist, medical physicist, and the stomatologist.
6. Conclusion
The use of individualized intraoral stents showed promising results in decreasing the adverse effects of radiation therapy in patients with early stage lip cancer, and highlights the importance of consulting a multidisciplinary team during oncological therapy.
Authors’ contribution
Study concepts: Breno A. Rocha, Mário R. de Melo Filho, Lívia M.R. Paranaíba, Maria Betânia de O. Pires.
Study design: Breno A. Rocha, Camilla V. Vilas Boas, Mário R. de Melo Filho.
Data acquisition: Breno A. Rocha, Angel da S. Martinez, Lucianne M.C. Lima, Camilla V. Vilas Boas.
Quality control of data and algorithms: Breno A. Rocha, Lucianne M.C. Lima, Angel da S. Martinez.
Data analysis and interpretation: Breno A. Rocha, Lucianne M.C. Lima, Lívia M.R. Paranaíba, Edimilson M. de Freitas.
Manuscript preparation: Breno A. Rocha, Lucianne M.C. Lima, Angel da S. Martinez, Lívia M.R. Paranaíba.
Manuscript editing: Breno A. Rocha, Lívia M.R. Paranaíba.
Manuscript review: Breno A. Rocha, Angel da S. Martinez, Lucianne M.C. Lima, Lívia M.R. Paranaíba, Maria Betânia de O. Pires, Edimilson M. de Freitas, Camilla V. Vilas Boas, Mário R. de Melo Filho.
Ethical approval
This article has been approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out.
Conflict of interest
None declared.
Financial disclosure
The Minas Gerais State Research Foundation-FAPEMIG State financed the materials and the entire infrastructure for patient care [grant numbers: CDS APQ – 03125/10].
References
- 1.Czerninski R., Zini A., Sgan-Cohen H.D. Lip cancer: incidence, trends, histology and survival: 1970–2006. Br J Dermatol. 2010;162:1103–1109. doi: 10.1111/j.1365-2133.2010.09698.x. [DOI] [PubMed] [Google Scholar]
- 2.Biasoli É.R., Valente V.B., Mantovan B. Lip cancer: a clinicopathological study and treatment outcomes in a 25-year experience. J Oral Maxillofac Surg. 2016;74(7):1360–1367. doi: 10.1016/j.joms.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Souza R.L., Fonseca-Fonseca T., Oliveira-Santos C.C. Lip squamous cell carcinoma in a Brazilian population: epidemiological study and clinicopathological associations. Med Oral Patol Oral Cir Bucal. 2011;16(6):e757–e762. doi: 10.4317/medoral.16954. [DOI] [PubMed] [Google Scholar]
- 4.Ozkul Y., Songu M., Imre A. Early stage squamous cell carcinoma of the lower lip: predictive factors for recurrence. J Laryngol Otol. 2016;130(4):369–372. doi: 10.1017/S0022215116000311. [DOI] [PubMed] [Google Scholar]
- 5.Vieira R.A., Minicucci E.M., Marques M.E., Marques S.A. Actinic cheilitis and squamous cell carcinoma of the lip: clinical, histopathological and immunogenetic aspects. An Bras Dermatol. 2012;87(1):105–114. doi: 10.1590/s0365-05962012000100013. [DOI] [PubMed] [Google Scholar]
- 6.Guibert M., David I., Vergez S. Brachytherapy in lip carcinoma: long-term results. Int J Radiat Oncol Biol Phys. 2011;81(5):e839–e843. doi: 10.1016/j.ijrobp.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Thanh Pham T., Cross S., Gebski V., Veness M.J. Squamous cell carcinoma of the lip in Australian patients: definitive radiotherapy is an efficacious option to surgery in select patients. Dermatol Surg. 2015;41(2):219–225. doi: 10.1097/DSS.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 8.Migliorati C.A., Seneda L.M., Burton E.L. Oral complications of cancer therapy: a summary guide for the clinician. J Tenn Dent Assoc. 2015;95(1):24–32. quiz 33-4. [PubMed] [Google Scholar]
- 9.Verrone J.R., Alves F.A., Prado J.D. Impact of intraoral stent on the side effects of radiotherapy for oral cancer. Head Neck. 2013;35(7):E213–E217. doi: 10.1002/hed.23028. [DOI] [PubMed] [Google Scholar]
- 10.Verrone J.R., Alves F.A., Prado J.D. Benefits of an intraoral stent in decreasing the irradiation dose to oral healthy tissue: dosimetric and clinical features. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(5):573–578. doi: 10.1016/j.oooo.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Goel A., Tripathi A., Chand P., Singh S.V., Pant M.C., Nagar A. Use of positioning stents in lingual carcinoma patients subjected to radiotherapy. Int J Prosthodont. 2010;23(5):450–452. [PubMed] [Google Scholar]
- 12.Aggarwal H., Kumar P. Radiation stents: minimizing radiation-induced complications. South Asian J Cancer. 2014;3(3):185. doi: 10.4103/2278-330X.136812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yangchen K., Siddharth R., Singh S.V. A pilot study to evaluate the efficacy of cerrobend shielding stents in preventing adverse radiotherapeutic effects in buccal carcinoma patients. J Cancer Res Ther. 2016;12(1):314–317. doi: 10.4103/0973-1482.154015. [DOI] [PubMed] [Google Scholar]
- 14.Eisbruch A., Rhodus N., Rosenthal D., Murphy B., Rasch C., Sonis S. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol. 2003;3:226–234. doi: 10.1016/S1053-4296(03)00033-X. [DOI] [PubMed] [Google Scholar]
- 15.Lalla R.V., Bowen J., Barasch A. Mucositis guidelines leadership group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) Cancer. 2014;120(10):1453–1461. [Google Scholar]