Abstract
Background
Motor abnormalities (MAs) of severe mental disorders have been traditionally neglected both in clinical practice and research, although they are an increasing focus of attention because of their clinical and neurobiological relevance. For historical reasons, most of the literature on MAs has been focused to a great extent on schizophrenia, and as a consequence their prevalence and featural properties in other psychiatric or neuropsychiatric disorders are poorly known. In this article, we evaluated the extent to which catatonic, extrapyramidal and neurological soft signs, and their associated clinical features, are present transdiagnostically.
Methods
We examined motor-related features in neurodevelopmental (schizophrenia, obsessive compulsive disorder, autism spectrum disorders), “functional” (nonschizophrenic nonaffective psychoses, mood disorders) and neurodegenerative (Alzheimer’s disease) disorders. Examination of the literature revealed that there have been very few comparisons of motor-related features across diagnoses and we had to rely mainly in disorder-specific studies to compare it transdiagnostically.
Results
One or more motor domains had a substantial prevalence in all the diagnoses examined. In “functional” disorders, MAs, and particularly catatonic signs, appear to be markers of episode severity; in chronic disorders, although with different degree of strength or evidence, all motor domains are indicators of both disorder severity and poor outcome; lastly, in Alzheimer’s disease they are also indicators of disorder progression.
Conclusions
MAs appear to represent a true transdiagnostic domain putatively sharing neurobiological mechanisms of neurodevelopmental, functional or neurodegenerative origin.
Keywords: schizophrenia, psychosis, mood disorders, obsessive-compulsive disorder, autism spectrum disorders, Alzheimer’s disease
Introduction
Motor abnormalities (MAs) of psychiatric disorders have been traditionally a neglected topic in both clinical practice and research, and modern taxonomies of psychopathology continue to ignore them.1–3 MAs are included among diagnostic criteria of many psychiatric disorders4 and are present as cardinal or associated features in many others (supplementary table 1); and they also have important implications for the etiology,5–7 nosology,5,8,9 pathophysiology,10–12 and management13–15 of psychiatric disorders. Furthermore, MAs cut-across many psychiatric, neuropsychiatric and neurological disorders16 and they have been proposed as a core phenotype dimension of major psychiatric disorders17,18 and as a putative domain within the NIMH Research Domain Criteria framework (RoDC).19
Despite the fact that MAs are a ubiquitous condition of many psychiatric disorders, for historical reasons, most studies on the topic have been mainly focused on psychotic disorders and more specifically on schizophrenia, and only a minority of studies have compared MAs across diagnoses. Thus, the extent to which MAs and their associated clinical features are either disorder-specific or have a transdiagnostic character remains an open question. The present article attempts to meet this challenge through a systematic review of empirical studies examining MAs in several diagnoses. In this article, we consider 3 domains of MAs: catatonic signs, extrapyramidal symptoms (EPS) and neurological soft signs (NSS). Catatonia is a neuropsychiatric psychomotor syndrome characterized not only by a broad range of MAs, but also by affectivity disturbance and complex behaviors including disturbance of will.15,20,21 Insofar as catatonia does not exclusively affect motility, it can be distinguished from pure MAs such as EPS. EPS may present as either abnormal excess or paucity of movements, and commonly used terms for the former are dyskinesia, hyperkinesias, and abnormal involuntary movements and for the latter hypokinesia, bradykinesia, and parkinsonism.22,23 Hereinafter, we use mainly the terms dyskinesia and parkinsonism. The NSS comprise a wide range of subtle abnormalities that are usually grouped into sensory integration, motor coordination and sequencing of complex motor tasks.24 The rationale for selecting these motor domains was that catatonic and extrapyramidal signs represent well-known phenotypic manifestations of many psychiatric disorders with important clinical and treatment implications. On the contrary, NSS are subclinical features that are not usually assessed in clinical practice, as they need to be elicited by neurological examination; however, they are of research relevance because of their value as endophenotype candidates for many psychiatric disorders.25
The goal of this study was 2-fold. We first examined the phenomenology and factor structure of MAs, in order to describe their core clinical phenotype. We next examined the main featured characteristics of MAs across disorders of neurodevelopmental, “functional” or neurodegenerative origin. Among neurodevelopmental disorders we specifically examined the diagnoses of schizophrenia, obsessive-compulsive disorder (OCD) and autism spectrum disorders (ASD); functional disorders included nonschizophrenic nonaffective psychoses (NSNAP) and major mood disorders; lastly, as a neurodegenerative disorder we examined Alzheimer’s disease (AD). Neurodevelopmental disorders are a group of conditions with onset, or early manifestations, in the developmental period as a result of early brain dysfunction, which usually follow a chronic course. Neurodegenerative disorders are characterized by late-onset neurodegenerative processes in the brain in which the clinical course is progressive rather than chronic. In opposition to these groups of disorders, “functional” disorders are mainly characterized by onset in early or middle adulthood and by an episodic/remitting course that is putatively tied to a mostly reversible brain dysfunction. The rationale for selecting these diagnoses of varying origin was that they represent major and prevalent psychiatric or neuropsychiatric disorders, in which MAs have been often described. Such an approach may inform on points of commonality and divergence of MAs across diagnostic classes and mechanisms. Before examining these questions, and to illustrate the complexity of motor dysfunction influencing clinical practice and research, it is necessary to address some relevant conceptual and methodological issues.
Conceptual and Methodological Issues
We lack of a unifying theory about normal and abnormal motility and there is no guiding principle of what makes a motor sign or behavior.26 As a consequence, both the definition and boundaries of abnormal motility have become unclear and changing according to different theoretical backgrounds.27 For example, we lack universally accepted concepts and assessment tools for catatonia4,15,16,28–35 and NSS24,36–40 (supplementary tables 2 and 3), and research is fragmented according to the predefined motor domains, although a comprehensive instrument for rating the 3 motor domains is available.36
Daniel Rogers41 advanced the idea of a “conflict of paradigms” to refer to the psychiatric vs neurologic view of MAs; nonetheless, there are several other conflicting views involving broad vs restrictive definitions, categorical vs dimensional approaches, cross-sectional vs longitudinal views and primary vs drug-induced MAs, issues to which we’ll refer briefly. Historically, catatonia was broadly defined as a psychomotor syndrome characterized by the most remarkable signs such as stupor, excitation, negativism, mutism, paratonia and waxy flexibility, but also by less dramatic manifestations including tic- and dyskinesia-like movements, choreo-athetoid movements, rigidity, bradykinesia, release signs and difficulties in motor coordination and balance.5,42–44 These less dramatic signs are now recognized as EPS or NSS and in general are not included within the catatonia syndrome, which has led to a more restrictive view of the syndrome.41
The complexity of the catatonia concept is further highlighted by uncertainty as to whether it is a discrete pathology or a dimensional cluster of symptoms, a question that needs to be framed within the broader context of categorical vs dimensional representations of psychopathology. Dimensions cut-across diagnostic categories, tend to exhibit more predictive value than categories and are an essential component of the RdoC matrix. Catatonia is usually defined as a discrete category and identified with the most striking manifestations; however, catatonia ratings, particularly when scales with broad item coverage are used, tend to follow a continuous distribution in severe mental disorders18,45–47; thus, choosing a determined cut-off point to make a diagnosis is a relatively arbitrary question that may reflect underlying severity rather than true categorical distinctions. An additional problem is that current catatonia rating scales and diagnostic criteria essentially define a cross-sectional disorder. Catatonia may vary over time according to specific patterns of symptoms and illness-related factors, by which a longitudinal perspective has been emphasized by all classical5,42–44 and some modern authors.48,49 Unlike catatonia, EPS and NSS lacked of nosological formulations, and they are usually rated dimensionally and eventually categorized according to specific cut-off points or criteria. A specific problem of NSS is that they lack a validate criterion to define abnormality, which favors great discrepancy between studies in prevalence rates in healthy controls and those with psychiatric disorders.24
Regarding the motor effects of antipsychotic medication, it is worth noting that Steck, who was one of the first in reporting these effects50 and had been involved in studying MAs of severe mental disorders in the preneuroleptic era,10 suggested that antipsychotic drugs may be acting by modifying the expression of disease-based motor disorder.50 This view was ignored by contemporary and subsequent authors and the contribution of drugs to motor disorders was seen as paramount importance. Antipsychotics may cause a broad range of acute and chronic MAs,51 although it appears that illness-related factors,52,53 and particularly preexisting MAs53,54 play also a role. Studies of drug-naïve subjects showed that spontaneous MAs may be an indigenous feature of severe mental disorders tied to the underlying pathophysiology,53,55–57 and that antipsychotics, in addition to produce drug-emergent MAs in some subjects, they may improve, worsen or left unchanged preexisting catatonic,58 extrapyramidal58 and neurological signs.59 Thus, in subjects on antipsychotics, MAs and particularly NSS and EPS, likely represent a mixture of primary and drug-induced motor features, and currently, a balanced view of MAs in treated subjects is one of antipsychotic medication interacting with or modifying the disease-based motor disorder.13,57–61
Systematic Review
We first identified relevant historical literature on MAs in psychiatric and neuropsychiatric disorders. Then we completed an initial search of MeSH terms for “Catatonia” OR “Abnormal involuntary movements” or “Parkinsonism” OR “Extrapyramidal symptoms” OR “Neurological soft signs” AND “Mental disorder” OR “Medical disease” OR “Factor Analysis”. Search dates were not constrained, and pertinent cohort studies and review articles were identified. Using this initial information, numerous subsequent searches of specific terms were made in order to examine MAs in the disorders of interest. MeSH terms for specific diagnoses included “Schizophrenia” OR “Psychotic disorder” OR “Mood disorder” OR “Obsessive-compulsive disorder” OR “Autism Spectrum Disorder” OR “Alzheimer’s disease”. Many variations on each search for the individual diagnoses were also conducted. Exclusion criteria were as follows: studies determining MAs exclusively by instrumental measures, studies of drug-induced motor disorders, and case reports. Studies in English, German, French, Italian or Spanish were included. Because of this study was mainly concerned with primary MA, we tried to prioritize studies including subjects who were drug-naïve, minimally treated, drug-free, or treated subjects in which the antipsychotic medication was controlled for.
The great majority of studies had a focus on schizophrenia spectrum disorders, which can be explained by historical trends. Most of the remaining studies examined MA within specific diagnoses and a total of 46 studies examined MAs in 2 or more diagnoses, one of them usually involving schizophrenia. Of these 46 studies, 22 exclusively addressed the prevalence of catatonia in hospital samples and 24 examined some featural properties of MAs, which should be the main focus of this review55,62–85 (supplementary table 4). Most of these articles, however, mainly examined the distribution of motor features and were of such variable methodology and quality that we’ll refer to some of them in the following narrative review. Thus, we had to rely on findings mainly coming from disorder-specific studies. To maintain a balance between findings in schizophrenia and in other diagnoses, those of schizophrenia were kept at a minimum according to their relevance. Lastly, because of motor dysfunction is a key component of abnormal neurodevelopment and at-risk states, this issue was also briefly addressed.
Phenomenology and Syndromic Structure of MAs
The phenomenology of MAs has been mainly addressed in classical text books5,42–44 and articles11,86–91 not included in PubMed. This literature was largely based on close clinical scrutiny of patients followed-up over years and laid the foundation of current descriptions. However, classical and current approaches highly differ in that the former is clinically-based and longitudinally-oriented, and the latter, clinometrically-based and cross-sectionally-oriented. The classical approach is best represented by the Wernicke-Kleist-Leonhard school of psychiatry,92 which set the MAs at the forefront of psychotic disorders because of their clinical, nosological and neurobiological relevance. Kleist11 was the first in elaborating a systematic account of catatonic phenomena and in relating them to specific brain circuitry dysfunctions. Leonhard, categorized human motility into spontaneous, expressive, reflex, and reactive movements,93,94 and articulated the most clinically detailed description and classification of motor phenomena ever produced (supplementary table 5).5 According to Leonhard, MAs need to be considered in relation to the entire illness course, and distinguished between quantitative increase or decrease of motor activity and qualitatively distinct motor disturbances. Indeed, Leonhard’s scheme allows ordering the varied MAs along a continuum of axial characteristics such as bipolarity, bizarreness and complexity of motor behaviors. Leonhard’s nosology has been validated to some extent by other authors,95–101 but it had little international impact mainly due to its complexity. Some attempts have been made to render this classification more simple and operational,102–104 but with limited success in terms of further use. Notwithstanding this, Leonhard’s classification remains of high heuristic value and of potential research interest to address heterogeneity of MAs in psychiatric disorders.92,101
When catatonia ratings are examined in the context of other psychopathological symptoms, a catatonia dimension consistently emerges as a highly differentiated domain.105 The 15 existing factor analytical studies of catatonia greatly vary about the number of factors obtained (from 2 to 7) and their item composition (supplementary table 6).18,30,33,63,72,106–115 The mean number of reported factors was 4.1 and the most replicated ones were motor excitement and motor retardation (13 and 11 studies, respectively), followed to a great distance by an involuntary movements factor (5 studies). Variability in the factor structure may be explained by item composition of the rating scales, method for determining the factor solution and sample issues; in fact, a somewhat different factor structure can be obtained by using different rating scales in the same sample18,113 and by using the same rating scale in different samples.18,110
Studies addressing the factor structure of EPS are lacking, but when EPS ratings are analyzed together with ratings of catatonia, they usually form part of overarching hyperkinetic and hypokinetic dimensions.18 A consistent multidimensionality of NSS has been observed (from 3 to 5 factors) in both exploratory39,79,116 and confirmatory factor analysis117 and most studies provided face validity for the 3 predefined NSS domains.24,118–121 The only study examining conjointly the factor structure of all 3 motor domains resulted in 5 dimensions: motor coordination, movement abnormalities, increased reflexes, dyskinesias, and catatonia.69
Strong associations between catatonia and EPS ratings have been reported,106,122 and to a less degree between these and NSS.123,124 Most importantly, these associations have also been observed in drug-naïve subjects,30,53,116 this indicating that associations are genuine and not due to chronicity or drug effects.
Schizophrenia
NSS and dyskinesias, but not catatonic signs, may appear long before the beginning of the first-episode of psychosis125–128; hence, these MAs may be better understood in the context of neurodevelopment deviations, as results of brain insults or dysfunctions during pregnancy and perinatal periods129 and genetic or epigenetic factors.130,131 Normal neurodevelopment evolves through a chronological schedule that is closely entwined with the age-associated stage and intrinsically linked to the development of motility, cognitive functions and social behavior.132–137 Early motor dysfunctions may be a risky step for the development of schizophrenia138–140 and might predict subsequent negative symptoms141 and cognitive performance.142–144 In addition, spontaneous dyskinesias may predict transition to psychosis in at-risk individuals,145,146 neuromotor dysfunction in children and adolescents may precede the prodrome and onset of schizophrenia,147 and deviant achievement of motor milestones may serve to recognize individuals at risk of psychosis.148
Compared to healthy controls, both dyskinesias131,149 and NSS150,151 are significantly more prevalent in subjects with schizotypy or at-risk individuals. Furthermore, meta-analytic evidence indicates that EPS152–154 and NSS155 capture a moderate proportion of psychosis proneness, which supports the endophenotype hypothesis of motor dysfunction by associating it with neurodevelopmental deviance. However, some caution is warranted since at-risk individuals are not just for schizophrenia but, more broadly, for other disorders with poor developmental outcomes.131
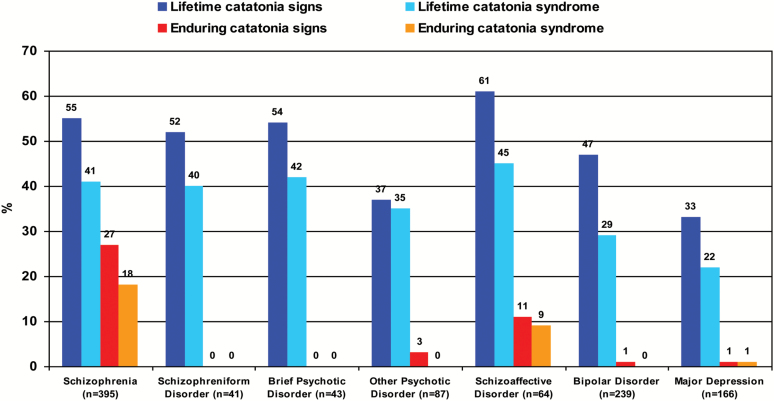
The catatonic subtype of schizophrenia was dropped from DSM-5 due to low diagnostic stability and validity.156 However; no less important is the poor validity of the DSM schizophrenia concept itself in resolving clinical and etiological heterogeneity,101 and that motor signs have been de-emphasized in current diagnostic criteria compared with earlier definitions.157,158 Indeed, Leonhard’s and Bleuler’s classifications diagnose as much as 2.5 times more catatonias than current consensus diagnoses.157 Catatonic signs are by no means confined to the catatonic subform of the disorder, and they appear to cut-across schizophrenia subtypes. The Iowa-500 study showed that 32% of people with schizophrenia had catatonia signs and only 6.5% met criteria for the catatonic subtype.159 From a lifetime perspective, the prevalence of a catatonia syndrome in schizophrenia raises up to 41% (figure 1), and Manschreck73 noted that most subjects with chronic schizophrenia exhibit mild catatonia-like movements that do not qualify for a catatonia diagnosis.
Fig. 1.
Data were re-analyzed from Peralta et al.101 This sample population was derived from a family study comprising 1094 subjects affected with psychotic or mood disorders, who were recruited from out and inpatients facilities in Navarra (Spain) between years 1990 and 2014. Subjects were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition), and catatonia was assessed by means of the Comprehensive Assessment of Symptoms and History (CASH).
Converging evidence indicates that catatonia signs are linked to severity of schizophrenia. Most,160–163 but not all164 modern outcome studies of schizophrenia examining baseline catatonic signs showed that they were related to a poor outcome, and most subjects with severe impairment present with catatonic symptoms.41,43,44,165,166 Likewise, motor domains have been consistently linked to negative or deficit schizophrenia,167 even in drug-naïve subjects,168 and to poor cognition.166,169 There is a lack of prospective studies examining catatonia sings across illness stages, but studies using the same rating instrument in samples with differing illness stages, provide indirect evidence for an overall increase of catatonia signs with illness chronicity and severity (supplementary table 7).18,41,110
Several systematic reviews have reported prevalence rates for spontaneous dyskinesia between 9%56 and 13%56 and for spontaneous parkinsonism between 17%56 and 25%,12 although rates up to 30% have been reported using broader definitions of EPS.18 Both dyskinesias and parkinsonism have been described in about two-thirds of subjects with chronic schizophrenia,169–171 and although they likely represent a mixture of primary and drug-induced phenomena, the contribution of antipsychotic drugs appears to be of minor relevance, particularly for dyskinesias.170 An outstanding longitudinal study comparing schizophrenia subjects, who were mostly drug-naïve, with and without spontaneous dyskinesias showed that the group with dyskinesias had poorer premorbid adjustment, earlier illness onset, more severe disorganized and catatonic signs, and much more poor functioning.172 Another study reported that subjects with chronic schizophrenia and dyskinesias had greater negative and disorganization symptoms, more voluntary MAs, lower premorbid IQ and higher cognitive impairment.173
NSS are prominent during the acute exacerbations of schizophrenia and to a less extent during the stabilization phase174–177; furthermore, their decreasing during the episode remission runs parallel to remission of symptomatology,37 even in drug-naïve samples.176,178 Duration of illness is significantly associated with NSS,124 and some evidence indicates that NSS are related to both poor cognitive functioning,179,180 although subscale motor scores may differ in this regard,74 and poor social functioning.81
Nonschizophrenic Nonaffective Psychoses
This diagnostic grouping entails the diagnoses of schizophreniform disorder, schizoaffective disorder, brief psychotic disorder and other unspecified psychotic disorder; and Kalbaum’s catatonia original concept mainly corresponds with NSNAP.181 Within NSNAP, catatonia may appear either in association with other psychotic syndromes or as the main manifestation of the disorder. Disorders with episodic-remitting course in which abnormal motility is the predominant manifestation have been historically acknowledged as periodic psychoses with disturbed motility43 or motility psychoses,5,182,183 and more recently as idiopathic or recurrent catatonia.184–186 Catatonia cut-across all NSNAP with lifetime prevalence rates of 35%–45% (figure 1). Motility psychoses represent the 18% of all NSNAP,5 and the idiopathic/recurrent catatonias have been described in 4%–46% of case series of subjects with catatonia.186 When catatonia is the main manifestation of the psychotic disorder, it is usually much more severe than catatonia in other psychiatric conditions.5,185
The relevance of catatonia within NSNAP contrasts with the paucity of studies of catatonia and other motor domains within this diagnostic grouping. Only one study reported prevalence rates of spontaneous dyskinesias in schizoaffective (11.4%) and schizophreniform disorder (0%).55 Consistent evidence indicates that levels of NSS in NSNAP did not differ from those observed in schizophrenia74,80 or psychotic mood disorder.74,81
Mood Disorders
The prevalence of catatonia in manic episodes ranges from 17%187 to 31%188 (figure 1), these rates being much higher in mixed mania: between 28%187 and 61%.189 The major MAs in mood disorders are psychomotor agitation and retardation, which are closely tied to mood states. Catatonic signs are related to severity of the manic episode188,190; and some,68,189 but not all190 studies revealed that catatonic manics displayed higher levels of comorbidity and poorer global functioning compared with their noncatatonic counterparts; furthermore, the poor prognosis of manic subjects with catatonia appears to be mediated by the higher comorbidity associated with the mixed states.191,192
The only study examining the prevalence of spontaneous dyskinesias in bipolar disorder (BD) reported a figure of 14.3%.55 Additionally, mood disorders may present a risk factor for developing tardive dyskinesias (TD),193 and a relationship appears to exist between affective states and TD as increased severity of depression often is coupled with TD worsening and TD often diminish with mania.194,195 Bipolar subjects show significantly more NSS than healthy controls,196–199 though without clear detachment from nonaffective psychoses.24,74,79–81 NSS occur in decreasing degree in psychotic mania,77 nonpsychotic mania,77 euthymic bipolars,200 unaffected first-degree relatives,200 and healthy controls.199,200 Thus, NSS appear to represent both severity and trait deficits in BD.
As many as 20% of depressed subjects may present with a catatonia syndrome,201 and compared with noncatatonic depressed they were older, more cognitively impaired and presented more severe depression.201 Psychomotor retardation indicates episode severity202 and is central to the melancholic subtype of depression203; although it may also appear in mixed states as inhibited mania.204 Psychomotor agitation in major depression has been reported to be a strong predictor of mood switching.205
The prevalence of spontaneous dyskinesias in major depression appears to be as low as 6.3%.55 The only study specifically addressing parkinsonism in depression reported a 20% prevalence rate, and it was related to older age, severity of depression and cognitive impairment.206 Levels of NSS in major depression are significantly lower than in psychotic disorders and BD and do not meaningfully differ from those observed in healthy controls207; however, psychotic depression exhibit NSS levels comparable to other psychotic disorders.77
Obsessive-Compulsive Disorder
Despite poor and nonfunctional motor behavior has been acknowledged in most subjects with OCD,208,209 only one study has examined catatonia ratings in subjects with this diagnosis66; and it reported that OCD subjects had significantly lower catatonia ratings than those with schizophrenia but higher ratings than healthy controls. A phenomenological overlap exists between catatonia and OCD regarding complex repetitive compulsions and catatonic mannerisms and repetitive/perseverative behaviors210; and evidence for a relationship between OCD and catatonia comes from the study of the so-called schizo-obsessive disorder.211 Compared with their schizophrenia counteparts, the majority of schizo-obsessive subjects exhibit both catatonia (83%) and EPS (58%),210 a clinical picture highly resembling Leonhard’s manneristic catatonia.5 The only study systematically examining catatonia in Tourette’s syndrome, a disorder highly comorbid with OCD, found that it was present in 87% of the subjects.212
About one-third of subjects with OCD exhibit both dyskinesias other than tics213 and parkinsonism,214 although studies differ in their comparative levels in relation to schizophrenia.66,76 The most common movement disorders comorbid with OCD are tics, now recognized as a diagnostic specifier in DSM5. On a lifetime basis, tics have been reported to co-occur in 22%–44% of subjects with OCD, and inversely, the 20%–40% of subjects with tic disorders have OCD.215 Subjects with OCD and comorbid tics exhibit earlier age of onset, male preponderance, greater likelihood of family members also having OCD, chronic course of symptoms and a poorer response to treatment.216 Furthermore, subjects with OCD, and particularly those with tic disorder, are more likely to have comorbid conditions characterized by abnormal motility such as attention deficit hyperactivity disorder (ADHD),217 ASD,218 and basal ganglia disorders.219,220
Subjects with OCD and parkinsonism differ from those without parkinsonism in having more severe compulsions, lower IQ scores and poorer cognitive performance.214 A subgroup of severely impaired OCD subjects present obsessional slowness,221,222 a concept highly overlapping with parkinsonism.223,224 The majority of subjects with obsessional slowness (76%) also present with a broad range of mild catatonia-like signs, EPS and NSS.222 Subjects with OCD consistently exhibit lower NSS ratings than those with schizophrenia,66,76,84 but higher ratings than their first-degree relatives225 and healthy controls.66,224,226
Autism Spectrum Disorders
As a typical neurodevelopmental disorder, the majority of children with ASD exhibit varying degrees of MAs. The association of ASD with psychosis and catatonia has long been recognized,227,228 since they share many abnormal patterns of movement. In their study of 117 cases of catatonic schizophrenia in children, Leonhard held that they “correspond to many of the autistic children studied by Kanner”.229 Based on this overlap, some modern authors conceptualize catatonia in ASDs as a deterioration of the previous level of motor behavior or the emergence of “new” motor signs.45,230,231 Although up to 20% of subjects with ASD develop a catatonia syndrome,231 this syndrome is poorly recognized in the clinical practice as there is a general bias to diagnose catatonia in its severe form.231 For example, Wing and Shah45 examined 28 catatonia-like behaviors and reported that the lifetime prevalence of at least 1 motor sign in subjects with ASDs, learning disabilities and typically developing children was 100%, 93%, and 33%, respectively. The finding of catatonia signs in typically developing children is of interest and may reflect age-dependent reversible developmental traits. Levels of catatonia in ASD are inversely related to IQ,45,230–232 a finding in line with the frequently reported association between catatonia and intellectual disability.233–236
The only studies examining EPS in autism reported prevalence rates of 25% for parkinsonism237 and 18% for dyskinesias.238 Levels of NSS in ASD are similar to those reported in early-onset schizophrenia,82,83 and are related to low IQ or cognitive impairment.239–241
Alzheimer’s Disease
A broad range of catatonia-like signs, which are generally described as neurological or EPS, have been extensively documented in AD.242,243 However, these MAs have been often poorly described,244 and no single study has addressed the prevalence of catatonia in AD. Nevertheless, factor-analytical studies of the neuropsychiatric inventory, which comprises some catatonia-like signs within the “aberrant behavior” item, have consistently identified a psychomotor factor, which has been reported to be as clinically significant in 11% of newly diagnosed subjects with AD245 and in 32% of subjects with varying severity of the disorder246; furthermore, this factor has been related to poor functional outcome, rapidity of evolution and severity of AD.245 On the other hand, in the neurological literature, the single most recognized catatonic feature in AD is paratonia (gegenhalten), which was first described by Dupré247 in subjects with intellectual disability and afterwards by Kleist in dementia.89 Paratonia usually occurs with other catatonia signs such as automatic obedience, motor perseveration, echopraxia and frontal release signs.248 Paratonia has been found in 10% of early stages and in 90% of late stages of AD,249 and it is a robust independent indicator of severity and progression of the illness.250 Thus, if we consider aberrant motor behavior and paratonia as proxy indicators of catatonia, the prevalence of a catatonia syndrome in AD appears to be substantial and of most clinical relevance.
Although cognitive impairment is the signature feature of AD, EPS are extremely common with a prevalence rate ranging from 12% in mild stages251 up to 92% in severe stages,252 with parkinsonism being much more prevalent than dyskinesias.253 Furthermore, mild parkinsonism in the elderly has been reported as a risk factor for developing dementia.254 Based on the presence or absence of EPS 2 AD subphenotypes can be recognized: “cognitive/pure” and “cognitive/motor,” mirroring the classification of Parkinson disease (PD) into “motor” and “motor/cognitive” forms. Such distinction is extremely important, as individuals with AD and EPS show a faster course of the disorder.253,255 Furthermore, EPS share a pattern of presentation similar to that seen in PD, suggesting common pathogenic mechanisms across the 2 neurodegenerative disorders.256
There are few studies of NSS in AD, but they consistently show that AD exhibit higher NSS compared with mild cognitive impairment257 and healthy old controls.257,258 Furthermore, NSS appear to increase with progression of AD and cognitive deterioration.258
Discussion
This revision raised some relevant findings that could eventually inform the transdiagnostic issue of MAs in psychiatric disorders. First, MAs represent an overarching concept entailing inter-related motor domains, which in turn can be further differentiated into several subdomains. Of particular concern was the lack of a consistent syndromic structure of catatonia beyond the excitement and retarded factors, which poorly account for the multidimensional structure of this motor domain. Given that highly differentiated symptoms tend to display a hierarchical arrangement,259,260 likely the catatonia syndrome may be viewed as a higher-order dimension with 2 middle-order dimensions and various lower-order dimensions that are close to the item level. This view appears to fit well how catatonia is currently approached, since on the one side, it is considered as an unspecific syndrome with similar phenomenological presentation in psychiatric, medical and neurological conditions15,261–263; and on the other side, clinical lore and distribution of signs across diagnoses indicates that lower-order dimensions are to some extent disorder-specific.
Second, one or more motor domains had a substantial prevalence across all the examined diagnoses. Notwithstanding this, prevalence rates are highly contingent on the methodological issues described above. Catatonic signs had a substantial prevalence in all diagnoses excepting OCD, a diagnosis in which they have been poorly examined. Particularly high rates of catatonic signs were observed across all classes of psychotic disorders, although enduring signs were mainly confined to schizophrenia; this suggesting that non-enduring catatonia signs are a hallmark transdiagnostic feature of psychotic illness, while enduring catatonic signs are specific to schizophrenia. EPS had a substantial prevalence in disorders of neurodevelopmental or neurodegenerative origin; and NSS showed similar high levels across diagnoses excepting OCD and depression. The transdiagnostic prevalence of MAs is in a small part definitional since only catatonic signs are included in the diagnostic criteria of psychotic and ASD.
Third, MAs were a severity marker of either the illness episode or the disorder. In “functional” disorders, MAs, and particularly catatonia, appear to be markers of episode severity. In neurodevelopmental and neurodegenerative (ie, chronic) disorders, but with different degree of strength or evidence, all motor domains were indicators of both illness severity and poor outcome. Dementia is an interesting case in relation to transdiagnostic mechanisms, since it has an established neuropathology. In AD, abnormal motility in addition to being a marker of severity was also a marker of illness progression.
A subordinate but interesting finding was that one or more motor domains were consistently related with poor cognition in neurodevelopmental disorders; furthermore, in schizophrenia both motor and cognitive dysfunctions appear to be stable traits long before illness onset. Motor and cognitive dysfunctions are also inextricably linked in AD and other widespread neurodegenerative disorders such as PD256; thus, it could be argued that the 2 domains are intimately related in both neurodevelopmental and neurodegenerative disorders, which further support the transdiagnostic character of MAs. Interestingly, in schizophrenia, dyskinesias may appear long before illness onset as a manifestation of deviant neurodevelopment125–128 (see Schiffman, this issue), and they increase with age,55 particularly after age 65,264 this suggesting that for this particular motor domain 2 overlapping and age-dependent neurobiological mechanisms are possible.
Although not object of this review, some etiological, therapeutic and neurobiological questions can also illustrate the transdiagnostic issue of MAs. Regarding familial-genetic factors, catatonic schizophrenia appears to exhibit higher familial loading of psychotic disorders than noncatatonic schizophrenia,265,266 and Leonhard’s periodic catatonia has been consistently considered as highly familial5,97 with a morbidity risk of 26.9% and major gene effect and anticipation.267 As showed in this review, compared to healthy controls, higher levels of NSS have been found in the first-degree relatives of subjects with schizophrenia, BD, and OCD, while in the other diagnoses there was a lack of studies thereof. Thus, familiality of catatonia may be disorder-specific, while that of NSS cut-across several diagnoses. Regarding treatment, lorazepam and electroconvulsive therapy are highly effective in treating acute catatonia regardless the underlying etiology, with response rates of 70%–90%.268,269 Although these treatments appear to be somewhat less effective in neurodevelopmental and medical conditions,270 their overall effectiveness for catatonia is one of the major arguments for considering this syndrome transdiagnostically.
Lastly, given the transdiagnostic character of MAs in most of the realms addressed in this review, it could be argued that they share abnormal brain circuitry functioning involving the motor system, which may be viewed as common pathway of the various motor loop networks related to different disorders and brain mechanisms. In this regard, some plausible models of brain dysfunction underlying motor domains have been proposed271–273 (also see Pantelis and Mittal et al, this issue). For example, Northoff,271 attempted to explain catatonic and extrapyramidal signs by integrating dysfunction of brain circuitry and neurotransmission; to explain catatonia, he suggested a deranged “top-down” modulation of cortical-subcortical connections (as reflected, eg, in the modulation of basal ganglia by lateral orbito-frontal cortex) related to cortical GABAergic-mediated dysfunction; and to explain EPS, he suggested a “bottom-up” modulation of cortical-subcortical connections related to basal ganglia dopamine dysfunction. More recently, Hiryak272 developed a 3-stage severity model of catatonic, extrapyramidal and neurological signs in schizophrenia on the basis of a graded dysfunction of the cortico-cerebelar-thalamo-cortical loop. The 2 models appear to work well across the 3 neurobiological mechanisms involved in the diagnoses examined: stable dysfunction in neurodevelopmental disorders, reversible dysfunction in functional disorders, and progressive dysfunction in AD.
Limitations
Several limitations apply to the present study. First, while our study covered the major clinical domains of MAs observed in psychiatric disorders, we excluded studies using instrumental measures274,275 and experimental paradigms276,277 of motor dysfunction. These measures and paradigms are increasingly used to disentangle mechanisms underlying abnormal motility and ideally they should be used together with clinical ratings of MAs. Second, some disorders defined by abnormal motility such as ADHD and tic disorders were not included in this review; these diagnoses, however, are important targets for transdiagnostic research that should be examined in future studies. Third, we largely relied on articles focused on specific disorders, which had a highly variable methodology; therefore, some subjectivity in selecting the most informative articles cannot be excluded; furthermore, the range of MAs-related features reviewed here was necessarily limited and highly dependent on the quality of individual studies. Fourth, in analyzing the existing data we mainly focused on general measures of catatonic, extrapyramidal and neurological signs, and it is possible that focusing on more specific dimensions of MAs might reveal a somewhat different pattern of MAs-related features across disorders. Lastly, the studies reviewed adopted widely varying measures and designs, and further progress in transdiagnostic comparison of MAs arguably requires consistency of measures across the groups studied.
Conclusions and Future Directions
Despite their clinical and neurobiological relevance, MAs continue to be a neglected area in clinical practice and research. Domains of MAs cut-across neurodevelopmental, “functional” and neurodegenerative disorders, although with different expressivity or prevalence. They are markers of episode or illness severity across diagnoses, and in AD are also indicators of illness progression. MAs appear to represent a true transdiagnostic domain putatively sharing neurobiological mechanisms of neurodevelopmental, functional or neurodegenerative origin, this being a strong argument for studying MAs on their own, irrespective of diagnostic categories. MAs are closely tied to neurobiological mechanisms, and thus, they are a window to the brain mechanisms of psychiatric and neuropsychiatric disorders, but we know virtually nothing about the shared and disorder-specific mechanisms. Thus, unraveling these mechanisms may share light on the nature of both motor dysfunction and the underlying diagnoses.
Given the complexities surrounding the definition and assessment of abnormal motility in psychiatry, we first need a unified theory and assessment tool for MAs, and the 3 motor domains should be examined concurrently along with instrumental measures of motor function. The ideal methodological requirements would be the prospective, independent screening and evaluation of MAs in a large sample with mixed diagnoses, coupled with risk factors, neurobiological measures, treatment response, and follow-up studies. This endeavor is undoubtedly arduous and may seem insurmountable; however, it appears to be a necessary step for unraveling the transdiagnostic and disorder-specific features of motor dysfunction in (neuro)psychiatric disorders. Indeed, MAs fit good the criteria for RDoC277 (also see Garvey and Cuthbert and Mittal et al, this issue); thus, they should be incorporated as an additional RDoC domain, which undoubtedly would boost knowledge of that under-researched clinical phenotype.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by Instituto de Salud Carlos III (PI16/02148).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotov R, Watson D, Bagby RM et al. The hierarchical taxonomy of psychopathology (HiTOP): adimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126:454–477. [DOI] [PubMed] [Google Scholar]
- 3. Wigman JT, de Vos S, Wichers M, van Os J, Bartels-Velthuis AA. A transdiagnostic network approach to psychosis. Schizophr Bull. 2017;43:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 5. Leonhard K. The classification of endogenous psychoses. Translated by R, Berman (1979). New York, NY: Irvington Publishers Inc; 1957. [Google Scholar]
- 6. McNeil TF, Cantor-Graae E. Neuromotor markers of risk for schizophrenia. Aust N Z J Psychiatry. 2000;34:65–73. [DOI] [PubMed] [Google Scholar]
- 7. Wolff AL, O’Driscoll GA. Motor deficits and schizophrenia: the evidence from neuroleptic-naïve patients and populations at risk. J Psychiatry Neurosci. 1999;24:304–314. [PMC free article] [PubMed] [Google Scholar]
- 8. Peralta V, Cuesta MJ, Serrano JF, Martinez-Larrea JA. Classification issues in catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 1):14–66. [DOI] [PubMed] [Google Scholar]
- 9. Pfuhlmann B, Stöber G. The different conceptions of catatonia: historical overview and critical discussion. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 1):4–7. [DOI] [PubMed] [Google Scholar]
- 10. Steck H. Les syndromes extrapiramidaus dans les maladies mentales. Arch Suiss Neurol Psychiatr. 1926;19:195–233. [Google Scholar]
- 11. Kleist K. Die Katatonien. Nervenarzt. 1943;16:1–10. [Google Scholar]
- 12. Torrey EF. Studies of individuals with schizophrenia never treated with antipsychotic medications: a review. Schizophr Res. 2002;58:101–115. [DOI] [PubMed] [Google Scholar]
- 13. Blumer D. Catatonia and the neuroleptics: psychobiologic significance of remote and recent findings. Compr Psychiatry. 1997;38:193–201. [DOI] [PubMed] [Google Scholar]
- 14. Ungvary GS, Kau LS, Wai-Kwong T, Shing NF. The pharmacological treatment of catatonia: an overview. Eur Arch Psychiatry Clin Neurosci. 2001;251 (suppl 1):31–34. [DOI] [PubMed] [Google Scholar]
- 15. Fink M, Taylor MA.. Catatonia. A clinicians Guide to Diagnosis and Treatment.Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- 16. Lohr JB, Wisniewski AA.. Movement disorders. A neuropsychiatric approach.New York, NY: The Guilford Press; 1987. [Google Scholar]
- 17. Ungvari GS, Caroff SN, Gerevich J. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophr Bull. 2010;36:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 19. Bernard JA, Mittal VA. Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 2015;45:2685–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carroff SN, Mann SC, Francis A, Fricchione GL.. Catatonia, from Psychopathology to Neurobiology.Washington, DC: American Psychiatric Publishing, Inc; 2004. [Google Scholar]
- 21. Northoff G. Katatonie Einfürung in die Phänomenologie, Klinik und Pathophysiologie eines psychomotorischen Syndroms.Stuttgart, Germany: Enke; 1997. [Google Scholar]
- 22. Ha AD, Jankovic J. An introduction to dyskinesia–the clinical spectrum. Int Rev Neurobiol. 2011;98:1–29. [DOI] [PubMed] [Google Scholar]
- 23. Fahn S. Hypokinesia and hyperkinesia. In Goetz C, Pappert EJ, Schmitt B, eds. Textbook of Clinical Neurology. New York, NY: Saunders Company; 1999:266–284. [Google Scholar]
- 24. Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145:11–18. [DOI] [PubMed] [Google Scholar]
- 25. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 26. Ungvari GS. Catatonia in DSM 5: controversies regarding its psychopathology, clinical presentation and treatment response. Neuropsychopharmacol Hung. 2014;16:189–194. [PubMed] [Google Scholar]
- 27. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57:524–537. [DOI] [PubMed] [Google Scholar]
- 28. Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: assessment using the modified rogers scale. Br J Psychiatry. 1991;158:323–327, 333. [DOI] [PubMed] [Google Scholar]
- 29. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. [DOI] [PubMed] [Google Scholar]
- 30. Northoff G, Koch A, Wenke J et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14:404–416. [DOI] [PubMed] [Google Scholar]
- 31. Bräunig P, Krüger S, Shugar G, Höffler J, Börner I. The catatonia rating scale I–development, reliability, and use. Compr Psychiatry. 2000;41:147–158. [DOI] [PubMed] [Google Scholar]
- 32. Carroll BT, Kirkhart R, Ahuja N et al. Katatonia: a new conceptual understanding of catatonia and a new rating scale. Psychiatry (Edgmont). 2008;5:42–50. [PMC free article] [PubMed] [Google Scholar]
- 33. Benarous X, Consoli A, Raffin M et al. Validation of the pediatric catatonia rating scale (PCRS). Schizophr Res. 2016;176:378–386. [DOI] [PubMed] [Google Scholar]
- 34. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry. 1990;51:357–362. [PubMed] [Google Scholar]
- 35. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86:825–832. [DOI] [PubMed] [Google Scholar]
- 36. Chen EY, Shapleske J, Luque R et al. The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res. 1995;56:183–204. [DOI] [PubMed] [Google Scholar]
- 37. Schröder J, Niethammer R, Geider FJ et al. Neurological soft signs in schizophrenia. Schizophr Res. 1991;6:25–30. [DOI] [PubMed] [Google Scholar]
- 38. Merriam AE, Kay SR, Opler LA, Kushner SF, van Praag HM. Neurological signs and the positive-negative dimension in schizophrenia. Biol Psychiatry. 1990;28:181–192. [DOI] [PubMed] [Google Scholar]
- 39. Krebs MO, Gut-Fayand A, Bourdel M, Dischamp J, Olié J. Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res. 2000;45:245–260. [DOI] [PubMed] [Google Scholar]
- 40. Rossi A, De Cataldo S, Di Michele V et al. Neurological soft signs in schizophrenia. Br J Psychiatry. 1990;157:735–739. [DOI] [PubMed] [Google Scholar]
- 41. Rogers D. Motor disorder in psychiatry. Towards a neurological psychiatry. Chichester, UK: John Wiley & Sons; 1992. [Google Scholar]
- 42. Kahlbaum K. Catatonia. Translated by Levij Y and Pridon T (1973). Baltimore, MD: Johns Hopkins University Press; 1874. [Google Scholar]
- 43. Kraepelin E. Dementia praecox and Paraphrenia. Translated by RM, Barklay (1971). Edinburgh, UK: E. & S. Livingstone; 1919. [Google Scholar]
- 44. Bleuler E. Dementia Praecox or the Group of Schizophrenias. Translated by Zinkin J. (1950). New York, NY: International University Press; 1908. [Google Scholar]
- 45. Wing L, Shah A. A systematic examination of catatonia-like clinical pictures in autism spectrum disorders. Int Rev Neurobiol. 2006;72:21–39. [DOI] [PubMed] [Google Scholar]
- 46. Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164:256–262. [DOI] [PubMed] [Google Scholar]
- 47. Peralta V, Cuesta MJ. Motor features in psychotic disorders. II. Development of diagnostic criteria for catatonia. Schizophr Res. 2001;47:117–126. [DOI] [PubMed] [Google Scholar]
- 48. Pfuhlmann B, Stöber G. The importance of a differentiated psychopathology of catatonia. Acta Psychiatr Scand. 1997;95:357–359. [DOI] [PubMed] [Google Scholar]
- 49. Ungvary GS, Chow LY, Leung HCM, Lau BST. Rating catatonia: discrepancy between cross-sectional and longitudinal assessment. Rev Psiq Clín. 1999;26:56–61. [Google Scholar]
- 50. Steck H. The syndrome extrapyramidal et diencephalique au cours des traitments au largactil et ou serpasil. Ann Med Psychol. 1954;112:737–743. [PubMed] [Google Scholar]
- 51. Owens CO. A Guide to the Extrapyramidal Side-Effects of Antipsychotic Drugs.Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 52. Ismail B, Cantor-Graae E, McNeil TF. Neurodevelopmental origins of tardivelike dyskinesia in schizophrenia patients and their siblings. Schizophr Bull. 2001;27:629–641. [DOI] [PubMed] [Google Scholar]
- 53. Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011;52:139–145. [DOI] [PubMed] [Google Scholar]
- 54. Tenback DE, van Harten PN, van Os J. Non-therapeutic risk factors for onset of tardive dyskinesia in schizophrenia: a meta-analysis. Mov Disord. 2009;24:2309–2315. [DOI] [PubMed] [Google Scholar]
- 55. Fenton WS, Blyler CR, Wyatt RJ, McGlashan TH. Prevalence of spontaneous dyskinesia in schizophrenic and non-schizophrenic psychiatric patients. Br J Psychiatry. 1997;171:265–268. [DOI] [PubMed] [Google Scholar]
- 56. Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naïve patients with schizophrenia: a systematic review. Psychol Med. 2009;39:1065–1076. [DOI] [PubMed] [Google Scholar]
- 57. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peralta V, Cuesta MJ. The effect of antipsychotic medication on neuromotor abnormalities in neuroleptic-naive nonaffective psychotic patients: a naturalistic study with haloperidol, risperidone, or olanzapine. Prim Care Companion J Clin Psychiatry. 2010;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cuesta MJ, Campos MS, García-Jalón E, Sánchez-Torres AM, Peralta V. Treatment response of neurological soft signs in drug-naïve patients with a first psychotic episode. Schizophr Res. 2012;139:144–150. [DOI] [PubMed] [Google Scholar]
- 60. Rogers D. Neuropsychiatry of movement disorders. Curr Op Psychiatry. 1992;5:84–87. [Google Scholar]
- 61. Chakos MH, Alvir JM, Woerner MG et al. Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Arch Gen Psychiatry. 1996;53:313–319. [DOI] [PubMed] [Google Scholar]
- 62. Bonner CA, Kent GH. Overlapping symptoms in catatonic excitement and manic excitement. Am J Psychiatry. 1936;92:1311–1322. [Google Scholar]
- 63. Abrams R, Taylor MA, Coleman Stolurow KA. Catatonia and mania: patterns of cerebral dysfunction. Biol Psychiatry. 1979;14:111–117. [PubMed] [Google Scholar]
- 64. Ungvari GS, Leung HC, Lee TS. Benzodiazepines and the psychopathology of catatonia. Pharmacopsychiatry. 1994;27:242–245. [DOI] [PubMed] [Google Scholar]
- 65. Oulis P, Lykouras L, Tomaras V, Panayotopoulou V, Gournellis R, Stefanis C. DSM-IV catatonic features among psychiatric inpatients: a preliminary study. Eur Psychiatry. 1997;12:412–414. [DOI] [PubMed] [Google Scholar]
- 66. Bolton D, Gibb W, Lees A et al. Neurological soft signs in obsessive compulsive disorder: standardised assessment and comparison with schizophrenia. Behav Neurol. 1998;11:197–204. [DOI] [PubMed] [Google Scholar]
- 67. Krishna KR, Maniar RC, Harbishettar VS. A comparative study of “Idiopathic catatonia” with catatonia in schizophrenia. Asian J Psychiatr. 2011;4:129–133. [DOI] [PubMed] [Google Scholar]
- 68. Krüger S, Cooke RG, Spegg CC, Bräunig P. Relevance of the catatonic syndrome to the mixed manic episode. J Affect Disord. 2003;74:279–285. [DOI] [PubMed] [Google Scholar]
- 69. Boks MP, Liddle PF, Burgerhof JG, Knegtering R, van den Bosch RJ. Neurological soft signs discriminating mood disorders from first episode schizophrenia. Acta Psychiatr Scand. 2004;110:29–35. [DOI] [PubMed] [Google Scholar]
- 70. Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010;118:168–175. [DOI] [PubMed] [Google Scholar]
- 71. Usman DM, Olubunmi OA, Taiwo O, Taiwo A, Rahman L, Oladipo A. Comparison of catatonia presentation in patients with schizophrenia and mood disorders in lagos, Nigeria. Iran J Psychiatry. 2011;6:7–11. [PMC free article] [PubMed] [Google Scholar]
- 72. Grover S, Chakrabarti S, Ghormode D, Agarwal M, Sharma A, Avasthi A. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229:919–925. [DOI] [PubMed] [Google Scholar]
- 73. Manschreck TC, Maher BA, Rucklos ME, Vereen DR. Disturbed voluntary motor activity in schizophrenic disorder. Psychol Med. 1982;12:73–84. [DOI] [PubMed] [Google Scholar]
- 74. Dazzan P, Lloyd T, Morgan KD et al. Neurological abnormalities and cognitive ability in first-episode psychosis. Br J Psychiatry. 2008;193:197–202. [DOI] [PubMed] [Google Scholar]
- 75. Sweet RA, Akil M, Mulsant BH, Ulrich R, Pasternak RE, Zubenko GS. Determinants of spontaneous extrapyramidal symptoms in elderly psychiatric inpatients diagnosed with Alzheimer’s disease, major depressive disorder, or psychotic disorders. J Neuropsychiatry Clin Neurosci. 1998;10:68–77. [DOI] [PubMed] [Google Scholar]
- 76. Jaafari N, Baup N, Bourdel MC et al. Neurological soft signs in OCD patients with early age at onset, versus patients with schizophrenia and healthy subjects. J Neuropsychiatry Clin Neurosci. 2011;23:409–416. [DOI] [PubMed] [Google Scholar]
- 77. Owoeye O, Kingston T, Scully PJ et al. Epidemiological and clinical characterization following a first psychotic episode in major depressive disorder: comparisons with schizophrenia and bipolar I disorder in the Cavan-Monaghan First Episode Psychosis Study (CAMFEPS). Schizophr Bull. 2013;39:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Manschreck TC, Ames D. Neurologic features and psychopathology in schizophrenic disorders. Biol Psychiatry. 1984;19:703–719. [PubMed] [Google Scholar]
- 79. Cox SM, Ludwig AM. Neurological soft signs and psychopathology: incidence in diagnostic groups. Can J Psychiatry. 1979;24:668–673. [DOI] [PubMed] [Google Scholar]
- 80. Keshavan MS, Sanders RD, Sweeney JA et al. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am J Psychiatry. 2003;160:1298–1304. [DOI] [PubMed] [Google Scholar]
- 81. Whitty P, Clarke M, McTigue O et al. Diagnostic specificity and predictors of neurological soft signs in schizophrenia, bipolar disorder and other psychoses over the first 4 years of illness. Schizophr Res. 2006;86:110–117. [DOI] [PubMed] [Google Scholar]
- 82. Mayoral M, Merchán-Naranjo J, Rapado M et al. Neurological soft signs in juvenile patients with Asperger syndrome, early-onset psychosis, and healthy controls. Early Interv Psychiatry. 2010;4:283–290. [DOI] [PubMed] [Google Scholar]
- 83. Hirjak D, Wolf RC, Koch SC et al. Neurological abnormalities in recent-onset schizophrenia and asperger-syndrome. Front Psychiatry. 2014;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tripathi R, Soni A, Tyagi A, Mehta S, Gupta S. Comparative study of neurological soft signs in patients with schizophrenia or obsessive-compulsive disorder, and healthy controls. East Asian Arch Psychiatry. 2015;25:64–72. [PubMed] [Google Scholar]
- 85. Chrobak AA, Siwek GP, Siuda-Krzywicka K et al. Neurological and cerebellar soft signs do not discriminate schizophrenia from bipolar disorder patients. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:96–101. [DOI] [PubMed] [Google Scholar]
- 86. Bostroem A. Katatone Störungen. In: O., Bumke, ed. Handbuch der Geisteskankheiren.Vol. II Berlin, Germany: Springer; 1928:134–205. [Google Scholar]
- 87. Bleuler E. Zur Theorie des schizophrenen Negati vismus. Psychiatrisch-Neurologische Wochenschrift. 1910–1911; 171–176,184-187,189-191,195-198. [Google Scholar]
- 88. Giraud P. Conception neurologique du syndrome catatonique. Encephale. 1924;19:571–579. [Google Scholar]
- 89. Kleist K. Gegenhalten (motorisher Negativismus), Zwangsgreifen und Thalamus Opticus. Monatschr Psychiat Neurol. 1927;65:317–396. [Google Scholar]
- 90. Spoerri T. Motorische Schablonen und Stereotypien bei schizophrenen Endzustanden. Psychiat Neurol Basel. 1967;153:81–127. [Google Scholar]
- 91. Pauleikhoff B. Die Katatonie (1868–1968). Fortschr Neurol Psychiat. 1969;37:461–496. [PubMed] [Google Scholar]
- 92. Ungvari GS. The Wernicke-Kleist-Leonhard school of psychiatry. Biol Psychiatry. 1993;34:749–752. [DOI] [PubMed] [Google Scholar]
- 93. Fontenelle LF, Mendlowicz MV. The Wernicke-Kleist-Leonhard “short-circuiting”: a missing link between attention deficit hyperactivity disorder, Tourette syndrome, and obsessive-compulsive disorder? Med Hypotheses. 2008;71:418–425. [DOI] [PubMed] [Google Scholar]
- 94. Solé-Sagarra J, Leonhard K.. Manual de Psiquiatría.Madrid, Spain: Ediciones Morata; 1953. [Google Scholar]
- 95. Fish F, Astrup C. The classification of chronic schizophrenia. A follow-up study. Folia Psychiatr Neurol Jpn. 1964;18:17–23. [DOI] [PubMed] [Google Scholar]
- 96. Gjessing LR. A review of periodic catatonia. Biol Psychiatry. 1974;8:23–45. [PubMed] [Google Scholar]
- 97. Franzek E, Beckmann H. Different genetic background of schizophrenia spectrum psychoses: a twin study. Am J Psychiatry. 1998;155:76–83. [DOI] [PubMed] [Google Scholar]
- 98. Meyer J, Huberth A, Ortega G et al. A missense mutation in a novel gene encoding a putative cation channel is associated with catatonic schizophrenia in a large pedigree. Mol Psychiatry. 2001;6:302–306. [DOI] [PubMed] [Google Scholar]
- 99. Stöber G, Saar K, Rüschendorf F et al. Splitting schizophrenia: periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet. 2000;67:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Beckmann H, Neumarker KJ.. Endogenous psychoses. Leonhard’s impact on modern psychiatry.Berlin, Germany: Ullstein Mosby; 1995. [Google Scholar]
- 101. Peralta V, Goldberg X, Ribeiro M, Sanchez-Torres AM, Fañanás L, Cuesta MJ. Familiality of psychotic disorders: a polynosologic study in multiplex families. Schizophr Bull. 2016;42:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Guy W. Manual for the classification of endogenous psychoses. 1979. International Network for the History of Neuropsychopharmacology. 2014. http://inhn.org/archives/guy-collection/manual-for-the-leonhard-classification-of-endogenous-psychoses.html. [Google Scholar]
- 103. Pethö B, Ban TA. DCR Budapest-Nashville in the diagnosis and classification of functional psychoses. Psychopathology. 1988;21:149–240. [DOI] [PubMed] [Google Scholar]
- 104. Fritze J, Lanzik M. Schedule for operationalized diagnosis according to the Leonhard classification of endogenous psychoses. Psychopathology. 1990;23:303–315. [DOI] [PubMed] [Google Scholar]
- 105. Peralta V, Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophr Res. 2001;49:269–285. [DOI] [PubMed] [Google Scholar]
- 106. McKenna PJ, Lund CE, Mortimer AM, Biggins CA. Motor, volitional and behavioural disorders in schizophrenia 2: the ‘conflict of paradigms’ hypothesis. Br J Psychiatry. 1991;158:328–336. [DOI] [PubMed] [Google Scholar]
- 107. Parker G, Hadzi-Pavlovic D, Brodaty H et al. Psychomotor disturbance in depression: defining the constructs. J Affect Dis. 1993;27:255–265. [DOI] [PubMed] [Google Scholar]
- 108. Starkstein SE, Petracca G, Tessón A et al. Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiat. 1996;60:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hoeffler J, Braeunig P, Boerner I, Krueger S. Factor-Analysis of Catatonic Schizophrenia. Proceedings and summary. American Psychiatric Association annual meeting May 30–June 4, 1998: 204–205. [Google Scholar]
- 110. Peralta V, Cuesta MJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr Res. 2001a;47:107–116. [DOI] [PubMed] [Google Scholar]
- 111. Krüger S, Bagby RM, Höffler J, Bräunig P. Factor analysis of the catatonia rating scale and catatonic symptom distribution across four diagnostic groups. Compr Psychiatry. 2003;44:472–482. [DOI] [PubMed] [Google Scholar]
- 112. Ungvari GS, Goggins W, Leung S-K, Gerevich J. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’) II. Factor analysis of the catatonic syndrome. Prog Neuropsychopharm Biol Psychiatry. 2007;31:462–468. [DOI] [PubMed] [Google Scholar]
- 113. Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010;118:168–175. [DOI] [PubMed] [Google Scholar]
- 114. Stuivenga M, Morrens M. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry. 2014;5:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164:256–262. [DOI] [PubMed] [Google Scholar]
- 116. Peralta V, de Jalón EG, Campos MS, Basterra V, Sanchez-Torres A, Cuesta MJ. Risk factors, pre-morbid functioning and episode correlates of neurological soft signs in drug-naive patients with schizophrenia-spectrum disorders. Psychol Med. 2011;41:1279–1289. [DOI] [PubMed] [Google Scholar]
- 117. Ojagbemi A, Akpa O, Esan O, Emsley R, Gureje O. The confirmatory factor structure of neurological soft signs in Nigerians with first episode schizophrenia. Neurosci Lett. 2015;589:110–114. [DOI] [PubMed] [Google Scholar]
- 118. Dazzan P, Murray RM. Neurological soft signs in first episode psychosis: a systematic review. Br J Psychiatry. 2002;43:50–57. [DOI] [PubMed] [Google Scholar]
- 119. Dazzan P, Morgan KD, Orr KG et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–153. [DOI] [PubMed] [Google Scholar]
- 120. Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull. 2005;31:962–977. [DOI] [PubMed] [Google Scholar]
- 121. Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ungvari GS, Leung S-K, Shing F, Cheung H-K, Leung T. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharm Biol Psychiatry. 2005;29:27–38. [DOI] [PubMed] [Google Scholar]
- 123. Docx L, Morrens M, Bervoets C et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126:256–265. [DOI] [PubMed] [Google Scholar]
- 124. Chen EY, Kwok CL, Au JW, Chen RY, Lau BS. Progressive deterioration of soft neurological signs in chronic schizophrenic patients. Acta Psychiatr Scand. 2000;102:342–349. [DOI] [PubMed] [Google Scholar]
- 125. Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. [DOI] [PubMed] [Google Scholar]
- 126. Walker E, Lewis N, Loewy R, Palyo S. Motor dysfunction and risk for schizophrenia. Dev Psychopathol. 1999;11:509–523. [DOI] [PubMed] [Google Scholar]
- 127. Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, Cannon TD. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophr Bull. 2000;26:395–410. [DOI] [PubMed] [Google Scholar]
- 128. Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. Am J Psychiatry. 2004;161:2021–2027. [DOI] [PubMed] [Google Scholar]
- 129. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dazzan P, Griffiths TD. Neurological abnormalities in patients with schizophrenia from singly- and multiply-affected families and their relatives. Chapter Six. In: McDonald, ed. The Maudsley Family Study of Psychosis: A Quest for Intermediate Phenotypes (Maudsley Series).Hove and New York: Psychology Press, Taylor and Francis; 2008:133–154. [Google Scholar]
- 131. Hameed MA, Lewis AJ. Offspring of parents with schizophrenia: a systematic review of developmental features across childhood. Harv Rev Psychiatry. 2016;24:104–117. [DOI] [PubMed] [Google Scholar]
- 132. Cioni G, Sgandurra G. Normal psychomotor development. Handb Clin Neurol. 2013;111:3–15. [DOI] [PubMed] [Google Scholar]
- 133. Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. [DOI] [PubMed] [Google Scholar]
- 134. Marín O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med. 2016;22:1229–1238. [DOI] [PubMed] [Google Scholar]
- 135. Shaffer D, Schonfeld I, O’Connor PA et al. Neurological soft signs. Their relationship to psychiatric disorder and intelligence in childhood and adolescence. Arch Gen Psychiatry. 1985;42:342–351. [DOI] [PubMed] [Google Scholar]
- 136. Martins I, Lauterbach M, Slade P et al. A longitudinal study of neurological soft signs from late childhood into early adulthood. Develop Med Child Neurol. 2008;50:602–607. [DOI] [PubMed] [Google Scholar]
- 137. Fellick JM, Thomson AP, Sills J, Hart CA. Neurological soft signs in mainstream pupils. Arch Dis Child. 2001;85:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Fish B. Neurobiologic antecedents of schizophrenia in children. Evidence for an inherited, congenital neurointegrative defect. Arch Gen Psychiatry. 1977;34:1297–1313. [DOI] [PubMed] [Google Scholar]
- 139. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect. A review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–235. [DOI] [PubMed] [Google Scholar]
- 140. McNeil TF, Harty B, Blennow G, Cantor-Graae E. Neuromotor deviation in offspring of psychotic mothers: a selective developmental deficiency in two groups of children at heightened psychiatric risk? J Psychiatr Res. 1993;27:39–54. [DOI] [PubMed] [Google Scholar]
- 141. Mittal VA, Dean DJ, Bernard JA et al. Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull. 2014;40:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Burton BK, Thorup AA, Jepsen JR et al. Impairments of motor function among children with a familial risk of schizophrenia or bipolar disorder at 7 years old in Denmark: an observational cohort study. Lancet Psychiatry. 2017;4:400–408. [DOI] [PubMed] [Google Scholar]
- 143. Murray GK, Jones PB, Kuh D, Richards M. Infant developmental milestones and subsequent cognitive function. Ann Neurol. 2007;62:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. [DOI] [PubMed] [Google Scholar]
- 145. Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116:796–803. [DOI] [PubMed] [Google Scholar]
- 146. Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dickson H, Laurens KR, Cullen AE, Hodgins S Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42:743–755. [DOI] [PubMed] [Google Scholar]
- 148. Filatova S, Koivumaa-Honkanen H, Hirvonen N et al. Early motor developmental milestones and schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2017. doi:10.1016/j.schres.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 149. Walker E, Lewis N, Loewy R, Palyo S. Motor dysfunction and risk for schizophrenia. Dev Psychopathol. 1999;11:509–523. [DOI] [PubMed] [Google Scholar]
- 150. Barkus E, Stirling J, Hopkins R, Lewis S. The presence of neurological soft signs along the psychosis proneness continuum. Schizophr Bull. 2006;32:573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Barrantes-Vidal N, Fañanás L, Rosa A, Caparrós B, Dolors Riba M, Obiols JE. Neurocognitive, behavioural and neurodevelopmental correlates of schizotypy clusters in adolescents from the general population. Schizophr Res. 2003;61:293–302. [DOI] [PubMed] [Google Scholar]
- 152. Chan RC, Xu T, Heinrichs RW, Yu Y, Gong QY. Neurological soft signs in non psychotic first-degree relatives of patients with schizophrenia: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2009;34:889–896. [DOI] [PubMed] [Google Scholar]
- 153. Koning JPF, Diederik E, Tenback DE. Dyskinesia and parkinsonism in antipsychotic-naive patients with schizophrenia, first-degree relatives and healthy controls: a meta-analysis. Schizophr Bull. 2010;36:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Koning JP, Kahn RS, Tenback DE, van Schelven LJ, van Harten PN Movement disorders in nonpsychotic siblings of patients with nonaffective psychosis. Psychiatry Res. 2011;30:133–137. [DOI] [PubMed] [Google Scholar]
- 155. Chan RC, Xie W, Geng FL et al. Clinical utility and lifespan profiling of neurological soft signs in schizophrenia spectrum disorders. Schizophr Bull. 2016;42:560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Braff DL, Ryan J, Rissling AJ, Carpenter WT. Lack of use in the literature from the last 20 years supports dropping traditional schizophrenia subtypes from DSM-5 and ICD-11. Schizophr Bull. 2013;39:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Stompe T, Ortwein-Swodoba G, Ritter K, Marquart B, Schanda RH. The impact of diagnostic criteria on the prevalence of schizophrenia subtypes. Comp Psychiatry. 2005;46:433–439. [DOI] [PubMed] [Google Scholar]
- 158. Kendler KS. Phenomenology of schizophrenia and the representativeness of modern diagnostic criteria. JAMA Psychiatry. 2016;73:1082–1092. [DOI] [PubMed] [Google Scholar]
- 159. Winokur W, Tsuang MT.. The Natural History of Mania, Depression and Schizophrenia.Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 160. Jönsson SAT, Jonsson H. Outcome in untreated schizophrenia: a search for symptoms and traits with prognostic meaning in patients admitted to a mental hospital in the preneuroleptic era. Acta Psychiatr Scand. 1992;85:313–320. [DOI] [PubMed] [Google Scholar]
- 161. Bland RC. Predicting the outcome in schizophrenia. Can J Psychiatry. 1982;27:52–62. [DOI] [PubMed] [Google Scholar]
- 162. World Health Organization. Schizophrenia: an International Follow-up Study.New York, NY: John Wiley and Sons Inc; 1979. [Google Scholar]
- 163. van Os J, Fahy TA, Jones P et al. Psychopathological syndromes in the functional psychoses: associations with course and outcome. Psychol Med. 1996;26:161–176. [DOI] [PubMed] [Google Scholar]
- 164. McGlashan TH. The prediction of outcome in chronic schizophrenia. IV. The Chestnut follow-up study. Arch Gen Psychiatry. 1986;43:167–176. [DOI] [PubMed] [Google Scholar]
- 165. Pfhol B, Winokur G. The evolution of symptoms in institutionalized hebephrenic/catatonic schizophrenics. Br J Psychiatry. 1982;141:567–572. [DOI] [PubMed] [Google Scholar]
- 166. Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TR. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med. 1990;20:357–365. [DOI] [PubMed] [Google Scholar]
- 167. Kay SR, Kanofsky D, Lindenmayer JP, Opler LA. The changing presentation of catatonia. Am J Psychiatry. 1987;144:834–835. [DOI] [PubMed] [Google Scholar]
- 168. Peralta V, Moreno-Izco L, Sanchez-Torres A, García de Jalón E, Campos MS, Cuesta MJ. Characterization of the deficit syndrome in drug-naive schizophrenia patients: the role of spontaneous movement disorders and neurological soft signs. Schizophr Bull. 2014;40:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sachdev P, Hume F, Toohey P, Doutney C. Negative symptoms, cognitive dysfunction, tardive akathisia and tardive dyskinesia. Acta Psychiatr Scand. 1996;93:451–459. [DOI] [PubMed] [Google Scholar]
- 170. Owens DGC, Johnstone EC, Frith CD. Spontaneous involuntary disorders of movement. Their prevalence, severity, and distribution in chronic schizophrenics with and without treatment with neuroleptics. Arch Gen Psychiatry. 1982;39:452–461. [DOI] [PubMed] [Google Scholar]
- 171. Johnstone EC, Owens DGC, Frith CD, Leary J. Disabilities and circumstances of schizophrenic patients. A follow-up study. III. Clinical findings. Abnormalities of the mental state and movement disorder and their correlates. Br J Psychiatry. 1991;suppl 13:21–25. [PubMed] [Google Scholar]
- 172. Yarden PE, Discipio WJ. Abnormal movements and prognosis in schizophrenia. Am J Psychiatry. 1971;128:97–103. [DOI] [PubMed] [Google Scholar]
- 173. Manschreck TC, Keuthen NJ, Schneyer ML, Celada MT, Laugheri J, Collins P. Abnormal involuntary movements and chronic schizophrenic disorders. Biol Psychiatry. 1990;27:150–158. [DOI] [PubMed] [Google Scholar]
- 174. Bachmann S, Bottmer C, Schroder J. Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry. 2005;162:2337–2243. [DOI] [PubMed] [Google Scholar]
- 175. Prikryl R, Ceskova E, Kasparek T, Kucerova H. Neurological soft signs, clinical symptoms and treatment reactivity in patients suffering from first episode schizophrenia. J Psychiatr Res. 2006;40:141–146. [DOI] [PubMed] [Google Scholar]
- 176. Cuesta MJ, Campos MS, García-Jalón E, Sánchez-Torres AM, Peralta V. Treatment response of neurological soft signs in drug-naïve patients with a first psychotic episode. Schizophr Res. 2012;139:144–150. [DOI] [PubMed] [Google Scholar]
- 177. Bachmann S, Degen C, Geider FJ, Schröder J. Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Front Psychiatry. 2014;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Schröder J, Silvestri S, Bubeck B et al. D2 dopamine receptor up-regulation, treatment response, neurological soft signs, and extrapyramidal side effects in schizophrenia: a follow-up study with 123I-iodobenzamide single photon emission computed tomography in the drug-naive state and after neuroleptic treatment. Biol Psychiatry. 1998;43:660–665. [DOI] [PubMed] [Google Scholar]
- 179. Chan RC, Wang Y, Wang L et al. Neurological soft signs and their relationships to neurocognitive functions: a re-visit with the structural equation modeling design. PLoS One. 2009;4:e8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Cuesta MJ, Peralta V, Zarzuela A, Calvo R, García M, Serrano F. Neurological soft-signs in psychosis: threshold criteria for discriminating normal controls and for predicting cognitive impairment. Schizophr Res. 2002;58:263–271. [DOI] [PubMed] [Google Scholar]
- 181. Peralta V, Cuesta MJ, Serrano JF, Mata I. The Kahlbaum syndrome: a study of its clinical validity, nosological status, and relationship with schizophrenia and mood-disorder. Comp Psychiatry. 1997;38:61–67. [DOI] [PubMed] [Google Scholar]
- 182. Wernicke C. Grundniss der Psychiatrie in klinischen Vorlesungen.Leipzig, Germany: Thieme; 1990. [Google Scholar]
- 183. Kleist K. Die klinische Stellung der Motilitätspsychosen. Allg Z Psychiatr. 1912;69:109–112. [Google Scholar]
- 184. Benegal V, Hingorani S, Khanna S. Idiopathic catatonia: validity of the concept. Psychopathology. 1993;26:41–46. [DOI] [PubMed] [Google Scholar]
- 185. Krishna KR, Maniar RC, Harbishettar VS. A comparative study of “Idiopathic catatonia” with catatonia in schizophrenia. Asian J Psychiatr. 2011;4:129–133. [DOI] [PubMed] [Google Scholar]
- 186. Caroff SN, Hurford I, Bleier HR, Gorton GE, Campbell EC. Recurrent idiopathic catatonia: implications beyond the diagnostic and statistical manual of mental disorders 5th Edition. Clin Psychopharmacol Neurosci. 2015;13:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lange J. Katatonishe Erscheinungen im Rahmen manish-depressiver Erkrankungen. Monographien aus dem Gesamtgebiete de Neurologie und Psychiatrie 31.Berlin, Germany: Springer; 1922. [Google Scholar]
- 188. Bräuning P, Krüger S, Shugar G. Prevalence and clinical significance of catatonic symptoms in mania. Comp Psychiatry. 1998;39:35–46. [DOI] [PubMed] [Google Scholar]
- 189. Krüger S, Cooke RG, Spegg CC, Bräunig P. Relevance of the catatonic syndrome to the mixed manic episode. J Affect Disord. 2003;74:279–285. [DOI] [PubMed] [Google Scholar]
- 190. Taylor ME, Abrams R. Catatonia. Prevalence and importance and importance in the manic phase of manic-depressive illness. Arch Gen Psychiatry. 1977;34:1223–1225. [DOI] [PubMed] [Google Scholar]
- 191. Krüger S, Bräuning P. Catatonia in affective disorder: new findings and a review of the literature. CNS Spectrums. 2000;5:48–53. [DOI] [PubMed] [Google Scholar]
- 192. Abrams R, Taylor MA. Catatonia. A prospective clinical study. Arch Gen Psychiatry. 1976;33:579–581. [DOI] [PubMed] [Google Scholar]
- 193. Casey DE. Mood-dependent tardive dyskinesia. In: Joseph AB, Young RR, ed. Movement disorders in neurology and neuropsychiatry.2nd ed Malden, MA: Blackwell Science; 1999:376–379. [Google Scholar]
- 194. de Potter RW, Linkowski P. State-dependent tardive dyskinesia in manic-depressive illness. J Neurol Neurosurg Psychiatry. 1983;46:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yazici O, Kantemir E, Tastaban Y et al. Spontaneous improvement of tardive dystonia during mania. Br J Psychiatry. 1991;158:847–850. [DOI] [PubMed] [Google Scholar]
- 196. Nasrallah HA, Tippin J, McCalley-Whitters M. Neurological soft signs in manic patients. A comparison with Schizophrenic and control groups. J Affect Disord. 1983;5:45–50. [DOI] [PubMed] [Google Scholar]
- 197. Negash A, Kebede D, Alem A et al. Neurological soft signs in bipolar I disorder patients. J Affect Disord. 2004;80:221–230. [DOI] [PubMed] [Google Scholar]
- 198. Baş TÖ, Poyraz CA, Baş A, Poyraz BÇ, Tosun M. The impact of cognitive impairment, neurological soft signs and subdepressive symptoms on functional outcome in bipolar disorder. J Affect Disord. 2015;174:336–341. [DOI] [PubMed] [Google Scholar]
- 199. Goswami U, Sharma A, Udayan A et al. Neuropsychological dysfunction, soft neurological signs and social disability in euthymic patients with bipolar disorder. Br J Psychiatry. 2006;188:366–373. [DOI] [PubMed] [Google Scholar]
- 200. Mrad A, Wassim Krir M, Ajmi I, Gaha L, Mechri A. Neurological soft signs in euthymic bipolar I patients: a comparative study with healthy siblings and controls. Psychiatry Res. 2016;236:173–178. [DOI] [PubMed] [Google Scholar]
- 201. Starkstein S, Gellar S, Parlier M et al. High rates of parkinsonism in adults with autism. J Neurodevelop Dis. 2015;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bennabi D, Vandel P, Papaxanthis C, Pozzo T, Haffen E. Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. BioMed Res Int. 2013;2013:158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Parker G, Melancholia HPD.. A Disorder of Movement and Mood.Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 204. Kraepelin E. Manic-Depressive Insanity and Paranoia. Translated by RM, Barklay (1971). Edinbugh, UK: E. & S. Livingstone; 1921. [Google Scholar]
- 205. Iwanami T, Maeshima H, Baba H et al. Psychomotor agitation in major depressive disorder is a predictive factor of mood-switching. J Affect Disord. 2015;170:185–189. [DOI] [PubMed] [Google Scholar]
- 206. Starkstein SE, Petracca G, Chemerinski E, Merello M. Prevalence and correlates of parkinsonism in patients with depression. Neurology. 2001;57:553–555. [DOI] [PubMed] [Google Scholar]
- 207. Zhao Q, Ma YT, Lui SS et al. Neurological soft signs discriminate schizophrenia from major depression but not bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:72–78. [DOI] [PubMed] [Google Scholar]
- 208. Zor R, Keren H, Hermesh H, Szechtman H, Mort J, Eliam D. Obsessive-compulsive disorder: a disorder of pessimal (non-functional) motor behavior. Acta Psychiat Scand. 2009;120:288–298. [DOI] [PubMed] [Google Scholar]
- 209. Zor R, Fineberg N, Hermesh H et al. Are between-country differences in motor behavior of obsessive-compulsive disorder patients? CNS Spect. 2010;15:445–455. [DOI] [PubMed] [Google Scholar]
- 210. Krüger S, Bräunig P, Höffler J, Shugar G, Börner I, Langkrär J. Prevalence of obsessive-compulsive disorder in schizophrenia and significance of motor symptoms. J Neuropsychiatry Clin Neurosci. 2000;12:16–24. [DOI] [PubMed] [Google Scholar]
- 211. Poyurowsky M, Zoar J, Glick I et al. Obsessive-compulsive symptoms in schizophrenia: implications for future psychiatric classifications. Comp Psychiatry. 2012;53:480–483. [DOI] [PubMed] [Google Scholar]
- 212. Cavanna AE, Robertson MM, Critchley HD. Catatonic signs in Gilles de la Tourette syndrome. Cog Behav Neurol. 2008;21:34–37. [DOI] [PubMed] [Google Scholar]
- 213. Hollander E, Schiffman E, Cohen B et al. Signs of central nervous system dysfunction in obsessive compulsive disorder. Arch Gen Psychiatry. 1990;47:27–32. [DOI] [PubMed] [Google Scholar]
- 214. Pasquini M, Fabbrini G, Moretti G et al. Bradykinesia in patients with obsessive-compulsive disorder. Eur Psychiatry. 2010;25:378–381. [DOI] [PubMed] [Google Scholar]
- 215. Franklin ME, Harrison JP, Benavides KL. Obsessive-compulsive and tic-related disorders. Child Adolesc Psychiatr Clin N Am. 2012;21:555–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gomes de Alvarenga P, de Mathis MA, Dominguez Alves AC et al. Clinical features of tic related obsessive-compulsive disorder: results from a large multicenter study. CNS Spectrums. 2012;17:87–93. [DOI] [PubMed] [Google Scholar]
- 217. Wanderer S, Roessner V, Freeman R et al. Relationship of obsessive-compulsive disorder to age-related comorbidity in children and adolescents with Tourette syndrome. J Develop & Behav Pediatr. 2012;33:124–133. [DOI] [PubMed] [Google Scholar]
- 218. de Vries FE, Cath DC, Hoogendoorn AW et al. Tic-related versus tic-free obsessive-compulsive disorder: clinical picture and 2-year natural course. J Clin Psychiatry. 2016;77:1240–1247. [DOI] [PubMed] [Google Scholar]
- 219. Rogers D. Psychiatric consequences of basal ganglia disease. Semin Neurol. 1990;10:262–266. [DOI] [PubMed] [Google Scholar]
- 220. Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384:523–531. [DOI] [PubMed] [Google Scholar]
- 221. Ratnasuriya RH, Marks IM, Forshaw DM, Hymas NF. Obsessive slowness revisited. Br J Psychiatry. 1991;159:273–274. [DOI] [PubMed] [Google Scholar]
- 222. Hymas N, Lees A, Bolton D, Epps K, Head D. The neurology of obsessional slowness. Brain. 1991;114:2203–2233. [DOI] [PubMed] [Google Scholar]
- 223. Rachman SJ. Primary obsessional slowness. Behav Res Ther. 1974;12:9–18. [DOI] [PubMed] [Google Scholar]
- 224. Veale D. Classification and treatment of obsessional slowness. Br J Psychiatry. 1993;162:198–203. [DOI] [PubMed] [Google Scholar]
- 225. Peng ZW, Xu T, Miao GD et al. Neurological soft signs in obsessive-compulsive disorder: the effect of co-morbid psychosis and evidence for familiality. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:200–205. [DOI] [PubMed] [Google Scholar]
- 226. Jaafari N, Fernández de la Cruz L, Grau M et al. Neurological soft signs in obsessive-compulsive disorder: two empirical studies and meta-analysis. Psychol Med. 2013;43:1069–1079. [DOI] [PubMed] [Google Scholar]
- 227. Neumärker KJ. Classification matters for catatonia and autism in children. Int Rev Neurobiol. 2006;72:3–19. [DOI] [PubMed] [Google Scholar]
- 228. Shorter E, Watchel LE. Childhood catatonia, autism and psychosis past and present: is there an “iron triangle”? Acta Psychiatr Scand. 2013;128:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Leonhard K. Über kindliche Katatonien. Psychiatr Neurol Med Psychol. 1960;12:1–17. [PubMed] [Google Scholar]
- 230. Stoppelbein L, Greening L, Kakooza A. The importance of catatonia and stereotypies in autistic spectrum disorders. Int Rev Neurobiol. 2006;72:103–118. [DOI] [PubMed] [Google Scholar]
- 231. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125:33–38. [DOI] [PubMed] [Google Scholar]
- 232. Breen J, Hare DJ. The nature and prevalence of catatonic symptoms in young people with autism. J Intellect Disabil Res. 2017;. 61:580-593. [DOI] [PubMed] [Google Scholar]
- 233. Walon H. Syndromes d’insuffisance psychomotrice et types psycho-moteurs. Ann Med Psychol. 1932;4:240–251. [Google Scholar]
- 234. Earl CJC. The primitive catatonic psychosis of idiocy. Br J Med Psychol. 1934;14:111–230. [Google Scholar]
- 235. Rollings HR. Personality in mongolism with special reference to the incidence of catatonic psychosis. Am J of Mental Deficiency. 1946;2:219–237. [PubMed] [Google Scholar]
- 236. Rogers D, Karki C, Bartlett C, Pocock P. The motor disorders of mental handicap. An overlap with the motor disorders of severe psychiatric illness. Br J Psychiatry. 1991;158:97–102. [DOI] [PubMed] [Google Scholar]
- 237. Starkstein S, Gellar S, Parlier M, Payne L, Piven J. High rates of parkinsonism in adults with autism. J Neurodev Disord. 2015;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Campbell M, Locascio JJ, Chorocco MC et al. Stereotypies and tardive dyskinesia in: abnormal movements in autistic children. Psychopharmacol Bull. 1990;26:260–266. [PubMed] [Google Scholar]
- 239. Jones V, Prior M. Motor imitation abilities and neurological signs in autistic children. J Autism Dev Disord. 1985;15:37–46. [DOI] [PubMed] [Google Scholar]
- 240. Tani P, Lindberg N, Appelberg B, Nieminen-von Wendt T, von Wendt L, Porkka-Heiskanen T. Clinical neurological abnormalities in young adults with Asperger syndrome. Psychiatry Clin Neurosci. 2006;60:253–255. [DOI] [PubMed] [Google Scholar]
- 241. Paquet A, Olliac B, Bouvard MP, Golse B, Vaivre-Douret L. The semiology of motor disorders in autism spectrum disorders as highlighted from a standardized neuro-psychomotor assessment. Front Psychol. 2016;7:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Franssen EH, Kluger A, Torossian CL, Reisberg B. The neurologic syndrome of severe Alzheimer’s disease: relationship to functional decline. Arch Neurol. 1993;50:1029–1039. [DOI] [PubMed] [Google Scholar]
- 243. Funkenstein HH, Albert MS, Cook NR et al. Extrapyramidal signs and other neurologic findings in clinically diagnosed Alzheimer’s disease: a community-based study. Arch Neurol. 1993;50:51–56. [DOI] [PubMed] [Google Scholar]
- 244. Hobbelen JSM, Koopmans RTCM, Verhey FRJ et al. Diagnosing paratonia in the demented elderly: reliability and validity of the Paratonia Assessment Instrument (PAI). Int Pchychogeriat. 2008;20:840–852. [DOI] [PubMed] [Google Scholar]
- 245. Spalletta G, Mussico M, Padovani A et al. Neuropsychiatric symptoms and síndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer Disease. Am J Geriatr Psychiatry. 2010;18:1026–1035. [DOI] [PubMed] [Google Scholar]
- 246. Zhao QF, Tan L, Wang H-F et al. The prevalence of neuropsychiatric symptoms in Alzheimer disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. [DOI] [PubMed] [Google Scholar]
- 247. Dupré E. Débilité mentale et débilité motrice asocies. Rev Neurol. 1910;20:54–56. [Google Scholar]
- 248. Diederich NJ. Paratonia (gegenhalten). In: Kompoliti K, Mentman LV, eds. The Encyclopedia of Movement Disorders. Oxford UK, San Diego, CA: Elsevier, United Press; 2010:376–379. [Google Scholar]
- 249. Souren LE, Franssen EH, Reisberg B. Neuromotor changes in Alzheimer’s disease: implications for patients care. J Geriat Psychiatry Neurol. 1997;10:93–98. [DOI] [PubMed] [Google Scholar]
- 250. Vahia I, Cohen CI, Prehogan A, Memmon Z. Prevalence and impact of paratonia in Alzheimer’s disease in a multiracial sample. Am J Geriat Psychiatry. 2007;15:351–353. [DOI] [PubMed] [Google Scholar]
- 251. Portet F, Scarmeas N, Cosentino S, Helzner EP, Stern Y. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Mitchell SL. Extrapyramidal features in Alzheimer’s disease. Age Ageing. 1999;28:401–409. [DOI] [PubMed] [Google Scholar]
- 253. Tsolaki M, Kokarida K, Iakovidou V, Stilopoulos E, Meimaris J, Kazis A. Extrapyramidal symptoms and signs in Alzheimer’s disease: prevalence and correlation with the first symptom. Am J Alzheimers Dis Other Demen. 2001;16:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Louis ED, Tang MX, Schupf N. Mild parkinsonian signs are associated with increased risk of dementia in a prospective, population-based study of elders. Mov Dis. 2010;30:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Scarmeas N, Albert M, Brandt J et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Tosto G, Monsell SE, Hawes SE, Mayeux R. Pattern of extrapyramidal signs in Alzheimer’s disease. J Neurol. 2015;262:2548–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Urbanowitsch N, Degen C, Toro P, Schröder J. Neurological soft signs in aging, mild cognitive impairment, and Alzheimer’s disease - the impact of cognitive decline and cognitive reserve. Front Psychiatry. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Seidl U, Thomann PA, Schröder J. Neurological soft signs in nursing home residents with Alzheimer’s disease. J Alzheimers Dis. 2009;18:525–532. [DOI] [PubMed] [Google Scholar]
- 259. Cuesta MJ, Peralta V, Gil P, Artamendi M. Psychopathological dimensions in first-episode psychoses. From the trunk to the branches and leaves. Eur Arch Psychiatry Clin Neurosci. 2003;253:73–79. [DOI] [PubMed] [Google Scholar]
- 260. Peralta V, Moreno-Izco L, Calvo-Barrena L, Cuesta MJ. The low- and higher-order factor structure of symptoms in patients with a first episode of psychosis. Schizophr Res. 2013;147:116–124. [DOI] [PubMed] [Google Scholar]
- 261. Schneider K. Über und Bedeuting katatonisher Symptome. Zeitschrift für die Gesamte Neurol und Psychiatr. 1914;22:486–505. [Google Scholar]
- 262. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66:1173–1177. [DOI] [PubMed] [Google Scholar]
- 263. Tandon R, Heckers S, Bustillo J et al. Catatonia in DSM-5. Schizophr Res. 2013;150:26–30. [DOI] [PubMed] [Google Scholar]
- 264. Quinn J, Meagher D, Murphy P, Kinsella A, Mullaney J, Waddington JL. Vulnerability to involuntary movements over a lifetime trajectory of schizophrenia approaches 100%, in association with executive (frontal) dysfunction. Schizophr Res. 2001;49:79–87. [DOI] [PubMed] [Google Scholar]
- 265. Mimica N, Folnegović-Smalc V, Folnegović Z. Catatonic schizophrenia in Croatia. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 1):17–20. [DOI] [PubMed] [Google Scholar]
- 266. Stöber G. Genetics. In Caroff S, Mann SC, Francis A, Friccione GL, ed. Catatonia. From Psychopathology to Neurobiology.Washington, DC: American Psychiatric Publishing; 2004:173–188. [Google Scholar]
- 267. Stöber G, Saar K, Rüschendorf F et al. Splitting schizophrenia: periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet. 2000;67:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Lucchini F, Medda P, Mariani MG, Mauri M, Toni C, Perugi G. Electroconvulsive therapy in catatonic patients: efficacy and predictors of response. World J Psychiat. 2015;22:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Abrams R, Taylor MA. Catatonia: prediction of response to somatic treatments. Am J Psychiatry. 1977;134:78–80. [DOI] [PubMed] [Google Scholar]
- 271. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25:555–577. [DOI] [PubMed] [Google Scholar]
- 272. Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. 2015;169:217–233. [DOI] [PubMed] [Google Scholar]
- 273. Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233:293–298. [DOI] [PubMed] [Google Scholar]
- 274. Walther S, Horn H, Razavi N, Koschorke P, Müller TJ, Strik W. Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology. 2009;60:80–86. [DOI] [PubMed] [Google Scholar]
- 275. Torres EB, Isenhower RW, Nguyen J et al. Toward precision psychiatry: statistical platform for the personalized characterization of natural behaviors. Front Neurol. 2016;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Walther S, Mittal VA. Why we should take a closer look at gestures. Schizophr Bull. 2016;42:259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pilars of RDoC. BMC Medicine. 2013;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.