Abstract
Cognitive deficits in schizophrenia have been hypothesized to reflect N-methyl-D-aspartate receptor (NMDAR) dysfunction. However, the mechanisms through which the NMDAR contributes to individual cognitive functions differ. To explore how NMDAR signaling relates to specific cognitive deficits in schizophrenia, we tested the effects of enhancing NMDAR signaling on working memory and experience-dependent plasticity using d-cycloserine (DCS). Plasticity was assessed using an EEG paradigm that utilizes high-frequency visual stimulation (HFvS) to induce neural potentiation, and 2 learning tasks, the information integration (IIT) and weather prediction (WPT) tasks. Working memory was assessed using an N-back task. Forty-five schizophrenia patients were randomized to receive a single 100 mg DCS dose (SZ-DCS; n = 24) or placebo (SZ-PLC; n = 21) in a double-blind, between-groups design. Testing occurred on a single day after placebo or DCS administration; baseline values were not obtained. DCS did not affect plasticity, as indicated by similar neural potentiation, and similar IIT and WPT learning between groups. However, among patients who successfully engaged in the working memory task (ie, performed above chance), SZ-DCS (n = 17) showed superior 2-back performance compared to SZ-PLC (n = 16). Interestingly, SZ-DCS also showed larger pre-HFvS neural responses during the LTP task. Notably, this pattern of DCS effects is the opposite of those found in our prior study of healthy adults. Results are consistent with target engagement of the NMDAR by DCS, but suggest that NMDAR signaling was not translated into synaptic plasticity changes in schizophrenia. Results highlight the importance of considering how distinct NMDAR-associated processes contribute to individual cognitive deficits in schizophrenia.
Keywords: cognition, NMDA receptor, psychosis, long-term potentiation, learning, memory
Introduction
Cognitive impairment is a core feature of schizophrenia and has been postulated to reflect dysfunction at the N-methyl-D-aspartate receptor (NMDAR).1 Cognitive deficits are present prior to psychosis onset, may be the most enduring feature of schizophrenia, and are the best predictor of long-term outcome.2–4 Expression of NMDAR transcripts and NMDAR-associated proteins are aberrant in schizophrenia5–10 and genomic studies identified genes intrinsic to the NMDAR and genes modulating NMDAR function as among those most robustly associated with schizophrenia.11–15 Notably, in healthy individuals, NMDAR antagonists induce not only positive and negative symptoms, but also cognitive symptoms similar to those in schizophrenia.16,17 Given extensive evidence from animal studies demonstrating a primary role for NMDARs in working memory, learning, and memory,18 it is frequently hypothesized that cognitive deficits in schizophrenia arise from NMDAR dysfunction. However, recent findings suggest that the NMDAR contributes to individual cognitive functions through distinct mechanisms. Thus, further research is needed to explore how NMDAR signaling relates to individual cognitive functions in schizophrenia.
NMDARs are unique receptors and are involved in a range of essential brain functions.19 They require concurrent binding of glutamate and glycine or d-serine, as well as membrane depolarization from activation of non-NMDARs such as amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptors (AMPARs) to relieve blockade from a magnesium ion at resting potential. NMDARs bind to scaffold and signaling molecules in the postsynaptic density (PSD), yielding a vast protein complex that physically links the NMDAR to kinases, cytoskeleton, and downstream signaling pathways.20 NMDAR opening leads to influx of sodium (Na+), which contributes to postsynaptic depolarization, and calcium (Ca2+), which promotes synaptic plasticity by activating signaling cascades in the PSD.21 Relative to AMPARs, the speed at which NMDARs activate and deactivate is markedly slow. The slow kinetics of the NMDAR channel facilitates the generation of sustained excitation in local neural circuits.19,21
These unique characteristics confer the NDMAR a specialized role in working memory and experience-dependent plasticity. Working memory is the ability to transiently hold and manipulate information to guide immediate goal-directed behavior. Experience-dependent plasticity is the capacity of the brain to encode environmental input and learning via lasting structural changes that guide future behavior.25,26 However, the properties that underlie NMDAR involvement in working memory and experience-dependent plasticity differ.23 Studies in nonhuman primates showed that during spatial working memory, short-term representation of stimuli depends on recurrent excitation in dorsolateral prefrontal cortex (dlPFC) microcircuits.23 These microcircuits involve glutamatergic pyramidal neurons with similar spatial tuning that excite each other via AMPARs and NMDARs, and GABAergic interneurons that inhibit neurons with dissimilar spatial tuning. While AMPAR activation is thought to generate the membrane depolarization that permits NMDAR activation, it is the slow NMDAR channel kinetics that is thought to sustain neural excitation over delay periods for working memory function.24 In contrast, in experience-dependent plasticity, influx of Ca2+ following NMDAR activation initiates intracellular signaling cascades in the PSD. These signaling cascades regulate structural synaptic changes such as AMPAR insertion in the membrane and enlargement of dendritic spines on which synapses are located, and thus produce long-term potentiation (LTP) or depression (LTD) of synaptic strength. Other forms of plasticity exist in the brain (see Citri and Malenka25 for review); however, LTP/LTD is considered the classical mechanism underlying most forms of learning in the brain.25,26 NMDAR-mediated LTP has been observed at various subcortical and sensory cortex synapses (eg, hippocampus, amygdala, striatum, visual, and somatosensory cortex), with LTP in each region thought to subserve distinct forms of learning.25–30
Consistent with NMDAR dysfunction, schizophrenia patients show deficits in working memory across diverse methods of assessment and in both visual-spatial and verbal domains.31,32 Schizophrenia patients also show deficits across behavioral and electrophysiological measures of experience-dependent plasticity. Patients show impaired simple reinforcement, probabilistic classification, and explicit and implicit learning.33–38 Patients also show deficits on tasks that use repetitive sensory stimulation to induce LTP in sensory neurons, as measured by changes in electroencephalograph (EEG) sensory evoked potentials. Animal studies demonstrated that potentiated neural responses following repetitive sensory stimulation show the cardinal features of synaptic LTP, including persistence, input specificity, and NMDAR-dependency. Thus, while high-frequency stimulation potentiated neural responses in healthy adults,41,42 in patients with schizophrenia, such responses were impaired.43,44
In the current study, we explored the effects of augmenting NMDAR signaling on working memory and experience-dependent plasticity in patients with schizophrenia using the partial NMDAR agonist, d-cycloserine (DCS). DCS binds to the NMDAR glycine-modulatory site and augments NMDAR signaling at low doses by increasing channel open time and open probability.45,46 Given evidence that repeated DCS dosing may desensitize the NMDAR complex,47–49 we used a single dose of DCS. In our prior study among healthy adults, acute DCS enhanced experience-dependent plasticity,50 as demonstrated by enhanced visual evoked potentials (VEPs) following high-frequency visual stimulation (HFvS) and enhanced learning on 2 cortico-striatal dependent learning tasks: the weather prediction task (WPT)51 and information integration task (IIT).52 In contrast, DCS did not affect performance on a N-back working memory task that was identical to the IIT in stimuli and trial structure. The beneficial effects of DCS across electrophysiological and behavioral measures of plasticity in healthy adults with no effect on working memory is consistent with evidence that beyond a threshold of NMDAR activity necessary to produce recurrent excitation in working memory circuits, further NMDAR activation has limited benefits.24 The current study closely paralleled our prior study to facilitate comparison of DCS effects in schizophrenia versus healthy adults.
Methods
Participants and Procedures
A complete description of participants and procedures is provided in Supplementary Information. Briefly, 45 patients with a psychotic disorder were randomized to receive a single 100 mg dose of DCS (SZ-DCS; n = 24) or placebo (SZ-PLC; n = 21) in a double-blind, between-groups design, followed by assessments using the Weschler Abbreviated Scale of Intelligence (WASI)53 and Brief Psychiatric Rating Scale (BPRS).54 Testing occurred on 1 day. EEG testing for the LTP task began approximately 3 hours after DCS or placebo administration, followed by the cognitive tasks in random order. The primary outcome measures were performance on the N-back, IIT, and WPT, and plasticity on the LTP EEG task (ie, change in VEPs following HFvS). Diagnosis was confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV).55
LTP Task
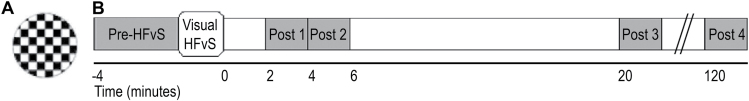
The LTP task assessed VEPs to a standard checkerboard stimulus presented at 0.83 Hz in 2-minute blocks before HFvS (ie, providing a pre-HFvS assessment) and after exposure to HFvS (figures 1A and 1B).43 For HFvS, the standard checkerboard was presented at approximately 8.87 Hz for 2 minutes. See Supplementary Information for task and EEG procedure details.
Fig. 1.
(A) Standard circle black and white checkerboard stimulus presented at 0.83 Hz during visual evoked potential (VEP) assessment blocks and at ~8.87 Hz during high-frequency visual stimulation (HFvS). (B) Time course of the long-term potentiation (LTP) paradigm.
Cognitive Tasks
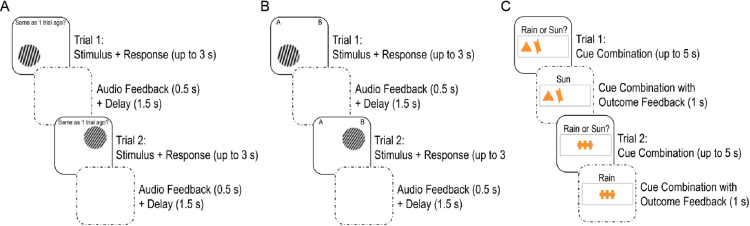
The cognitive tasks are shown in figure 2 and described in detail in Supplementary Information. Briefly, the N-back is a spatial working memory task with 3 memory loads: 0- to 2-back. On each trial, a single sine-wave grating stimulus was presented in 1 of 4 quadrants of the screen for a maximum of 3 seconds. In the 0-back control condition, participants indicated whether stimuli were on the left or right. In the 1- and 2-back working memory conditions, participants indicated whether stimuli were in the same location as the stimulus shown 1 or 2 trials ago, respectively. The IIT is a category learning task in which participants learned whether circular sine-wave grating stimuli that varied in bar width and orientation belonged to category A or B.52 Following response, participants were provided auditory feedback for 500 ms. Stimuli and trial structure were identical between the IIT and N-back. The WPT is a probabilistic classification task during which participants predicted the weather based on cue combinations that probabilistically predicted “sun” or “rain.”51 Cues were presented for a maximum of 5 s. Following response, feedback showing the cue combination and actual outcome for the trial was presented for 1 s.
Fig. 2.
(A) Two example trials for the 1-back condition of the N-back task. (B) Two example information integration task (IIT) trials. (C) Two example weather prediction task (WPT) trials. The N-back and IIT were identical in stimuli and trial structure such that the only difference participants experienced was whether they were asked to recall whether stimuli were in the same location on the screen as recently shown stimuli (ie, for the N-back) or learn about the stimuli (ie, for the IIT).
Statistical Analyses
Statistical analyses were conducted with SPSS, version 22.0 (SPSS). Independent samples t-tests and chi-square tests assessed group differences in demographic and clinical characteristics.
LTP Analyses.
C1 and P2 components were analyzed using the channel showing the largest amplitudes, Oz. One SZ-PLC who had a blind spot and one SZ-DCS who was unable to keep his eyes open had unusual VEPs; Oz data were unavailable for 2 additional SZ-DCS due to EEG equipment problems. LTP data for these 4 participants were excluded.
Independent samples t-tests assessed group differences in pre-HFvS C1 and P2 amplitude. Drug group × block repeated measures ANOVA followed by simple contrasts compared to pre-HFvS amplitude characterized the timecourse of HFvS effects on C1 and P2 and assessed group differences in component amplitudes across blocks. To further assess plasticity, pre-HFvS amplitude was subtracted from each post-HFvS amplitude; group differences in plasticity across post-HFvS blocks were assessed using group × block repeated measures ANOVA.
Cognitive Analyses.
Percent correct responses per 80-trial blocks were calculated for each task; group differences in accuracy and reaction time were assessed using drug group × block repeated measures ANOVA. Patients who missed more than 5% of trials on a given task were excluded for that task, leaving 20 SZ-PLC and 23 SZ-DCS on the N-back, 21 SZ-PLC and 22 SZ-DCS on the IIT, and 20 SZ-PLC and 21 SZ-DCS on the WPT. To ensure that null group effects were not due to difficulties understanding or engaging in a task, analyses for each task were re-run restricted to patients performing above chance. For the N-back, this was defined as ≥75% accuracy during the 0-back control condition and ≥55% during the 1- and 2-back, leaving 16 SZ-PLC and 17 SZ-DCS. For the IIT and WPT, this was defined as ≥55% accuracy during the last block of trials, leaving 15 SZ-PLC and 16 SZ-DCS on the IIT and 20 SZ-PLC and 19 SZ-DCS on the WPT.
Parallel analyses including sex, age, IQ, and antipsychotic dose as covariates were conducted for all measures and yielded similar results. Mauchly’s test of sphericity was violated on the following group × block ANOVA: N-back accuracy when all patients were included in the analysis; WPT reaction time; all group × block LTP analyses. P values for effects involving block for these analyses are reported using Greenhouse–Geiser correction. For clarity, noncorrected degrees of freedom are reported. An alpha of 0.05 was used for all analyses.
Results
Patient Characteristics
Randomization yielded groups that were similar in age, sex, IQ, antipsychotic status and dose, diagnosis, and symptom severity (table 1).
Table 1.
Characteristics of Schizophrenia Patients Who Received Placebo (SZ-PLC) and DCS (SZ-DCS)
| SZ-PLC (n = 21) | SZ-DCS (n = 24) | t or χ2 | df | P | |
|---|---|---|---|---|---|
| Age ± SD (range) | 28.14 ± 6.62 (18–39) | 26.88 ± 6.58 (18–42) | 0.64 | 43 | .52 |
| Sex | 5 F/16 M | 3 F/21 M | 0.98 | 1 | .32 |
| WASI ± SD (range) | 103.95 ± 13.64 (74–124) | 101.79 ± 16.74 (70–127) | 0.47 | 43 | .64 |
| BPRS positive symptoms ± SD | 5.81 ± 3.40 | 5.21 ± 2.98 | 0.06 | 43 | .95 |
| BPRS negative symptoms ± SD | 6.14 ± 3.86 | 6.76 ± 3.50 | 0.63 | 43 | .53 |
| BPRS total ± SD | 40.52 ± 10.87 | 40.75 ± 12.80 | 0.55 | 43 | .58 |
| Chlorpromazine equivalence (mg) ± SD | 229.89 ± 133.11 | 224.05 ± 170.74 | 0.05 | 42 | .96 |
| Antipsychotic-free (n) | 3 | 3 | 0.03 | 1 | .86 |
| Diagnosis | 0.50 | 2 | .78 | ||
| Schizophrenia (n) | 16 | 17 | |||
| Schizoaffective (n) | 3 | 3 | |||
| Schizophreniform (n) | 2 | 4 |
LTP Task
Larger Neural Responses But Similar Plasticity in Patients Who Received DCS.
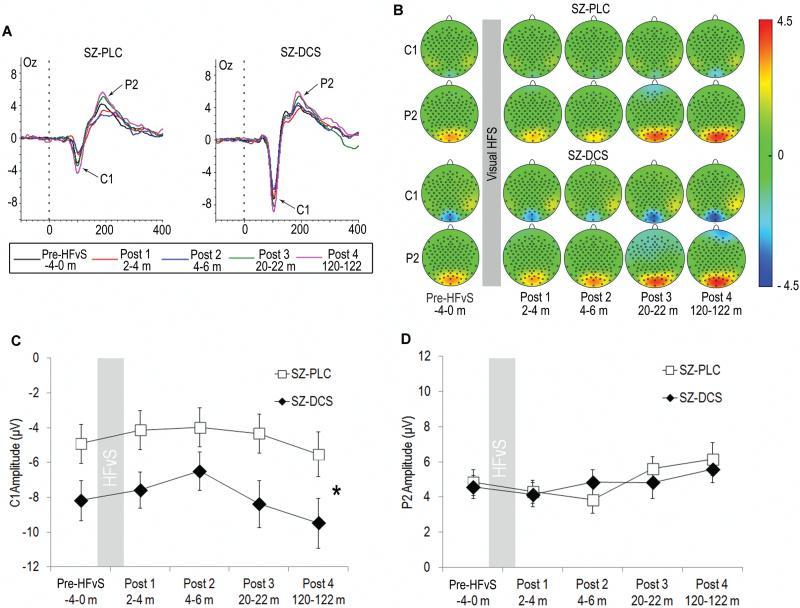
The VEP included a negative component, C1, that peaked at Oz at 96.10 ms (SD = 19.13) in SZ-PLC and 104.62 ms (SD = 6.48) in SZ-DCS, and a positive component, P2, that peaked at Oz at 200.70 ms (SD = 28.81) in SZ-PLC and 203.19 ms (SD = 24.07) in SZ-DCS (figure 3A).
Fig. 3.
(A) Average visual evoked potentials (VEPs) elicited by the checkerboard stimulus in Oz and (B) topography of VEPs for schizophrenia patients who received placebo (SZ-PLC) or DCS (SZ-DCS) across assessment blocks. The VEP included a negative component, C1, and a positive component, P2. (C) Mean (±SE) C1 amplitude was larger across blocks in SZ-DCS compared to SZ-PLC*. (D) Mean (±SE) P2 amplitude was similar in SZ-PLC and SZ-DCS across blocks.
Pre-HFvS C1 amplitude was significantly larger (ie, larger negative amplitude) in SZ-DCS compared to SZ-PLC, t(39) = 2.03, P = .049, indicating that SZ-DCS showed larger neural responses than SZ-PLC prior to HFvS. Repeated measures ANOVA across blocks similarly showed an effect of drug, F(1, 39) = 4.86, P = .03, with no drug × block interaction, F(4, 156) = 0.72, P = .50, indicating larger C1 amplitude throughout testing in SZ-DCS (figures 3B and 3C). There was a significant block effect, F(4, 156) = 5.45, P < .005, due to both groups showing depression of C1 at 4–6 minutes post-HFvS, P = .001. SZ-DCS and SZ-PLC tended towards depression at 2–4 minutes post-HFvS, P = .07, but showed no significant modulation of C1 at 20–22, P = .68, or 120–122 minutes post-HFvS, P = .10.
Pre-HFvS P2 amplitude was similar between SZ-DCS and SZ-PLC, t(39) = 0.25, P = .80. Repeated measures ANOVA across blocks showed no drug effect, F(1, 39) = 0.01, P = .92, or drug × block interaction, F(4, 156) = 2.14, P = .10 (figures 3B and 3D). There was a significant effect of block, F(4, 156) = 7.10, P < .001, due to both groups showing potentiation of P2 at 120–122 minutes post-HFvS, P < .001. However, there was no significant modulation of P2 at 2–4, P = .22, 4–6, P = .24, or 20–22 minutes post-HFvS, P = .13.
Repeated measures ANOVA on post-HFvS plasticity scores confirmed that SZ-PLC and SZ-DCS did not differ in C1 or P2 potentiation following HFvS. Thus, there was no drug effect, F(1, 39) = 0.14, P = .71, or drug × block interaction, F(3, 117) = .81, P = .44, on C1 potentiation. There was also no drug effect on P2 potentation, F(1, 39) = .18, P = .67. There was a trend toward a drug × block interaction for P2, F(3, 117) = 2.48, P = .08; however, this was not significant. SZ-PLC and SZ-DCS were also similar in C1-P2 peak-to-peak potentiation (Supplementary Information).
Cognitive Tasks
Superior Working Memory But Similar Learning in Patients Who Received DCS.
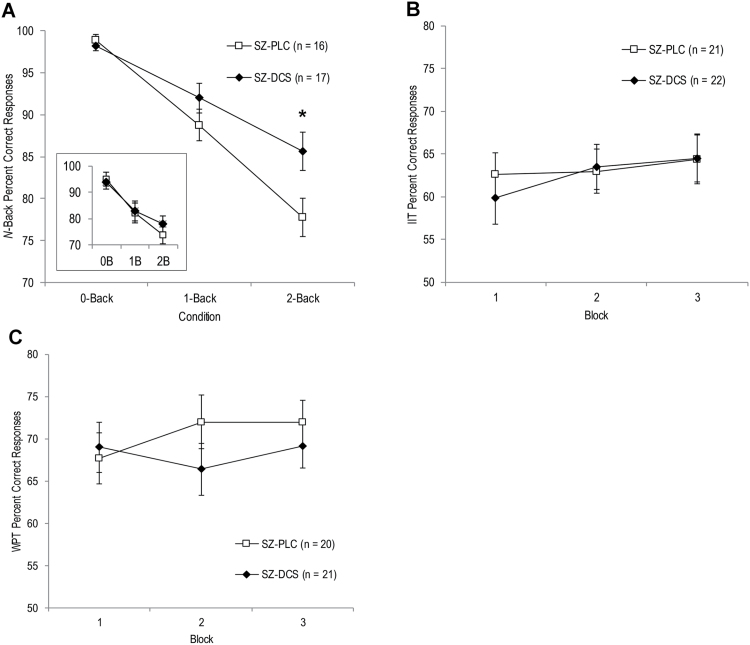
When all patients were included in the N-back analysis, both groups performed better at lower working memory loads, F(2, 82) = 40.97, P < .001, but the drug effect, F(1, 41) = .10, P = .75, and drug × condition interaction, F(2, 82) = 1.00, P = .36, were not significant. However, when analyses were restricted to patients who performed above chance, there was a significant drug × condition interaction, F(2, 62) = 3.24, P = .046, due to superior performance in SZ-DCS compared to SZ-PLC during the 2-back working memory condition, P = .045 (figure 4A). Although SZ-DCS also showed higher mean performance during the 1-back, this was not significant, P = .36; groups performed similarly during the 0-back control condition, P = .49. Exploratory analysis of reaction times comparing chance performers versus above-chance performers provided additional support that N-back chance performers were not adequately engaged in the working memory task. Specifically, patients who performed above chance showed progressively longer reaction times with increased working memory load, consistent with prior studies,56,57 whereas chance performers showed no increase in reaction times for the working memory loads relative to the 0-back control condition (Supplementary Information). Exploratory analysis also indicated that across drug groups, chance performers had significantly lower IQs than above-chance performers (Supplementary Information). Thus, among patients who successfully engaged in the N-back task, SZ-DCS showed superior working memory.
Fig. 4.
(A) Mean ± SE percent correct responses per 80-trial load on the N-back for schizophrenia patients who received placebo (SZ-PLC) or DCS (SZ-DCS) and performed above chance. *SZ-DCS performed significantly better than SZ-PLC during the 2-back. Inset shows percent correct responses when all SZ-PLC (n = 20) and SZ-DCS (n = 23) were included. Mean ± SE percent correct responses per 80-trial blocks for the (B) weather prediction task (WPT) and (C) information integration task (IIT) for SZ-PLC and SZ-DCS.
In contrast, SZ-PLC and SZ-DCS performed similarly on the learning measures of plasticity. On the IIT, there was a trend towards an effect of block, F(2, 82) = 2.79, P = .07, but no drug effect, F(1, 41) = .04, P = .84, or drug × block interaction, F(2, 82) = .78, P = .46 (figure 4B). Similarly, on the WPT, there was no effect of block, F(2, 78) = .98, P = .38, drug, F(1, 39) = .40, P = .53, or drug × block interaction, F(2, 78) = 2.37, P = .10 (figure 4C). Restricting analyses to patients performing above chance on each task yielded a similar lack of group differences in IIT and WPT learning.
Restricting analyses to patients who performed above chance across all 3 cognitive tasks (SZ-PLC n = 13, SZ-DCS n = 12) yielded the same pattern of effects, with SZ-DCS showing superior working memory compared to SZ-PLC, but similar plasticity across the LTP task, IIT, and WPT (Supplementary Information).
Reaction times were similar between SZ-PLC and SZ-DCS for each cognitive task (Supplementary Information).
Opposite Pattern of DCS Effects in Schizophrenia Versus Healthy Adults.
To facilitate comparison of the pattern of DCS effects in schizophrenia patients versus healthy adults, data from our prior healthy adult study were re-processed and analyzed to parallel the current study. Cohen’s d effect sizes for DCS were calculated for each study sample for working memory, electrophysiological and learning measures of plasticity, and pre-HFvS VEP amplitudes. Results highlighted an opposite pattern of DCS effects on working memory and pre-HFvS C1 amplitude versus plasticity in schizophrenia versus healthy adults (Supplementary Information).
Discussion
The present study used acute DCS to explore how augmenting NMDAR signaling affected working memory and experience-dependent plasticity in patients with schizophrenia. In contrast to our prior findings of DCS in healthy adults,50 schizophrenia patients who received DCS performed similarly to patients who received placebo across electrophysiological and learning measures of plasticity. Conversely, among patients who performed above chance, patients who received DCS showed superior 2-back working memory performance compared to patients who received placebo. Interestingly, patients who received DCS also showed larger C1 amplitudes across pre- and post-HFvS assessments. Exploratory analyses comparing the 2 schizophrenia groups to healthy adults from our prior study who received placebo suggested that 2-back performance and C1 amplitude were impaired in schizophrenia patients who received placebo relative to healthy adults, whereas 2-back performance and C1 amplitude were similar between patients who received DCS and healthy adults (Supplementary Information). DCS operates as an NMDAR agonist by increasing the open time and open probability of the NMDAR channel.45,46 Superior working memory and larger C1 amplitude in schizophrenia patients who received DCS versus placebo is consistent with this mechanism and suggests that DCS was successful in increasing signaling across the NMDAR. Conversely, the lack of group differences on any measure of plasticity in schizophrenia may indicate a failure in the translation of electrical signaling across the NMDAR into the synaptic changes that support plasticity.
One prior study found beneficial effects of DCS on working memory in schizophrenia;58 however, several others found no benefits.59–63 The majority of prior studies involved older and more chronic patients, used a 50 mg DCS dose, and assessed working memory after 2–8 weeks of daily or weekly DCS administration. Patients in the current study were relatively young and high-functioning, had low symptom levels, and were administered a single dose of 100 mg DCS. It is unclear which of these factors might account for the differential effects in the current study. However, it is notable that group differences on the N-back were only evident after patients who performed around or below chance were excluded. This suggests that positive effects of DCS were initially washed out by patients who had difficulty understanding and/or engaging in the task. For prior studies that included more chronic and low-functioning patients, this may have weakened power to detect benefits of DCS. Additionally, multiple rodent studies found that beneficial cognitive effects following a single dose of DCS did not persist with chronic dosing.47–49 This suggests that prolonged DCS administration may lead to desensitization of the NMDAR, which could also account for prior negative findings.
During spatial working memory, sufficient NMDAR activity is necessary to generate the transient, recurrent excitation in dlPFC microcircuits that represents stimuli over delay periods.24 Convergent evidence indicates that both spatial working memory31,32 and NMDAR signaling are impaired in schizophrenia.5–15 Although our finding of superior working memory in patients who received DCS compared to placebo requires independent replication, this suggests that DCS increased NMDAR signaling, possibly restoring recurrent excitation in working memory microcircuits.
Interestingly, C1 amplitude was also increased (ie, larger negative amplitude) in patients who received DCS relative to patients who received placebo. C1 is an early preattentive evoked potential generated by primary visual cortex neurons.64 Prior studies suggest that early VEP components are reduced in schizophrenia, including C1.65,66 To our knowledge, no studies have investigated mechanisms underlying early visual processing deficits in schizophrenia. However, EEG signals are thought to reflect excitatory postsynaptic potentials conveyed by NMDARs and AMPARs in the cortex.67 Thus, early VEP deficits could reflect reduced glutamate signaling or responsivity of NMDARs or AMPARs in visual cortex. Our finding of larger C1 amplitude in patients who received DCS compared to placebo is consistent with a role for NMDAR-mediated transmission in the generation of C1 and suggests the interesting possibility that increasing NMDAR neurotransmission may increase the early VEP in schizophrenia.
In contrast to the group differences in working memory and C1 amplitude, DCS and placebo patients performed similarly across electrophysiological and learning measures of experience-dependent plasticity. The LTP task, IIT, and WPT are diverse and indirect measures of synaptic plasticity; however, animal studies suggest that each require NMDAR-dependent LTP. Potentiation of neural responses following HFvS has been shown to share the cardinal features of synaptic LTP at visual cortex synapses, including persistence, input specificity, and NMDAR dependency.39,40 Similarly, the WPT and IIT depend on incremental, feedback-based learning which has been shown to require NMDAR-dependent LTP at dorsomedial striatal synapses.29,30 Indeed, we previously found that augmenting NMDAR signaling using DCS in healthy adults enhanced VEP potentiation following HFvS and IIT and WPT learning.50 Controlling for antipsychotic dose, sex, age, and IQ did not alter the similarity of placebo and DCS schizophrenia patients on the plasticity measures, nor did excluding patients who performed around chance for each learning task. Given that the current patients were relatively high-functioning, had low symptom levels, and that the same subgroup of DCS patients showed superior working memory performance over placebo, it is unlikely that confounding factors such as lack of motivation, inattention, or difficulty understanding the tasks accounts for the lack of group differences on plasticity. It is possible that DCS patients would have shown benefits over a longer period of practice on the WPT or IIT, or on other tasks assessing plasticity. However, we found positive effects of DCS in healthy adults over the same number of trials on each plasticity task (Supplementary Information). One recent study found that DCS improved performance on an auditory discrimination task on which patients were trained over 8 weeks.68 However, numerous studies found minimal effects of DCS on neuropsychological tests of short-term verbal or spatial learning in schizophrenia patients.61–63,68 Given that we did not measure NMDAR signaling or synaptic changes directly in the current study, any mechanistic explanations for our negative findings of DCS on plasticity in schizophrenia are speculative. However, the minimal effects of DCS on plasticity in the current study could reflect a breakdown in the translation of electrical signaling across the NMDAR into the signaling cascades and synaptic changes that underlie experience-dependent plasticity.
Indeed, impaired translation of NMDAR signaling into structural synaptic changes that support plasticity is consistent with growing evidence that the broader glutamatergic PSD is compromised in schizophrenia. Large-scale genomic studies indicate that schizophrenia is associated with common and rare genetic variants that converge on both the NMDAR and the broader PSD in which NMDARs are embedded. For example, schizophrenia was associated with loci spanning GRIN2A which encodes the NR2A NMDAR subunit and serine racemase which generates the NMDAR co-agonist d-serine from l-serine, as well as genetic variants encoding scaffold proteins involved in trafficking and clustering NMDARs and AMPARs at the PSD, and activity-regulated cytoskeleton-associated protein, which is involved in synaptic remodeling and long-term maintenance of synaptic changes.10–13 A recent review concluded that genetic risk for schizophrenia converges on NMDAR-associated pathways involved in synaptic plasticity.69 Additionally, a recent proteomic study found that 143 out of approximately 700 PSD proteins were differentially expressed in schizophrenia brains compared to controls, with NMDAR-interacting proteins showing the most notable alterations.70 If a breakdown in NMDAR signaling occurs in the broader machinery that is coupled to NMDARs in schizophrenia, increasing the opening of the NMDAR channel would be insufficient to ameliorate deficits in plasticity. This could explain the limited effects of DCS on plasticity in the current study. Alternatively, given that other neurotransmitter systems (eg, dopamine, serotonin) and neurotrophic factors that can modulate plasticity have also been implicated in schizophrenia,71 the lack of group differences on experience-dependent plasticity in the current study could also reflect aberrances in these systems or interactions of these systems with NMDAR signaling.
Several limitations to the current study should be noted. First, we utilized a between-subject design. Thus, it is possible that group differences in N-back performance and pre-HFvS C1 amplitude reflected preexisting group differences rather than effects of DCS. However, the 2 groups were similar in clinical and demographic characteristics, IQ, and performance on the WPT and IIT. Given that this was an initial exploratory study, a between-subject design was chosen over a within-subject design to parallel our prior study in healthy adults and to avoid learning/practice effects on plasticity measures from repeat testing that could confound our ability to parse the effects of DCS. In healthy adults, we previously found that participant performance on a second day of WPT and IIT testing started at the same level as the end of practice on the first day of testing.50 Thus, we viewed a between-subject design as the most practical approach given the practice effects observed on these experience-dependent plasticity tasks. However, given this limitation, the modest sample size, and the fact that beneficial effects of DCS on the N-back were only found in the subgroup of patients who were defined behaviorally as adequately engaging in the task, independent replication is essential, including using a within-subject design for nonplasticity measures. Second, the majority of patients were taking antipsychotic medication, which could interact with the effects of DCS. Most prior studies of DCS in schizophrenia involved patients who were taking antipsychotics, including those showing beneficial effects of DCS.59,68 Patients in the current study were on relatively low antipsychotic doses and controlling for antipsychotic dose did not alter the effects of DCS. Additionally, patients who were antipsychotic-free performed similarly to those who were not and showed the same pattern of DCS effects across working memory and plasticity measures. Nevertheless, it is possible that different DCS effects would be found in a larger sample of un-medicated patients. Third, while our findings suggested differential effects of DCS on working memory versus experience-dependent plasticity in schizophrenia, we do not have information on the sensitivity or discriminating power of these tasks to DCS and these tasks are less commonly used than clinical neuropsychological tests. We created a N-back task with identical stimuli and trial structure to the IIT to allow us to compare effects of augmenting NDMAR signaling on working memory versus experience-dependent plasticity with greater clarity than neuropsychological tasks assessing these domains. Indeed, given that the N-back and IIT were identical in stimuli and trial structure, and that we previously found beneficial effects of DCS across the same plasticity measures in healthy adults, it is unlikely that the current lack of DCS effects on our plasticity measures was due to low discriminating power of these tasks. However, other tasks assessing these cognitive domains may have yielded different results. Finally, the agonist profile of DCS at NMDARs with different subunit compositions is complex. DCS has been shown to increase the channel open time at NMDARs containing the NR2C subunit with ~200% efficacy compared to glycine, whereas at NMDARs containing NR2A or 2B subunits, DCS has approximately 50% efficacy compared to glycine.72 Thus, while DCS is an agonist at NMDARs with NR2C subunits regardless of dose, at NMDARs with NR2B and NR2A subunits, DCS may act as an agonist at low doses (eg, 50–250 mg) by stimulating unoccupied glycine sites, but as an antagonist at high doses (eg 1000 mg)73 by displacing endogenous glycine. We chose a low DCS dose that was likely to act as an agonist across NMDAR subtypes and we previously found that 100 mg DCS enhanced plasticity in healthy adults. Thus, it is unlikely that the agonist profile of DCS depending on NMDAR subtype could account for the pattern of DCS effects in schizophrenia patients. Nevertheless, given that NMDARs with different subunits may differentially participate in various cognitive functions,74 further studies clarifying the involvement of NMDAR subtypes in various cognitive functions as well as the agonist profile of DCS at each NMDAR subtype would be useful.
The NMDAR has played a prominent role in theories explaining the cognitive deficits of schizophrenia. As such, numerous studies have attempted to improve cognition in schizophrenia by targeting the NMDAR, with relatively limited success.75 The present results require replication in a larger sample of patients and in additional studies comparing patients to age- and gender-matched controls. Nevertheless, our findings raise the intriguing possibility that enhancing NMDAR signaling in schizophrenia may partially restore processes that are closely linked to electrical signaling across the NMDAR. Conversely, targeting other components of the postsynaptic signaling complex or other factors involved in synaptic plasticity may be required to ameliorate deficits in experience-dependent plasticity. These results highlight the need for further research investigating how discrete NMDAR-associated processes relate to individual cognitive functions in schizophrenia.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
Della Martin Foundation (to Asarnow); American Psychological Association Dissertation Research Award (to Forsyth), Society for a Science of Clinical Psychology Dissertation Grant Award (to Forsyth).
Supplementary Material
Acknowledgments
The authors thank the UCLA Aftercare Program and Center for the Assessment and Prevention of Prodromal States for assistance with participant recruitment, Drs Gregory Ashby and Barbara Knowlton for consultation on the IIT and WPT, respectively, and Cheryl Li, Heather Hansen, Devin Deer, and Chantelle Kinzel for assistance with data collection and/or programming.
References
- 1. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. 1999;25:309–319. [DOI] [PubMed] [Google Scholar]
- 3. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. [DOI] [PubMed] [Google Scholar]
- 4. Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. [DOI] [PubMed] [Google Scholar]
- 5. Akbarian S, Sucher NJ, Bradley D, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. [DOI] [PubMed] [Google Scholar]
- 7. Funk AJ, Rumbaugh G, Harotunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. 2009;20:1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–747, 705. [DOI] [PubMed] [Google Scholar]
- 9. Emamian ES, Karayiorgou M, Gogos JA. Decreased phosphorylation of NMDA receptor type 1 at serine 897 in brains of patients with schizophrenia. J Neurosci. 2004;24:1561–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weickert CS, Fung SJ, Catts VS, et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2013;18:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayalew M, Le-Niculescu H, Levey DF, et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry. 2012;17:887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohi K, Hashimoto R, Ikeda M, et al. Glutamate networks implicate cognitive impairments in schizophrenia: genome-wide association studies of 52 cognitive phenotypes. Schizophr Bull. 2015;41:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. [DOI] [PubMed] [Google Scholar]
- 17. Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl). 2006;188:408–424. [DOI] [PubMed] [Google Scholar]
- 18. Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zito K, Scheuss V. NMDA receptor function and physiological modulation. Encyclopedia Neurosci. 2009;6:1157–1164. [Google Scholar]
- 20. Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. [DOI] [PubMed] [Google Scholar]
- 21. Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. [DOI] [PubMed] [Google Scholar]
- 22. Vargas-Caballero M, Robinson HP. Fast and slow voltage-dependent dynamics of magnesium block in the NMDA receptor: the asymmetric trapping block model. J Neurosci. 2004;24:6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Yang Y, Wang CJ, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. [DOI] [PubMed] [Google Scholar]
- 26. Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. [DOI] [PubMed] [Google Scholar]
- 29. Yin HH, Mulcare SP, Hilário MR, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. [DOI] [PubMed] [Google Scholar]
- 32. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. [DOI] [PubMed] [Google Scholar]
- 33. Murray GK, Cheng F, Clark L, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weickert TW, Terrazas A, Bigelow LB, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn Mem. 2002;9:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagshal D, Knowlton BJ, Cohen JR, et al. Deficits in probabilistic classification learning and liability for schizophrenia. Psychiatry Res. 2012;200:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 2008;22:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siegert RJ, Weatherall M, Bell EM. Is implicit sequence learning impaired in schizophrenia? A meta-analysis. Brain Cogn. 2008;67:351–359. [DOI] [PubMed] [Google Scholar]
- 38. Pedersen A, Siegmund A, Ohrmann P, et al. Reduced implicit and explicit sequence learning in first-episode schizophrenia. Neuropsychologia. 2008;46:186–195. [DOI] [PubMed] [Google Scholar]
- 39. Clapp WC, Eckert MJ, Teyler TJ, Abraham WC. Rapid visual stimulation induces N-methyl-D-aspartate receptor-dependent sensory long-term potentiation in the rat cortex. Neuroreport. 2006;17:511–515. [DOI] [PubMed] [Google Scholar]
- 40. Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teyler TJ, Hamm JP, Clapp WC, Johnson BW, Corballis MC, Kirk IJ. Long-term potentiation of human visual evoked responses. Eur J Neurosci. 2005;21:2045–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kirk IJ, McNair NA, Hamm JP, et al. Long-term potentiation (LTP) of human sensory-evoked potentials. Wiley Interdiscip Rev Cogn Sci. 2010;1:766–773. [DOI] [PubMed] [Google Scholar]
- 43. Cavuş I, Reinhart RM, Roach BJ, et al. Impaired visual cortical plasticity in schizophrenia. Biol Psychiatry. 2012;71:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mears RP, Spencer KM. Electrophysiological assessment of auditory stimulus-specific plasticity in schizophrenia. Biol Psychiatry. 2012;71:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dravid SM, Burger PB, Prakash A, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30:2741–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henderson G, Johnson JW, Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D-aspartate receptor. J Physiol. 1990;430:189–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257: 7–12. [DOI] [PubMed] [Google Scholar]
- 48. Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. [DOI] [PubMed] [Google Scholar]
- 49. Mickley GA, Remus JL, Ramos L, Wilson GN, Biesan OR, Ketchesin KD. Acute, but not chronic, exposure to d-cycloserine facilitates extinction and modulates spontaneous recovery of a conditioned taste aversion. Physiol Behav. 2012;105:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forsyth JK, Bachman P, Mathalon DH, et al. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc Natl Acad Sci U S A. 2015;112:15331–15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wagshal D, Knowlton BJ, Cohen JR, et al. Impaired automatization of a cognitive skill in first-degree relatives of patients with schizophrenia. Psychiatry Res. 2014;215:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waldschmidt JG, Ashby FG. Cortical and striatal contributions to automaticity in information-integration categorization. Neuroimage. 2011;56:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 54. Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97:129–135. [DOI] [PubMed] [Google Scholar]
- 55. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV® Axis I Disorders—Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 56. Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. [DOI] [PubMed] [Google Scholar]
- 57. Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. [DOI] [PubMed] [Google Scholar]
- 58. Goff DC, Tsai G, Manoach DS, Coyle JT. Dose-finding trial of D-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry. 1995;152:1213–1215. [DOI] [PubMed] [Google Scholar]
- 59. Goff DC, Tsai G, Levitt J, et al. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry. 1999;56:21–27. [DOI] [PubMed] [Google Scholar]
- 60. Evins AE, Amico E, Posever TA, Toker R, Goff DC. D-Cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schizophr Res. 2002;56:19–23. [DOI] [PubMed] [Google Scholar]
- 61. Goff DC, Cather C, Gottlieb JD, et al. Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophr Res. 2008;106:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goff DC, Herz L, Posever T, et al. A six-month, placebo-controlled trial of D-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berl). 2005;179:144–150. [DOI] [PubMed] [Google Scholar]
- 63. Buchanan RW, Javitt DC, Marder SR, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. [DOI] [PubMed] [Google Scholar]
- 64. Di Russo F, Martínez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2002;15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Butler PD, Martinez A, Foxe JJ, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kirschstein T, Köhling R. What is the source of the EEG? Clin EEG Neurosci. 2009;40:146–149. [DOI] [PubMed] [Google Scholar]
- 68. Cain CK, McCue M, Bello I, et al. d-Cycloserine augmentation of cognitive remediation in schizophrenia. Schizophr Res. 2014;153:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry. 2015;77:52–58. [DOI] [PubMed] [Google Scholar]
- 70. Föcking M, Lopez LM, English JA, et al. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol Psychiatry. 2015;20:424–432. [DOI] [PubMed] [Google Scholar]
- 71. Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. [DOI] [PubMed] [Google Scholar]
- 72. Goff DC. d-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull. 2012;38:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krystal JH, Petrakis IL, Limoncelli D, et al. Characterization of the interactive effects of glycine andd-cycloserine in men: further evidence for enhanced NMDA receptor function associated with human alcohol dependence. Neuropsychopharmacology. 2011;36:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. [DOI] [PubMed] [Google Scholar]
- 75. Iwata Y, Nakajima S, Suzuki T, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2015;20:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.