Abstract
Catatonia is a psychomotor syndrome that not only frequently occurs in the context of schizophrenia but also in other conditions. The neural correlates of catatonia remain unclear due to small-sized studies. We therefore compared resting-state cerebral blood flow (rCBF) and gray matter (GM) density between schizophrenia patients with current catatonia and without catatonia and healthy controls. We included 42 schizophrenia patients and 41 controls. Catatonia was currently present in 15 patients (scoring >2 items on the Bush Francis Catatonia Rating Scale screening). Patients did not differ in antipsychotic medication or positive symptoms. We acquired whole-brain rCBF using arterial spin labeling and GM density. We compared whole-brain perfusion and GM density over all and between the groups using 1-way ANCOVAs (F and T tests). We found a group effect (F test) of rCBF within bilateral supplementary motor area (SMA), anterior cingulate cortex, dorsolateral prefrontal cortex, left interior parietal lobe, and cerebellum. T tests indicated 1 cluster (SMA) to be specific to catatonia. Moreover, catatonia of excited and retarded types differed in SMA perfusion. Furthermore, increased catatonia severity was associated with higher perfusion in SMA. Finally, catatonia patients had a distinct pattern of GM density reduction compared to controls with prominent GM loss in frontal and insular cortices. SMA resting-state hyperperfusion is a marker of current catatonia in schizophrenia. This is highly compatible with a dysregulated motor system in catatonia, particularly affecting premotor areas. Moreover, SMA perfusion was differentially altered in retarded and excited catatonia subtypes, arguing for distinct pathobiology.
Keywords: motor system, subthalamic nucleus, arterial spin labeling, SMA, retarded catatonia, cerebellum
Introduction
Catatonia has been described and termed by Karl Kahlbaum in 1874 1as a complex syndrome of bizarre motor behavior, impaired volition, and vegetative abnormalities. This severe psychomotor syndrome is associated with various psychiatric disorders and medical conditions and may also occur in the context of schizophrenia. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) allows diagnosing catatonia in all major mood disorders, psychotic disorders, in general medical conditions, and as a separate entity not otherwise specified. In general, the symptom presentation of catatonia is broad and very diverse. In addition, symptoms may wax and wane. 2–6Most importantly, the catatonic syndrome may become a live-threatening condition. 7,8
Even though recent investigations demonstrated considerably high prevalence rates of catatonia across psychiatric conditions 3,4,9–13(estimated prevalence rates: 28% schizophrenia and 44% affective disorders), 13–15little is known on the pathophysiology. Only few reports with small sample sizes explored catatonia with neuroimaging methods. 16,17Investigations yielded inconsistent results with reports of decreased 18,19as well as increased 20resting-state perfusion in prefrontal brain areas and the motor cortex. In catatonia, neural activity in the supplementary motor area (SMA) and primary motor cortex was reduced during finger tapping tasks. 17,21,22Moreover, catatonic patients had reduced gamma amino butyric acid A (GABAA) receptor density in the primary motor cortex. 23Finally, the few structural neuroimaging studies (eg, cerebral computed tomography investigations) detected alterations in catatonia mainly in frontal cortical regions and the cerebellum. 19,24,25
The psychomotor symptoms of schizophrenia map on the cerebral motor circuit. 26Schizophrenia patients with psychomotor retardation suffer from critical disturbances of cortico-subcortical interactions in the motor loop, compensated by increased premotor cortical activity. 27–29Thus, current catatonia symptoms may lead to increased premotor neural activity.
In this study, we aimed to compare resting-state cerebral blood flow (rCBF) and gray matter (GM) density (voxel-based morphometry [VBM]) between schizophrenia patients with current catatonia and without catatonia and healthy controls. Based on previous findings, we hypothesized current catatonia to be linked to increased neural activity, ie, hyperperfusion in premotor areas. In order to focus on homogeneous underlying pathology, we restricted the investigation to acute catatonia in the context of schizophrenia spectrum disorders.
Methods
Subjects
We included 42 schizophrenia patients according to DSM-5 criteria, either with current catatonia or without lifetime history of catatonia, and 41 healthy controls participants. Patients were recruited at the inpatient and outpatient departments of the University Hospital of Psychiatry, Bern. Healthy control subjects were recruited via advertisement and among staff and students. Controls were matched for age, gender, and duration of education. All participants were right-handed according to the Edinburgh Handedness Inventory. 30General exclusion criteria for all subjects were substance abuse or dependence other than nicotine, history of motor impairments such as dystonia, idiopathic parkinsonism, multiple sclerosis or stroke, history of head trauma with concurrent loss of consciousness, or history of electroconvulsive treatment. Additional exclusion criteria for controls were a history of any psychiatric disorder, as well as any first-degree relatives with schizophrenia or schizoaffective disorder. All participants provided written informed consent. The study protocol adhered to the declaration of Helsinki and was approved by the local ethics committee.
All participants were interviewed with the Mini-International Neuropsychiatric Interview (MINI). 31Patients were further interviewed with the Comprehensive Assessment of Symptoms and History (CASH). 32All but 3 patients were on antipsychotic medication, mean chlorpromazine equivalents (CPZEs) were calculated according to Woods. 33Six patients (2 with catatonia and 4 without) received benzodiazepines within 24 hours prior to magnetic resonance imaging (MRI) scanning (see supplementary table S1A). Diazepam equivalents (DEs) were calculated according to Ashton. 34In addition, we assessed psychopathology with the Positive And Negative Syndrome Scale (PANSS) 35and catatonia symptoms with the Bush Francis Catatonia Rating Scale (BFCRS). 36Briefly, the BFCRS consists of 23 items (eg, mutism, excitement, preservation, or autonomic abnormality). The first 14 items constitute the BFCRS Screening Instrument (BFCRSI), rating presence of signs independent of severity. Presence of 2 or more BFCRSI items for more than 24 hours indicate current catatonia. 36Catatonia severity rating includes all 23 items. Severity ratings for all items range from 0 to 3, with higher scores indicating increased severity. In addition to catatonia, patients had parkinsonian symptoms as determined by the Unified Parkinson’s Disease Rating Scale (UPDRS) 37motor part (table 1).
Table 1.
Demographic and Clinical Data
| Controls (n = 41) | Catatonia Patients (n = 15) | Patients Without Catatonia (n = 27) | Tests | |||
|---|---|---|---|---|---|---|
| Gender (n) | Men/women | Men/women | Men/women | χ2 | df | P |
| 25/16 | 11/4 | 17/10 | 0.74 | 2 | .691 | |
| M (SD) | M (SD) | M (SD) | F/T | df | P | |
| Age (y) | 38.6 (13.6) | 35.9 (12.7) | 37.1 (10.6) | 0.47 | 2 | .762 |
| Education (y) | 14.1 (2.7) | 12.0 (4.0) | 14.2 (3.1) | 2.81 | 2 | .066 |
| Number of episodes | — | 7.7 (8.3) | 6.5 (6.9) | 0.48 | 40 | .634 |
| DOI (mo) | — | 153.4 (143.9) | 126.4 (127.2) | 0.63 | 40 | .533 |
| CPZE (mg) | — | 461.3 (346.4) | 373.6 (359.4) | 0.77 | 40 | .447 |
| DE (mg) | — | 3.4 (12.9) | 8.7 (28.2) | −0.682 | 40 | .499 |
| PANSS total | — | 77.1 (18.9) | 67.1 (16.3) | 1.79 | 40 | .080 |
| PANSS positive | — | 16.0 (8.1) | 17.9 (6.2) | −0.86 | 40 | .394 |
| PANSS negative | — | 21.9 (6.9) | 16.4 (4.1) | 2.87 | 40 | .010 |
| BFCRS total | — | 8.2 (5.2) | 0.0 (0.0) | — | — | — |
| BFCRS screening | — | 5.4 (3.6) | 0.0 (0.0) | — | — | — |
| UPDRS III (motor part) | — | 15.6 (7.9) | 4.4 (5.3) | 5.09 | 36 | <.001 |
Note: BFCRS, Bush Francis Catatonia Rating Scale; CPZE, chlorpromazine equivalence; DE, diazepam equivalent; DOI, duration of illness; PANSS, Positive And Negative Syndrome Scale; UPDRS, Unified Parkinson’s Disease Rating Scale. Significant values are highlighted in bold.
Catatonia was currently present in 15 patients (scoring at least at 2 items on the BFCSI for more than 24 h). Two groups of patients were not included in this study: first patients with past catatonia episodes according to the CASH interview and review of all case files who would not qualify for current catatonia according to the BFCRS and second patients with current catatonia symptoms below the BFCRS threshold. Patients with catatonia (n = 15) and without catatonia (n = 27) did not differ in medication dosage (CPZEs or DEs) or positive and total PANSS scores (see table 1). In addition, we classified catatonia patients into those with motor retardation (retarded catatonia: loading on “retarded” signs derived from the BFCSI: immobility/stupor, mutism, staring, echopraxia/echolalia, rigidity, negativism, and withdrawal) and those with motor excitation (excited catatonia: loading on “excited” signs on the BFCSI: excitement, grimacing, stereotypy, and verbigeration). 10Full list of catatonia symptoms presented is given in the supplementary table S1B.
Neuroimaging
Imaging was performed on a 3T MRI scanner (Siemens Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) with a 12-channel radio frequency head coil for signal reception. 3D-T1-weighted (Modified Driven Equilibrium Fourier Transform [MDEFT] pulse sequence) images for each subject have been obtained, providing 176 sagittal slices with 256 × 256 matrix points with a noncubic field of view (FOV) of 256 mm, yielding a nominal isotopic resolution of 1 mm3 (ie, 1 mm × 1 mm × 1 mm). Further scan parameters for the anatomical data were 7.92 ms repetition time (TR), 2.48 ms echo time (TE) and a flip angle of 16° (FA). In addition, 110 functional images (pseudocontinuous arterial spin labeling [ASL] sequence), providing 20 slices with 64 × 64 matrix points with a noncubic FOV of 230 mm, yielding a nominal isotopic resolution of 4.27 mm3 (ie, 3.6 mm × 3.6 mm × 6 mm) have been obtained. Further scan parameters for the functional images were TR of 4000 ms, TE of 18 ms, and a FA of 25°.
Structural and perfusion images were processed using SPM8 (Wellcome Trust Center for Neuroimaging, London; http://www.fil.ion.ucl.ac.uk/spm). Preprocessing of the perfusion images was conducted with an in-house-written MATLAB program toolbox. 29In detail, ASL images were first realigned. From the time series of these realigned ASL signal, the mean regional resting-state cerebral blood (rCBF) flow was calculated voxelwise and stored as a CBF map. Then all these CBS maps were co-registered to the T1 weighted images, normalized, and smoothed with 8-mm full-width at half maximum (FWHM) kernel.
All preprocessing steps of the VBM data were conducted using standard procedures as implemented in SPM8 (VBM toolbox). Images were normalized, segmented into tissue classes, modulated, and smoothed with 8-mm FWHM kernel.
Statistical Analyses
Statistical tests were performed using SPM routines and SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Two-sample t tests, 1-way ANOVAs, and chi-square tests (χ2) were used to test for group differences in continuous and categorical variables. Effects of categorical and continuous variables on rCBF and VBM were investigated using whole-brain 1-way ANCOVAs, 2-sample t tests, and multiple regression analyses, respectively.
The main focus of the analyses was the effect of catatonia on rCBF. Therefore, rCBF differences were calculated between all patients, patient subgroups (catatonia patients and patients without catatonia), and healthy controls using a whole-brain ANCOVA (F and T tests). We excluded all voxels with less than 10 ml/min blood flow and included age and motion parameters as covariates of no interest.
Within patients, we tested the association of catatonia severity and whole-brain rCBF by including BFCRS total values and rCBF in a multiple regression analysis (T tests). As in the group analyses, we excluded all voxels with less than 10 ml/min blood flow and included age and motion parameters as covariates of no interest.
Likewise group differences in GM density were calculated comparing VBM between all patients, patient subgroups, and controls using a whole-brain ANCOVA (F and T tests). Covariates included age and individual total GM volumes in order to correct for head size. In addition, we excluded all voxels with GM values of less than 0.1 (absolute threshold masking). Similarly to rCBF data, we tested the association of catatonia severity and whole-brain GM density within patients. We therefore included BFCRS total values and GM density using multiple regression analysis (T tests).
A uniform statistical threshold of P <.001 (uncorrected) with minimum cluster size of 100 voxels was applied to all analyses. For illustration purposes, we extracted the data from significant clusters of the whole-brain analyses for each subject with the SPM toolbox MarsBaR. 38
Exploratory analyses tested the effects of clinical parameters on the rCBF findings, including catatonia subtypes, medication, parkinsonism, or negative symptoms. First, to rule out the putative effects of medication on our whole-brain findings, we provided additional ANCOVAs of the main contrasts in the supplementary material with CPZEs and DEs as additional covariates. Second, we compared extracted mean perfusion values in the SMA cluster between catatonia subtypes (retarded type: n = 6 and excited type: n = 9), patients without catatonia, and healthy controls using an ANOVA with post hoc T tests corrected for multiple comparisons (Sidak). Third, we calculated partial correlations of extracted CBF values in patients and catatonia severity (BFCRS) controlling for UPDRS III, PANSS negative, CPZEs, and DEs.
Results
As expected, scores of catatonia (BFCRS), negative symptoms (PANSS negative), and parkinsonism (UPDRS III) were highly correlated (BFCRS and PANSS negative: r = .474, P = .002; BFCRS and UPDRS III: r = .655, P ≤ .001; PANSS negative and UPDRS III: r = .354, P = .021). 12,39,40Furthermore, in line with the literature, no catatonia symptom was confined exclusively to 1 catatonia subtype 41(supplementary table S1B and S1C).
SMA Hyperperfusion in Catatonia
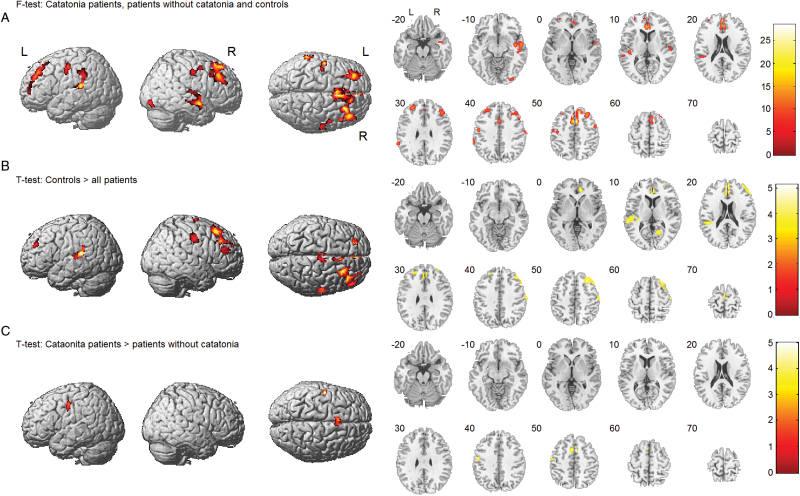
Motion parameters of CBF data demonstrated no group difference (see supplementary table S2). The ANCOVA of rCBF identified significant group effects in the bilateral SMA (P(FWE-corr.) < .001), anterior cingulate cortex (ACC, P(FWE-corr.) = .014), dorsolateral prefrontal cortex, left interior parietal lobe, left superior temporal gyrus (P(FWE-corr.) = .036), and left ventral premotor cortex (vPMC) comparing patients with catatonia, patients without catatonia, and healthy controls (see table 2 and figure 1A; supplementary table S3).
Table 2.
Resting-State Cerebral Blood Flow in Patients With and Without Catatonia and Controls (Regions Surviving Family-Wise Error Correction)
| Cluster | Peak | MNI Coordinates | ||||
|---|---|---|---|---|---|---|
| k | P (FWE-corr.) | F(2,73)/T(73) | x | y | z | |
| (A) F test: catatonia patients, patients without catatonia, and controls | ||||||
| L/R SMA | 1620 | <.001 | 28.525 | 4 | 12 | 52 |
| L/R ACC | 772 | .014 | 17.779 | 8 | 34 | 14 |
| L superior temporal gyrus | 402 | .036 | 16.192 | −40 | −30 | 14 |
| (B) T test: controls > all patients | ||||||
| L/R primary motor cortex (M1) | 118 | .022 | 5.141 | 0 | −16 | 76 |
| R superior extending to middle frontal gyrus | 526 | .041 | 4.961 | 28 | 24 | 56 |
| (C) T test: catatonia patients > patients without catatonia | ||||||
| L/R SMA | 193 | .037 | 4.991 | 2 | 12 | 50 |
| (D) T test: catatonia patients > controls | ||||||
| No clusters | ||||||
| (E) T test: patients without catatonia > catatonia patients | ||||||
| No clusters | ||||||
| (F) T test: controls > catatonia patients | ||||||
| No clusters | ||||||
Note: ACC, anterior cingulate cortex; SMA, supplementary motor area. Full table with all significant effects, see supplementary material covariates: age, motion parameters.
Fig. 1.
Cerebral blood flow in patients and healthy controls. (A) Group effect comparing patients with and without catatonia and healthy controls. (B) Decreased resting-state cerebral blood flow (rCBF) in patients comparing all patients independent of catatonia symptoms and healthy controls. (C) Increased rCBF in the supplementary motor area in patients with catatonia compared to patients without catatonia. Covariates: age and motion parameters.
The comparison of all schizophrenia patients independent of catatonia symptoms and healthy controls (T test) revealed several mainly frontal and temporal brain areas with reduced rCBF in patients. However, no effect appeared in the SMA and the vPMC contrasting all patients to controls (table 2 and figure 1B; supplementary table S3). Instead, subjects with current catatonia had significant hyperperfusion in the SMA and the left vPMC compared to patients without catatonia (table 2 and figure 1C; supplementary table S3).
Moreover, within patients, catatonia severity and rCBF were linearly associated within the SMA and the left vPMC. In fact, severer catatonia was associated with higher perfusion of brain regions including bilateral the SMA and the left vPMC (supplementary figure S1 and table S4).
Furthermore, the ANCOVA of rCBF including antipsychotic and benzodiazepine dosages as covariate (CPZEs and DEs) yielded substantially the same results confirming relative hyperperfusion of the SMA in catatonia patients vs noncatatonic patients (see supplementary table S5).
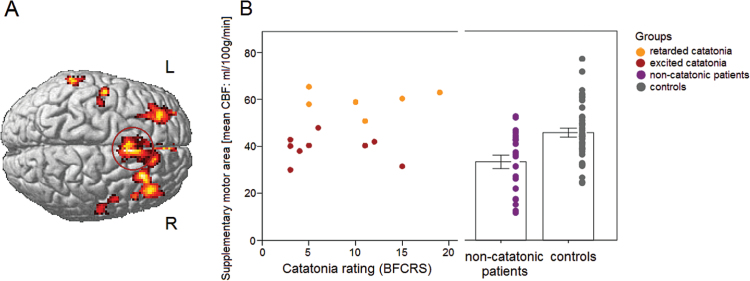
Among patients, relative hyperperfusion of the SMA appears to be specific to catatonia. But the type of catatonia presentation had a major influence. Exploratory tests of extracted rCBF values in the SMA cluster with significant (FWE-corrected) group effect in the ANCOVA (catatonia vs patients without catatonia vs healthy controls) substantiate increased rCBF in retarded catatonia compared to excited catatonia patients and compared to noncatatonic patients (T = 7.102, P = .034 and T = 6.082, P < .001) (figure 2B). Moreover, noncatatonic patients had reduced rCBF compared to healthy controls (T = 3.340, P < .001). A very similar finding emerged for the rCBF data extracted from the cluster within the vPMC (see supplementary figure S2).
Fig. 2.
(A) Group effect comparing resting-state cerebral blood flow (rCBF) between patients with and without catatonia and healthy controls. (B) Mean rCBF values in the supplementary motor area in all in patients with catatonia and motor excitation, in patients with retarded catatonia, noncatatonic patients and healthy controls. Values of scores of catatonia severity = 0 in noncatatonic patients and healthy controls. BFCRS, Bush Francis Catatonia Rating Scale.
Exploratory partial correlations indicated that extracted rCBF in the SMA and catatonia severity were positively correlated (r(40) = .514, P = .001) in patients. This association remained significant when controlling for parkinsonism, negative symptoms, and medication (r(36) = .366, P = .024) (see supplementary figure S3).
Reduced Insular and Frontal GM Density in Catatonia
A significant group effect in GM density appeared in various brain areas, including the right insula (P(FWE-corr.) = .012) and the frontal and temporal cortex (see supplementary figure S4A and table S6A). Patients with catatonia had reduced GM density compared to controls in frontal cortex and insula, but not in the temporal cortex (supplementary figure S4C and table S6C). In contrast, schizophrenia patients without catatonia had reduced GM density compared to controls in temporal and frontal cortex, but not in the insula (supplementary figure S4B and table S6B). In addition, patients with catatonia had increased cerebellar GM density compared to patients without catatonia in a large cluster including the cerebellar region VIIb.
Moreover, catatonia severity demonstrated a positive linear association with GM within patients within the cerebellum (supplementary figure S5A and table S7A) and an inverse association within the frontal cortex and the insula (supplementary figure S5B and table S7B). In fact, severer catatonia was associated with higher GM density of the cerebellum and lower GM density of the frontal cortex (superior frontal gyrus and ACC) and the right insula.
Finally, the exploratory ANCOVA of GM density including antipsychotic and benzodiazepine dosage (CPZEs and DEs) as covariates yielded substantially the same results confirming reduced GM density compared to controls in frontal cortex and insula, but not in the temporal cortex in catatonia as well as increased cerebellar GM density compared to noncatatonic patients (see supplementary table S8).
Discussion
Even though catatonia is a highly prevalent syndrome, 2,3the underlying pathobiology is still unknown. The existing studies investigated small clinically heterogeneous samples with very basic measures of rCBF. Here, we focused on state catatonia in schizophrenia as determined by a standard clinical rating scale, ie, the BFCRS. Furthermore, we applied a modern rCBF measure, ie, MRI with ASL. Indeed, ASL provides an absolute measure of rCBF, reflecting the level of state neuronal activity with superior spatial resolution compared to positron emission tomography. We confirmed our hypothesis of increased resting-state blood flow in premotor areas in catatonia compared to noncatatonic patients. In fact, among schizophrenia patients, increased perfusion in the SMA and vPMC, key regions for self-initiated movements, 26,42was specific for state catatonia. Particularly, SMA perfusion differed between excited and retarded catatonia, indicating increased SMA neural activity in the retarded state. Moreover, increased catatonia severity was associated with higher perfusion in SMA and vPMC. These findings held true even after controlling for antipsychotic and benzodiazepine dosage. Finally, we detected a specific pattern of GM decrease in catatonia patients compared to controls, which appeared different from the pattern in schizophrenia patients without catatonia.
The SMA as a Key Player
The SMA is tightly connected to the primary motor cortex, the pre-SMA, the striatum (including direct and indirect motor pathways), the subthalamic nucleus (STN) (hyperdirect pathway), the thalamus, and the corticospinal tract. 43The SMA is involved in selecting, preparing, and executing different modes of action, particularly internally generated movements. 43Most important, SMA activity may facilitate or inhibit ongoing action via the basal ganglia loops. 43,44Particularly, the hyperdirect pathway from SMA to the STN can rapidly stop ongoing movement. 45Aberrant SMA structure and function has been implicated in altered motor behavior in schizophrenia. For example, structural alterations in the SMA 46and connecting fibers 27,29were associated with the severity of motor impairments in schizophrenia. Furthermore, reduced SMA activation during motor tasks has been repeatedly reported in schizophrenia, in general, 47and in catatonic schizophrenia. 17,21Finally, increased rCBF in premotor areas, including the SMA, correlated with higher objectively assessed motor activity in schizophrenia. 29Hence, the present study adds to existing body of evidence supporting a key role of the SMA in psychomotor symptoms in schizophrenia. According to our model, patients presenting motor symptoms may use cortical pathways involving the SMA to compensate for insufficient or inhibitory basal ganglia output. 26Indeed, retarded catatonia states presented with hyperperfusion of the SMA, linking increased neural activity to massive motor inhibition. In line with this observation, motor retardation in acute catatonia is alleviated by lorazepam administration. 7In fact, one study demonstrated reduced iomazenil binding in the left sensorimotor cortex in akinetic catatonia, suggesting a decrease in the density of GABAA receptors in the primary motor cortex in patients without benzodiazepine administration in the prior 6 months. 23Thus, benzodiazepine effects on various neurotransmitter systems may normalize rCBF in the motor loop including SMA. However, the molecular mechanism underlying catatonic states associated with increased SMA perfusion remains to be elucidated.
There are several possible explanations for increased rCBF in schizophrenia patients with state catatonia. For instance, increased neural activity may results from attempts to overcome massive inhibitory basal ganglia output. Alternatively, SMA hyperactivity may also drive basal ganglia inhibitory effects via the hyperdirect pathway to the STN. 43,45However, based on our previous findings in schizophrenia, we suggest that SMA hyperperfusion in catatonia may be related to inefficient connectivity between basal ganglia and cortical motor areas probably giving rise to motor inhibition. 26–29
Studies in other neuropsychiatric disorders with reduced motor behavior point in a similar direction. Bradykinesia in Parkinson’s disease (PD) shares some of the abnormal motor behavior of catatonia. 2In PD, the balance within the corticobasal ganglia motor loop is shifted to hypoactivation of the motor and premotor cortices, 48based on diminished drive within basalganglia-thalamo-premotor circuits. Specifically, older functional MRI literature suggests that bradykinesia is related to hypoactivation of SMA, which is thought to be partially compensated by hyperactivation of the primary motor cortex, 49pointing to a dysbalance within premotor-motor networks. In line with our finding, abnormally increased metabolic activity was detected in the motor network (particularly the premotor and motor cortex) of PD patients at rest. 50Likewise, major depressive disorder frequently presents with psychomotor retardation. In patients with major depressive disorder, we detected increased perfusion of SMA with reduced motor behavior, an association that was paralleled in controls at trend level. 51As in retarded catatonia, behavioral inhibition was associated with increased SMA perfusion.
Structural Changes Linked to Catatonia
In line with the literature, schizophrenia was associated with reduced GM density in the bilateral insula, the anterior cingulate gyrus, and the left inferior frontal cortex. 52In addition, the pattern of relative GM density reductions differed between patients with catatonia and patients without catatonia. In fact, while temporal GM density was reduced in patients without catatonia symptoms compared to controls, this was not the case in the comparison of catatonia vs controls. A prominent loss of right hemispheric insular GM density was exclusively seen in catatonia patients compared to controls, along with frontal (eg, ACC) GM reductions. Finally, patients with catatonia had increased cerebellar GM density compared to patients without catatonia in a large cluster including the cerebellar region VIIb. This posterior cerebellar subregion has effective motor output on many body regions, most prominently the upper and lower extremities. 53,54Region VIIb sends output to striatal neurons of the indirect pathway. 55Furthermore, region VIIb has efferents to the motor part of the STN, which in turn is connected to SMA and M1. 55As noted above, STN is a key node of the indirect and the hyperdirect pathway within the motor corticobasal ganglia circuit. Aberrant increased STN activity would result in reduced motor output, as seen in PD. 55Therefore, increased GM of area VIIb may well fit in the hypothesis of inhibitory basal ganglia action in catatonia. 26In addition, this finding points towards a pathology in pathways including the STN in catatonia. Again, whether it is a direct inhibitory effect via motor cortex and STN or a compensation of inefficient connectivity within the corticobasal ganglia loop remains to be discovered. Some authors have suggested that cortico-cerebellar motor loops may compensate inhibition of the corticobasal ganglia loop in PD. 48This could also be the case in schizophrenia-associated catatonia. The GM density results held true after correction for antipsychotic and benzodiazepine dosage. Furthermore, our findings are in line with the few available structural neuroimaging studies showing prominent frontal as well as cerebellar structural changes linked to the catatonia syndrome. 24,25
Clinical Heterogeneity in Catatonia
Distinct types of catatonia have been proposed, as the clinical presentation of catatonia is characterized by multiple symptoms and varying course among patients. Interestingly, 2 classical catatonia types, 7differed in resting-state SMA perfusion: hyperperfusion in the retarded type and hypoperfusion in the excited type. The 2 types have been repeatedly identified in factor analyses 4,10and are considered to be opposing behavioral presentations. Our data suggest distinct pathobiology of the 2 subtypes within the same syndrome. Retarded catatonia includes symptoms such as stupor, mutism, rigidity, and negativism. Instead, the excited type is characterized by excitement, grimacing, stereotypy, and verbigeration. 10The SMA and related medial premotor areas, such as the cingulate motor area and pre-SMA, have been implicated in volitional aspects of behavior and drive. 2,26,43Most of the catatonia symptoms refer to disturbances in volition, action selection or motor planning. 2
This dissociation between excited and retarded catatonia may also explain the inconsistencies in brain imaging studies with cerebral hypoperfusion as well as hyperperfusion in catatonia. Moreover, our results suggest that different catatonia states have distinct pathobiology. In addition, the results on excited and retarded catatonia require careful interpretation, given the exploratory nature of such kind of analysis and the small sample size of these catatonia subtypes.
The diagnosis and nosology of the catatonia syndrome remains a matter of debate. There are probably more than the investigated 2 classic catatonia dimensions. In fact, factor analytic studies identified 3–6 factors. 4,10,56–59The ongoing debate on the definition and course of catatonia, however, hampers thorough neurobiological investigations. 4,60
Currently, there is no evidence supporting a differential treatment of catatonia subtypes in schizophrenia. Some clinical characteristics have been suggested to influence treatment response to electroconvulsive therapy (ECT) or benzodiazepines, among them chronicity, predominant affective state, age, and schizophrenia diagnosis. 61–63Case series reported rapid response to benzodiazepine administration in patients with acute stupor. 64,65Moreover, some experts suggested effectiveness of benzodiazepines in retarded catatonia. 61In contrast, a randomized placebo-controlled trial failed to demonstrate an effect of lorazepam on catatonia in chronic schizophrenia. 62Both catatonia subtypes (retarded and excited type) in nonaffective psychoses benefit from antipsychotic therapy. 9To our knowledge, no randomized controlled trial investigated the impact of catatonia subtypes on treatment response to ECT or benzodiazepines in schizophrenia. We may speculate that increased SMA perfusion in retarded catatonia could be reduced by benzodiazepine administration alleviating the motor inhibition; however, this has to be tested in future studies.
Possible Limitations
First of all, this study exclusively focused on catatonia in the context of schizophrenia spectrum disorders. Thus, our results must not be easily transferred to catatonia of different origin. In addition, patients requiring ECT were excluded. Patients with substantial negativism were not included if they could not provide informed consent. These limitations introduce some selection bias.
Furthermore, all patients with catatonia had received treatment for the catatonic syndrome. In fact, medication with antipsychotics and benzodiazepines remains one possible limitation of our study as all but 3 patients were medicated with antipsychotics and 6 patients received benzodiazepines, which may affect brain structure and function. However, reanalyzing our data including dosage of antipsychotics and benzodiazepines as covariates yielded substantially the same results. As expected, catatonia patients had more parkinsonian signs and negative symptoms compared to patients without catatonia. However, partial correlations indicated higher SMA perfusion associated with catatonia severity even when controlling for parkinsonism, negative symptoms, and medication dosage. In line with the literature, catatonia, parkinsonism, and negative symptoms are highly correlated in our sample. 12,39,40,66This might either reflect true comorbidity or more likely constitute a “conflict of paradigms,” ie, conceptual overlap when observing the same phenomenon as proposed by Rogers. 67Finally, a relatively small number of patients has been included in our analysis and exploratory analyses of catatonia subtypes have therefore to be interpreted with caution. However, to our knowledge, this is the largest homogeneous group of catatonia patients ever investigated with brain imaging.
Conclusions
SMA resting-state perfusion may serve as a marker of current catatonia in schizophrenia. This is highly compatible with a dysregulated motor system in catatonia, particularly affecting premotor areas and the STN. Furthermore, differentially altered SMA perfusion was shown in retarded and excited catatonia arguing for biologically distinct clinical subtypes. Further studies need to clarify whether the findings also hold true for catatonia of different origin. The distinct clinical and biological subtypes of catatonia may require different therapeutic approaches, which remain to be discovered.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This study received funding from the Bangerter-Rhyner Foundation (to S.W.) and the Swiss National Science Foundation (SNF grant 152619/1 to S.W., A.F., and S.B.).
Supplementary Material
Acknowledgments
We thank Nadja Razavi for help with data collection and Kay Jann who kindly provided the arterial spin labeling tool for our analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kahlbaum K.Die Katatonie oder das Spannungsirresein. Eine klinische Form psychischer Krankheit. Berlin, Germany: A Hirschwald; 1874. [Google Scholar]
- 2. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. [DOI] [PubMed] [Google Scholar]
- 3. Stuivenga M, Morrens M. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry. 2014;5:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164:256–262. [DOI] [PubMed] [Google Scholar]
- 5. Walther S, Stegmayer K, Horn H, et al. The longitudinal course of gross motor activity in schizophrenia—within and between episodes. Front Psychiatry. 2015;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walther S, Stegmayer K, Horn H, Razavi N, Müller TJ, Strik W. Physical activity in schizophrenia is higher in the first episode than in subsequent ones. Front Psychiatry. 2014;5:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66:1173–1177. [DOI] [PubMed] [Google Scholar]
- 8. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;127(suppl 441): 1–47. [DOI] [PubMed] [Google Scholar]
- 9. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 10. Grover S, Chakrabarti S, Ghormode D, Agarwal M, Sharma A, Avasthi A. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229:919–925. [DOI] [PubMed] [Google Scholar]
- 11. Compton MT, Fantes F, Wan CR, Johnson S, Walker EF. Abnormal movements in first-episode, nonaffective psychosis: dyskinesias, stereotypies, and catatonic-like signs. Psychiatry Res. 2015;226:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ungvari GS, Leung SK, Ng FS, Cheung HK, Leung T. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’): I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:27–38. [DOI] [PubMed] [Google Scholar]
- 13. Walther S, Strik W. Catatonia. CNS Spectr. 2016;21:341–348. [DOI] [PubMed] [Google Scholar]
- 14. Francis A, Fink M, Appiani F, et al. Catatonia in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. J ECT. 2010;26:246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Ameele S, Sabbe B, Morrens M. Characteristics of catatonia in schizophrenia and mood disorders. Tijdschr Psychiatr. 2015;57:94–98. [PubMed] [Google Scholar]
- 16. Northoff G, Kötter R, Baumgart F, et al. Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull. 2004;30:405–427. [DOI] [PubMed] [Google Scholar]
- 17. Scheuerecker J, Ufer S, Käpernick M, et al. Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res. 2009;43:607–614. [DOI] [PubMed] [Google Scholar]
- 18. Galynker I, Vilkas N, Dutta E, et al. Secondary negative syndrome and rCBF in prefrontal cortex. Biol Psychiatry. 1997;41:65–65.8988797 [Google Scholar]
- 19. Northoff G. Brain imaging in catatonia: current findings and a pathophysiologic model. CNS Spectr. 2000;5:34–46. [DOI] [PubMed] [Google Scholar]
- 20. Tsujino N, Nemoto T, Yamaguchi T, et al. Cerebral blood flow changes in very-late-onset schizophrenia-like psychosis with catatonia before and after successful treatment. Psychiatry Clin Neurosci. 2011;65:600–603. [DOI] [PubMed] [Google Scholar]
- 21. Payoux P, Boulanouar K, Sarramon C, et al. Cortical motor activation in akinetic schizophrenic patients: a pilot functional MRI study. Mov Disord. 2004;19:83–90. [DOI] [PubMed] [Google Scholar]
- 22. Northoff G, Braus DF, Sartorius A, et al. Reduced activation and altered laterality in two neuroleptic-naive catatonic patients during a motor task in functional MRI. Psychol Med. 1999;29:997–1002. [DOI] [PubMed] [Google Scholar]
- 23. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilcox JA. Cerebellar atrophy and catatonia. Biol Psychiatry. 1991;29:733–734. [DOI] [PubMed] [Google Scholar]
- 25. Northoff G, Waters H, Mooren I, et al. Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry Res. 1999;91:45–54. [DOI] [PubMed] [Google Scholar]
- 26. Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233:293–298. [DOI] [PubMed] [Google Scholar]
- 27. Bracht T, Schnell S, Federspiel A, et al. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res. 2013;143:269–276. [DOI] [PubMed] [Google Scholar]
- 28. Walther S, Federspiel A, Horn H, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011;42:276–283. [DOI] [PubMed] [Google Scholar]
- 29. Walther S, Federspiel A, Horn H, et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res. 2011;192:117–124. [DOI] [PubMed] [Google Scholar]
- 30. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 31. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 32. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 33. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 34. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158–165. [Google Scholar]
- 35. Kay SR, Fiszbein A, Opler LA. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 36. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. [DOI] [PubMed] [Google Scholar]
- 37. Fahn S, Elton RL. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Vol. 2 Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 38. Brett M, Anton J-L, Romain V, Poline J-B. Region of interest analysis using an SPM toolbox [abstract 497]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan, Vol. 16, No. 2. [Google Scholar]
- 39. Docx L, Morrens M, Bervoets C, et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126:256–265. [DOI] [PubMed] [Google Scholar]
- 40. Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011;52:139–145. [DOI] [PubMed] [Google Scholar]
- 41. Morrison JR. Catatonia. Retarded and excited types. Arch Gen Psychiatry. 1973;28:39–41. [DOI] [PubMed] [Google Scholar]
- 42. Fahn S, Jankovic J, Hallett M. Principles and Practice of Movement Disorders. 1st ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2007. [Google Scholar]
- 43. Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. [DOI] [PubMed] [Google Scholar]
- 44. Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci. 2010;30:14657–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. [DOI] [PubMed] [Google Scholar]
- 46. Stegmayer K, Horn H, Federspiel A, et al. Supplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res. 2014;223:49–51. [DOI] [PubMed] [Google Scholar]
- 47. Schröder J, Essig M, Baudendistel K, et al. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. Neuroimage. 1999;9:81–87. [DOI] [PubMed] [Google Scholar]
- 48. Martinu K, Monchi O. Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson’s disease: pathophysiology or compensation? Behav Neurosci. 2013;127:222–236. [DOI] [PubMed] [Google Scholar]
- 49. Haslinger B, Erhard P, Kämpfe N, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 2001;124:558–570. [DOI] [PubMed] [Google Scholar]
- 50. Ko JH, Mure H, Tang CC, et al. Parkinson’s disease: increased motor network activity in the absence of movement. J Neurosci. 2013;33:4540–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walther S, Höfle O, Federspiel A, et al. Neural correlates of disbalanced motor control in major depression. J Affect Disord. 2012;136:124–133. [DOI] [PubMed] [Google Scholar]
- 52. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. [DOI] [PubMed] [Google Scholar]
- 53. Mottolese C, Richard N, Harquel S, Szathmari A, Sirigu A, Desmurget M. Mapping motor representations in the human cerebellum. Brain. 2013;136:330–342. [DOI] [PubMed] [Google Scholar]
- 54. Batson MA, Petridou N, Klomp DW, Frens MA, Neggers SF. Single session imaging of cerebellum at 7 Tesla: obtaining structure and function of multiple motor subsystems in individual subjects. PLoS One. 2015;10:e0134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ungvari GS, Goggins W, Leung SK, Gerevich J. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’). II. Factor analysis of the catatonic syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:462–468. [DOI] [PubMed] [Google Scholar]
- 57. Krüger S, Bagby RM, Höffler J, Bräunig P. Factor analysis of the catatonia rating scale and catatonic symptom distribution across four diagnostic groups. Compr Psychiatry. 2003;44:472–482. [DOI] [PubMed] [Google Scholar]
- 58. Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010;118:168–175. [DOI] [PubMed] [Google Scholar]
- 59. Peralta V, Cuesta MJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr Res. 2001;47:107–116. [DOI] [PubMed] [Google Scholar]
- 60. Ungvari GS. Catatonia in DSM 5: controversies regarding its psychopathology, clinical presentation and treatment response. Neuropsychopharmacol Hung. 2014;16:189–194. [PubMed] [Google Scholar]
- 61. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142:393–398. [DOI] [PubMed] [Google Scholar]
- 63. van Waarde JA, Tuerlings JH, Verwey B, van der Mast RC. Electroconvulsive therapy for catatonia: treatment characteristics and outcomes in 27 patients. J ECT. 2010;26:248–252. [DOI] [PubMed] [Google Scholar]
- 64. Ungvari GS, Leung CM, Wong MK, Lau J. Benzodiazepines in the treatment of catatonic syndrome. Acta Psychiatr Scand. 1994;89:285–288. [DOI] [PubMed] [Google Scholar]
- 65. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry. 1990;51:357–362. [PubMed] [Google Scholar]
- 66. McKenna PJ, Lund CE, Mortimer AM, Biggins CA. Motor, volitional and behavioural disorders in schizophrenia. 2. The ‘conflict of paradigms’ hypothesis. Br J Psychiatry. 1991;158:328–336. [DOI] [PubMed] [Google Scholar]
- 67. Rogers D. The motor disorders of severe psychiatric illness: a conflict of paradigms. Br J Psychiatry. 1985;147:221–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.