Abstract
Neuroimaging studies investigating dopamine (DA) function widely support the hypothesis of presynaptic striatal DA hyperactivity in schizophrenia. However, published data on the striatal DA transporter (DAT) appear less consistent with this hypothesis, probably partly due to methodological limitations. Moreover, DAT in extrastriatal regions has been very poorly investigated in the context of schizophrenia. In order to address these issues, we used a high resolution positron emission tomograph and the selective DAT radioligand [11C]PE2I, coupled with a whole brain voxel-based analysis method to investigate DAT availability in striatal but also extra-striatal regions in 21 male chronic schizophrenia patients compared to 30 healthy male controls matched by age. We found higher DAT availability in schizophrenia patients in midbrain, striatal, and limbic regions. DAT availability in amygdala/hippocampus and putamen/pallidum was positively correlated with hallucinations and suspiciousness/persecution, respectively. These results are consistent with an increase of presynaptic DA function in patients with schizophrenia, and support the involvement of both striatal and extrastriatal DA dysfunction in positive psychotic symptoms. The study also highlights the whole brain voxel-based analysis method to explore DA dysfunction in schizophrenia.
Keywords: neuroimaging, DAT, psychosis, positive symptoms
Introduction
Striatal presynaptic dopaminergic hyperactivity is among the most widely replicated results in neurochemical imaging studies of schizophrenia. This finding is based on the repeatedly demonstrated increase of striatal dopamine (DA) synthesis capacity1,2 in studies using radiolabeled L-DOPA, the precursor of DA. Increases in baseline DA synaptic concentration and release have also been reported using D2 receptors imaging,3 thus offering further evidence of a striatal DA hyperactivity in schizophrenia. These results are consistent with the DA hypotheses of schizophrenia, which postulate that DA hyperactivity in subcortical brain regions may account for the positive symptoms of the disease.4,5 However, neuroimaging studies of the DA transporter (DAT), a critical presynaptic component of the DA function regulating synaptic DA concentration through high-affinity reuptake,6 appear less consistent. Since DAT is expressed exclusively in neurons synthesizing DA, it is also considered to reflect the density of DA neurons. Therefore, a number of neuroimaging studies have explored the DAT availability in schizophrenia patients as a putative biomarker of DA dysfunction associated with the disease. Overall, the results of these studies appear disappointing and recent meta-analyses lead to the conclusion of a lack of DAT abnormality in schizophrenia.2,7 For instance, a meta-analysis including 13 single photon emission tomography (SPECT) and positron emission tomography (PET) studies revealed no evidence of an alteration of striatal DAT availability in schizophrenia patients, and no influence of antipsychotic treatment, duration of illness or severity of psychotic symptoms.7
Some methodological issues might have impaired the reproducibility of the DAT neuroimaging studies outcomes. First, all these studies used predefined regions of interest (ROIs) for imaging data analysis, most often large ROIs directly drawn on the caudate and putamen nuclei or on the whole striatum. This method, mainly based on anatomic subdivisions, appears to be relatively coarse considering the complexity of the DA projections, especially in subcortical areas. Furthermore, it does not allow the examination of abnormalities involving only parts of caudate or putamen, or areas with mixed normal and abnormal DAT densities. Second, almost all studies have evaluated the DAT exclusively in the striatum since the low DAT density in extra-striatal structures precludes its detection using conventional PET and SPECT scanners. The only PET study that explored some extra-striatal regions, namely thalamus and subtantia nigra, found an augmentation of DAT availability in thalamus positively correlated with clinical symptoms,8 highlighting the interest of wider brain regional investigations. Finally, recent data suggest that tobacco smoking, a factor that has not been considered in most previous studies, could decrease DAT availability.9–11 Therefore, the relative higher prevalence of tobacco smokers in patients with respect to the general population12 could have confounded a schizophrenia-related effect.
To address these issues, we investigated the DAT availability in striatal and extra-striatal regions without anatomical a priori hypotheses in a group of male chronic schizophrenia patients compared to a group of healthy male subjects matched by age. We used a high resolution research tomograph (HRRT, Siemens Medical Solutions) with [11C]PE2I, a potent and highly selective DAT radioligand,13 and a whole brain voxel-based analysis (requiring no a priori defined ROIs). Previous studies using HRRT and [11C]PE2I in healthy controls reported good suitability and test/retest reproducibility, and better accuracy in quantifying DAT binding in the striatal and extrastriatal DA regions, as compared with conventional PET scans.14,15 Smoking status and age were used as confounding variables in all statistical analyses. We sought to identify DAT availability abnormalities consistent with the DA hyperactivity reported in schizophrenia. We also hypothesized these DAT abnormalities in striatal or extrastriatal areas could be related to clinical symptoms of the illness consistently with the DA hypotheses of schizophrenia.
Methods
Participants
The study was approved by the regional biomedical research ethics committee (CPP IDF04-38), and each subject gave written informed consent after receiving full information on the procedures, prior to the study. Insurance promotion and administrative conditions were established (N° DGS: 2004/0497; coordinator: J.L.M.).
Male healthy controls (HC) and male patients with schizophrenia (SCZ) who met criteria for a diagnosis of schizophrenia according to the DSM-IV-TR were included. SCZ were recruited by senior psychiatrists from psychiatric departments within the south Paris area.
The clinical assessment included the verification of the diagnosis by senior psychiatrists, review of hospital records, and an interview about personal psychiatric and medico-surgical antecedents, as well as former and current treatments. Antipsychotic medications were allowed in mono-therapy, since typical or atypical antipsychotic drugs are unlikely to interfere with DAT binding.16–21 No association with other treatment like antidepressant or mood regulators was allowed, except low dosage of benzodiazepines. In order to further ensure the absence of interference with the brain imaging data, patients’ treatment characteristics (compounds, doses) were recorded.
Exclusion criteria were: age over 60, alcohol or substance abuse or dependence (except tobacco) in the past 6 months, treatment with molecule susceptible to interfere directly with DAT, electroconvulsive therapy treatment in the past six months, any present medical condition, history of epileptic seizures, history of other psychiatric, neurological disorders or substantial brain damage, and contraindication to magnetic fields (metal objects, claustrophobia) according to established safety criteria.
Given the known action of psychoactive substances on the DA system, semi-quantitative urinary multi-screens for the detection of cocaine, amphetamine, methamphetamine, cannabis, methadone, opiates, ecstasy, barbiturates, benzodiazepines, and tricyclic antidepressants (BMD: Biomedical Diagnostics) were systematically performed prior to each PET scan to rule out multiple-drug users. In order to minimize differences in short-term nicotine effects on the DA system, all smoker participants were asked to smoke one of their own cigarettes just before imaging session.
Clinical Assessments
Clinical evaluations were performed on the day of the imaging session by senior psychiatrists (E.A., J.L.M., M.A.D.). Severity of clinical symptoms was assessed using the Positive and Negative Symptom Scale (PANSS).22 Tobacco dependence was assessed by the Fagerström test of nicotine dependence (FTND).23
Data Acquisition and Processing
PET Acquisition.
A second generation Siemens ECAT HRRT 3D-PET scanner was used to acquire PET images. The HRRT has a 31.2 cm transaxial and 25.5 cm axial field of view (207 slices of 1.2 mm thickness). Point source resolution varied across the field of view approximately from 2.3 to 3.2 mm (FWHM) in the transaxial direction and from 2.5 to 3.4 mm in the axial direction.24 The subjects were positioned in the scanner using laser alignment. A thermoplastic head mask was molded to each subject’s face to restrain head movements, and head position was checked every 10 min during acquisition. A 6-min brain transmission scan was performed using a 137Cs point source to correct the emission scan for tissue attenuation. The emission scan started with the intravenous bolus injection of about 300 MBq of [11C]PE2I and lasted for 60 min. Injected radioactivity and specific radioactivity of SCZ and HC are shown in table 1. [11C]PE2I was prepared using a TRACERlab FX-C Pro synthesizer.25 Twenty sequential frames ranging from 1 to 5 min in duration were acquired in list mode. Images were reconstructed with the statistical algorithm OP-OSEM (Ordinary Poisson–Ordered Subset Expectation Maximization, 10 iterations using 16 subsets) including Point Spread Function (PSF) modeling so that no further post-correction was needed for partial volume effect. The voxel size was 1.2 × 1.2 × 1.2 mm3.
Table 1.
Subjects’ Characteristics
| Characteristic | Schizophrenia Patients (N = 21) | Healthy Controls (N = 30) | t-Tests | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t Value | df | P | |
| Age | 34.19 | 10.23 | 30.17 | 9.65 | 1.43 | 49 | .16 |
| BMI, kg/m−2 | 25.80 | 4.67 | 23.86 | 4.48 | 1.49 | 49 | .14 |
| Injected dose (MBq) | 296.37 | 69.19 | 296.00 | 40.33 | 0.05 | 47 | .95 |
| Radioactivity (GBq/µmole) | 23.50 | 13.75 | 25.66 | 12.64 | 0.77 | 47 | .44 |
| Patients clinical characteristics | |||||||
| Age at onset (y) | 20.51 | 4.65 | |||||
| Illness duration (y) | 13.57 | 9.25 | |||||
| Antipsychotics dosage (mg/d)a (N = 20) | 310.95 | 197.54 | |||||
| PANSS positive subscale | 18.85 | 5.43 | |||||
| PANSS negative subscale | 20.33 | 7.96 | |||||
| PANSS general psychopathology | 40.23 | 10.90 | |||||
| PANSS total | 79.42 | 20.42 | |||||
Note: PANSS, Positive and Negative Symptom Scale.
aExpressed as chlorpromazine equivalents (8 patients were treated with risperidone, 6 with aripiprazole, 2 with olanzapine, 2 with haloperidol, 1 with clozapine, 1 with clopentixol, and 1 was untreated).
MRI Acquisition.
Structural images were acquired using a 1.5 Tesla whole-body system (Signa, General Electric). T1-weighted structural MRI scan was carried out with the following specifications: 3D Fourier-transform spoiled-gradient-recalled acquisition with TR = 12.5 ms, TE = 2.2 ms, 124 contiguous slices, 256 × 256 view matrix, voxel size = 0.9375 mm × 0.9375 mm × 1.3 mm, 16 bits/pixel.
Data Analysis.
Motion corrections during PET acquisition were carried out using a home-made tool within the BrainVISA/Anatomist software (http://brainvisa.info) consisting in frame by frame co-registration of the PET dynamic series using a mutual information method. In order to process parametric binding potential images, brain regions were determined by T1-MRI automatic parcellation and applied on dynamic co-registered PET images using PMOD. Time-activity curves obtained from bilateral dorsal caudate and putamen nuclei as high specific binding and crus1 cerebellar subregion as a reference tissue were exported to PMOD. Parametric maps of the regional [11C]PE2I nondisplaceable binding potential (BPND) were generated using Gunn’s basis function method,26 which is closely related to the Simplified Reference Tissue Model of Lammertsma and Hume.27 Previous studies have validated the suitability of quantification of specific [11C]PE2I binding using a compartmental approach with the cerebellum as a reference region.14,15,28
Statistical Parametric Mapping software29 (SPM8, http://www.fil.ion.ucl.ac.uk/spm/) and Matlab (Math Works) were used for image processing and statistical analysis. The BPND maps were spatially normalized using a “homemade” ligand-specific [11C]PE2I template generated according to an MRI-aided procedure.30 The normalized BPND maps were smoothed using a 10 mm FWHM Gaussian filter. The voxel size was 2 × 2 × 2 mm3.
Statistical Analysis.
A one-sample t-test of all BPND maps was performed to visualize the localization of DAT availability in the brain and was used to define a mask of analysis. This mask has been defined as all voxels with a minimum value of PET signal that was 50% superior to the maximum value of the cerebellar reference region (CRUS1) and thus was considered to represent brain regions with non-negligible specific binding of [11C]PE2I to DAT. It included bilateral striatum (caudate and putamen), insula, pallidum, claustrum, amygdala, thalamus, midbrain (substantia nigra [SN] and ventral tegmental area [VTA]), parts of anterior cingulate gyrus, inferior frontal cortex (gyrus rectus, olfactory, and orbitofrontal cortex) and temporal cortex (hippocampus, parahippocampal gyrus, heschl gyrus, superior temporal gyrus).
BPND maps were compared using an ANCOVA with groups (SCZ and HC) as between-subject factor and age and tobacco status as confounding covariates.
We examined correlations between the clinical scores of the PANSS items and BPND maps. For each clinical item, we performed a multiple regression analysis with age and tobacco status as confounding covariates.
For group comparisons, height and extent thresholds were set at P < .05 family wise error (FWE) corrected, in order to reduce type I errors introduced by potential noise. For correlation analyses, height threshold was set at P < .001 uncorrected, and extent threshold was set at P < .05 FWE-corrected (140 voxels).
Results
Subjects and Clinical Characteristics
Twenty-one right-handed male SCZ and 30 age-matched HC males took part in the study. Among patients, 20 were treated and 1 was untreated (table 1). Seventeen were tobacco smokers (21.94 ± 8.23 cig/day for 17.00 ± 9.62 years, FTND score: 6.29 ± 2.44) and 4 were nonsmokers. The control group was composed by 15 smokers (17.80 ± 5.19 cig/day for 12.33 ± 7.87 years, FTND score: 4.53 ± 2.61) and 15 nonsmokers. Among smokers, SCZ and HC were not statistically different for FTND score and tobacco use frequency and duration.
Demographic and radiochemical variables and patients’ clinical characteristics are available in table 1. No significant difference was found between SCZ and HC for age, BMI and radioactivity parameters.
Imaging Results
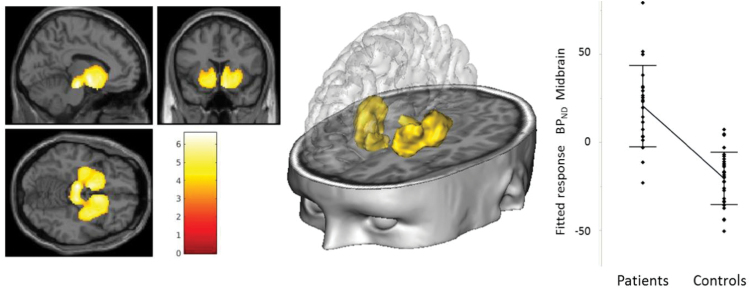
Between-groups comparisons (figure 1; table 2) revealed higher BPND levels (denoting higher DAT availability) in SCZ bilaterally in substantia nigra/ventral tegmental area, putamen, nucleus accumbens, caudate head, amygdala, parts of subcallosal and orbito-frontal gyrus, hippocampus, and in left thalamus.
Fig. 1.
Between-groups comparisons: higher DAT availability in patients with schizophrenia compared to healthy subjects. Height threshold and cluster significance (extent threshold) were set at P < .05 family wise error (FWE) corrected for multiple comparisons. Slice view, three-dimensional representation of the comparison patients > controls cluster(s) and plot at maximum peak voxel [−8, −20, −16] in the midbrain.
Table 2.
Between-Groups Comparison: Regions Where DAT Availability Is Higher in Schizophrenia Patients as Compared With Healthy Controls
| Region | Cluster Level | Peak Level | |||||
|---|---|---|---|---|---|---|---|
| Cluster Size (Voxels) | P FWE | Peak Voxel | P FWE | MNI Coordinates | |||
| Corrected | t Value | Corrected | x | y | z | ||
| L substantia nigra/VTA | 2887 | <.0001 | 6.59 | .00007 | −8 | −20 | −16 |
| L ventral limbica | 6.46 | .0001 | −8 | −2 | −6 | ||
| L putamen (ventral)/hippocampus | 6.15 | .0002 | −30 | −6 | −8 | ||
| R substantia nigra/VTA | 6.03 | .0004 | 8 | −22 | −16 | ||
| L putamen (dorsal) | 5.70 | .001 | −28 | −16 | 4 | ||
| L thalamus | 5.45 | .002 | −20 | −16 | −2 | ||
| L caudate head/nucleus accumbens | 5.34 | .003 | −8 | 10 | −4 | ||
| L hippocampus | 4.94 | .010 | −30 | −26 | −6 | ||
| R putamen (dorsal) | 2566 | <.0001 | 6.22 | .0002 | 30 | −14 | 4 |
| R olfactory cortex/nucleus accumbens | 6.09 | .0003 | 8 | 12 | −16 | ||
| R putamen | 5.99 | .0005 | 32 | 2 | −2 | ||
| R hippocampus | 5.32 | .003 | 26 | −6 | −20 | ||
Height and extent thresholds were set at P < .05 family wise error (FWE) corrected for multiple comparisons. L, left; R, right; MNI, Montreal Neurological Institute; VTA, ventral tegmental area.
aRegion including amygdala/subcallosal/orbitofrontal gyrus.
There was no region showing lower DAT availability in SCZ as compared with the HC.
In patients, the DAT availability correlated significantly with hallucinations, grandiosity, persecution (figure 2, table 3) and unusual thought content, as assessed by PANSS. We found no significant correlation(s) with negative symptoms, antipsychotic treatments (chlorpromazine equivalents), illness duration or age of illness onset.
Fig. 2.
Correlations between DAT availability and PANSS scores in patients with schizophrenia. Significance threshold set at 0.001 uncorrected for voxel level and extent threshold set at 0.05 family wise error (FWE) corrected for multiple comparisons. Three-dimensional representation of the PANSS items correlated regions. For each correlation, a slice view of the cluster(s), overlaid onto a MRI template is presented, with a plot of bivariate fit in the specific region peak voxel. For P6 in the putamen, [−24, −4, 18], R2 = 0.81 with P < .0001; for P5 in the putamen, [−20, 0, 0], R2 = 0.63 with P < .0001; for P3 in amygdala, [−30, 2, −16], R2 = 0.65 with P < .0001. P3, hallucinations; P5, grandiosity; P6, suspiciousness/persecution.
Table 3.
Positive Correlations Between DAT Availability in Patients and PANSS Scores
| PANSS Item | Region | Cluster Level | Peak Level | |||||
|---|---|---|---|---|---|---|---|---|
| Cluster Size (Voxels) | P FWE Corrected | Peak Voxel | P Uncorrected | MNI Coordinates | ||||
| t Value | x | y | z | |||||
| P3 | L amygdala | 187 | .028 | 4.96 | 6.0 e-5 | −30 | 2 | −16 |
| L hippocampus | 4.86 | 7.3 e-5 | −26 | −14 | −16 | |||
| P5 | L pallidum/putamen | 148 | .046 | 4.38 | 2.0 e-4 | −20 | 0 | 0 |
| P6 | L putamen (dorsal) | 2297 | <.0001 | 8.07 | 1.6 e-7* | −24 | −4 | 18 |
| L putamen | 6.49 | 2.8 e-6* | −20 | 12 | 4 | |||
| L subcallosal | 5.67 | 1.4 e-5* | −10 | 20 | −12 | |||
| L pallidum | 5.31 | 2.9 e-5 | −12 | 4 | 2 | |||
| R putamen (dorsal) | 2273 | <.0001 | 6.99 | 1.1 e-6* | 24 | −6 | 14 | |
| R putamen | 6.02 | 6.9 e-6* | 32 | 14 | −4 | |||
| R subcallosal | 5.89 | 2.5 e-5 | 14 | 22 | −12 | |||
| G9 | R amygdala | 471 | .001 | 4.90 | 6.7 e-5 | 22 | −2 | −14 |
| R hippocampus | 4.72 | 9.9 e-5 | 26 | −16 | −14 | |||
| Olfactory cortex/anterior cingulate | 4.29 | 2.5 e-4 | 4 | 8 | −12 | |||
Height threshold P < .001 and extent threshold at P < .05 family wise error (FWE) corrected. L, left; R, right; MNI, Montreal Neurological Institute; VTA, ventral tegmental area; P3, hallucinations; P5, grandiosity; P6, suspiciousness/persecution; G9, unusual thought content.
aHeight threshold P < .05 FWE corrected.
Discussion
The current study reports higher DAT availability in chronic male patients with schizophrenia as compared to healthy subjects, in striatum, midbrain, thalamus, and temporal limbic regions. Furthermore, DAT availability in a number of striatal and extrastriatal regions was positively correlated with positive symptoms of schizophrenia.
Our main finding is in line with 2 previous works that revealed higher DAT availability in striatum31 or thalamus8,31 of patients with schizophrenia compared to controls. However, it clearly contrasts with most of the previously published studies that found no significant difference of DAT availability.2,7 The use of a high-resolution research tomograph with a highly selective DAT radioligand and a voxel-wise approach which does not use a priori defined ROIs could account for the sensitivity of the present method to regional anomalies. The findings are not confined to the striatum as a whole, but delineate only parts of it with a predominant location in the putamen and the ventral striatum. Using this method, we also explored DAT availability in extra-striatal regions that have never been assessed before. Indeed, we detected higher DAT availability in the midbrain (ventral tegmental area and subtantia nigra), and unexpectedly in the hippocampus and parahippocampal areas in SCZ. The hippocampus has been previously reported to display low presence or even a lack of DAT protein in healthy controls in postmortem and imaging studies.32,33 However, hippocampus is known to be rich in DA receptors and to receive DA projections from the midbrain32–36 and measurable levels of DAT binding sites were observed postmortem in this region.37 While DAT protein density in hippocampus appears difficult to be detected in healthy controls, the high resolution, and sensitivity of HRRT might enabled us to reveal a significant increase in DAT availability in patients with schizophrenia.
Our results must be considered in the light of previous presynaptic DA function assessments in schizophrenia patients. First, the elevation of presynaptic striatal DA function previously reported in schizophrenia1,2 has been suggested to predominate in the putamen,2 which is in line with the pattern of DAT availability we report here. Second, DAT availability was also significantly increased in our patients in the midbrain (including VTA and SN) where most DA neurons are located. This observation is in accordance with recent evidences of an increase of DA synthesis capacity in substantia nigra in schizophrenia.38
The increase in DAT availability may reflect either a primary DAT density abnormality or an adaptive functional change related to DA dysfunction. An increase in DA neuron density appears unlikely since postmortem data report decreases of midbrain DA neurons in schizophrenia patients.39 The significant overlap between the brain areas where we found DAT availability augmentations and those where an increase of DA synthesis capacity or release has been previously described, rather supports the hypothesis of an adaptive functional change. Thus, we advance that higher DAT availability could be a marker of an excessive presynaptic DA activity in patients with schizophrenia.
DAT availability correlated positively with distinct positive symptoms in a number of striatal and extra-striatal regions (table 3; figure 2). In untreated schizophrenia patients, previous studies also reported positive relationships between DAT and positive symptoms severity.8,40 Arakawa et al8 found positive correlations between DAT density and total, negative and positive PANSS scores in thalamus of drug-free or naive patients with schizophrenia, whereas Schmitt et al40 found a positive striatal DAT levels correlations with the core symptoms of the acute psychotic syndrome in naïve patients with first acute schizophrenic episode. The use of different ROIs and composite scores of positive symptoms in drug-free patients could explain the differences of correlations among studies.
Interestingly, in our study correlated regions differ according to the symptoms, suggesting the involvement of different DA neuronal structures in different core positive symptoms of schizophrenia. Indeed, suspiciousness/persecution (P6) and grandiosity (P5) mainly correlated with DAT availability in the putamen and pallidum while hallucinatory behavior (P3) and unusual thought content (G9) related to amygdala and hippocampus. Although DA function has been scarcely assessed in the amygdala-hippocampus, a number of functional and anatomical MRI studies support the involvement of these regions in the positive symptoms of schizophrenia.41–49
It is also noteworthy that we did not find any correlation between negative symptoms and DAT availability in either striatal or extra-striatal regions. Negative symptoms have been suggested to be related to DA hypoactivity in the prefrontal cortex.4,50 However, the low DAT density in the frontal cortex prevented us from exploring this region.
Overall, considering high DAT availability as a marker of an excessive presynaptic DA activity, our findings are consistent with the “classical” hypothesis of a relationship between positive symptoms and DA hyperactivity in schizophrenia.4 Further, most of the regions where we found higher DAT availability and correlations with the symptoms belong to ventral striatum and the limbic system, thus supporting the hypothesis of an involvement of the DA mesolimbic pathway in the positive dimension of schizophrenia.
There are some methodological considerations regarding PET imaging.
First, we used a scanning time of 60 min after injection of [11C]PE2I, consistently with previous studies in other psychiatric samples.9,51 While 60 min PET acquisition allows extrastriatal DAT imaging with PE2I, a scan duration >70 min may be required to reach equilibrium in high DAT density regions such as the striatum.14,52 Since the HRRT provides optimal conditions for the examination of small regions with high sensitivity and spatial resolution (about 2.5 mm) and reduced partial volume effects,15,24 our method appears well suited for the examination of extra-striatal areas in patients. However, it cannot be excluded that the scanning duration used here increased the variability and consequently affected the reliability of BPND measures within the striatum. Yet this cannot account for the difference detected between putamen and caudate. Also, the present study has a case–control design and it is unlikely that this methodological bias differs between healthy controls and schizophrenia patients.
Second, we used crus 1 cerebellar subregion instead of the whole cerebellar grey matter as reference region, because it displays the lowest [11C]PE2I uptake (data not shown). We also improved signal to noise ratio using motion corrections and PSF modeling. Indeed, the partial volume effect was corrected by directly incorporating PSF modeling inside the iterative algorithm.53 Overall, this could partly explain why BPND values in extrastriatal regions such as thalamus are higher in our sample than in a previous study using the same PET scanner with [11C]PE2I.14
Third, the SRTM method has been widely applied in PET studies assessing the dopamine system. Because it does not require arterial measures, it is convenient for clinical samples in psychiatric populations. Still, this method underestimates the BPND and the inter-individual variability in DAT density, as compared to methods based on arterial input function.14,52,54,55 Consequently, the SRTM approach may have underestimated the group differences in the present study.
Fourth, [11C]PE2I produces radiometabolites that could pass the blood–brain barrier and possibly increase the nonspecific radioactivity throughout the brain, including the cerebellum.56 Consequently, a possible radiometabolite contamination could have impaired DAT quantification using SRTM.28 However, it seems unlikely that [11C]PE2I metabolism and clearance rates differ between patients and healthy controls in our study since we found no difference in crus1 cerebellar radioactivity when comparing SUVs between the 2 groups (data not shown).
Other Factors Could Be Considered as Limitations.
As all patients except one were receiving long-term antipsychotic treatment, the correlations between DAT and symptoms should be considered carefully. However, it is unlikely that treatments influenced the assessment of DAT availability. Indeed, we did not find any correlation between DAT availability and chlorpromazine equivalents. Most antipsychotics are commonly known to have negligible affinity for the DAT21 and studies in rodents revealed no change in striatal DAT density after acute or chronic treatment with antipsychotics.18,20 In humans, 2 longitudinal studies failed to find significant changes in DAT availability after subchronic treatment by olanzapine16 or risperidone,19 and studies and meta-analyses comparing treated and untreated patients repeatedly failed to show a significant difference between the 2 groups.7,17,57
Otherwise, recent data suggest that tobacco smoking could decrease DAT availability.9–11 Since both groups were not fully matched regarding tobacco use, this could have confounded schizophrenia-related effects. Thus, tobacco status (smokers or nonsmokers) was used as covariate in our statistical analyses. Furthermore, the higher prevalence of smokers in the patient group cannot explain the increase of DAT availability, since tobacco use and schizophrenia seem to have opposite effects on the DAT.
Last, only male subjects were included in the study. Considering gender differences in DAT expression in healthy individuals58–60 and gender differences as a well-known broad-spectrum characteristic of schizophrenia61 this selection precluded results regarding any possible gender effect.
In conclusion, this high-resolution PET study shows increased DAT availability in the midbrain and in most subcortical and limbic dopaminergic projection fields, in schizophrenia patients as compared to controls. Increased DAT in some of these regions, especially limbic areas such as amygdala, hippocampus, ventral striatum, and midbrain, was positively correlated with positive symptoms, underscoring their implication in schizophrenia. Assuming that increased DAT availability in patients reflects a presynaptic hyper-dopaminergic state, our findings also bring solid, novel, imaging evidence that support the DA hypothesis of schizophrenia and further postulate that hyperactivity of mesolimbic DA transmission underlies psychotic positive symptoms. Given the complexity of the DA system, this study also highlights the interest of using whole brain voxel-based analysis methods for exploring DA function in psychiatric diseases.
Funding
This study was supported by grants (SCHIZODAT, APV05143LSA) from the National Agency for Research (ANR); the National Institute of Health and Medical Research (INSERM); the Interministerial Mission in the Fight Against Drugs and Drug Addiction (MILDT, Grant AO2004-37); a postdoctoral grant from the French Foundation for Medical Research (FRM) to CL, and a join INSERM-APHP grant to ETT.
Acknowledgments
The authors thank Ms Christine Baron, Mrs Claude Comtat, Vincent Brulon, Yoann Fontyn, Frédéric Dollé, Philippe Gervais, and Renaud Maroy from the Service Hospitalier Frédéric Joliot for their efficient technical support in 11C radioligand preparation and PET image acquisition and processing. The authors thank Drs Martin Bouzel, Nicolas Gruel, Natalia Dos Santos Mascarenhas, and Annie Gauvain for their involvement in the recruitment of patients. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 2013;39:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. [DOI] [PubMed] [Google Scholar]
- 5. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 6. Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol. 2005;204:355–360. [DOI] [PubMed] [Google Scholar]
- 7. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, Part I: meta-analysis of dopamine active transporter (DAT) density. Schizophr Bull. 2013;39:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arakawa R, Ichimiya T, Ito H, et al. Increase in thalamic binding of [(11)C]PE2I in patients with schizophrenia: a positron emission tomography study of dopamine transporter. J Psychiatr Res. 2009;43:1219–1223. [DOI] [PubMed] [Google Scholar]
- 9. Leroy C, Karila L, Martinot JL, et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict Biol. 2012;17:981–990. [DOI] [PubMed] [Google Scholar]
- 10. Newberg A, Lerman C, Wintering N, Ploessl K, Mozley PD. Dopamine transporter binding in smokers and nonsmokers. Clin Nucl Med. 2007;32:452–455. [DOI] [PubMed] [Google Scholar]
- 11. Yang YK, Yao WJ, Yeh TL, et al. Decreased dopamine transporter availability in male smokers—a dual isotope SPECT study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:274–279. [DOI] [PubMed] [Google Scholar]
- 12. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. [DOI] [PubMed] [Google Scholar]
- 13. Halldin C, Erixon-Lindroth N, Pauli S, et al. [(11)C]PE2I: a highly selective radioligand for PET examination of the dopamine transporter in monkey and human brain. Eur J Nucl Med Mol Imaging. 2003;30:1220–1230. [DOI] [PubMed] [Google Scholar]
- 14. Hirvonen J, Johansson J, Teräs M, et al. Measurement of striatal and extrastriatal dopamine transporter binding with high-resolution PET and [11C]PE2I: quantitative modeling and test-retest reproducibility. J Cereb Blood Flow Metab. 2008;28:1059–1069. [DOI] [PubMed] [Google Scholar]
- 15. Leroy C, Comtat C, Trébossen R, Syrota A, Martinot JL, Ribeiro MJ. Assessment of 11C-PE2I binding to the neuronal dopamine transporter in humans with the high-spatial-resolution PET scanner HRRT. J Nucl Med. 2007;48:538–546. [DOI] [PubMed] [Google Scholar]
- 16. Kim C-E, Lee M-H, Lee P-G, Choe W-S, Pyo S-J. Correlation between psychopathology and dopamine transporter density in striatum before and after taking olanzapine assessed with IPT-SPECT in first episode schizophrenia. Korean J Psychopharmacol. 2004;15:8. [Google Scholar]
- 17. Laruelle M, Abi-Dargham A, van Dyck C, et al. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry. 2000;47:371–379. [DOI] [PubMed] [Google Scholar]
- 18. Lavalaye J, Knol RJ, de Bruin K, Reneman L, Janssen AG, Booij J. [123I]FP-CIT binding in rat brain after acute and sub-chronic administration of dopaminergic medication. Eur J Nucl Med. 2000;27:346–349. [DOI] [PubMed] [Google Scholar]
- 19. Mateos JJ, Lomeña F, Parellada E, et al. Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology (Berl). 2007;191:805–811. [DOI] [PubMed] [Google Scholar]
- 20. Tarazi FI, Zhang K, Baldessarini RJ. Olanzapine, quetiapine, and risperidone: long-term effects on monoamine transporters in rat forebrain. Neurosci Lett. 2000;287:81–84. [DOI] [PubMed] [Google Scholar]
- 21. Tatsumi M, Jansen K, Blakely RD, Richelson E. Pharmacological profile of neuroleptics at human monoamine transporters. Eur J Pharmacol. 1999;368:277–283. [DOI] [PubMed] [Google Scholar]
- 22. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 23. Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69:763–765. [PubMed] [Google Scholar]
- 24. de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA. Performance evaluation of the ECAT HRRT: an LSO-LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol. 2007;52:1505–1526. [DOI] [PubMed] [Google Scholar]
- 25. Dolle FB, Demphel M, Emond S, et al. Highly efficient synthesis of [11C]PE2I, a selective radioligand for the quantification of the dopamine transporter using PET. J Labelled Comp Radiopharm. 2000;43:7. [Google Scholar]
- 26. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. [DOI] [PubMed] [Google Scholar]
- 27. Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. [DOI] [PubMed] [Google Scholar]
- 28. Jucaite A, Odano I, Olsson H, Pauli S, Halldin C, Farde L. Quantitative analyses of regional [11C]PE2I binding to the dopamine transporter in the human brain: a PET study. Eur J Nucl Med Mol Imaging. 2006;33:657–668. [DOI] [PubMed] [Google Scholar]
- 29. Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapp. 1995;2:189–210. [Google Scholar]
- 30. Meyer JH, Gunn RN, Myers R, Grasby PM. Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage. 1999;9:545–553. [DOI] [PubMed] [Google Scholar]
- 31. Sjøholm H, Bratlid T, Sundsfjord J. 123I-beta-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology (Berl). 2004;173:27–31. [DOI] [PubMed] [Google Scholar]
- 32. Hall H, Halldin C, Guilloteau D, et al. Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. Neuroimage. 1999;9:108–116. [DOI] [PubMed] [Google Scholar]
- 33. Ito H, Takahashi H, Arakawa R, Takano H, Suhara T. Normal database of dopaminergic neurotransmission system in human brain measured by positron emission tomography. Neuroimage. 2008;39:555–565. [DOI] [PubMed] [Google Scholar]
- 34. Retailleau A, Boraud T. The Michelin red guide of the brain: role of dopamine in goal-oriented navigation. Front Syst Neurosci. 2014;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scatton B, Simon H, Le Moal M, Bischoff S. Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett. 1980;18:125–131. [DOI] [PubMed] [Google Scholar]
- 36. Verney C, Baulac M, Berger B, Alvarez C, Vigny A, Helle KB. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience. 1985;14:1039–1052. [DOI] [PubMed] [Google Scholar]
- 37. Little KY, Carroll FI, Cassin BJ. Characterization and localization of [125I]RTI-121 binding sites in human striatum and medial temporal lobe. J Pharmacol Exp Ther. 1995;274:1473–1483. [PubMed] [Google Scholar]
- 38. Howes OD, Williams M, Ibrahim K, et al. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 2013;136:3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bogerts B, Häntsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry. 1983;18:951–969. [PubMed] [Google Scholar]
- 40. Schmitt GJ, la Fougère C, Dresel S, et al. Dual-isotope SPECT imaging of striatal dopamine: first episode, drug naïve schizophrenic patients. Schizophr Res. 2008;101:133–141. [DOI] [PubMed] [Google Scholar]
- 41. Anticevic A, Tang Y, Cho YT, et al. Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr Bull. 2014;40:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bogerts B, Lieberman JA, Ashtari M, et al. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry. 1993;33:236–246. [DOI] [PubMed] [Google Scholar]
- 43. Duan HF, Gan JL, Yang JM, et al. A longitudinal study on intrinsic connectivity of hippocampus associated with positive symptom in first-episode schizophrenia. Behav Brain Res. 2015;283:78–86. [DOI] [PubMed] [Google Scholar]
- 44. Ford JM, Palzes VA, Roach BJ, et al. ; Functional Imaging Biomedical Informatics Research Network. Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr Bull. 2015;41:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goghari VM, Sponheim SR, MacDonald AW.III. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34:468–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kühn S, Musso F, Mobascher A, Warbrick T, Winterer G, Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl Psychiatry. 2012;2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pinkham AE, Liu P, Lu H, Kriegsman M, Simpson C, Tamminga C. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172:784–792. [DOI] [PubMed] [Google Scholar]
- 48. Vai B, Sferrazza Papa G, Poletti S, et al. Abnormal cortico-limbic connectivity during emotional processing correlates with symptom severity in schizophrenia. Eur Psychiatry. 2015;30:590–597. [DOI] [PubMed] [Google Scholar]
- 49. Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136:804–814. [DOI] [PubMed] [Google Scholar]
- 50. Abi-Dargham A, Kegeles LS, Zea-Ponce Y, et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55:1001–1006. [DOI] [PubMed] [Google Scholar]
- 51. Karila L, Leroy C, Dubol M, et al. Dopamine transporter correlates and occupancy by modafinil in cocaine-dependent patients: a controlled study with high-resolution PET and [C]-PE2I. Neuropsychopharmacology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seki C, Ito H, Ichimiya T, et al. Quantitative analysis of dopamine transporters in human brain using [11C]PE2I and positron emission tomography: evaluation of reference tissue models. Ann Nucl Med. 2010;24:249–260. [DOI] [PubMed] [Google Scholar]
- 53. Sureau FC, Reader AJ, Comtat C, et al. Impact of image-space resolution modeling for studies with the high-resolution research tomograph. J Nucl Med. 2008;49:1000–1008. [DOI] [PubMed] [Google Scholar]
- 54. DeLorenzo C, Kumar JS, Zanderigo F, Mann JJ, Parsey RV. Modeling considerations for in vivo quantification of the dopamine transporter using [(11)C]PE2I and positron emission tomography. J Cereb Blood Flow Metab. 2009;29:1332–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Odano I, Varrone A, Savic I, et al. Quantitative PET analyses of regional [11C]PE2I binding to the dopamine transporter–application to juvenile myoclonic epilepsy. Neuroimage. 2012;59:3582–3593. [DOI] [PubMed] [Google Scholar]
- 56. Shetty HU, Zoghbi SS, Liow JS, et al. Identification and regional distribution in rat brain of radiometabolites of the dopamine transporter PET radioligand [11C]PE2I. Eur J Nucl Med Mol Imaging. 2007;34:667–678. [DOI] [PubMed] [Google Scholar]
- 57. Lavalaye J, Linszen DH, Booij J, et al. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res. 2001;47:59–67. [DOI] [PubMed] [Google Scholar]
- 58. Laakso A, Vilkman H, Bergman J, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52:759–763. [DOI] [PubMed] [Google Scholar]
- 59. Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. [DOI] [PubMed] [Google Scholar]
- 60. Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. [DOI] [PubMed] [Google Scholar]
- 61. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. [DOI] [PubMed] [Google Scholar]