Abstract
Signs of motor dysfunction are evidenced across a range of psychiatric disorders including schizophrenia. Historically, these features have been neglected but emerging theoretical and methodological advancements have shed new light on the utility of considering movement abnormalities. Indeed, the National Institute of Mental Health Research Domain Criteria initiative has recently met to develop a Motor Systems Domain. This reflects a growing appreciation for the enhanced reliability and validity that can come along with evaluating disturbances relevant to psychiatric illnesses from multiple levels of analysis, and conceptualizing these domains with respect to the complexity of their role in a broader integrated system (ie, weighing contributions and interactions between the cognitive, affective, and motor domains). This article discusses motor behaviors and seeks to explain how research into basal ganglia, cerebellar, and cortico-motor circuit function/dysfunction, grounded in brain circuit-motor behavior relationships, can elucidate our understanding of pathophysiology, provide vital links to other key systems of interest, significantly improve identification and classification, and drive development of targeted individualized treatments.
Keywords: schizophrenia, psychosis, RDoC, motor, movement abnormalities, basal ganglia, cerebellum, cortico-motor circuits
Introduction
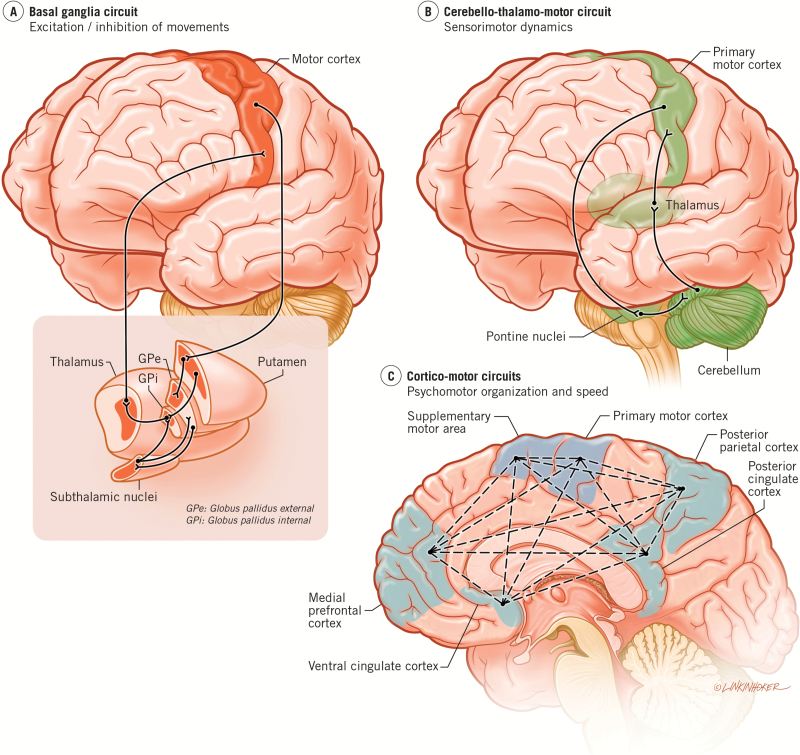
Psychiatric disorders like psychosis and bipolar disorder (BD) show cognitive, social, affective, sensory, thought and motor symptoms. While significant attention has been devoted to cognitive and affective symptoms, motor changes are often neglected. This phenomenon, as well as the rich history of research into motor dysfunction across psychiatric illnesses, is discussed comprehensively in Peralta and Cuesta’s review (this issue). Until recently, such neglect has gone along with limited integration with research supporting the contribution of the underlying basal ganglia, cerebello-thalamo-cortical and cortico-motor circuits involved in the various aspects of motor function such as excitation/inhibition, timing, and psychomotor modulation (figure 1). The aim in the present paper is to show the relevance of these 3 circuits for different aspects of “normal” motor function and their neural-behavioral-motor alterations in psychosis.
Fig. 1.
The figure shows the relevant motor circuits for psychosis. (A) Basal ganglia circuit. GPi and e: Globus pallidus internal and external; STN = subthalamic nucleus. (B) Cerbellar-thalamo-cortical circuit. Here, we show the cerebello-thalamo-motor. While the cerebello-thalamo-frontal circuit was originally noted by Andreasen and colleagues28 as being implicated in patients with schizophrenia, more recent work, including our own, has demonstrated that the cerebello-thalamo-motor circuit is also impacted in this population, and relates to deficits in sensorimotor integration. (C) Cortico-motor circuits. Multiple areas of the frontal and parietal cortices interact to control and influence movement. Notably, these regions also interact with the subcortical systems in (A) and (B).
Further underscoring the potential importance of motor symptoms in psychosis, and across psychopathology more broadly, is the new addition of the Motor Systems domain to the National Institute of Mental Health Research Domain Criteria (RDoC) matrix. A primary goal of the RDoC framework is to shift psychopathology research towards a pathophysiology-based framework.1,2 Just as in other RDoC matrix domains, the Motor Systems domain is comprised of multiple constructs and subconstructs meant to guide research in this area from the cellular-molecular level, up to observable behavior and reports, including brain-circuit based measures (see editorial by Garvey and Cuthbert in this issue). As we put the function of key motor circuits into context, we do so with an eye towards the RDoC matrix, and describe how investigations of these circuits in psychotic disorders may be especially informative for our understanding of the disease.
Basal Ganglia Circuit and Motor Excitation/Inhibition Abnormalities
Particular motor behaviors, including spontaneous dyskinesias (hyper/hypokinetic movements that occur as a result of pathophysiology), or more basic motor behaviors, elicited by instrumental tasks designed to engage the same underlying circuit component dysfunction,3 are closely tied to basal ganglia circuits, and as a result, can be powerful tools for understanding vulnerability, progression, and treatment response and further, for refining theories such as the dopamine (DA) hypothesis. By governing the selection or gating of a subset of representations that have been activated by the cortex, parallel basal ganglia circuits are responsible for modulating a range of higher order behaviors.4,5 Basal ganglia circuits each share the same general organization, originating in specific cortical areas, passing though portions of the basal ganglia and thalamus, and then projecting back into the frontal cortical area of origin in a closed loop. Each circuit is also comprised of direct and indirect pathways that work in synchrony to balance inhibitory restraint on the thalamocortical output leading back to the cortex.6 This intricate system is governed by a specific chemoarchitecture and multiple neurotransmitter interactions, with DA activity representing the primary modulatory chemical messenger.6,7 As a result, the circuits are highly sensitive to DA abnormalities, and what is reflected by dysfunction in one BG circuit may speak to common factors that would impair functions of other BG circuits as well.8 Thus, parameterizing motor behaviors or tracking changes in motor activity over time, or in response to treatment, may serve as sensitive outward marker of changes in an underlying system that modulates functions ranging from motor, cognitive, and emotional behavior to perception, affect, motivation and action.4,5,9
Within the context of the new RDoC domain, there are a number of promising constructs/subconstructs that hold significant relevance to mapping markers of basal ganglia circuit dysfunction. Constructs/subconstructs that tap into processes involved with initiation of a selected action plan or the inhibition of motor plans will provide a context for evaluating hypokinetic and hyperkinetic movements characteristic of basal ganglia circuit dysfunction in psychosis. For example, work from our research program has suggested that velocity scaling and force variability are highly sensitive to basal ganglia dysfunction in individuals with varying psychosis vulnerability.10,11 There is additional evidence suggesting that these abnormalities are also present in schizophrenia patients.12,13 Currently, we are working to evaluate how these experimental paradigms map on to other units of analysis across Motor Systems sub/constructs. This is an important line of inquiry as these motor behaviors clearly tie into underlying pathophysiology that drives clinically relevant outcomes. Indeed, recent work indicates that motor behaviors specific to the basal ganglia, as well as those that tap into basal ganglia function in addition to other networks (eg, gesture behavior), predict onset of psychosis in risk populations,14–16 or functional outcome in individual with schizophrenia17,18 (also see Commentary by Schiffman, this issue).
This system will also provide building blocks for examining circuit-motor behavior relationships in the context of investigating links with other existing RDoC domains. For example, basal ganglia circuits also contribute to functions included in the Cognitive Systems domain, allowing for flexible modulation of internally generated/externally evoked behavioral responses to environmental cues.19,20 If related dysfunction prevents the execution of an initiated order, or does not effectively inhibit unintended orders, this can negatively impact cognitive function across domains.8 In this framework, it is likely that movement abnormalities that are closely linked to underlying basal-ganglia circuit dysfunction may serve as useful components for integrated study; in this context these motor behaviors (eg, dyskinesias, as well as stereotypies, catatonic immobility, perseveration), or related experimental paradigms might be used to evaluate specificity or highlight informative underlying commonalities between RDoC domains.
Cerebellar-Subcortical Circuits and Alterations in Sensorimotor Dynamics
In parallel with the significant contributions to our understanding of psychosis in the context of basal ganglia circuits, cerebellar circuits are also notable in their understanding of the disease. The initial nonhuman primate literature demonstrated distinct motor and prefrontal closed-loop circuits arising from different regions of the cerebellum, via distinct thalamic nuclei. More recently, such dissociable circuits have been demonstrated in the human brain as well, using21,22 both diffusion tensor imaging23 and resting state functional connectivity analysis.24,25 Dysfunction in this closed-loop circuitry could thus give rise to deficits in both the motor and cognitive domains, consistent with the deficits seen in psychosis. Indeed, the first suggestions of a role for the cerebellum in psychosis were instantiated through the cognitive dysmetria framework, wherein dysfunctional activation in cerebello-thalamo-prefrontal circuit was related to cognitive deficits.26,27
While the initial suggestions of a role for the cerebellum in psychosis were more cognitively focused,28,29 more recently there has been a great deal of work in both clinical high-risk groups and patients with schizophrenia that has focused on the motor domain. With respect to its role in motor control, the cerebellum is known to be important for the smooth control and online updating of our movements. This structure allows for the use of internal models of behavior that are formed and modified through the processes of learning.30 In psychosis, deficits in a variety of motor behaviors are present, including postural control,31,32 motor learning,33 and eye-blink conditioning.34 Recently, there is also work to suggest that deficits in these domains are present in both clinical high-risk groups, as well as unaffected siblings of patients with the disease25,35,36 suggesting that in these circuits, much like the basal ganglia circuits, motor deficits are present prior to formal disease onset, and in the absence of antipsychotic medication. Thus, they may be related to the underlying etiology of the disease. Our work37,38 and that of others39 has suggested that internal model deficits may contribute to the diverse symptomatology in patients with psychosis.
With the recent addition of the Motor Systems domain to the RDoC matrix, this focus on motor behavior in psychosis with respect to the cerebellum represents an interesting and important avenue of research. For example, much of the work to date with respect to postural control in psychosis can be encompassed under a proposed RDoC subconstruct covering sensorimotor dynamics. The body is reliant upon sensorimotor inputs to provide updated information about the position of the body in space. Perturbations in things like the sway area are indicative of deficits in these dynamics, and as noted, have been demonstrated in both clinical high risk and patients with schizophrenia.32 However, other paradigms, such as sensorimotor adaptation,40 also provide an excellent means of quantifying sensorimotor dynamics. Such paradigms have been very successful in the study of autism,41 but have yet to be widely applied to psychosis. Further, the cerebellum and related circuits tie closely into functions that will fall under RDoC subconstructs involving inhibition and termination. Future work would greatly benefit from investigating choice reaction time paradigms, motor timing function, and stop signal reaction time with the cerebellum in mind, as suggested by the RDoC matrix. Though these tasks are well studied and characterized in healthy individuals, their application to psychosis stands to provide important new insights into cerebellar motor deficits that may also be related to broader cognitive and affective symptomatology.
Importantly, the cerebello-thalamo-motor circuits do not act in isolation. Intriguingly, and perhaps of great importance for our understanding of motor deficits in psychosis, the cerebellum and BG also share direct connections.42,43 Thus, the anatomical connections are in place to allow for communication between these important multi-modal systems, both of which have been implicated in many of the motor deficits experienced by patients with psychosis more. While the BG loops help to select a particular action or sequence, the cerebellum works to fine tune, or add skill to these actions; these circuits work in close concert through both cortical and direct connections,42 and it is not possible to have a comprehensive understanding of the contributions of one, without examining the other. Our group is currently at work on evaluating how overlapping and distinct areas of circuit dysfunction may provide important clues for understanding and treating psychosis. For example, it remains to be seen if unique subtypes of patients can be highlighted on the basis of particular motor behaviors (tapping into potentially different etiological pathways, and pointing to the need to employ different treatment approaches), or if patients with some motor deficits will be more likely to have other types of motor abnormalities (speaking to a more generalized dysfunction). The RDoC matrix provides a sound framework for examining these questions and further, for enriching conceptual understanding by integrating perspectives across critical systems in this population.
Cortico-Motor Circuits and Changes in Psychomotor Organization and Speed
Both the basal ganglia and cerebellum provide major inputs to cortical regions including motor cortex and other regions like the prefrontal cortex.5–7,23 This accounts for bottom-up modulation of cortical motor regions by subcortical regions which, as we have seen, accounts for motor excitation/inhibition and timing. Additionally, the reverse modulation also takes place, namely from cortical to subcortical motor regions. Specifically, cortical regions implicated in cognitive, social, and affective functions exert top-down modulatory effects on cortical and subcortical regions implicated in motor function.44 Though research is still in its infancy in this regard, we describe such circuits as cortico-motor circuits and assume them to be closely related to psychomotor function. In the following, we will give 2 examples of cortico-motor circuits (ie, orbitofrontal-motor connectivity, and default-mode network [DMN]—sensorimotor balance) and how they are involved in different forms of psychomotor modulation (ie, psychomotor organization and speed).
Anterior medial prefrontal cortical regions like the ventromedial prefrontal cortex and pre/subgenual anterior cingulate cortex are strongly implicated in emotional processing.45 At the same time these regions show strong functional connectivity with premotor, supplementary, and motor cortex.44 One can thus speak of a medial prefrontal—motor cortico-cortical circuit (MPMCCC) which, functionally, may allow to link emotion and movements allowing for psychomotor modulation. The MPMCCC has been found abnormal in patients with catatonia who, correspondingly, show both affective-emotional and motor abnormalities: they remain unable to control their abnormal emotional intensity and show abnormal hypo- and hyperkinetic movements with catalepsy, flexibilitas cerea, posturing, and hyperkinetic changes.44,46,47 This perspective is also supported by multimodal imaging studies that have pinpointed an important role for the supplementary motor area in catatonia.48,49 Moreover, data in catatonia indicate that the MPMCCC can be modulated by GABA-ergic drugs such as Lorazepam in both healthy44 and catatonic50 subjects—this is of high interest given that Lorazepam has been proven therapeutically highly beneficial in acute catatonic patients.51 Accordingly, the MPMCCC may serve as a possible candidate for a construct that can be related to both specific behavior (ie, psychomotor organization) and biochemical levels (ie, GABA).
Yet another example of psychomotor modulation can be found in a related circuit, ie, the relationship between different neural networks in the brain’s spontaneous activity.52,53 Among these are the DMN and the sensorimotor network (SMN). While the SMN is related to motor and sensory functions,53 the DMN is strongly implicated in internal cognition including self-related processing,54 spontaneous thoughts or mind wandering,55 and mental time travel or episodic simulation.56 A recent investigation57 demonstrated reciprocal balance between DMN and SMN in BD: high levels of neuronal variability in DMN are accompanied by low variability levels in SMN in depressed BD while the reverse constellation (ie, low DMN and high SMN variability) can be observed in manic BD. Both constellations exert major impact on psychomotor function: manic BD is featured by psychomotor agitation while depressed BD shows psychomotor retardation. Hence, the balance between DMN and SMN (DMN-SMN ratio) is related to psychomotor speed while both neuronal and behavioral levels are abnormally altered in opposite ways in manic and depressed BD. Recent work designed to also understand the role structural connectivity abnormalities play in contributing to slowing in affective disorder populations will also be important for providing a more comprehensive perspective.58
In sum, domain construction is warranted for the psychomotor features of psychiatric disorders. Psychological changes in affective, social, and/or cognitive functions can affect and modulate motor functions in an abnormal way. This may be related to cortico-motor circuits like MPMCCC and DMN-SMN ratio. However, the investigation of such psychomotor circuits has been largely neglected so far. They may be crucial in order to understand the motor changes including their close relation to psychological abnormalities in psychosis and various other psychiatric disorders though. Of course, it is also possible that motor systems affected across psychiatric illness may also drive deficits in important high-order functions as well. Future work aimed at teasing apart if motor abnormalities are secondary or primary to psychological symptoms is sorely needed, and will certainly provide a more holistic perspective for understanding aberrant human behavior.
Conclusion
We pointed out the fruitfulness of the RDoC concept for exploring and understanding different motor functions in the context of psychiatric illnesses. First, we demonstrated how motor excitation/inhibition may provide important clues for understanding basal ganglia circuit pathology in psychosis. Secondly, the cerebello-thalamo-cortical circuits are relevant for motor timing and sensorimotor dynamics which, again, are abnormally altered in schizophrenia and perhaps other psychotic disorders. Finally, we determined cortico-motor or psychomotor circuits that appear relevant for (different forms of) psychomotor modulation such as psychomotor organization and speed which are abnormally altered in catatonia and BD. Though much works remains to be done in the future development of RDoC in the motor domain, our commentary supports the utility of such an approach for defining and determining the often neglected motor function and its various motor/psychomotor symptoms in psychiatric disorders.
More broadly, we need to view motor circuits as inter-related. At the same time, while each motor circuit is active during any given activity, it will be important to understand how dysfunction in a specific circuit may yield dissociable behavioral abnormalities. In addition, it will also be important to map the extent to which motor circuit abnormalities directly impact other systems such as cognition. There has been compelling evidence for such a link in schizophrenia.59 The RDoC system, providing tools to look across systems in a mechanistically informed way, offers particular strengths in this regard.60 Furthermore, although RDoC provides a theoretically and empirically informed Matrix, centered on brain-behavioral relationships, it will be informative to continue to view incoming studies within the context of prominent etiological models of psychosis. It is important to consider that motor “circuits” (central to any RDoC domain), speak to a variety of connected brain regions, and can therefore be interpreted through the lens of the disconnectivity hypothesis.60–62 Indeed, there is already an accumulating body of studies indicating links between motor impairment and aberrant connectivity.63 Future work evaluating relationships between RDoC motor constructs/subconstructs (eg, motor execution, action perception), will be particularly informative in this regard. The new RDoC Motor Systems constructs also show promise to fit well within developmental hypotheses,64 cognitive dysmetria,27 as well as DA and resource processing theories.65,66 In addition, it will be important for future work to consider bi-directionality between healthy and psychiatric states. While understanding of the basal ganglia circuit is well developed on the basis of both animal and human research in the healthy brain, this is less the case in the second, cerebello-thalamo-cortical circuit, and even less so in the third circuit, the cortico-motor or psychomotor circuits. The latter in particular provides an example where we may want to start in the reverse. That is, from psychiatric symptoms and their motor alterations to the “normal” function of a circuit—the psychiatric abnormalities may thus pave the way and teach us a lesson about the “healthy brain.”67
Funding
V.A.M. was supported by R01MH094650, R21/R33MH103231, and R21MH110374. J.A.B. was supported in part by a Brain and Behavior Research Foundation NARSAD Young Investigator Award as the Donald and Janet Boardman Family Investigator.
Acknowledgments
G.N. acknowledges financial support from Canada Research Chair (CRC), the CIHR, the Michael Smith Foundation, the University of Ottawa Brain and Mind Research Institute, the Seventh Hospital for Mental Health in Hangzhou/China, and the National Science Foundation of China (NSF). V.A.M. is a consultant to Takeda Pharmaceuticals and no author authors have any disclosures.
References
- 1. Cuthbert BN. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology. 2014;51:1205–1206. [DOI] [PubMed] [Google Scholar]
- 2. Insel T, Cuthbert B, Garvey M et al. . Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. [DOI] [PubMed] [Google Scholar]
- 3. Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75:65–75. [DOI] [PubMed] [Google Scholar]
- 4. Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–935. [DOI] [PubMed] [Google Scholar]
- 5. Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. [DOI] [PubMed] [Google Scholar]
- 6. DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. [DOI] [PubMed] [Google Scholar]
- 7. Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 8. Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384:523–531. [DOI] [PubMed] [Google Scholar]
- 9. Howes OD, Montgomery AJ, Asselin MC et al. . Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 10. Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ Schizophr. 2015;1 https://www.nature.com/articles/npjschz20146/fig_tab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 2011;132:194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caligiuri MP, Lohr JB. A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry. 1994;35:104–111. [DOI] [PubMed] [Google Scholar]
- 13. Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry. 1993;150:1343–1348. [DOI] [PubMed] [Google Scholar]
- 14. Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116:796–803. [DOI] [PubMed] [Google Scholar]
- 15. Mittal VA, Walker EF, Bearden CE et al. . Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010;68:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walther S, Eisenhardt S, Bohlhalter S et al. . Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull. 2016;42:1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mittal VA, Walker EF. Abnormal movements and the longitudinal course of role and social functioning in adolescents at high-risk for psychosis. Schizophr Res. 2011;130:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. [DOI] [PubMed] [Google Scholar]
- 20. Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. [DOI] [PubMed] [Google Scholar]
- 22. Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernard JA, Orr JM, Mittal VA. Differential motor and prefrontal cerebello-cortical network development: evidence from multimodal neuroimaging. Neuroimage. 2016;124:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernard JA, Dean DJ, Kent JS et al. . Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Brain Mapp. 2014;35:4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. [DOI] [PubMed] [Google Scholar]
- 27. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 28. Andreasen NC, O’Leary DS, Cizadlo T et al. . Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Dysfunctional cortico-cerebellar circuits cause ‘cognitive dysmetria’ in schizophrenia. Neuroreport. 1998;9:1895–1899. [DOI] [PubMed] [Google Scholar]
- 30. Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. [DOI] [PubMed] [Google Scholar]
- 31. Kent JS, Hong SL, Bolbecker AR et al. . Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One. 2012;7:e41808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marvel CL, Schwartz BL, Rosse RB. A quantitative measure of postural sway deficits in schizophrenia. Schizophr Res. 2004;68:363–372. [DOI] [PubMed] [Google Scholar]
- 33. Marvel CL, Turner BM, O’Leary DS et al. . The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology. 2007;21:761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bolbecker AR, Steinmetz AB, Mehta CS et al. . Exploration of cerebellar-dependent associative learning in schizophrenia: effects of varying and shifting interstimulus interval on eyeblink conditioning. Behav Neurosci. 2011;125:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolbecker AR, Kent JS, Petersen IT et al. . Impaired cerebellar-dependent eyeblink conditioning in first-degree relatives of individuals with schizophrenia. Schizophr Bull. 2014;40:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dean DJ, Kent JS, Bernard JA et al. . Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res. 2015;162:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bernard JA, Mittal VA. Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 2015;45:2685–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3:545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry. 2014;71:28–35. [DOI] [PubMed] [Google Scholar]
- 40. Bernard JA, Seidler RD. Cerebellar contributions to visuomotor adaptation and motor sequence learning: an ALE meta-analysis. Front Hum Neurosci. 2013;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldberg MC, Mostow AJ, Vecera SP et al. . Evidence for impairments in using static line drawings of eye gaze cues to orient visual-spatial attention in children with high functioning autism. J Autism Dev Disord. 2008;38:1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. [DOI] [PubMed] [Google Scholar]
- 44. Northoff G. What catatonia can tell us about “top-down modulation:” a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25:555–577; discussion 578–604. [DOI] [PubMed] [Google Scholar]
- 45. Phan KL, Fitzgerald DA, Gao K, Moore GJ, Tancer ME, Posse S. Real-time fMRI of cortico-limbic brain activity during emotional processing. Neuroreport. 2004;15:527–532. [DOI] [PubMed] [Google Scholar]
- 46. Northoff G, Kötter R, Baumgart F et al. . Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull. 2004;30:405–427. [DOI] [PubMed] [Google Scholar]
- 47. Fink M, Fricchione G, Rummans T, Shorter E. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133:250–251. [DOI] [PubMed] [Google Scholar]
- 48. Walther S, Schappi L, Federspiel A et al. . Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull. 2016. https://academic.oup.com/schizophreniabulletin/article/doi/10.1093/schbul/sbw140/2512107/Resting-State-Hyperperfusion-of-the-Supplementary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheuerecker J, Ufer S, Käpernick M et al. . Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res. 2009;43:607–614. [DOI] [PubMed] [Google Scholar]
- 50. Richter A, Grimm S, Northoff G. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol. 2010;25:55–62. [DOI] [PubMed] [Google Scholar]
- 51. Northoff G. Options for the treatment of febrile catatonia. J Psychiatry Neurosci. 2010;35:E5–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Northoff G. Resting state activity and the “stream of consciousness” in schizophrenia–neurophenomenal hypotheses. Schizophr Bull. 2015;41:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- 54. Northoff G. How does the ‘rest-self overlap’ mediate the qualitative and automatic features of self-reference? Cogn Neurosci. 2016;7:18–20. [DOI] [PubMed] [Google Scholar]
- 55. Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci. 2016;17:718–731. [DOI] [PubMed] [Google Scholar]
- 56. Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martino M, Magioncalda P, Huang Z et al. . Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci U S A. 2016;113:4824–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walther S, Hügli S, Höfle O et al. . Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis. 2012;47:13–19. [DOI] [PubMed] [Google Scholar]
- 59. Matthews N, Gold BJ, Sekuler R, Park S. Gesture imitation in schizophrenia. Schizophr Bull. 2013;39:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Camchong J, MacDonald AW III, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Friston K. Disconnection and cognitive dysmetria in schizophrenia. Am J Psychiatry. 2005;162:429–432. [DOI] [PubMed] [Google Scholar]
- 63. Straube B, Green A, Sass K, Kircher T. Superior temporal sulcus disconnectivity during processing of metaphoric gestures in schizophrenia. Schizophr Bull. 2014;40:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 65. Luck SJ, McClenon C, Beck VM et al. . Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Northoff G. Neuro-Philosophy and the Healthy Mind: Learning from the Unwell Brain. 1st ed. New York: W. W. Norton & Company; 2016. [Google Scholar]