Abstract
Background
Enzymatically modified isoquercitrin (EMIQ) is produced from rutin using enzymatic hydrolysis followed by treatment with glycosyltransferase in the presence of dextrin to add glucose residues. EMIQ is absorbed in the same way as quercetin, a powerful antioxidant reported to prevent disused muscle atrophy by targeting mitochondria and to have ergogenic effects. The present study investigated the effect of EMIQ on skeletal muscle hypertrophy induced by functional overload.
Methods
In Study 1, 6-week-old ICR male mice were divided into 4 groups: sham-operated control, sham-operated EMIQ, overload-operated control, and overload-operated EMIQ groups. In Study 2, mice were divided into 3 groups: overload-operated whey control, overload-operated whey/EMIQ (low dose), and overload-operated whey/EMIQ (high dose) groups. The functional overload of the plantaris muscle was induced by ablation of the synergist (gastrocnemius and soleus) muscles. EMIQ and whey protein were administered with food. Three weeks after the operation, the cross-sectional area and minimal fiber diameter of the plantaris muscle fibers were measured.
Results
In Study 1, functional overload increased the cross-sectional area and minimal fiber diameter of the plantaris muscle. EMIQ supplementation significantly increased the cross-sectional area and minimal fiber diameter of the plantaris muscle in both the sham-operated and overload-operated groups. In Study 2, EMIQ supplementation combined with whey protein administration significantly increased the cross-sectional area and minimal fiber diameter of the plantaris muscle.
Conclusion
EMIQ, even when administered as an addition to whey protein supplementation, significantly intensified the fiber hypertrophy of the plantaris muscle in functionally overloaded mice. EMIQ supplementation also induced fiber hypertrophy of the plantaris in sham-operated mice.
Keywords: Enzymatically modified isoquercitrin, Rutin, Quercetin, Muscle, Hypertrophy, Functional overload, Whey protein
Background
Rutin is a flavonoid glycoside that is ubiquitously present in a variety of fruits and vegetables, such as onions and buckwheat. Rutin is converted into quercetin and its metabolites before absorption [1]. Rutin and quercetin are known as vitamin P and play various roles in cardiovascular and metabolic disease [2]. As for the role of rutin in muscle, Mukai et al. reported that quercetin injection suppressed muscle atrophy by attenuating the induction of ubiquitin ligases [3]. They also found that quercetin intake prevented muscle atrophy by targeting mitochondrial function [4]. According to Seo et al., rutin intake increased the size of mitochondria and mitochondrial DNA content, as well as stimulated mitochondrial biogenesis [5]. Therefore, rutin and quercetin have a beneficial effect in muscle atrophy. It has also been reported that quercetin supplementation has an ergogenic effect [6]. However, the effects of rutin and its metabolites in muscle hypertrophy have remained unknown.
Rutin and quercetin are poorly absorbed when administered orally, which diminishes their positive health effects [1]. Therefore, several trials have been conducted to increase the bioavailability of these compounds. Since quercetin is nearly insoluble in water (<0.1 g/100 mL at 21 °C), enzymatic glucosyl conjugation has been performed to enhance its water solubility [7, 8]. Enzymatically modified isoquercitrin (EMIQ) (Fig. 1) is one of water soluble glucoside derivatives of quercetin. EMIQ is produced from rutin via enzymatic hydrolysis, which removes the rhamnosyl group, followed by treatment of the product with glycosyltransferase in the presence of dextrin to add glucose residues. The bioavailability of EMIQ, which is absorbed as quercetin and metabolized like rutin, is about 17-fold greater than that of quercetin [8]. It is therefore reasonable to expect considerably greater health benefits with EMIQ. In Japan, EMIQ is approved as a food additive [9], and the U.S. FDA concluded that EMIQ is generally regarded as safe (GRAS) for use as an antioxidant. The level of EMIQ in a food product is recommended to be no greater than 150 mg/kg, with the exception of chewing gum, in which the antioxidant may be present up to 1500 mg/kg [10].
Fig. 1.
Chemical structures
Skeletal muscle mass is maintained by a balance between synthesis and breakdown, with disproportionally increased protein synthesis leading to muscle fiber hypertrophy. Resistance training is known to induce muscle fiber hypertrophy, which is important to enhance exercise capacity and locomotive power in athletes and the elderly [11]. Protein and especially essential amino acids supplementation augments the muscle hypertrophy during resistance training [12, 13]. During resistance training, many athletes consume whey protein, which is known to augment muscle hypertrophy.
In this study, we used a mouse model of functional overload created by synergist ablation surgery. Surgical removal of the gastrocnemius and soleus muscles resulted in functional overload of the remaining plantaris muscle, leading to myofiber hypertrophy mimicking the effect of resistance training [14]. The purpose of this study was to evaluate the effect of EMIQ supplementation on muscle hypertrophy in these functionally overloaded mice. The effect of EMIQ supplementation simultaneously with whey protein was also evaluated.
Methods
Animals and experimental design
Male ICR mice (6 weeks old, n = 59) were obtained from Clea Japan Inc. Animals were maintained under standard conditions (temperature: 23 ± 2 °C, humidity: 50 ± 10%, 12:12-h light-dark cycle; lights on at 7:00 a.m.) with ad libitum access to food (Study 1: CE2, Clea Japan Inc.; Study 2: CE2 and AIN-93G, Oriental Yeast Co.) and water. After a 14-day acclimatization to the laboratory conditions, ablation of the synergistic gastrocnemius and soleus muscles was performed, and mice were maintained on standard bedding for one week. Thereafter, mice were individually housed on wire floor. They lived as they did in the cage previously. The body weight and food intake in each group were measured thrice a week. All animal experiments were carried out in a humane manner after receiving approval from the Institutional Animal Experiment Committee of the University of Tsukuba (identification number: 09–058) and in accordance with the Regulations for Animal Experimentation of the University and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of Ministry of Education, Culture, Sports, Science and Technology of Japan.
Surgical procedure
In order to initiate overload-induced hypertrophy of the plantaris muscle, ablation of the synergistic gastrocnemius and soleus muscles was performed as described previously [15, 16]. Briefly, under pentobarbital sodium anesthesia (0.5%; 10 μL/g body weight), a skin incision was made from the popliteal to the Achilles tendon. The soleus muscle was completely removed except for a small portion at the proximal end, where it attaches to the plantaris. Both the lateral and medial gastrocnemius muscles were also completely removed. Care was taken to avoid trauma to the plantaris. In control groups, a sham operation was performed by making the skin incision only. The animals were sacrificed 3 weeks after the surgery, and the plantaris muscles of both hind limbs were excised and measured for weight. The samples were embedded in tissue-freezing medium (OCT compound, Sakura Finetek Japan), flash-frozen in liquid nitrogen, and stored at −80°C until use.
EMIQ supplementation
EMIQ was obtained from Sanei-gen FFI Inc. The dosage of EMIQ supplementation was determined according to the previous study [17]. EMIQ was administered with diet at an average dose of approximately 4.0 mg/kg of body weight in study 1, and approximately 4.0 and 40 mg/kg of body weight in +EMIQ (L) and +EMIQ (H) groups, respectively, in study 2.
Study 1. The mice were randomly divided into four groups: sham-operated control (“Sham,” n = 9), sham-operated EMIQ (“Sham + EMIQ,” n = 9), overload-operated control (“Overload,” n = 10), and overload-operated EMIQ (“Overload + EMIQ,” n = 9). The control mice were kept on a normal diet (CE-2, Clea Japan Inc.), whereas mice in the EMIQ groups received the same diet and EMIQ (0.003%, Clea Japan Inc.) for 3 weeks (Table 1).
Table 1.
Compositions of diets in Study 1
| Ingredients(%) | CE-2 | CE-2 + EMIQ |
|---|---|---|
| Carbohydrate | 50.500 | 50.490 |
| Protein | 25.000 | 24. 995 |
| Fat | 4.700 | 4.699 |
| Mineral mixture | 6.800 | 6.799 |
| Fiber | 4.000 | 3.999 |
| Water | 9.000 | 8. 998 |
| EMIQ | - | 0.003 |
| Total | 100 | 100 |
| Energy (kcal/100 g) | 344 | 344 |
Study 2. The mice were randomly divided into three groups: overload-operated control mice that received whey protein supplementation (“Overload + W,” n = 7), overload-operated mice that received whey protein and a low dose of EMIQ (“+EMIQ (L),” n = 7), and overload-operated mice that received whey protein and a high dose of EMIQ (“+EMIQ (H),” n = 8). The mice in the whey control group received a diet in which milk casein in AIN-93G was replaced with whey protein (Clea Japan Inc.), whereas the mice in the whey EMIQ groups were fed the same whey diet and EMIQ (low dose: 0.003%; high dose: 0.03%) for 3 weeks (Table 2).
Table 2.
Compositions of diets in Study 2
| Ingredients(%) | Whey | +EMIQ(L) | +EMIQ(H) |
|---|---|---|---|
| Cornstarch | 39.7486 | 39.7407 | 39.6693 |
| Whey protein | 20.0000 | 19.9960 | 19.9601 |
| α-Cornstarch | 13.2000 | 13.1974 | 13.1737 |
| Sucrose | 10.0000 | 9.9980 | 9.9800 |
| Soybean oil | 7.0000 | 6.9986 | 6.9860 |
| Cellulose | 5.0000 | 4.9990 | 4.9900 |
| Mineral mixture | 3.5000 | 3.4993 | 3.4930 |
| Vitamin mixture | 1.0000 | 0.9998 | 0.9980 |
| L-Cystine | 0.3000 | 0.2999 | 0.2994 |
| Choline bitartrate | 0.2500 | 0.2500 | 0.2495 |
| tert-Butylhidroquinone | 0.0014 | 0.0014 | 0.0014 |
| EMIQ | - | 0.003 | 0.03 |
| Total | 100 | 100 | 100 |
| Energy(Kcal/100 g) | 373 | 373 | 372 |
Immunohistochemical analysis
For immunohistochemical analysis, serial cross-sections (10-μm thick) of plantaris muscles were cut and stained with hematoxylin and eosin (H&E). The sections were observed using a fluorescent microscope (OLYMPUS BX51) and analyzed with the ImageJ software (the National Institutes of Health, USA) to calculate the mean cross-sectional area and the minimal fiber diameter. As described previously, cross-sectional areas of at least 100 randomly selected myofibers were measured [18]. Myofibers with central nuclei were excluded from analysis because they could be regenerated myofibers.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using the SPSS software (version 22). In Study 1, the groups were compared by two-way analysis of variance (ANOVA). One-way ANOVA was used in Study 2. Tukey’s test was performed for post-hoc comparisons. A P-value of <0.05 or <0.01 was considered to indicate a statistically significant difference.
Results
Study 1
The food intake, body weight, and plantaris muscle weight data are summarized in Table 3. The food intake and initial body weight were similar in all the groups. The final body weight was slightly lower in the Overload group compared to the Sham group (P < 0.05). In contrast, the weight of each plantaris muscle in the Overload groups was significantly higher than that in the Sham groups (P < 0.01). EMIQ supplementation slightly increased the plantaris muscle weight in the Overload + EMIQ and Sham + EMIQ groups, although the difference was not statistically significant (Fig. 1).
Table 3.
Body and plantaris muscle weight in Study 1
| Sham | Sham + EMIQ | Ovld | Ovld + EMIQ | P value | |||
|---|---|---|---|---|---|---|---|
| Ovld | EMIQ | Ovld × EMIQ | |||||
| Food intake (g day−1) | 5.1 ± 0.2 | 5.2 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 | 0.388 | 0.711 | 0.849 |
| Initial body weight (g) | 35.9 ± 0.5 | 35.5 ± 0.7 | 36.0 ± 0.5 | 35.0 ± 0.6 | 0.749 | 0.222 | 0.609 |
| Final body weight (g) | 39.3a ± 0.6 | 39.7a ± 0.7 | 38.1b ± 0.9 | 37.6b ± 0.6 | 0.032 | 0.964 | 0.547 |
| Left Plantaris (mg) | 21.6a ± 1.0 | 22.7a ± 0.9 | 43.4b ± 2.3 | 46.0b ± 3.2 | 0.000 | 0.388 | 0.727 |
| Right Plantaris (mg) | 21.8a ± 0.9 | 23.3a ± 0.8 | 41.5b ± 3.0 | 45.3b ± 3.7 | 0.000 | 0.298 | 0.638 |
| Av. Plantaris (mg) | 21.7a ± 0.6 | 23.0a ± 0.8 | 42.4b ± 2.3 | 45.6b ± 3.2 | 0.000 | 0.290 | 0.648 |
| Plantaris/B.W. (%) | 0.055a ± 0.002 | 0.058a ± 0.002 | 0.111b ± 0.005 | 0.121b ± 0.007 | 0.000 | 0.290 | 0.648 |
Values are mean ± SEM. Means without a common letter are statistically different (P < 0.05, two-way ANOVA (analysis of variance) followed by Tukey’s test). Sham: sham-operated mice, Sham + EMIQ: sham-operated mice receiving EMIQ, Ovld: functionally overloaded mice, Ovld + EMIQ: functionally overloaded mice receiving EMIQ
To investigate the effect on the plantaris muscle, the cross-sectional area and minimal diameter of the myofibers were determined (Fig. 2). The cross-sectional area was 1559 ± 30 μm2, 1706 ± 51 μm2, 1820 ± 96 μm2, and 2278 ± 100 μm2 in the Sham, Sham + EMIQ, Overload, and Overload + EMIQ groups, respectively. The cross-sectional areas in the Overload groups were higher than those in the Sham groups (P < 0.01). The cross-sectional areas in the EMIQ-treated groups were higher than those in the untreated groups (P < 0.01). The minimal fiber diameter was 35.9 ± 0.4 μm, 38.6 ± 0.6 μm, 37.7 ± 0.7 μm, and 43.4 ± 0.7 μm in the Sham, Sham + EMIQ, Overload, and Overload + EMIQ groups, respectively. The minimal fiber diameters in the Overload groups were higher than those in the Sham groups (P < 0.01), and the minimal fiber diameters in the EMIQ-treated groups were higher than those in the untreated groups (P < 0.01). Two-way ANOVA detected an interaction between overload operation and EMIQ treatment.
Fig. 2.
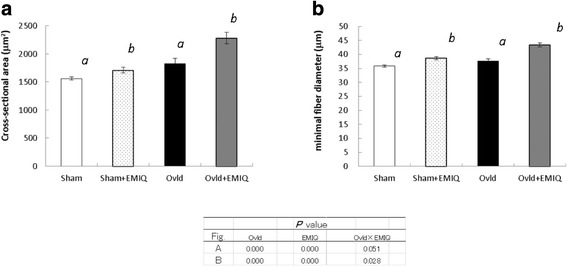
Cross-sectional area (a) and minimal fiber diameter (b) of the plantaris muscle in Study 1. Values are mean ± SEM. Means without a common letter are statistically different (P < 0.05, two-way ANOVA (analysis of variance) followed by Tukey’s test). Sham: sham-operated mice, Sham + EMIQ: sham-operated mice receiving EMIQ, Ovld: functionally overloaded mice, Ovld + EMIQ: functionally overloaded mice receiving EMIQ
Study 2
To examine the cumulative effect of EMIQ/whey protein supplementation, we changed the protein source from milk casein to whey protein and examined overload-operated mice that received whey only and whey combined with EMIQ at two concentrations. The food intake, body weight, and plantaris muscle weight were similar in all the three groups (Table 4).
Table 4.
Body and plantaris muscle weight in Study 2
| Ovld + W | Ovld + W + EMIQ(L) | Ovld + EMIQ(H) | |
|---|---|---|---|
| Total food intake (g) | 98.1 ± 2.3 | 99.0 ± 3.1 | 98.0 ± 1.6 |
| Body mass (g) | 37.2 ± 0.7 | 36.6 ± 0.8 | 37.3 ± 0.7 |
| Change of body mass (g) | 3.9 ± 0.7 | 2.9 ± 0.7 | 3.6 ± 0.4 |
| Left Plantaris (mg) | 46.9 ± 1.6 | 43.1 ± 2.6 | 43.0 ± 3.1 |
| Right Plantaris (mg) | 44.7 ± 4.3 | 46.3 ± 3.1 | 43.6 ± 1.7 |
| Av. Plantaris (mg) | 45.8 ± 2.9 | 44.7 ± 2.2 | 43.3 ± 2.1 |
| Plantaris/B.W. (%) | 0.124 ± 0.009 | 0.123 ± 0.008 | 0.117 ± 0.007 |
Values are mean ± SEM. Ovld + W: functionally overloaded mice receiving whey protein, Ovld + W + EMIQ (L): functionally overloaded mice receiving whey protein and EMIQ at a low concentration, Ovld + W + EMIQ (H): functionally overloaded mice receiving whey protein and EMIQ at a high concentration
To further investigate the effect on the plantaris muscle, the cross-sectional area and minimal diameter of myofibers were determined (Fig. 3). The cross-sectional area in the +EMIQ (H) group was significantly higher than in the Overload + W and +EMIQ (L) groups (P < 0.01; Overload + W: 1713 ± 58 μm2, +EMIQ (L): 1795 ± 114 μm2, +EMIQ (H): 2052 ± 73 μm2). Moreover, the minimal fiber diameter in the +EMIQ (H) group was higher than in the Overload + W and +EMIQ (L) groups (P < 0.05; Overload + W: 38.3 ± 0.8 μm, +EMIQ (L): 38.5 ± 1.4 μm, +EMIQ (H): 41.3 ± 0.9 μm).
Fig. 3.
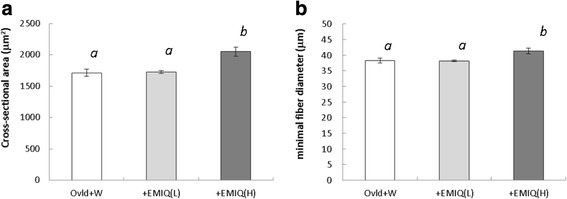
Cross-sectional area (a) and minimal fiber diameter (b) of the plantaris muscle in Study 2. Values are mean ± SEM. Means without a common letter are statistically different (P < 0.05, one-way ANOVA (analysis of variance) followed by Tukey’s test). Ovld + W: functionally overloaded mice receiving whey protein, +EMIQ (L): functionally overloaded mice receiving whey protein and EMIQ at a low concentration, +EMIQ (H): functionally overloaded mice receiving whey protein and EMIQ at a high concentration
Discussion
The present study demonstrated that EMIQ supplementation intensifies muscle fiber hypertrophy in functionally overloaded mice. Moreover, EMIQ supplementation in sham-operated mice and EMIQ/whey protein supplementation also intensified muscle fiber hypertrophy in this model. Quercetin has been reported to prevent disused muscle atrophy [3, 5]. EMIQ is a quercetin with improved absorption. In the present study, we showed that EMIQ can stimulate muscle fiber hypertrophy. Few studies have found that food ingredients, except proteins, amino acids, and their metabolites, can induce muscle hypertrophy [19–21].
One possible mechanism of muscle fiber hypertrophy stimulation by EMIQ is via its antioxidant activity. Functional overlord and eccentric exercise induce inflammation and oxidative stress in the muscle. According to many reports, antioxidant supplementation can reduce the inflammation and oxidative damage, although it is unclear whether supplementation of antioxidants influences muscle hypertrophy [22]. Antioxidants are expected to prevent muscle damage, oxidative stress, and muscular fatigue, leading to prolonged exercising capacity and muscle hypertrophy. On the contrary, reactive oxygen species activates important cell signaling pathways that mediate skeletal muscle adaptions to exercise, such as hypertrophy [23]. Vitamin C, a common antioxidant, has been reported to attenuate overload-induced skeletal muscle hypertrophy [24], though an antioxidant mixture including rutin promoted muscle protein synthesis by restoring the impaired leucine stimulation [25]. Therefore, specific antioxidants may positively affect muscle hypertrophy.
Another possible mechanism is related to the effects of rutin on mitochondria. Recent studies have suggested that quercetin exerts its beneficial effects independently of its antioxidant activity. Thus, quercetin modulates pathways related to mitochondria biogenesis, elevation of mitochondria membrane potential, oxidative respiration and ATP anabolism, and intra-mitochondrial redox status [26]. Muscle protein synthesis is a process that requires high utilization of ATP and mitochondria is an important regulator of intracellular signaling cascades that modulate skeletal muscle size and function [27]. Dysfunctional mitochondria trigger proteolytic pathways leading to muscle atrophy during aging. In contrast, exercise training can induce mitochondrial biogenesis and dynamics, and increase muscle protein synthesis that favors myofiber and whole muscle hypertrophy. Overexpression of ATP citrate lyase, which improves mitochondrial function, is sufficient to induce hypertrophy in human myotubes [28]. It is therefore possible that EMIQ induces muscle fiber hypertrophy by affecting mitochondria, although the detailed mechanism needs to be explored in the future.
A remarkable finding was that EMIQ supplementation induced muscle fiber hypertrophy even in the sham-operated group. This result indicates that EMIQ intake may induce muscle fiber hypertrophy without functional overload mimicking resistant training. Moreover, EMIQ supplementation combined with whey protein supplementation also induced muscle fiber hypertrophy in overload-operated mice. Protein intake after resistance exercise augments muscle protein synthesis and can lead to muscle fiber hypertrophy. Whey protein, which contains high amounts of essential branched amino acids and is absorbed rapidly, is especially effective in this regard [12, 13], resulting in its widespread use by athletes. Our results suggest that EMIQ supplementation might have a beneficial effect in athletes; however, it needs to be validated in a human study.
It has to be noted that the effective concentration of EMIQ in Study 2 was 10-fold higher than in Study 1, suggesting that effective EMIQ concentration depends on the food content. Whey contained in Study 2 is reported to augment muscle hypertrophy dependent on high content of leucine. Therefore, it is speculated that the amount of EMIQ to be effective needs to be high. Study 1 identified an interaction between overload operation and EMIQ treatment, indicating that the effective concentration of EMIQ is affected by presence or absence of functional overload. We speculate that the effective concentration of EMIQ may depend on specific details of exercise and/or nutrition. EMIQ can induce muscle fiber hypertrophy in resistance training and daily life. Additional studies will be needed to determine the exact mechanisms and conditions.
Conclusions
EMIQ supplementation intensified the fiber hypertrophy of the plantaris muscle in mice induced by compensatory overload. Moreover, both EMIQ supplementation alone and in combination with whey protein intake increased muscle fiber hypertrophy. Given that muscle fiber hypertrophy is beneficial not only in athletes but also in the elderly with locomotive syndrome, EMIQ can be an effective supplement for various sub-populations in need of muscle fiber hypertrophy and maintenance.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Data are all contained within the article.
Abbreviations
- +EMIQ (H)
overload-operated mice that received whey protein and a high dose of EMIQ
- +EMIQ (L)
overload-operated mice that received whey protein and a low dose of EMIQ
- ANOVA
analysis of variance
- EMIQ
enzymatically modified isoquercitrin
- Overload + EMIQ
overload-operated mice that received EMIQ
- Overload + W
overload-operated control mice that received whey protein
- Overload
overload-operated control mice
- Sham + EMIQ
sham-operated mice that received EMIQ
- Sham
sham-operated control mice
Authors’ contributions
AK, MM, HI, TI, NO, and TT developed the study protocol. AK was the principle investigator and TT was the project leader of this study. AK, MM, YS, MS, RI, and TT performed the research. AK, HM-U, NO, and TT carried out the statistical analysis and wrote the manuscript. All the authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were carried out in a humane manner after receiving approval from the Institutional Animal Experiment Committee of the University of Tsukuba (identification number: 09–058) and in accordance with the Regulations for Animal Experimentation of the University and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of Ministry of Education, Culture, Sports, Science and Technology of Japan.
NOTE: This statement is also found in the ‘Animals and experimental design’ section of the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Akiko Kohara, Email: a_kohara@yakult-hf.co.jp.
Masanao Machida, Email: mmasanao@cc.saga-u.ac.jp.
Yuko Setoguchi, Email: y-setoguchi-ia@morinaga.co.jp.
Ryouichi Ito, Email: r-ito-ih@morinaga.co.jp.
Masanori Sugitani, Email: m-sugitani-gh@morinaga.co.jp.
Hiroko Maruki-Uchida, Email: h-uchida-ji@morinaga.co.jp.
Hiroyuki Inagaki, Email: h-inagaki-jj@morinaga.co.jp.
Tatsuhiko Ito, Email: kazoku5mei@yahoo.co.jp.
Naomi Omi, Email: mi.naomi.gn@u.tsukuba.ac.jp.
Tohru Takemasa, Phone: +81 29 853 2622, Email: takemasa.tohru.gm@u.tsukuba.ac.jp.
References
- 1.Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409(1):12–16. doi: 10.1016/S0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 2.Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Investig. 2014;37(9):783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 3.Mukai R, Nakao R, Yamamoto H, Nikawa T, Takeda E, Terao J. Quercetin prevents unloading-derived disused muscle atrophy by attenuating the induction of ubiquitin ligases in tail-suspension mice. J Nat Prod. 2010;73(10):1708–10. doi: 10.1021/np100240y. [DOI] [PubMed] [Google Scholar]
- 4.Mukai R, Matsui N, Fujikura Y, Matsumoto N, Hou DX, Kanzaki N, Shibata H, Horikawa M, Iwasa K, Hirasaka K, Nikawa T, Terao J. Preventive effect of dietary quercetin on disuse muscle atrophy by targeting mitochondria in denervated mice. J Nutr Biochem. 2016;31:67–76. doi: 10.1016/j.jnutbio.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Seo S, Lee MS, Chang E, Shin Y, Oh S, Kim IH, Kim Y. Rutin increases muscle mitochondrial biogenesis with AMPK activation in high-fat diet-induced obese rats. Nutrients. 2015;7(9):8152–8169. doi: 10.3390/nu7095385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casuso RA, Martínez-Amat A, Martínez-López EJ, Camiletti-Moirón D, Porres JM, Aranda P. Ergogenic effects of quercetin supplementation in trained rats. J Int Soc Sports Nutr. 2013;10(1):3. doi: 10.1186/1550-2783-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoi K, Yoshizumi K, Kido T, Usui Y, Yumoto T. Absorption and urinary excretion of quercetin, rutin, and alphaG-rutin, a water soluble flavonoid, in rats. J Agric Food Chem. 2003;51(9):2785–2789. doi: 10.1021/jf026108a. [DOI] [PubMed] [Google Scholar]
- 8.Makino T, Shimizu R, Kanemaru M, Suzuki Y, Moriwaki M, Mizukami H. Enzymatically modified isoquercitrin, alpha-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol Pharm Bull. 2009;32(12):2034–2040. doi: 10.1248/bpb.32.2034. [DOI] [PubMed] [Google Scholar]
- 9.Japan Food Additives Association . Japanese specifications and standards for food additives. 8. Tokyo: Japan Food Additives Association; 2007. [Google Scholar]
- 10.U.S. Food and Drug Administration GRAS Notice 000220: alpha-Glycosyl isoquercitrin. www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm269110.pdf
- 11.Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37(3):225–264. doi: 10.2165/00007256-200737030-00004. [DOI] [PubMed] [Google Scholar]
- 12.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 13.Hulmi JJ, Lockwood CM, Stout JR. Effect of protein/essential amino acids and resistance training on skeletal muscle fiber hypertrophy: A case for whey protein. Nutr Metab (Lond). 2010;7:51. [DOI] [PMC free article] [PubMed]
- 14.Alway SE, Siu PM, Murlasits Z, Butler DC. Muscle fiber hypertrophy models: applications for research on aging. Can J Appl Physiol. 2005;30(5):591–624. doi: 10.1139/h05-143. [DOI] [PubMed] [Google Scholar]
- 15.Plyley MJ, Olmstead BJ, Noble EG. Time course of changes in capillarization in hypertrophied rat plantaris muscle. J Appl Physiol. 1998;84(3):902–907. doi: 10.1152/jappl.1998.84.3.902. [DOI] [PubMed] [Google Scholar]
- 16.Miller GR, Stauber WT. Use of computer-assisted analysis for myofiber size measurements of rat soleus muscles from photographed images. J Histochem Cytochem. 1994;42(3):377–382. doi: 10.1177/42.3.8308255. [DOI] [PubMed] [Google Scholar]
- 17.Motoyama K, Koyama H, Moriwaki M, Emura K, Okuyama S, Sato E, Inoue M, Shioi A, Nishizawa Y. Atheroprotective and plaque-stabilizing effects of enzymatically modified isoquercitrin in atherogenic apoE-deficient mice. Nutrition. 2009;25(4):421–427. doi: 10.1016/j.nut.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan HS, Goldspink G. Fibre number and fibre size in a surgically overloaded muscle. J Anat. 1979;129(Pt 2):293–303. [PMC free article] [PubMed] [Google Scholar]
- 19.Maganaris CN, Maughan RJ. Creatine supplementation enhances maximum voluntary isometric force and endurance capacity in resistance trained men. Acta Physiol Scand. 1998;163:279–287. doi: 10.1046/j.1365-201x.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 20.Escalante G, Alencar M, Haddock B, Harvey P. The effects of phosphatidic acid supplementation on strength, body composition, muscular endurance, power, agility, and vertical jump in resistance trained men. J Int Soc Sports Nutr. 2016;13:24. doi: 10.1186/s12970-016-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durkalec-Michalski K1, Jeszka J. The Effect of β-Hydroxy-β-Methylbutyrate on Aerobic Capacity and Body Composition in Trained Athletes. J Strength Cond Res.2016;30:2617–26. [DOI] [PubMed]
- 22.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Cabrera MC, Viña J, Ji LL. Role of Redox Signaling and Inflammation in Skeletal Muscle Adaptations to Training. Antioxidants (Basel). 2016; 5(4). pii: E48. [DOI] [PMC free article] [PubMed]
- 24.Makanae Y, Kawada S, Sasaki K, Nakazato K, Ishii N. Vitamin C administration attenuates overload-induced skeletal muscle fiber hypertrophy in rats. Acta Physiol (Oxf) 2013;208(1):57–65. doi: 10.1111/apha.12042. [DOI] [PubMed] [Google Scholar]
- 25.Marzani B, Balage M, Vénien A, Astruc T, Papet I, Dardevet D, Mosoni L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J Nutr. 2008;138(11):2205–11. doi: 10.3945/jn.108.094029. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF. Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv. 2016;34(5):532–549. doi: 10.1016/j.biotechadv.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Konopka AR, Harber MP. Skeletal muscle fiber hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42(2):53–61. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanello V, Sandri M. Mitochondrial quality control and muscle mass maintenance. Front Physiol. 2016;6:422. doi: 10.3389/fphys.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are all contained within the article.


