Summary
The outer membrane (OM) of the pathogenic diderm spirochete, Borrelia burgdorferi, contains integral β-barrel outer membrane proteins (OMPs) in addition to its numerous outer surface lipoproteins. Very few OMPs have been identified in B. burgdorferi, and the protein machinery required for OMP assembly and OM localization is currently unknown. Essential OM BamA proteins have recently been characterized in Gram-negative bacteria that are central components of an OM β-barrel assembly machine and are required for proper localization and insertion of bacterial OMPs. In the present study, we characterized a putative B. burgdorferi BamA orthologue encoded by open reading frame bb0795. Structural model predictions and cellular localization data indicate that the B. burgdorferi BB0795 protein contains an N-terminal periplasmic domain and a C-terminal, surface-exposed β-barrel domain. Additionally, assays with an IPTG-regulatable bb0795 mutant revealed that BB0795 is required for B. burgdorferi growth. Furthermore, depletion of BB0795 results in decreased amounts of detectable OMPs in the B. burgdorferi OM. Interestingly, a decrease in the levels of surface-exposed lipoproteins was also observed in the mutant OMs. Collectively, our structural, cellular localization and functional data are consistent with the characteristics of other BamA proteins, indicating that BB0795 is a B. burgdorferi BamA orthologue.
Introduction
Borrelia burgdorferi is a pathogenic spirochete that is the causative agent of Lyme disease (Benach et al., 1983; Steere et al., 1983). Similar to Gram-negative proteobacteria, B. burgdorferi is a diderm bacterium possessing both an inner cytoplasmic membrane and an outer membrane (OM). However, in addition to having a unique phospholipid and glycolipid content (Eiffert et al., 1991; Belisle et al., 1994; Hossain et al., 2001; Ben-Menachem et al., 2003; Kinjo et al., 2006), the B. burgdorferi OM is distinct from that of a typical Gram-negative organism with respect to its overall protein composition. Whereas B. burgdorferi contains numerous surface-anchored amphiphilic lipoproteins (Howe et al., 1985; Brandt et al., 1990; Fuchs et al., 1992; Norris et al., 1992; Lam et al., 1994; Guo et al., 1995; Fraser et al., 1997; Zhang et al., 1997; Probert and Johnson, 1998; Casjens et al., 2000; Wallich et al., 2005), the spirochete has relatively few membrane-spanning integral outer membrane proteins (OMPs), as compared with a typical enteric Gram-negative organism, such as Escherichia coli (Bunikis et al., 1995; Probert et al., 1995; Noppa et al., 2001; Brooks et al., 2006; Bunikis et al., 2008). For instance, freeze-fracture electron microscopy data have shown that there are approximately 12 000 integral OMPs per μm2 in the OM of E. coli (Lugtenberg and van Alphen, 1983), whereas virulent B. burgdorferi have approximately 1200 OMPs per μm2 (Radolf et al., 1994). Although the B. burgdorferi OM contains approximately 10-fold fewer OMPs than that found in the E. coli OM (Lugtenberg and van Alphen, 1983; Radolf et al., 1994), B. burgdorferi still possesses numerous membrane-spanning OMPs, all of which must be properly folded within the OM. Because all other diderm bacteria identified to date contain a protein complex specifically dedicated to chaperoning and localizing integral OMPs, it seems likely that B. burgdorferi contains a similar OMP localization system.
In Gram-negative bacteria, precursor integral OMPs typically progress through a Sec-mediated translocation process (Pugsley, 1993). After inner membrane translocation through the SecYEG channel, the signal peptide is cleaved and the mature protein must then be transported through the periplasm and properly inserted into the OM (Pugsley, 1993; Papanikou et al., 2007). Until recently, the machinery and mechanisms utilized for OM localization and insertion of proteins, such as porins, remained largely uncharacterized in Gram-negative bacteria. In recent years, however, the descriptions of the Neisseria meningitidis and the E. coli BamA proteins (previously referred to as Omp85 and YaeT, respectively) have provided functional evidence for a bacterial protein that is required for efficient OMP localization (Voulhoux et al., 2003; Doerrler and Raetz, 2005; Werner and Misra, 2005; Wu et al., 2005). Data from these studies indicated that BamA is essential for viability in both N. meningitidis and in E. coli, and that depletion of BamA causes a decrease in the levels of properly assembled OMPs (Voulhoux et al., 2003; Doerrler and Raetz, 2005; Werner and Misra, 2005; Wu et al., 2005). Combined genetic and biochemical studies in E. coli have also revealed that BamA exists as a central channel protein in a multicomponent OMP complex, termed the β-barrel assembly machine (BAM). This complex is composed of BamA and four accessory lipoproteins, BamB, BamC, BamD and BamE (previously known as YfgL, NlpB, YfiO and SmpA, respectively), of which only BamA and BamD are essential (Wu et al., 2005; Sklar et al., 2007; Vuong et al., 2008; Knowles et al., 2009).
Although the primary amino acid sequence homology is poorly conserved between the putative BamA orthologues identified to date (approximately 40–50% similarity), all BamA proteins appear to perform a similar function (Knowles et al., 2009), which is likely the result of their conserved three-dimensional structure and membrane topology (Kim et al., 2007; Gatzeva-Topalova et al., 2008; Knowles et al., 2008). The bacterial BamA orthologues are predicted to contain an N-terminal periplasmic region composed of five polypeptide transport-associated (POTRA) domains, as well as a C-terminal integral OM β-barrel region (Sanchez-Pulido et al., 2003; Voulhoux et al., 2003; Gentle et al., 2005; Robert et al., 2006; Stegmeier and Andersen, 2006; Knowles et al., 2009). The crystal structure of POTRA domains 1–4 (residues 21–351) from E. coli BamAwas resolved by both Kim et al. (2007) and Gatzeva-Topalova et al. (2008), while the three-dimensional structure of POTRA domain 5 was characterized by Knowles et al. (2008) using small angle X-ray scatter. Studies have indicated that these periplasmic POTRA domains bind OMP precursors before they are folded into β-barrels and inserted into the OM (Robert et al., 2006; Knowles et al., 2008). The exact mechanism(s) of how the BAM complex functions in OMP folding and insertion is still not entirely understood, although several models have been proposed (Knowles et al., 2009). In any event, it is now clear that all members of the BAM complex are involved, to some degree, in OMP assembly, and the essential BamA protein is a conserved core component in this process (Voulhoux et al., 2003; Doerrler and Raetz, 2005; Wu et al., 2005; Malinverni et al., 2006; Bos et al., 2007; Kim et al., 2007; Sklar et al., 2007; Knowles et al., 2009).
The recent identification of BamA proteins in several other diderms led us to investigate whether there is a BamA orthologue with conserved function in B. burgdorferi. After scanning the borrelial genome, we identified a putative B. burgdorferi BamA orthologue encoded by open reading frame (ORF) bb0795. Subsequent structural model predictions and cellular localization assays resulted in a topographical model of BB0795 that consists of five conserved periplasmic POTRA domains and a C-terminal, surface-exposed, transmembrane β-barrel domain. To characterize the function of BB0795, we also generated a regulatable bb0795 mutant using the IPTG-inducible promoter system previously reported by Gilbert et al. (2007). The mutant revealed that BB0795 is essential for B. burgdorferi growth and viability in vitro, and that BB0795-depleted cells contained decreased amounts of integral OMPs in the B. burgdorferi OM. Interestingly, a reduction in the levels of surface-exposed lipoproteins was also observed in the OM of the BB0795 mutant. The combined structural, cellular localization and functional data presented here indicate that BB0795 is the B. burgdorferi BamA orthologue.
Results
Identification of a putative B. burgdorferi BamA orthologue
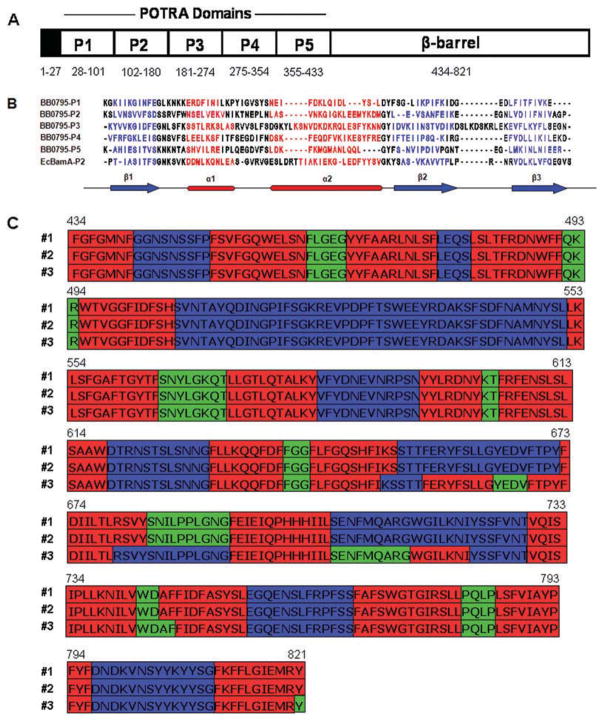
All Gram-negative bacteria whose genomes have been sequenced encode a putative orthologue of the BAM protein, BamA (Reumann et al., 1999; Gentle et al., 2004; Voulhoux and Tommassen, 2004). Because all spirochete genomes sequenced to date are also predicted to encode a putative BamA orthologue (Fraser et al., 1997; Fraser et al., 1998; Voulhoux et al., 2003), we searched the B. burgdorferi protein database to determine whether an orthologous protein was present in the borrelial genome. This analysis resulted in the identification of a predicted B. burgdorferi protein with 41% similarity to the E. coli BamA sequence. The 94 kDa protein, designated BB0795, is encoded by a 2467 bp chromosomal ORF, bb0795. Hydrophilicity and SignalP3.0 (Bendtsen et al., 2004) analyses on the BB0795 protein indicated that it contains a putative 27-amino-acid (aa) hydrophobic signal peptide at its N-terminus (data not shown). Additionally, secondary structure predictions failed to identify any other hydrophobic regions of significant length that could act as a stop-transfer sequence and anchor the protein to the B. burgdorferi inner membrane. Therefore, BB0795 is most likely translocated through the Sec-mediated transport system and may be further exported to the B. burgdorferi OM.
After the initial genome peptide scan and hydrophilicity analyses, we examined the structural organization of the BB0795 protein. Prior studies have determined that the mature N-terminus of E. coli BamA is composed of five repeating structural elements, termed POTRA domains (Sanchez-Pulido et al., 2003; Kim et al., 2007; Gatzeva-Topalova et al., 2008; Knowles et al., 2008). We therefore postulated that if BB0795 is a BamA orthologue, then BB0795 should be structurally similar and also contain five POTRA domains at its N-terminus. Accordingly, aa 28–433 of the BB0795 N-terminal sequence (chosen from peptide sequence alignment with the E. coli BamA POTRA domains) were submitted to the Robetta server for protein domain prediction (http://robetta.bakerlab.org). The Robetta algorithms predicted that aa 28–433 of the BB0795 peptide sequence contained five repeating putative POTRA domains, with each domain predicted to be ~75–80 residues (Fig. 1A). Similar to what was observed in the E. coli BamA structure, however, P3 is predicted to be longer in sequence, containing ~100 residues (Kim et al., 2007). These POTRA domains collectively contained a very high confidence value of 56 (http://robetta.bakerlab.org). The confidence value of 56 from the Robetta server indicates that the similarity identified in the known E. coli POTRA domain structure and that modelled by the Robetta programme for the B. burgdorferi POTRA domains has an expected probability value of 1 × 10−57 (http://robetta.bakerlab.org).
Fig. 1.
Domain organization and predicted structural features of BB0795.
A. Schematic diagram of the BB0795 protein. The N-terminal region is composed of a putative signal peptide (amino acids 1–27; indicated by a black box) followed by five putative POTRA domains, indicated as P1–P5 (amino acids 28–433). The BB0795 C-terminal region (amino acids 434–821) is composed of a putative β-barrel domain.
B. Comparison of the B. burgdorferi putative POTRA domains P1–P5 with E. coli POTRA domain P2. The canonical β1-α1-α2-β2-β3 POTRA domain secondary structure is predicted to be conserved between all of the putative BB0795 POTRA domains and the known E. coli BamA POTRA domain. Secondary structure predictions were performed as indicated in the Experimental procedures. Regions predicted to be in β-sheet are indicated in blue and regions predicted to be in α-helical conformation are shown in red. At bottom is the consensus secondary structure regions identified in the comparison.
C. Predicted topology of the BB0795 β-barrel domain. Amino acids 434–821 of the BB0795 protein (corresponding to the C-terminal putative β-barrel domain) were subjected to the PRED-TMBB β-barrel prediction programme (http://bioinformatics.biol.uoa.gr/PRED-TMBB) (Bagos et al., 2004a,b). Topology results from the Viterbi, N-Best and Posterior decoding algorithms used by PRED-TMBB are indicated as #1, #2 and #3 respectively. Predicted transmembrane regions (red), extracellular domains (blue) and periplasmic domains (green) are indicated for each topology prediction algorithm.
All POTRA domains are defined by a specific order and number of β-sheet and α-helix structural elements, which form a conserved β1-α1-α2-β2-β3 motif (Sanchez-Pulido et al., 2003; Clantin et al., 2007; Kim et al., 2007). Therefore, although the peptide sequences are not well conserved between individual POTRA domains, the canonical secondary structural motif was observed in all five putative POTRA domains of B. burgdorferi BB0795, when compared with the known E. coli POTRA domain 2 (Fig. 1B). The BB0795 C-terminal region was also examined using the transmembrane β-barrel prediction algorithm (PRED-TMBB) (Bagos et al., 2004b), which predicted aa 434–821 to comprise a membrane-integrated β-barrel containing 18 membrane-spanning regions (Fig. 1A and C).
POTRA modelling and BB0795 predicted topology
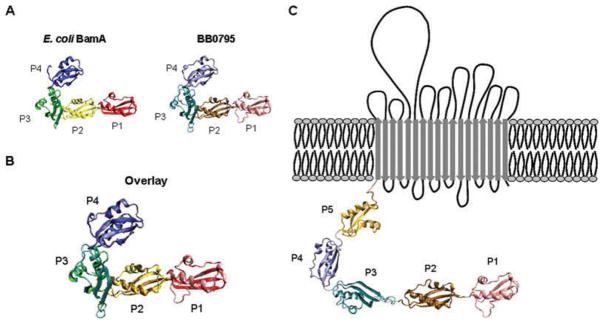
After examining the predicted structural organization of BB0795, we next wanted to determine whether BB0795 shares a similar three-dimensional structure and topology as BamA. Because the X-ray crystal structure of the E. coli BamA POTRA domains P1–P4 was previously determined (Kim et al., 2007), we were able to use those structural coordinates to create a three-dimensional model of the predicted BB0795 POTRA domains. Accordingly, we submitted aa 28–354 of BB0795 (the peptide sequence corresponding to predicted POTRA domains P1–P4) to the Swiss-Model platform for modelling. Ribbon images of BB0795 (Fig. 2A, right), and the adjacent E. coli BamA POTRA structure (Fig. 2A, left), are displayed. As with the Robetta programme prediction, the structural model indicates that the N-terminal peptides of BB0795 comprise repeating POTRA domains. Similar to E. coli BamA, each of the POTRA domains (labelled as P1–P4 and indicated by different colours) are composed of the β1-α1-α2-β2-β3 motif and are folded so that the three β-sheets are overlaid with two α-helices. The predicted existence of the repeating POTRA domains suggests that the N-terminal region of BB0795 possesses a similar tertiary structure as E. coli BamA. The superimposed models (Fig. 2B) show that both the BamA structure and the BB0795 model align very closely. The differences that exist between the two POTRA structures are most apparent in the less structured regions between the β-strands and α-helices (see Fig. 2A and B).
Fig. 2.
BB0795 structural model and predicted membrane topology.
A. X-ray crystal structure co-ordinates from E. coli BamA POTRA domains 1–4 (pdb co-ordinates: 2qdf) were used to model the structure of the putative BB0795 POTRA domains 1–4. The known E. coli BamA POTRA crystal structure (left) and the predicted structure of POTRA domains 1–4 from BB0795 (right) are displayed in ribbon model format.
B. The ribbon models for the known E. coli structure and the predicted BB0795 POTRA domains were overlaid to demonstrate structural similarity.
C. Predicted topology of the BB0795 protein. The BB0795 P1–P4 model and a structural model of POTRA domain P5, predicted by the Robetta programme (see Experimental procedures), were combined with the BB0795 β-barrel prediction to create the topographical model displayed. The BB0795 protein is predicted to contain five soluble POTRA domains and an 18-stranded outer membrane β-barrel domain (indicated by anti-parallel arrows) with eight periplasmic and nine surface-exposed loops.
Finally, after obtaining a POTRA domain 5 structural prediction for BB0795 using the Robetta programme (http://robetta.bakerlab.org), we combined the N-terminal BB0795 POTRA domains P1–P5 with the predicted C-terminal transmembrane β-barrel (Bagos et al., 2004b) to create a final topographical model of the mature B. burgdorferi BB0795 protein (Fig. 2C). Consistent with other BamA orthologues, the combined analyses predicted that the mature BB0795 protein contains a large periplasmic, soluble domain containing five POTRA domains at its N-terminus (aa 28–433), followed by an OM β-barrel domain (aa 434–821). The exact physiological orientations of the POTRA domains remains unclear, but in contrast to the first E. coli BamA POTRA structure provided by Kim et al. (2007) (shown in Fig. 2A and B), recent small angle X-ray scatter (SAXS) structural predictions indicate that POTRA domain 3 may exist in an extended confirmation in line with POTRA domains 1 and 2 (Gatzeva-Topalova et al., 2008; Knowles et al., 2008), as displayed in the final topographical model generated here for the B. burgdorferi BB0795 protein (Fig. 2C).
BB0795 cellular localization assays
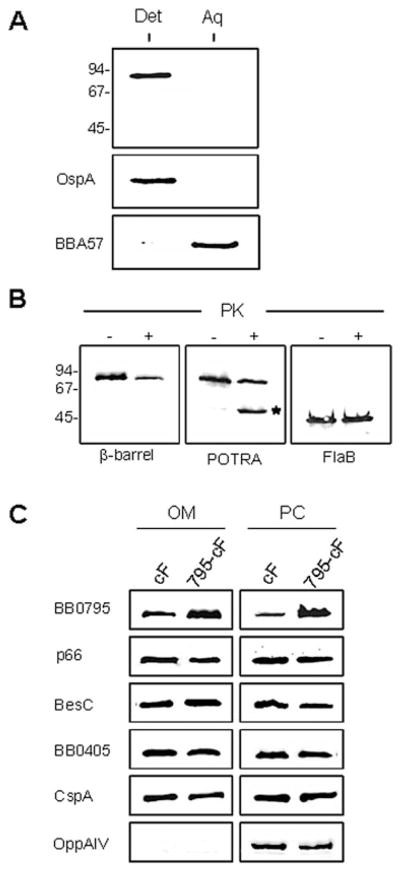
The previous modelling and topological prediction results provided us with a model of BB0795 that is structurally similar to E. coli BamA, suggesting that the two proteins are orthologues. Because BamA is localized to the E. coli OM, we subsequently wanted to determine the cellular location of BB0795 in B. burgdorferi. To test our working topological model of BB0795, we first performed Triton X-114 (TX-114) phase partitioning studies. TX-114 phase partitioning is a commonly used method for separating membrane proteins from soluble cytoplasmic and peri-plasmic proteins, which makes it possible to determine if a protein is membrane-integrated (Bordier, 1981). We therefore subjected B. burgdorferi B31cF cells to TX-114 detergent solubilization, and the resulting aqueous-enriched and detergent-enriched fractions were immunoblotted with BB0795 antisera. As shown in Fig. 3A, BB0795 partitioned exclusively into the detergent phase, which would be expected of an integral OMP (Bordier, 1981; Brusca and Radolf, 1994). As controls for the phase separation technique, the amphiphilic membrane-anchored lipoprotein OspA and the soluble periplasmic BBA57 protein were also subjected to TX-114 phase partitioning. As expected, OspA was identified in the membrane-enriched phase and BBA57 was only identified in the aqueous-enriched phase, as we have shown previously (Brooks et al., 2006).
Fig. 3.
BB0795 is a surface-exposed OMP.
A. BB0795 is amphipathic and partitions into the detergent-enriched phase after Triton X-114 phase partitioning. Whole-cell lysates of B. burgdorferi B31cF were subjected to Triton X-114 phase partitioning, and equal amounts of the detergent-enriched and aqueous phases were subjected to immunoblot analysis with BB0795 antisera. Molecular weight standards, in kDa, are indicated at left. To ensure proper phase separation, a known detergent phase protein and a soluble aqueous phase protein, OspA and BBA57, respectively, were included as controls.
B. BB0795 contains surface-exposed epitopes. Whole-cell lysates of B. burgdorferi B31cF cells overexpressing BB0795 were either mock-treated (−) or proteinase K-treated (+) before being immunoblotted with rat anti-β-barrel domain antibodies (left panel), rat anti-POTRA domain antibodies (middle panel) or rabbit anti-FlaB antibodies (right panel). The asterisk in the middle panel indicates the liberated ~47 kDa periplasmic POTRA domain that is protected from protease treatment. An identical membrane transfer was immunoblotted with rabbit anti-FlaB antibodies to ensure equal loading of the mock-treated and proteinase K-treated whole-cell lysates. Molecular weight standards, in kDa, are indicated at left.
C. BB0795 is localized to the B. burgdorferi OM. Strains B. burgdorferi B31cF (cF) and the BB0795 overexpressing strain (795-cF) were separated into OM and PC fractions. Whole-cell equivalents of each fraction were subjected to immunoblot analysis with rat anti-BB0795 antibodies. To determine the effect of BB0795 overexpression on the OM levels of other surface-localized proteins, the OM and PC samples were also probed with antisera to various known integral OMPs (p66, BesC and BB0405) and to the surface-exposed lipoprotein CspA. As a control for the fractionation procedure, antisera to the inner membrane-anchored OppAIV lipoprotein was also included to ensure OM fractions were not contaminated with PC components.
To examine the cellular location of BB0795 in more detail, we next performed Proteinase K (PK) accessibility assays to determine if the BB0795 protein is surface-localized in B. burgdorferi. After incubating whole B. burgdorferi B31cF cells in the presence or absence of PK, we immunoblotted cell lysates with BB0795 antisera. The immunoblot profiles generated from PK-treated cells appeared to have an increased banding pattern, indicating that BB0795 was at least partially degraded and PK-accessible (data not shown). However, the results from multiple experiments were not conclusive. Similar results were also obtained when cells were treated with trypsin instead of PK. This observation was not entirely surprising, given the fact that other BamA orthologues have been shown to be at least partially resistant to protease treatment, a phenomenon that has also been noted for other bacterial OMPs, such as porins (Robert et al., 2006; Stegmeier and Andersen, 2006; Sun et al., 2007). Additionally, it is possible that very little of the total cellular content of BB0795 is actually surface-localized, and that most of the protein is still retained in the periplasmic compartment. We postulated that if this latter scenario were true, then an increase in BB0795 expression might increase the amount of BB0795 protein found on the borrelial surface. Therefore, in order to investigate this possibility, we overexpressed bb0795 from the strong flgB promoter of B. burgdorferi. This allowed us to overexpress BB0795 in B. burgdorferi strain B31cF, generating a strain designated 795-cF. Immunoblot analysis of mock-treated (left lanes) or PK-treated 795-cF cells (right lanes) are displayed in Fig. 3B. When equivalent amounts of whole-cell lysates were blotted with antibodies to the C-terminal half of BB0795 (the putative surface-exposed β-barrel region), there was a dramatic decrease in the amount of detectable full-length BB0795, as compared with the mock-treated cells (Fig. 3B, left panel). Additionally, scanning densitometric analyses of the chemiluminescent immunoblots revealed that 70% of the detectable BB0795 protein was degraded after PK treatment. When the same samples were immunoblotted with antisera to the N-terminal POTRA region (i.e. the ~47 kDa periplasmic domain), we again observed a decrease in detectable full-length BB0795 in the PK-treated cells (Fig. 3B, middle panel). Interestingly, in this PK-treated sample, which was immunoblotted with antibodies specific for the periplasmic BB0795 domain, there was a dramatic increase in intensity of a ~47 kDa band (indicated in the middle panel by asterisk). The migration range of this particular band corresponds well with the estimated size of the five putative POTRA domains from BB0795, which would be ~46.5 kDa. These results are therefore consistent with the BB0795 topographical model proposed above. That is, PK-mediated degradation of the surface-exposed BB0795 caused the periplasmic POTRA domains to be liberated from the β-barrel domain, resulting in accumulation of the ~47 kDa periplasmic fragment, which was detected by the N-terminal anti-POTRA antibodies (Fig. 3B, middle panel). Identical results were also obtained when B. burgdorferi 795-cF cells were incubated with trypsin instead of PK (data not shown). To ensure equal loading of all samples, mock- or PK-treated lysates were also immunoblotted with the constitutively expressed FlaB protein (Fig. 3B, right panel).
To further verify that BB0795 is localized to the borrelial OM, and that overexpression of the protein results in increased amounts of BB0795 in the B. burgdorferi OM, we next purified OMs from B. burgdorferi B31cF and from the overexpressing strain, B. burgdorferi 795-cF. Subsequently, whole-cell equivalents of purified OM and protoplasmic cylinder (PC) preparations from each strain were immunoblotted with BB0795 antisera. As shown in Fig. 3C, the overexpressing 795-cF strain contained increased amounts of BB0795 in both fractions as compared with the parental B31cF strain (Fig. 3C, top panels). The purified OM and PC fractions were also probed for other known B. burgdorferi integral OMPs p66, BesC and BB0405 (Probert et al., 1995; Brooks et al., 2006; Bunikis et al., 2008), as well as for the surface lipoprotein CspA (Kraiczy et al., 2004; Brooks et al., 2005; Kenedy et al., 2009), to demonstrate that BB0795 overexpression did not cause a global dysregulation of protein localization into the B. burgdorferi OM. Interestingly, increased amounts of BB0795 in the OM did not increase the amounts of these other proteins in either the OM or the PC fractions. As a control, the OM and PC fractions were also immunoblotted with antisera to the inner membrane-localized OppAIV lipoprotein (bottom panels) to verify that the B. burgdorferi OM fractions were not contaminated with proteins from the PC fraction. Combined, the TX-114 phase partitioning, PK surface localization and OM purification studies strongly suggest that B. burgdorferi BB0795, similar to the E. coli BamA protein, contains an N-terminal periplasmic domain and a C-terminal trans-membrane domain that is localized to the B. burgdorferi OM.
Generation of a regulatable bb0795 mutant
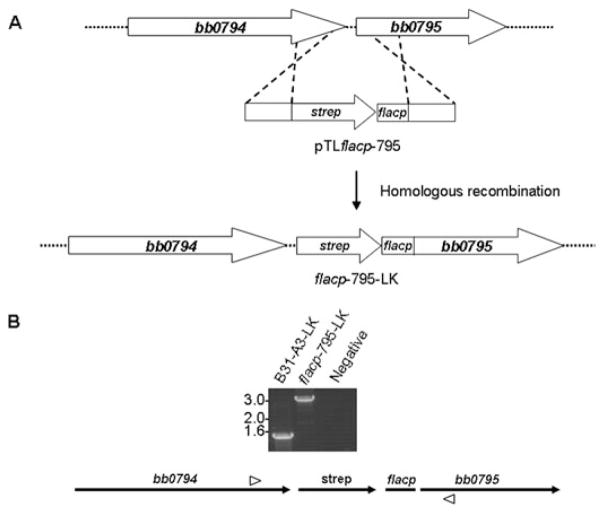
Previous studies on BamA orthologues from other bacteria have demonstrated that these proteins are essential for viability (Reumann et al., 1999; Voulhoux et al., 2003; Doerrler and Raetz, 2005; Werner and Misra, 2005). Consistent with the essential nature of BamA in other bacteria, we were unsuccessful on multiple attempts to inactivate the bb0795 ORF in B. burgdorferi. Therefore, in order to study the function of the BB0795 protein, we generated a B. burgdorferi strain containing a regulatable bb0795 gene. As shown in Fig. 4A, we replaced the native bb0795 promotor with an IPTG-responsive promoter, designated flacp, in B. burgdorferi B31-A3-LK, a strain previously engineered to express the LacI repressor protein from plasmid lp25 (Gilbert et al., 2007). The recombination construct (pTLflacp-795) also included a streptomycin resistance cassette upstream of the flacp promoter for selection (Fig. 4A). After electroporation of B. burgdorferi B31-A3-LK with the pTLflacp-795 cassette, we identified several streptomycin-resistant transformants and chose one for further study, which was designated B. burgdorferi flacp-795-LK. To confirm proper insertion of the B. burgdorferi pTLflacp-795 recombination cassette upstream of bb0795, PCR analysis was performed on both the parental B31-A3-LK and the flacp-795-LK strains using primers bb0794 (KpnI) F and bb0795 (784–762) R (Fig. 4B, primer positions are indicated by arrowheads in lower schematic). As expected, the parental B. burgdorferi B31-A3-LK parental strain produced a ~1.5 kb amplicon (Fig. 4B, left lane). However, PCR amplification from the B. burgdorferi flacp-795-LK strain produced a ~3 kb amplicon (Fig. 4B, middle lane), which was consistent with insertion of both the streptomycin resistance cassette and the flacp promoter into the B31-A3-LK chromosome between ORFs bb0794 and bb0795.
Fig. 4.
Construction of an IPTG-regulated bb0795 gene in B. burgdorferi B31-A3-LK.
A. The native bb0795 promotor was replaced with the IPTG-regulatable promotor, flacp, in the B. burgdorferi B31-A3-LK strain. The pTLflacp-795 construct was composed of a streptomycin resistance cassette fused to flacp and flanked by homologous DNA from bb0794 and bb0795. The construct was inserted upstream of the bb0795 start codon by homologous recombination.
B. Insertion of the streptomycin resistance cassette fused to the flacp promoter upstream of bb0795 was verified by PCR using primers BB0794 (KpnI) F and BB0795 (784–762) R (arrowheads). A 1.5 kb amplicon was amplified from the wild-type B. burgdorferi B31-A3-LK strain (lane B31-A3-LK), as expected. Amplification of the mutant flacp-795-LK DNA produced a 3 kb amplicon, consistent with insertion of the streptomycin-flacp insert between the bb0794 and bb0795 genes. A PCR reaction with no template DNA was also included as a negative control. Molecular weight standards, in kb, are indicated at left.
B. burgdorferi flacp-795-LK growth is dependent on BB0795 expression
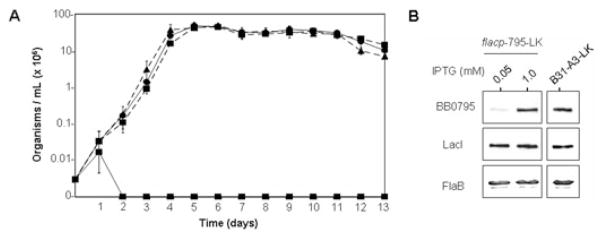
To examine the role of BB0795 expression on cell viability, we performed growth curves with the parental B. burgdorferi B31-A3-LK and with the IPTG-regulatable B. burgdorferi flacp-795-LK strains. To perform the growth curves, mid-logarithmic cultures of both strains, resurrected from frozen stocks in 1.0 mM IPTG, were gently pelleted and washed in IPTG-deplete media. Subsequently, the IPTG-regulatable B. burgdorferi flacp-795-LK strain was incubated in complete Barbour-Stoenner-Kelly (BSK)-II containing 0 mM, 0.1 mM, 0.025 mM, 0.05 mM or 1 mM IPTG. The parental B. burgdorferi B31-A3-LK strain was cultured in either 1 mM IPTG or in IPTG-deplete media. All cultures were seeded at 3000 organisms per ml, and growth was followed for 13 days post inoculation. These preliminary growth experiments revealed that organisms cultivated in 0 mM, 0.01 mM and 0.025 mM IPTG were not viable (data not shown). Therefore, as shown in Fig. 5A, the growth of the parental strain (in IPTG-deplete media) and the B. burgdorferi flacp-795-LK strain were examined by cultivation in 0.05 mM or 1.0 mM IPTG. While there was no significant difference observed in viability between organisms cultivated in 0.05 mM IPTG and the wild-type strain, the B. burgdorferi flacp-795-LK strain cultivated in IPTG-deplete media was no longer viable after 24 h. Growth of the parental B31-A3-LK strain was not affected by the addition of IPTG to the growth media (data not shown). This was also previously demonstrated for B31-A3-LK by Gilbert et al. (2007). While the B. burgdorferi flacp-795-LK strain was viable for approximately 24 h in the absence of IPTG, this was likely due to the fact that complete repression of bb0795 was delayed until all residual intracellular IPTG was depleted from these spirochetes. In any event, the B. burgdorferi flacp-795-LK was not viable after 24 h in IPTG-deplete media, indicating that BB0795 is essential for B. burgdorferi growth.
Fig. 5.
BB0795 is an essential protein in B. burgdorferi.
A. IPTG is required for growth of the B. burgdorferi flacp-795-LK strain. flacp-795-LK cultures were seeded at 3000 organisms per ml and incubated in media containing 0 mM (
 ), 0.05 mM (
), 0.05 mM (
 ) or 1 mM (
) or 1 mM (
 ) IPTG in triplicate. The parental B31-A3-LK strain (
) IPTG in triplicate. The parental B31-A3-LK strain (
 ) was seeded in IPTG-deplete media. Spirochetes were enumerated daily by dark-field microscopy for 13 days post inoculation.
) was seeded in IPTG-deplete media. Spirochetes were enumerated daily by dark-field microscopy for 13 days post inoculation.
B. IPTG dose-dependent expression of BB0795 in B. burgdorferi strain flacp-795-LK. Whole-cell lysates of B. burgdorferi flacp-795-LK cultivated in media containing 0.05 mM or 1 mM IPTG were subjected to immunoblot analysis using rat anti-BB0795 antibodies. Lysates were also probed with a LacI monoclonal antibody and FlaB antisera to ensure equivalent amounts of the LacI repressor protein and equivalent amounts of whole-cell lysates were loaded, respectively. Immunoblots of whole-cell lysates of the parental B31-A3-LK strain (grown in IPTG-deplete media) are shown for comparison.
In addition to performing IPTG dose-dependent growth assays, we also examined the expression level of BB0795 in both the parental strain grown in complete BSK-II and in the IPTG-regulatable strain grown in media containing 0.05 mM or 1 mM IPTG. As shown in Fig. 5B, when whole-cell lysates of mid-logarithmic phase organisms were examined for expression of BB0795, the parental B. burgdorferi B31-A3-LK strain and the IPTG-regulatable flacp-795-LK strain cultivated in 1.0 mM IPTG expressed similar levels of BB0795. The IPTG-regulatable strain cultivated in 0.05 mM IPTG, however, expressed very low, almost undetectable levels of BB0795. Because there was not a significant difference observed in growth or viability between the parental strain and the B. burgdorferi flacp-795-LK strain cultivated in 0.05 mM IPTG (see Fig. 5A), this strongly suggests that only a minimal level of BB0795 expression is required by B. burgdorferi for viability in complete BSK-II cultivation media. Immunoblot analysis for the LacI repressor was also performed to ensure that BB0795 expression was specifically regulated by IPTG and not due to aberrant expression of the LacI repressor between the various strains and conditions analysed (Fig. 5B, middle panels). Additionally, all cultures were probed with FlaB antisera to confirm that equivalent amounts of whole-cell lysates were loaded for each sample (Fig. 5B, lower panels).
BB0795 depletion affects localization of B. burgdorferi integral OMPs and lipid-anchored lipoproteins
Previous studies on bacterial BamA orthologues from E. coli and N. meningitidis have demonstrated a role for these proteins in proper localization of β-barrel OMPs (Voulhoux et al., 2003; Gentle et al., 2004; Doerrler and Raetz, 2005). To determine if BB0795 also functions to enhance localization of proteins into the B. burgdorferi OM, we next isolated OM fractions from the B. burgdorferi flacp-795-LK strain grown in either 0.05 mM or 1.0 mM IPTG or from the wild-type parental B31-A3-LK strain. Immunoblot analyses revealed that the OM fractions (Fig. 6, left panels) of B. burgdorferi flacp-795-LK grown in 0.05 mM IPTG contained dramatically lower levels of BB0795 protein as compared with the same strain cultivated in 1.0 mM IPTG and the parental B31-A3-LK strain. Similarly, OM levels of the other known B. burgdorferi integral OMPs p66, BesC and BB0405 were also observed to be dramatically decreased when the IPTG-regulatable strain was cultivated in 0.05 mM IPTG. In contrast to the OM fractions, there was no observable difference in the p66, BesC or BB0405 levels when PC cell equivalents were compared (Fig. 6, right panels). Interestingly, in the B. burgdorferi flacp-795-LK mutant strain cultivated in 0.05 mM and 1.0 mM IPTG there was also a distinct decrease in the detectable levels of two lipoproteins, CspA and OspA, as compared with the parental B31-A3-LK strain, although this difference was most discernable in OMs purified from organisms cultivated in 0.05 mM IPTG (Fig. 6, left panels). The PC cell equivalents, however, contained similar levels of the CspA and OspA lipoproteins (Fig. 6, right panels). This observation was notable as lipoproteins are tethered by lipids to the outer leaflet of the B. burgdorferi OM and are not anchored in the OM by membrane-spanning β-barrel domains. As a control for the fractionation procedure, antisera to the inner membrane-anchored OppAIV lipoprotein was also included to ensure that the OM fractions analysed were not contaminated with PC components.
Fig. 6.
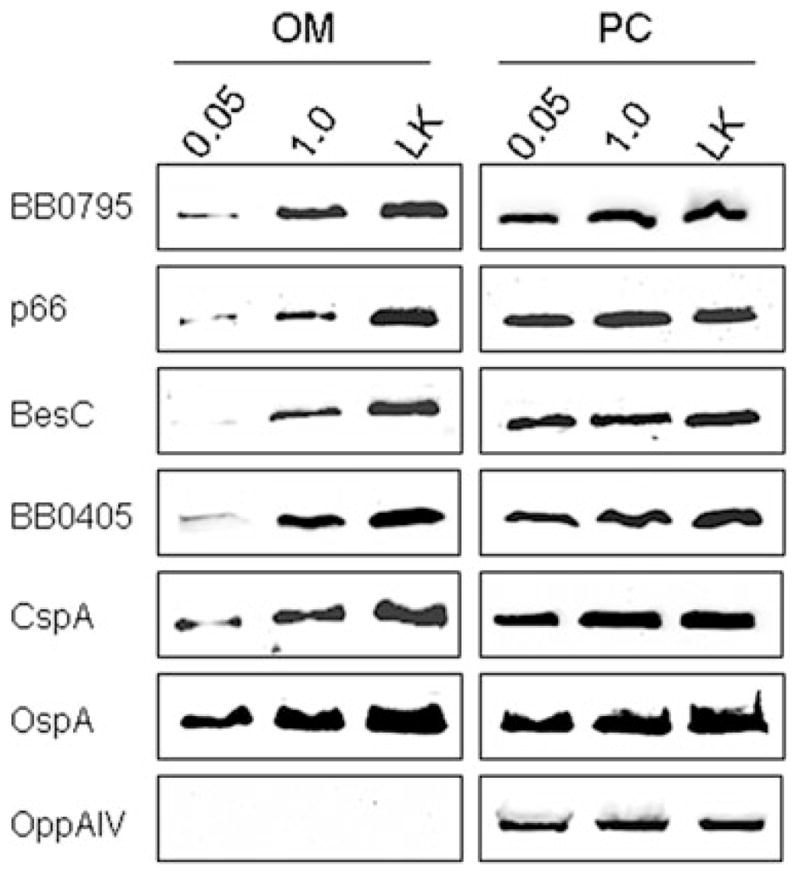
BB0795 depletion inhibits proper localization of known B. burgdorferi outer membrane proteins and lipoproteins into the OM. OM and PC fractions were isolated from B. burgdorferi flacp-795-LK cultures grown in either 0.05 mM or 1.0 mM IPTG, and from B31-A3-LK cultures grown in IPTG-deplete media. Equivalent amounts of OMs (left panels) and PCs (right panels) from each culture condition were subjected to immunoblot analysis with rat anti-BB0795 antibodies, and with antisera specific to known integral OMPs p66, BesC and BB0405, as well as to the surface lipoproteins CspA and OspA. As a control for the fractionation procedure, antisera to the inner membrane-anchored OppAIV lipoprotein was also included to ensure OM fractions were not contaminated with PC components.
Discussion
Members of the BamA family of proteins, which are predicted to be present in all Gram-negative bacteria sequenced to date (Reumann et al., 1999; Voulhoux et al., 2003; Gentle et al., 2005), are essential components of the core BAM complex (Knowles et al., 2009). Structurally, these orthologous BamA proteins contain a poreforming C-terminus that resides as a β-barrel in the OM. In diderm bacteria, the BamA N-terminus is typically composed of five periplasmic POTRA domains. These POTRA domains are important because they have also been shown to exhibit chaperone activity, presumably to aid in the proper localization and insertion of integral OMPs into the bacterial OM. Here we described the characterization of a putative B. burgdorferi BamA orthologue, encoded by locus bb0795. The BB0795 protein was found: (i) to be essential for cell viability, (ii) to contain five N-terminal POTRA domains composed of the canonical β1-α1-α2-β2-β3 secondary structure motif, (iii) to contain a putative C-terminal membrane-spanning β-barrel domain, and (iv) to exhibit functional properties of a BamA orthologue (i.e. enhance localization of OMPs into the borrelial OM). In line with these findings, the BB0795 protein sequence is predicted to encode a putative signal peptide, which would be expected of an OMP that is destined for export to the OM through the general secretory pathway (Pugsley, 1993).
Because BamA is an OMP, it has been examined as a potential vaccinogen in a number of bacterial species. The BamA orthologue in Haemophilus ducreyi, designated D15, was shown to be localized to the OM, and a recombinant form of D15 was shown to provide partial protection against infection in an experimental rabbit model (Thomas et al., 2001). In spirochetes, putative BamA orthologues from two Treponema spp. have also been examined. Antibodies generated against a putative BamA orthologue from Treponema pallidum subspecies pallidum, designated Tp92 (which is 43% similar to the E. coli BamA protein), was shown by Cameron and co-workers to promote opsonization and phagocytosis of T. pallidum by rabbit macrophages (Cameron et al., 2000). Additionally, immunization with recombinant Tp92 partially protected rabbits from subsequent T. pallidum challenge, which led these authors to conclude that Tp92 may represent a potential vaccine candidate for syphilis (Cameron et al., 2000). In a more recent study focusing on conserved surface proteins of oral spirochetes, the putative T. denticola BamA orthologue, designated Td92, was characterized by indirect immunofluorescence and shown to be highly expressed and surface-exposed (Jun et al., 2008). A recombinant form of the Td92 protein, which is 43% similar to E. coli BamA, was shown to bind human epithelial cells. Td92 was also capable of stimulating various cytokines and immune modulators involved in inflammation and osteoclastogenesis, such as IL-1β, TNF-α, IL-6, prostaglandin E2 and matrix metalloproteinase 9 in host cells (Jun et al., 2008). While these prior studies revealed the virulence properties and vaccine potential for the Tp92 and Td92 surface-exposed proteins, neither study examined the structural or functional characteristics of these proteins as they relate to other known BamA orthologues (Cameron et al., 2000; Jun et al., 2008). Our cellular localization data indicated that BB0795, like Tp92 and Td92, is also a surface-exposed protein. Therefore, future studies examining the potential role of BB0795 in B. burgdorferi virulence, and its possible use as a novel Lyme disease vaccinogen, are warranted.
Although the TX-114 phase-partitioning data revealed that BB0795 is a membrane protein, and the cellular localization data clearly showed that BB0795 is an OM protein, it was somewhat surprising that we were unable to obtain reproducible protease degradation of BB0795 until we generated a strain that overexpressed the protein. It is possible that the surface-located loops of the β-barrel domain of BamA are resistant to proteases, especially as BamA has been shown to multimerize in vitro (Robert et al., 2006). Additionally, our surface accessibility assays may have been confounded by the potentially small number of BB0795 molecules exposed on the B. burgdorferi surface. In a proteomic study performed with E. coli, Church and co-workers calculated that the cellular abundance of BamA (previously referred to as YaeT) was approximately only 200 molecules in the OM (Link et al., 1997). By contrast, in the same study, it was determined that there are over 10-fold more molecules (~2500 per cell) of the OmpA porin in the E. coli OM. Therefore, it seems likely that the low abundance of BamA in the OM of B. burgdorferi could have confounded the protease accessibility experiments and that overexpression was required to increase the sensitivity of the assay. While we fully recognize that the overexpressing strain contains increased amounts of BB0795 compared with the wild-type strain, it seems unlikely that overexpression of the protein could result in an aberrant cellular localization by somehow forcing BB0795 into the OM. Finally, all of the combined physicochemical properties and homologies identified between BB0795 and other BamA orthologues known to be located in the OM of other bacteria, including other spirochetes, is consistent with BB0795 being located in the borrelial OM.
Previous studies on bacterial BamA orthologues have shown that depletion of these essential proteins results in a decrease in properly inserted β-barrel OMPs (Voulhoux et al., 2003; Doerrler and Raetz, 2005; Werner and Misra, 2005; Wu et al., 2005). We observed similar results with our IPTG-regulatable bb0795 mutant. When we cultivated the mutant in the low IPTG concentration (0.05 mM), we detected a dramatic decrease in the OM levels of known borrelial OMPs p66, BesC and BB0405. It was somewhat surprising, however, to find that the decreased level of these OMPs being localized to the OM did not significantly affect B. burgdorferi growth (see Fig. 5A). Although we would have expected to see some defect in the growth rate of the flacp-795-LK mutant when cultivated at the low IPTG concentration, it seems that a depletion of BB0795 and these other OMPs from the OM is apparently well tolerated for B. burgdorferi cultivation in vitro. However, more pronounced growth or behavioural defects may be observed during in vivo cultivation of the mutant in the arthropod vector or in infected mice.
In addition to observing a decrease in the amount of OMPs in the OM of the regulatable strain incubated in 0.05 mM IPTG, we also observed that depletion of BB0795 resulted in a considerable decrease in the amount of two surface-exposed lipoproteins, OspA and CspA, in isolated B. burgdorferi OMs. Because bacterial lipoproteins are anchored to the membrane solely by N-terminal lipid moieties, and they do not contain the classical amphipathic β-sheet structure of typical membrane-spanning OMPs, it was surprising to us that depletion of a BamA orthologue in B. burgdorferi would affect OM levels of both β-barrel OMPs and surface lipoproteins. To our knowledge, the role of BamA in surface lipoprotein localization has not been examined in E. coli or in N. meningitidis, presumably because most OM lipoproteins in diderm proteobacteria are localized to the periplasmic compartment. This was therefore a novel observation with regard to the possible role(s) of BamA, most notably in Borrelia spp., because they have more surface-localized lipoproteins than any other organism studied to date (Brandt et al., 1990; Fraser et al., 1997; Casjens et al., 2000; Brooks et al., 2006). Although it is currently not known how lipoproteins are transported and localized to the B. burgdorferi surface, it seems logical that the differences in structure and folding tendencies between lipoproteins and nascent OMPs might require separate localization systems, as is the case with other diderm bacteria (Bos et al., 2007; Tokuda, 2009). This is relevant as it has recently been shown that the E. coli BamA POTRAs bind β-sheet-containing peptides from the β-barrel OMP PhoE, but not to peptides derived from the α-helical periplasmic protein MalE (Knowles et al., 2008). Because BB0795 is predicted to possess a POTRA domain structure that is highly similar to the E. coli BamA POTRA domains, it seems unlikely that BB0795 would bind non-amphipathic β-barrel precursors such as the OspA lipoprotein (Li et al., 1997), or lipoproteins comprised almost exclusively of α-helix secondary structure, such as CspA (Cordes et al., 2005).
In all other diderm bacteria examined to date, lipoproteins are sorted and transported by the lipoprotein localization (Lol) pathway. The Lol system is composed of an inner membrane ABC transporter complex (LolCDE), a periplasmic chaperone (LolA) and an OM lipoprotein receptor (LolB) that inserts lipoproteins into the inner leaflet of the OM (Matsuyama et al., 1995; Yokota et al., 1999; Tokuda, 2009). In a study of lipoprotein export and localization in B. burgdorferi, it was noted by Schulze and Zuckert (2006) that the putative inner membrane LolCDE transporter and the periplasmic LolA proteins were identified in B. burgdorferi, but an orthologous gene encoding the OM LolB lipoprotein is apparently not present in the borrelial genome. The lack of an obvious LolB orthologue in B. burgdorferi led the authors to speculate that lipoprotein export in B. burgdorferi likely diverges from other diderm bacteria after LolA transport through the periplasm. In lieu of LolB, B. burgdorferi may instead use an unidentified OM protein or protein complex to facilitate translocation of lipoproteins to the B. burgdorferi surface (Schulze and Zuckert, 2006). If this assumption is correct, then part of this novel lipoprotein export system may include a currently unidentified integral OMP with amphipathic β-barrel structure. Because we predict that BB0795 is the B. burgdorferi BamA orthologue, the unknown integral OMP, which is utilized for lipoprotein export to the borrelial surface, would require BB0795 for its proper insertion into the OM. Thus, depletion of BB0795 most likely inhibits lipoprotein export to the borrelial surface through an indirect mechanism. That is, when BB0795 is depleted, the lipoprotein export protein complex is also depleted, which ultimately inhibits the proper localization of lipoproteins to the OM of B. burgdorferi. This inference is entirely consistent with our observation that OMs isolated from the bb0795 mutant cultivated in 0.05 mM IPTG contained decreased levels of the lipoproteins CspA and OspA as compared with OMs isolated from the wild-type parental strain. A similar situation was observed in E. coli, when it was first thought that BamA was involved in the insertion of LPS into the OM (Genevrois et al., 2003). In fact, it was later discovered that the defect observed in LPS assembly was indirect, because BamA depletion resulted in improper folding and localization of the OM-integrated LPS biosynthetic protein LptD (formerly named Imp) (Bos et al., 2004). Alternatively, it is also possible that BB0795-mediated depletion of assembled β-barrel OMPs could cause the fragile B. burgdorferi OM to become unstable, resulting in the depletion of all proteins from the OM.
In summary, the results of this study indicate that BB0795 is similar both in its structure, cellular localization and function to other known bacterial BamA proteins. Additionally, consistent with BB0795 being a B. burgdorferi BamA orthologue, our depletion studies indicate that BB0795 plays an essential role in OM localization of B. burgdorferi OMPs. Further functional studies will be required to determine the exact role of BB0795 in β-barrel OMP localization and to what extent it may be involved in lipoprotein localization or OM biogenesis. Future studies should also focus on whether BB0795, like BamA, exists in a complex with proteins that perform accessory functions. Although B. burgdorferi does not encode distinct homologues of any of the known E. coli BamA accessory proteins, the spirochete may encode proteins with conserved structural motifs that could provide a related function. Characterization of these accessory proteins, in conjunction with additional BB0795 functional studies, should not only provide a better understanding of spirochete protein transport, but could also aid in the identification of novel B. burgdorferi OMPs that are involved in virulence and/or Lyme disease pathogenesis.
Experimental procedures
Bacterial strains and growth conditions
Borrelia burgdorferi strains B31cF (Eggers et al., 2002), B31-A3-LK (Gilbert et al., 2007), 795-cF and flacp-795-LK were cultivated at 34°C in BSK liquid medium containing 6% heat-inactivated rabbit serum (complete BSK-II) (Barbour, 1984). The B31-A3-LK strain, an infectious B31-A3 derivative (Elias et al., 2002), has been described (Gilbert et al., 2007). The B31-A3-LK strain expresses LacI from the bbe02 locus of lp25 and contains a gentamicin resistance cassette on plasmid lp28-1 that allows for selection of lp28-1, which is required for mammalian infection (Purser and Norris, 2000; McDowell et al., 2001; Purser et al., 2003). Strains B31cF, B31-A3-LK and the derivatives generated from the parental strains (described below) were cultivated in complete BSK-II supplemented with kanamycin (200 μg ml−1), streptomycin (100 μg ml−1), gentamicin (40 μg ml−1) or erythromycin (80 ng ml−1), and/or isopropyl-β-D-thiogalactopyranoside (IPTG; 0.05 mM or 1.0 mM) as needed. All cloning vectors were propagated using E. coli strains DH5α or SCS110 (Stratagene, La Jolla, CA) grown in Luria-Bertani (LB) broth or agar supplemented with appropriate antibiotics.
BB0795 sequence analysis and modelling
The E. coli BamA protein sequence was used to search the B. burgdorferi B31 peptide database using the J. Craig Venter Comprehensive Microbial Resource Blast server (http://blast.jcvi.org/cmr-blast/). Hydrophilicity analyses were performed using MacVector version 10.0 sequence analysis software (MacVector, Cary, NC) according to the method of Kyte and Doolittle (1982). Amino acid sequence analysis and secondary structure predictions for the BB0795 POTRA regions were determined using the Ginzu domain prediction method within the Robetta Full-Chain Protein Prediction Server (http://robetta.bakerlab.org) (Chivian et al., 2003; 2005; Kim et al., 2004; 2005). The putative BB0795 POTRA domains and E. coli BamA POTRA domain P2 were aligned using the MacVector version 10.0 multiple sequence alignment programme (MacVector), followed by manual editing using the Jalview 2 multiple alignment editor (Waterhouse et al., 2009). BB0795 POTRA domains P1–P4 were modelled against the E. coli BamA POTRA domains P1–P4 (pdb co-ordinates: 2qdf) using the Swiss-Model platform (http://swissmodel.expasy.org) (Arnold et al., 2006). The resulting pdb file was loaded into the Visual Molecular Dynamics (VMD) programme for graphical representation (Humphrey et al., 1996). The BB0795 structural model for POTRA domain P5 was generated using the Robetta comparative modelling algorithm (Chivian et al., 2003; Rohl et al., 2004; Chivian and Baker, 2006) followed by graphical representation with VMD. The membrane topography of the BB0795 C-terminal β-barrel region was predicted using the PRED-TMBB server (http://bioinformatics.biol.uoa.gr/PRED-TMBB) (Bagos et al., 2004a,b).
Generation of BB0795 recombinant proteins
DNA sequences corresponding to the BB0795 N-terminal POTRA region encoding aa 24–441, the BB0795 C-terminal β-barrel region (aa 441–821) and the full-length mature protein lacking the putative signal peptide (aa 24–821) were PCR-amplified from B31 genomic DNA. Primers used for amplifying the POTRA domain (bb0795 F1 and bb0795 R1), β-barrel domain (bb0795 F2 and bb0795 R2) and the full-length mature region (bb0795 F1 and bb0795 R2), respectively, are listed in Table 1. All amplicons were ligated into the Topo-TA pBAD/Thio vector (Invitrogen, Carlsbad, CA), and the resulting constructs were transformed into electro-competent E. coli DH5α cells. Prior to protein purification, selected transformants were verified to contain the correct insert sequence by restriction digest and by nucleotide sequence analysis. Recombinant proteins corresponding to the BB0795 POTRA domain, the BB0795 β-barrel domain and the full-length mature BB0795 protein were purified as thioredoxin fusions using a solubilization protocol as follows. One litre cultures of transformed E. coli were grown to an optical density at 600 nm (OD600) of 0.8 in LB broth supplemented with ampicillin (100 μg ml−1) prior to overnight induction with 0.5% arabinose. Cells were harvested by centrifugation at 8000 g for 20 min, followed by sonication in lysis buffer [6 M guanidine, 20 mM Tris-HCl (pH 7.9), 500 mM NaCl, 4 mM octylglucoside]. The lysed cells were centrifuged at 17 000 g for 30 min, and the resulting supernatant was layered onto a column (Bio-Rad Laboratories, Hercules, CA) loaded with equilibrated Ni-NTA agarose beads (Qiagen, Valencia, CA). After draining the column of supernatant, the beads were washed with 25 ml of wash buffer [6 M urea, 20 mM Tris-HCl (pH 7.9), 500 mM NaCl, 20 mM imidazole] eight times before the proteins were eluted off the beads with 9 ml of elution buffer [6 M urea, 20 mM Tris-HCl (pH 7.9), 500 mM NaCl, 20 mM imidazole, 50 mM EDTA]. All recombinant proteins were subjected to SDS-PAGE to verify purity.
Table 1.
Oligonucleotide primers used in this study.
| Primer | Sequence (5′ to 3′)a | Description |
|---|---|---|
| bb0795 F1 | GTTGAAAATTACAAGGGGAAAATAAT | Nucleotides 70–96 of bb0795 |
| bb0795 R1 | TCCAAAATTCATACCAAATCCAAA | Complementary to nucleotides 1300–1323 of bb0795 |
| bb0795 F2 | GGCAATTCAAATTCTTCATTTCC | Nucleotides 1324–1346 of bb0795 |
| bb0795 R2 | ATATCTCATCTCAATTCCTAAGAA | Complementary to nucleotides 2440–2463 of bb0795 |
| ermC (NcoI) F | GCGCCATGGGATCTTGCAGTATAAATTTAACG | Nucleotides 140–162 of pJD50 (−210 to −190 upstream of the ermC start codon) plus the NcoI site |
| ermC (NcoI) R | GCGCCATGGTTACTTATTAAATAATTTATAGCTATT | Complementary to nucleotides 922–948 of the ermC gene plus the NcoI site |
| flgB (HindIII) F | GCGAAGCTTTACCCGAGCTTCAAGGAAG | Nucleotides 1–19 of the flgB promoter plus the HindIII site |
| flgB (PstI) R | GCGCTGCAGATGGAAACCTCCCTCATTTAAA | Complementary to nucleotides 386–407 of the flgB promoter plus the PstI site |
| bb0795 (PstI) F | GCGCTGCAGATGGGTTCAATTAGAGGTTTGTTTTT | Nucleotides 1–26 of bb0795 plus the PstI site |
| bb0795 (XbaI) R | GCGTCTAGATCAATATCTCATCTCAATTCCTAAG | Complementary to nucleotides 2442–2466 of bb0795 plus the XbaI site |
| bb0794 (KpnI) F | GCGGGTACCCAAGAGGGGATAAACTTG | Nucleotides 3605–3622 of bb0794 plus the KpnI site |
| bb0794 (XhoI) R | GCGCTCGAGTTAATATTTAAATTTCCAAGAAATG | Complementary to nucleotides 4398–4374 of bb0794 plus the XhoI site |
| bb0795 (4–26) F | GCGGAATCCCATATGGGTTCAATTAGAGGTTTGTTTTT | Nucleotides 4–26 of bb0795 plus the EcoRI and NdeI sites |
| bb0795 (784–762) R | GCGACTAGTCTAGTCTTTTAGAATCTTTAAGG | Complementary to nucleotides 784–762 of bb0795 plus the SpeI site |
| flgB (XhoI) F | GCGCTCGAGTACCCGAGCTTCAAGGAAG | Nucleotides 1–19 of the flgB promoter plus the XhoI site |
| flgB (MluI) R | GCGACGCGTATGGAAACCTCCCTCATTTAAA | Complementary to nucleotides 386–407 of the flgB promoter plus the MluI site |
| Strep F | GCGACGCGTATGAGGGAAGCGGTGATCG | Nucleotides 1–19 of the aadA1 gene plus the MluI site |
| Strep R | GCGGAATTCGACGTCTTATTTGCCGACTACCTTGG | Complementary to nucleotides 1176–1199 of the aadA1 gene plus the EcoRI and AatII site |
Restriction sites indicated in bold.
Generation of antibodies
Antibodies to the recombinant POTRA domain, the β-barrel domain and the full-length BB0795 were generated in rats as previously described (Brooks et al., 2006). Briefly, 30 μg of each recombinant protein was combined with 200 μl of PBS (pH 7.4) and 200 μl of Freund’s complete adjuvant (Difco Laboratories, Detroit, MI). The protein-adjuvant mixture was injected intraperitoneally into Sprague-Dawley rats (Harlan, Indianapolis, IN), and booster immunizations were performed at 2 and 4 weeks post primary immunization. Two weeks after the final immunization, the rats were exsanguinated to collect the antisera. Antibody specificity was determined using B. burgdorferi whole-cell lysates and the purified recombinant proteins. A LacI mouse monoclonal antibody (Mab) was purchased (United States Biological, Swampscott, MA), and p66 mouse monoclonal antibody supernatant was kindly provided by Dr Michael Norgard, U.T. Southwestern Medical Center at Dallas, Dallas, TX. Rabbit anti-BesC antibodies were provided by Dr Sven Bergstrom and Dr Ignas Bunikis, Umeå University, and rat anti-OppAIV antibodies were provided by Dr Justin Radolf and Dr Melissa Caimano, University of Con-necticut Health Center, Farmington, CT. Monospecific poly-clonal rat anti-BB0405, anti-BBA57, anti-CspA and anti-OspA antibodies were generated as described (Brooks et al., 2005; 2006).
Electrophoresis and immunoblot analyses
Borrelia burgdorferi whole-cell lysates, membrane fractions or purified recombinant proteins were boiled for 8 min in final sample buffer [62 mM Tris-HCl (pH 6.8), 10% v/v glycerol, 100 mM DTT, 2% SDS, 0.001% bromophenol blue] and subjected to SDS-PAGE using a 4.8% stacking gel and a 12.5% separating gel before being electrophoretically transferred to nitrocellulose membranes. For FlaB immunoblots, the transferred membranes were probed with rabbit anti-FlaB antisera (Brooks et al., 2006) followed by incubation in a 1:1000 dilution of horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA) prior to chromogenic development using 4-chloronapthol and hydrogen peroxide. For all other immunoblots, enhanced chemiluminescence was used as previously described (Kenedy et al., 2009). After primary antibody incubation, membranes were incubated in a 1:10 000 dilution of horseradish peroxidase-conjugated goat anti-rat (for BB0795, BB0405, CspA, OspA and OppAIV blots), goat anti-mouse (for p66 and LacI blots) or goat-anti rabbit (for BesC blots) secondary antibodies. Following secondary antibody incubation, the washed membranes were developed using SuperSignal West Pico enhanced chemiluminescent reagent according to manufacturer’s instructions (Thermo Fischer Scientific, Rockford, IL).
TX-114 phase partitioning
To determine whether BB0795 has the amphiphilic properties expected of an integral membrane protein, B. burgdorferi strain B31cF was subjected to TX-114 phase partitioning as described previously (Brusca and Radolf, 1994; Brooks et al., 2006). The resulting aqueous- and detergent-enriched proteins were precipitated with 10 volumes of ice-cold acetone and recovered by centrifugation at 15 000 g for 15 min at 4°C. The resulting pellets were prepared immediately or stored at −20°C for subsequent SDS-PAGE and immunoblot analysis.
PK surface accessibility
To determine whether BB0795 contains surface-exposed domains, PK experiments were performed as described by Brooks et al. (2006). Final protein pellets from the PK-treated and the mock-treated samples were subjected to SDS-PAGE and immunoblot analysis with rat anti-POTRA domain and rat anti-β-barrel domain antibodies, as described above.
Generation of B. burgdorferi strain 795-cF overexpressing BB0795
Generation of a B. burgdorferi strain overexpressing BB0795 was performed by first generating an erythromycin resistant derivative of the borrelial shuttle vector pBSV2 (Stewart et al., 2001). This was accomplished by amplifying the ermC gene from pJD50 (generously provided by Dr Michael Norgard) using primers ermC (NcoI) F and ermC (NcoI) R. After digestion with NcoI, the purified ermC amplicon was ligated into the NcoI restriction site of pBSV2. Next, the kanamycin resistance cassette was inactivated by cleaving the vector with PvuI, which liberates an 881 bp fragment that encodes the flgB promoter and the first 425 bp of the kanamycin resistance gene. The resulting vector, designated pBSVE, was transformed into E. coli DH5α and incubated on LB agar plates containing 250 μg ml−1 erythromycin for selection. The pBSVE vector was then modified to express the bb0795 gene fused to the borrelial flgB promoter. Briefly, the flgB promoter was amplified from pBSV2 using primers flgB (HindIII) F and flgB (PstI) R. The resulting flgB amplicon was next ligated into the pBSVE HindIII and PstI restriction sites, which resulted in vector flgB-pBSVE. Finally, the bb0795 gene was amplified from B. burgdorferi B31 genomic DNA using primers bb0795 (PstI) F and bb0795 (XbaI) R and cloned into the corresponding restriction sites in the flgB-pBSVE vector. The final construct, designated pTL795-1, is erythromycin resistant and contains a bb0795 gene regulated by the flgB promoter. Fifteen micrograms of the pTL795-1 vector was transformed into electrocompetent B31cF as previously described (Samuels, 1995), and erythromycin-resistant clones were identified and analysed for BB0795 overexpression using the rat anti-BB0795 antibodies generated above. One clone was used for subsequent studies and was designated B. burgdorferi 795-cF.
Generation of B. burgdorferi strain flacp-795-LK containing an IPTG-regulated promoter
The bb0795 regulatable mutant was generated by transforming a clonal isolate of the B. burgdorferi B31-A3-LK strain (generously provided by Dr D. Scott Samuels, University of Montana) (Gilbert et al., 2007) with the pTLflacp-795 construct (see Fig. 4A). The pTLflacp-795 construct was created as follows. The 3′ region of the bb0794 gene (nucleotides 3601–4398) and the 5′ region of the bb0795 gene (nucleotides 4–784) were amplified from B. burgdorferi B31 genomic DNA using primers bb0794 (KpnI) F and bb0794 (XhoI) R, and bb0795 (4–26) F and bb0795 (784–762) R respectively. The bb0794 and bb0795 amplicons were individually cloned into pBluescript-II KS± (Stratagene) using the KpnI and XhoI sites, and the SpeI and EcoRI sites respectively. This resulted in a construct containing DNA regions sufficient for homologous recombination between the B. burgdorferi bb0794 and bb0795 ORFs. Next, the flgB promoter and the streptomycin resistance gene (aadA1) were amplified from the borrelial shuttle vector pKFSS-1 (Frank et al., 2003) using primers flgB (XhoI) F and flgB (MluI) R, and Strep F and Strep R respectively. The flgB and the aadA1 amplicons were individually digested using the appropriate restriction enzymes, and the purified fragments were ligated together before being cloned into the XhoI and EcoRI sites of pBluescript-II KS± (Stratagene). The resulting vector contained the flgB::aadA1 streptomycin resistance cassette flanked by bb0794 and bb0795. Finally, the flacp promoter (containing the Lac operator) was digested from the pTA-flacp vector (provided by Dr D. Scott Samuels) (Gilbert et al., 2007) using AatII and NdeI, and the liberated fragment was cloned into the corresponding restriction sites in the pBluescript-II vector from above (which contains flgB::aadA1 flanked by bb0794 and bb0795). The final vector construct, designated pTLflacp-795, was composed of a pBluescript-II KS± vector backbone carrying a 797 bp region of the 3′ end of the bb0794 gene followed by the streptomycin resistance cassette and an IPTG-inducible flacp promoter fused to the first 780 bp of bb0795. Next, pTLflacp-795 was used as template for PCR amplification with primers bb0794 (KpnI) F and bb0795 (784–762) R. The resulting 3177 bp amplicon, which was verified by nucleotide sequence analysis, was purified and transformed into electrocompetent B. burgdorferi B31-A3-LK cells using the methods described above. Electropo-rated spirochetes were incubated in complete BSK-II supplemented with 200 μg ml−1 kanamycin, 40 μg ml−1 gen-tamicin and 100 μg ml−1 streptomycin. Additionally, 1 mM IPTG was also added to the media to induce bb0795 expression from the flacp promotor. Transformants were analysed by PCR using the primer set bb0794 (KpnI) F and bb0795 (784–762) R to amplify the region of DNA between base 3605 of bb0794 and base 784 of bb0795. One PCR-positive clone was confirmed to have undergone homologous recombination between bb0794 and bb0795, resulting in the insertion of the streptomycin resistance cassette and replacement of the native bb0795 promotor with the regulatable flacp promoter (see Fig. 4B). The B. burgdorferi isolate, designated flacp-795-LK, was subjected to a complete plasmid analysis and was determined to contain all plasmids except cp9, which was originally absent from the parental B31-A3-LK strain (Elias et al., 2002; Brooks et al., 2005; Gilbert et al., 2007).
Growth curve assays
Borrelia burgdorferi IPTG dose-dependent growth curves were performed in triplicate using the IPTG-inducible strain flacp-795-LK and the parental strain B31-A3-LK. The flacp-795-LK clone was resurrected in complete BSK-II supplemented with kanamycin (200 μg ml−1), streptomycin (100 μg ml−1), gentamicin (40 μg ml−1) and 1 mM IPTG, and then harvested and washed in IPTG-deplete media. Subsequently, the washed cells were seeded at 3000 organisms per ml into 4 ml volumes of prewarmed media (containing the appropriate antibiotics) that was supplemented with IPTG to a final concentration of 0 mM, 0.01 mM, 0.025 mM, 0.05 mM or 1 mM prior to inoculation. The B31-A3-LK parental strain was resurrected in complete BSK-II supplemented with kana-mycin (200 μg ml−1) and gentamicin (40 μg ml−1) before being seeded into prewarmed media containing 0 or 1.0 mM IPTG. Both the parental B31-A3-LK cultures and the IPTG-regulated flacp-795-LK cultures were incubated in triplicate for all IPTG concentrations utilized. Spirochetes were enumerated by dark-field microscopy at 24 h intervals throughout the analysis.
IPTG dose-dependent expression assays
For IPTG dose-dependent BB0795 expression assays, the B. burgdorferi flacp-795-LK strain was resurrected in 2 ml of complete BSK-II supplemented with 200 μg ml−1 kanamycin, 40 μg ml−1 gentamicin, 100 μg ml−1 streptomycin and 1 mM IPTG. At late-logarithmic phase (~3 × 107 per ml), spirochetes were centrifuged for 5 min at 4000 g, washed once in pre-warmed IPTG-deplete media and subsequently seeded into 50 ml volumes of prewarmed media at 3000 spirochetes per ml. Each culture was supplemented with IPTG to a final concentration of 0.05 mM or 1 mM prior to inoculation. Whole-cell lysates from late-logarithmic phase B. burgdorferi flacp-795-LK (containing the IPTG regulatable bb0795) and the parental B31-A3-LK strain (cultivated without IPTG) were separated by SDS-PAGE, transferred to nitrocellulose membranes and immunoblotted with rat anti-full-length BB0795 antibodies. Additionally, whole-cell lysates were probed for LacI expression using a mouse anti-LacI Mab (United States Biological) followed by immunoblot with rabbit anti-FlaB antibodies to verify that each lane was loaded with equivalent amounts of whole-cell lysate.
Isolation of B. burgdorferi OM vesicles and PCs
Borrelia burgdorferi OM and PCs were isolated as previously described (Skare et al., 1995; Mulay et al., 2007) with minor modifications. Five hundred millilitres to 1 L cultures of B. burgdorferi B31-A3-LK, flacp-795-LK (in 0.05 mM or 1 mM IPTG), B31cF and 795-cF were grown in complete BSK-II to late-log phase and harvested by centrifugation at 5800 g for 20 min. After one wash in PBS (pH 7.4) containing 0.1% BSA, the pellet (corresponding to 5.7 × 1010 spirochetes for B31-A3-LK and flacp-795-LK, or 9.4 × 1010 spirochetes for B31cF and 795-cF) was resuspended in 90 ml of ice-cold 25 mM citrate buffer (pH 3.2) containing 0.1% BSA. The spirochetes were agitated at room temperature for 2 h with a 1 min vortex every 30 min, followed by centrifugation at 20 000 g for 20 min. The resulting pellet, containing the OM and PC fractions, was resuspended in 5.5 ml of 25 mM citrate buffer (pH 3.2) containing 0.1% BSA and layered onto a discontinuous sucrose gradient in 25 mM citrate buffer (pH 3.2) composed of 5 ml of 56% (wt/wt), 15.5 ml of 42% (wt/wt) and 12.5 ml of 25% (wt/wt) sucrose. The discontinuous gradient was centrifuged overnight at 100 000 g, and the buoyant OM band was removed using a Gradient Station 153 fraction collector (BioComp Instruments, New Brunswick, Canada). The heavier band containing the PC material was collected and diluted eightfold in PBS (pH 7.4), pelleted at 10 000 g for 20 min and resuspended in 1–1.5 ml of PBS (pH 7.4) prior to storage at −80°C for subsequent analyses. The OMs isolated from the discontinuous gradient were resuspended in PBS (pH 7.4), divided between two 14 × 89 mm centrifuge tubes (Beckman Instruments) and subjected to centrifugation at 141 000 g for 4 h at 5°C. Following centrifugation, the OM pellets were combined and resuspended in 1 ml of 25 mM citrate buffer (pH 3.2). Next, a 10–41% (wt/wt) continuous sucrose gradient, in 25 mM citrate buffer (pH 3.2), was prepared using a Gradient Master 107 gradient maker according to manufacturer’s instructions (BioComp Instruments). For further OM purification, the 1 ml of OM sample was layered onto the prechilled continuous gradient and centrifuged overnight at 100 000 g at 5°C. The resultant membrane band was collected and pelleted as described above, and the final OM pellet was resuspended in 150–350 μl of PBS containing 1 mM PMSF and stored at −80°C. To ensure proper fractionation technique and to verify that the OM and PC fractions were not cross contaminated, all OM and PC fractions were immunoblotted with antisera to the known inner membrane-anchored lipoprotein OppAIV (Bono et al., 1998; Nowalk et al., 2006; Mulay et al., 2007).
Acknowledgments
We would like to thank Dr Scott Samuels and Dr Michael Gilbert for providing the B. burgdorferi B31-A3-LK strain and the pTA-flacp vector. We also would like to thank Dr Michael Norgard for providing vector pJD50 and p66 antibodies, Dr Justin Radolf and Dr Melissa Caimano for providing OppAIV antibodies, and Dr Sven Bergstrom and Dr Ignas Bunikis for providing BesC antibodies. We also wish to thank Daniel Desrosiers and Vishwaroop Mulay for valuable discussions and correspondence regarding the Robetta modelling programme and the OM isolation protocol respectively. We also would like to thank Melisha Kenedy for critical review of the manuscript. This work was supported by grants AI059373 and AI064629 from the National Institutes of Health (NIAID) to DRA.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Bagos P, Liakopoulos T, Spyropoulos I, Hamodrakas S. A Hidden Markov Model method, capable of predicting and discriminating beta-barrel outer membrane proteins. BMC Bioinformatics. 2004a;5:29. doi: 10.1186/1471-2105-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagos PG, Liakopoulos TD, Spyropoulos IC, Hamo-drakas SJ. PRED-TMBB: a web server for predicting the topology of beta-barrel outer membrane proteins. Nucleic Acids Res. 2004b;32:W400–W404. doi: 10.1093/nar/gkh417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Belisle JT, Brandt ME, Radolf JD, Norgard MV. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176:2151–2157. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono JL, Tilly K, Stevenson B, Hogan D, Rosa PA. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology. 1998;144:1033–1044. doi: 10.1099/00221287-144-4-1033. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Bos MP, Tefsen B, Geurtsen J, Tommassen J. Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci USA. 2004;101:9417–9422. doi: 10.1073/pnas.0402340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Robert V, Tommassen J. Biogenesis of the gram-negative bacterial outer membrane. Ann Rev Microbiol. 2007;61:191–214. doi: 10.1146/annurev.micro.61.080706.093245. [DOI] [PubMed] [Google Scholar]
- Brandt ME, Riley BS, Radolf JD, Norgard MV. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, Akins DR. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- Brooks CS, Vuppala SR, Jett AM, Akins DR. Identification of Borrelia burgdorferi outer surface proteins. Infect Immun. 2006;74:296–304. doi: 10.1128/IAI.74.1.296-304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca JS, Radolf JD. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- Bunikis I, Denker K, Ostberg Y, Andersen C, Benz R, Bergstrom S. An RND-type efflux system in Borrelia burgdorferi is involved in virulence and resistance to antimicrobial compounds. PLoS Pathog. 2008;4:e1000009. doi: 10.1371/journal.ppat.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000;181:1401–1413. doi: 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- Casjens S, Palmer N, van Vugt R, Huang WM, Steven-son B, Rosa P, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- Chivian D, Baker D. Homology modeling using parametric alignment ensemble generation with consensus and energy-based model selection. Nucleic Acids Res. 2006;34:e112. doi: 10.1093/nar/gkl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivian D, Kim DE, Malmstrom L, Bradley P, Robert-son T, Murphey P, et al. Automated prediction of CASP-5 structures using the Robetta server. Proteins. 2003;53:524–533. doi: 10.1002/prot.10529. [DOI] [PubMed] [Google Scholar]
- Chivian D, Kim DE, Malmstrom L, Schonbrun J, Rohl CA, Baker D. Prediction of CASP6 structures using automated Robetta protocols. Proteins. 2005;61:157–166. doi: 10.1002/prot.20733. [DOI] [PubMed] [Google Scholar]
- Clantin B, Delattre AS, Rucktooa P, Saint N, Meli AC, Locht C, et al. Structure of the Membrane Protein FhaC: A Member of the Omp85-TpsB Transporter Superfamily. Science. 2007;317:957–961. doi: 10.1126/science.1143860. [DOI] [PubMed] [Google Scholar]
- Cordes FS, Roversi P, Kraiczy P, Simon MM, Brade V, Jahraus O, et al. A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat Struct Mol Biol. 2005;12:276–277. doi: 10.1038/nsmb902. [DOI] [PubMed] [Google Scholar]
- Doerrler WT, Raetz CRH. Loss of outer membrane proteins without inhibition of lipid export in an Escherichia coli YaeT mutant. J Biol Chem. 2005;280:27679–27687. doi: 10.1074/jbc.M504796200. [DOI] [PubMed] [Google Scholar]
- Eggers CH, Caimano MJ, Clawson ML, Miller WG, Samuels DS, Radolf JD. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol Microbiol. 2002;43:281–295. doi: 10.1046/j.1365-2958.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- Eiffert H, Jarecki-Khan K, Thomssen R. Iden-tification of an immunoreactive nonproteinaceous component in Borrelia burgdorferi. Med Microbiol Immunol. 1991;180:229–237. doi: 10.1007/BF00202557. [DOI] [PubMed] [Google Scholar]
- Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. aadA confers streptomycin resistance in Borrelia burgdorferi. J Bacteriol. 2003;185:6723–6727. doi: 10.1128/JB.185.22.6723-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GC, Dodson R, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992;6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Gatzeva-Topalova PZ, Walton TA, Sousa MC. Crystal structure of YaeT: conformational flexibility and substrate recognition. Structure. 2008;16:1873–1881. doi: 10.1016/j.str.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevrois S, Steeghs L, Roholl P, Letesson JJ, van der Ley P. The Omp85 protein of Neisseria meningitidis is required for lipid export to the outer membrane. EMBO J. 2003;22:1780–1789. doi: 10.1093/emboj/cdg174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol. 2004;164:19–24. doi: 10.1083/jcb.200310092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I, Burri L, Lithgow T. Molecular architecture and function of the Omp85 family of proteins. Mol Microbiol. 2005;58:1216–1225. doi: 10.1111/j.1365-2958.2005.04906.x. [DOI] [PubMed] [Google Scholar]
- Gilbert MA, Morton EA, Bundle SF, Samuels DS. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol Microbiol. 2007;63:1259–1273. doi: 10.1111/j.1365-2958.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- Guo B, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain H, Wellensiek HJ, Geyer R, Lochnit G. Structural analysis of glycolipids from Borrelia burgdorferi. Biochimie. 2001;83:683–692. doi: 10.1016/s0300-9084(01)01296-2. [DOI] [PubMed] [Google Scholar]
- Howe TR, Mayer LW, Barbour AG. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985;227:645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jun HK, Kang YM, Lee HR, Lee SH, Choi BK. Highly conserved surface proteins of oral spirochetes as adhesins and potent inducers of proinflammatory and osteoclastogenic factors. Infect Immun. 2008;76:2428–2438. doi: 10.1128/IAI.01128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, Akins DR. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun. 2009;77:2773–2782. doi: 10.1128/IAI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DE, Chivian D, Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DE, Chivian D, Malmstrom L, Baker D. Automated prediction of domain boundaries in CASP6 targets using Ginzu and RosettaDOM. Proteins. 2005;61:193–200. doi: 10.1002/prot.20737. [DOI] [PubMed] [Google Scholar]
- Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC, Kahne D. Structure and function of an essential component of the outer membrane protein assembly machine. Science. 2007;317:961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Knowles TJ, Jeeves M, Bobat S, Dancea F, McClelland D, Palmer T, et al. Fold and function of polypeptide transport-associated domains responsible for delivering unfolded proteins to membranes. Mol Microbiol. 2008;68:1216–1227. doi: 10.1111/j.1365-2958.2008.06225.x. [DOI] [PubMed] [Google Scholar]
- Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009;7:206–214. doi: 10.1038/nrmicro2069. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, et al. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem. 2004;279:2421–2429. doi: 10.1074/jbc.M308343200. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lam TT, Nguyen TPK, Montgomery RR, Kantor FS, Fikrig E, Flavell RA. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AJ, Robison K, Church GM. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coli K-12. Electrophoresis. 1997;18:1259–1313. doi: 10.1002/elps.1150180807. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other Gram-negative bacteria. Biochim Biophys Acta. 1983;737:51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- McDowell JV, Sung SY, Labandeira-Rey M, Skare JT, Marconi RT. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect Immun. 2001;69:3670–3677. doi: 10.1128/IAI.69.6.3670-3677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinverni JC, Werner J, Kim S, Sklar JG, Kahne D, Misra R, Silhavy T. YfiO stabilizes the YaeT complex and is essential for outer membrane protein assembly in Escherichia coli. Mol Microbiol. 2006;61:151–164. doi: 10.1111/j.1365-2958.2006.05211.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Tajima T, Tokuda H. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 1995;14:3365–3372. doi: 10.1002/j.1460-2075.1995.tb07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulay V, Caimano M, Liveris D, Desrosiers DC, Radolf JD, Schwartz I. Borrelia burgdorferi BBA74, a periplasmic protein associated with the outer membrane, lacks porin-like properties. J Bacteriol. 2007;189:2063–2068. doi: 10.1128/JB.01239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppa L, Ostberg Y, Lavrinovicha M, Bergstrom S. P13, an integral membrane protein of Borrelia burgdorferi, is C-terminally processed and contains surface-exposed domains. Infect Immun. 2001;69:3323–3334. doi: 10.1128/IAI.69.5.3323-3334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ, Carter CJ, Howell JK, Barbour AG. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowalk AJ, Gilmore RD, Jr, Carroll JA. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun. 2006;74:3864–3873. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol. 2007;5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- Probert WS, Johnson BJB. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- Probert WS, Allsup KM, LeFebvre RB. Iden-tification and characterization of a surface-exposed 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Bourell KW, Akins DR, Brusca JS, Norgard MV. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J Bacteriol. 1994;176:21–31. doi: 10.1128/jb.176.1.21-31.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Davila-Aponte J, Keegstra K. The evolutionary origin of the protein-translocating channel of chloroplastic envelope membranes: Identification of a cyanobacterial homolog. Proc Natl Acad Sci USA. 1999;96:784–789. doi: 10.1073/pnas.96.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Volokhina EB, Senf F, Bos MP, Gelder PV, Tommassen J. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 2006;4:e377. doi: 10.1371/journal.pbio.0040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohl CA, Strauss CE, Chivian D, Baker D. Modeling structurally variable regions in homologous proteins with Rosetta. Proteins. 2004;55:656–677. doi: 10.1002/prot.10629. [DOI] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol. 1995;47:253–259. doi: 10.1385/0-89603-310-4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pulido L, Devos D, Genevrois S, Vicente M, Valencia A. POTRA: a conserved domain in the FtsQ family and a class of beta-barrel outer membrane proteins. Trends Biochem Sci. 2003;28:523–526. doi: 10.1016/j.tibs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Schulze RJ, Zuckert WR. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol Microbiol. 2006;59:1473–1484. doi: 10.1111/j.1365-2958.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- Skare JT, Shang ES, Foley DM, Blanco DR, Champion CI, Mirzabekov T, et al. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Invest. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar JG, Wu T, Gronenberg LS, Malinverni JC, Kahne D, Silhavy TJ. Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli. Proc Natl Acad Sci USA. 2007;104:6400–6405. doi: 10.1073/pnas.0701579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stegmeier JF, Andersen C. Characterization of pores formed by YaeT (Omp85) from Escherichia coli. J Biochem. 2006;140:275–283. doi: 10.1093/jb/mvj147. [DOI] [PubMed] [Google Scholar]
- Stewart P, Thalken R, Bono J, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001;39:714–721. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, et al. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol. 2007;189:6222–6235. doi: 10.1128/JB.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Leduc I, Olsen B, Thomas CE, Cameron DW, Elkins C. Cloning, overexpression, purification, and immunobiology of an 85-kilodalton outer membrane protein from Haemophilus ducreyi. Infect Immun. 2001;69:4438–4446. doi: 10.1128/IAI.69.7.4438-4446.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H. Biogenesis of outer membranes in Gram-negative bacteria. Biosci Biotechnol Biochem. 2009;73:465–473. doi: 10.1271/bbb.80778. [DOI] [PubMed] [Google Scholar]
- Voulhoux R, Tommassen J. Omp85, an evolutionarily conserved bacterial protein involved in outer-membrane-protein assembly. Res Microbiol. 2004;155:129–135. doi: 10.1016/j.resmic.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Voulhoux R, Bos MP, Geurtsen J, Mols M, Tom-massen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- Vuong P, Bennion D, Mantei J, Frost D, Misra R. Analysis of YfgL and YaeT interactions through bioinformatics, mutagenesis, and biochemistry. J Bacteriol. 2008;190:1507–1517. doi: 10.1128/JB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R, Pattathu J, Kitiratschky V, Brenner C, Zipfel PF, Brade V, et al. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect Immun. 2005;73:2351–2359. doi: 10.1128/IAI.73.4.2351-2359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J, Misra R. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol Microbiol. 2005;57:1450–1459. doi: 10.1111/j.1365-2958.2005.04775.x. [DOI] [PubMed] [Google Scholar]
- Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Yokota N, Kuroda T, Matsuyama S, Tokuda H. Characterization of the LolA-LolB system as the general lipoprotein localization mechanism of Escherichia coli. J Biol Chem. 1999;274:30995–30999. doi: 10.1074/jbc.274.43.30995. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]