Abstract
Survival of allogeneic hematopoietic stem cell transplant (aHSCT) recipients in the intensive care unit (ICU) has been poor. We retrospectively analyzed the short- and long-term outcomes of aHSCT patients admitted to the ICU over a 12-year period. Of 1235 adult patients who had aHSCT between 2002 and 2013, 161 (13%) were admitted to the ICU. The impact of clinical parameters was assessed and outcomes were compared for the periods 2002–2007 and 2008–2013. The ICU, in-hospital, 1- and 5-year survival rates were 64.6 %, 46%, 33% and 20%, respectively. Mechanical ventilation and vasopressor use predicted for worse hospital- and overall survival. After 2008, the requirement for mechanical ventilation and vasopressors, and the diagnosis of sepsis were reduced. While hospital mortality decreased from 69% to 44%, long-term survival remained unchanged. Late deaths, due to causes not associated with the ICU such as relapse and graft-versus-host disease, increased. As thresholds for transplant are lowered, improvements in ICU outcomes for aHSCT recipients may be limited.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (aHSCT) has evolved as a potentially curative treatment modality for a variety of hematologic disorders [1]. The well-described morbidity and mortality associated with this treatment is commonly attributed to a variety of transplant-related factors such as patient age, intensity of conditioning, type of graft and spectrum of infections, many of which have changed significantly over the past two decades [2, 3]. Although several studies have addressed outcomes of HSCT patients admitted to the intensive care unit (ICU), these have been difficult to interpret and apply to clinical practice due to the inclusion of both autologous and allogeneic HSCT recipients [4, 5, 6, 7, 8, 9, 10], early and late post-transplant admissions to the ICU [11, 12, 13, 14, 15], and limited numbers of patients [16, 17].
The objectives of this study were three-fold: 1) to identify clinical predictors of adverse outcomes in a homogeneous population of aHSCT patients emergently admitted to the ICU in the peri-transplant period; 2) to determine short- and long-term survival rates to assist healthcare providers in counseling families; and 3) to compare the outcomes in two distinct time periods to evaluate the impact of advances in transplantation practice and supportive care.
SUBJECTS AND METHODS
A retrospective review was conducted of all adult patients who underwent aHSCT at Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY between January 2002 and December 2013. The study cohort included those admitted to the ICU emergently during their transplant hospitalization. For patients with multiple ICU admissions, only data from the first ICU admission was analyzed. Comparisons were made to the control group transplanted during the same period which did not require ICU admission. The study was granted a limited waiver of authorization by the Institutional Review Board.
Demographic and clinical data (including transplant- and ICU-specific parameters) were obtained from the institutional, bone marrow transplant and ICU databases as well as individual case records. Renal and hepatic function at the time of ICU admission were assessed respectively, based on the changes in serum creatinine and total bilirubin levels from baseline (pre- conditioning) to the time of ICU admission. Baseline pulmonary function and cardiac function were not taken into account as all patients were required to have a diffusing lung capacity for carbon monoxide of at least 50% of predicted and a resting left ventricular ejection fraction by echocardiogram or multiple gated acquisition scan (MUGA) of at least 50% to be eligible for aHSCT. The Mortality Probability Model-II (MPM-II) score [18] at ICU admission was used to estimate probability of hospital mortality. Definitions for myeloablative versus non-myeloablative and reduced intensity conditioning (RIC) were as outlined in the workshop convened by the Center for International Blood and Marrow Transplantation research [19]. Antithymocyte globulin (ATG) was given as additional immunosuppression to patients receiving TCD grafts (except for those on one protocol beginning in 2001 which excluded ATG for a group receiving matched related donor grafts), to aplastic anemia patients and to patients receiving mismatched adult donor conventional grafts. Ex vivo T-cell depletion (TCD) of bone marrow (BM) grafts was accomplished by sequential soybean lectin agglutination and sheep red blood cell (sRBC)-rosette depletion (SBA-E-) as previously described [20]. Peripheral blood stem cells (PBSC) were obtained by G-CSF mobilization. CD34+ cells were positively selected using the ISOLEX 300i Magnetic Cell Selection System, followed by sRBC-rosette depletion of T cells until 2010,4 and beginning in 2010, the CliniMACS system was used [21]. This achieved an approximate 3–5 log10 depletion of CD3+ cells with the patient receiving ~103 CD3+/kg of patient weight or less. No pharmacologic GVHD prophylaxis was given to the patients receiving TCD grafts. There were no conventional haploidentical-only grafts used in any patient included in this study cohort. TCD haplografts were used only in conjunction with CB transplants where the TCD haplograft was used as a bridge to neutrophil recovery of the CB graft. For conventional PBSC and BM transplants the GVHD prophylaxis was either tacrolimus/methotrexate [22], or tacrolimus/sirolimus/methotrexate [23]. For cord blood transplants cyclosporine/mycophenolate mofetil was used [24].
Analyses were performed for the entire group over the 12-year period as well as subgroup analyses for outcomes before and after December 2007 to assess the impact of changing patterns of practice and the opening of a new and larger ICU. Outcome determinations included: rate of admission to the ICU, survival to discharge from the ICU, survival to hospital discharge, 1- and 5- year long-term survival (LTS). The point of reference was the day of ICU admission.
Statistical analysis
Differences based on year group, admission to the ICU, and ICU and hospital survival status were examined using Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Overall survival (OS) was estimated using the method of Kaplan-Meier. The log-rank test for categorical variables and Cox proportional hazards regression for continuous variables were utilized to determine associations between patient variables and OS. The associations of time-dependent covariates on survival (e.g. hemodialysis, vasopressors, mechanical ventilation) were examined using the Cox proportional hazards model. Multivariate logistic regression was used to evaluate the association of a selected group of clinical and disease characteristics on ICU and hospital survival status while multivariate Cox proportional hazards regression was used for OS. A stepwise selection procedure was used for the multivariate models with a p-value threshold of 0.05 to enter the model and a threshold of 0.05 to stay in the model. The adequacy of fit of the final logistic regression models for ICU and hospital survival status was checked using the Hosmer-Lemeshow test [25]. Both p-values were not significant (both p>0.30), indicating adequate fit. We checked the assumption of proportional hazards for the variables in the final Cox regression model that were not time dependent using a Kolmogorov-type supremum test [26]. The assumption for proportional hazards of the variables was not violated (all p>0.10). All computations were performed using SAS 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Patients and Transplants
During the study period, 1235 patients underwent their first aHSCT. Of these, 161 (13%) patients were admitted to the ICU peri-transplant. Patient demographics and transplant characteristics are summarized in Table 1. The study group had greater percentages of patients with unrelated- (p=0.005) and mismatched donors (p=0.006). The overall ICU admission rates for the patients who received myeloablative versus non-myeloablative/RIC aHSCTs were 14% (115/803) and 11% (46/432), respectively (p=0.07). The median time from aHSCT to ICU transfer was 10 days (interquartile range (IQR) 5–22) and their median MPM-II score was 34.7 (IQR 21.6–55).
Table 1.
Patient- and transplant characteristics
| Variable | Category | Study group N=161 N (%) |
Control Group N=1074 N (%) |
|---|---|---|---|
| Age | Median (range) | 52 (21–73) | 52 (18–76) |
| Sex | F | 68 (42) | 425 (40) |
| M | 93 (58) | 649 (60) | |
| Disease | AML/ALL/ABL/MDSCR | 70 (44) | 398 (37) |
| AML/ALL/ABL/MDS active disease | 45 (28) | 189 (18) | |
| NHL/HD/MM | 36 (22) | 397 (37) | |
| AA/CML/other | 10 (6) | 90 (8) | |
| Relation | Related | 44 (27) | 436 (41) |
| Unrelated | 117 (73) | 638 (59) | |
| Matched | HLA-matched | 97 (60) | 777 (72) |
| HLA-mismatched | 64 (40) | 297 (28) | |
| Stem cells | PBSC | 121 (75) | 890 (83) |
| BM | 12 (8) | 57 (5) | |
| CB | 28 (17) | 127 (12) | |
| TCD | Y | 71 (44) | 547 (51) |
| N | 90 (56) | 527 (49) | |
| Conditioning | Myeloablative | 115 (71) | 688 (64) |
| Non-myeloablative | 46 (29) | 386 (36) | |
| TBI/dose | No TBI | 99 (62) | 544 (51) |
| Low dose | 22 (14) | 256 (24) | |
| High dose | 40 (25) | 274 (26) |
AML = acute myelogenous leukemia, ALL = acute lymphocytic leukemia, MDS = myelodysplastic syndrome, ABL = acute biphenotypic leukemia, NHL = non-Hodgkin’s lymphoma, HD = Hodgkin’s disease, MM= multiple myeloma, AA = aplastic anemia, CML = chronic myelogenous leukemia, CR = complete remission, PBSC = peripheral blood stem cells, BM = bone marrow, CB= cord blood TCD = T cell depleted, TBI = total body irradiation
Causes for ICU admission
The causes for ICU transfer were organized by primary organ failure. The majority (68%) were transferred for respiratory failure, and less frequently for hemodynamic instability (24%) alone. Primary neurologic, renal or hepatic failure accounted for <10% of the diagnoses. Single organ failure occurred in 47% of patients; 33% had 2 failing organs and 21% had 3 or more. Thirty-eight percent had an underlying diagnosis of sepsis.
Survival
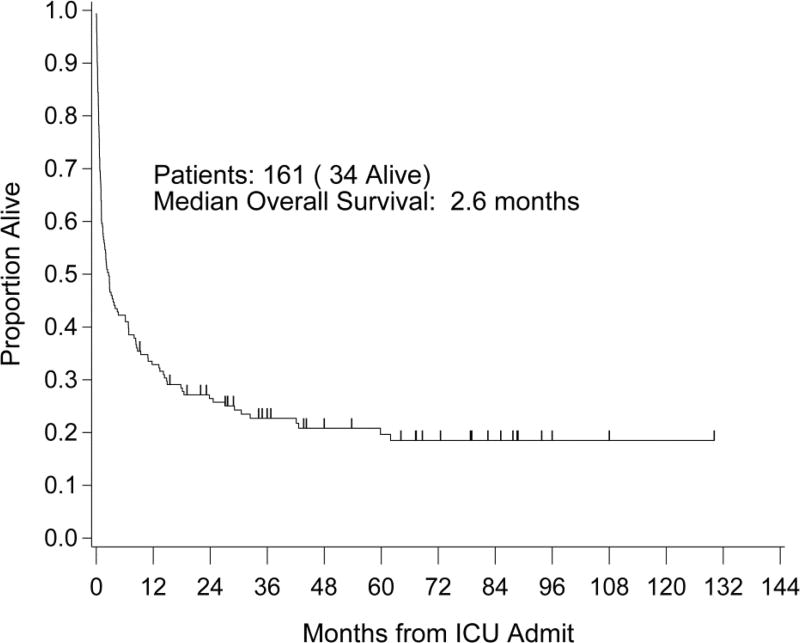
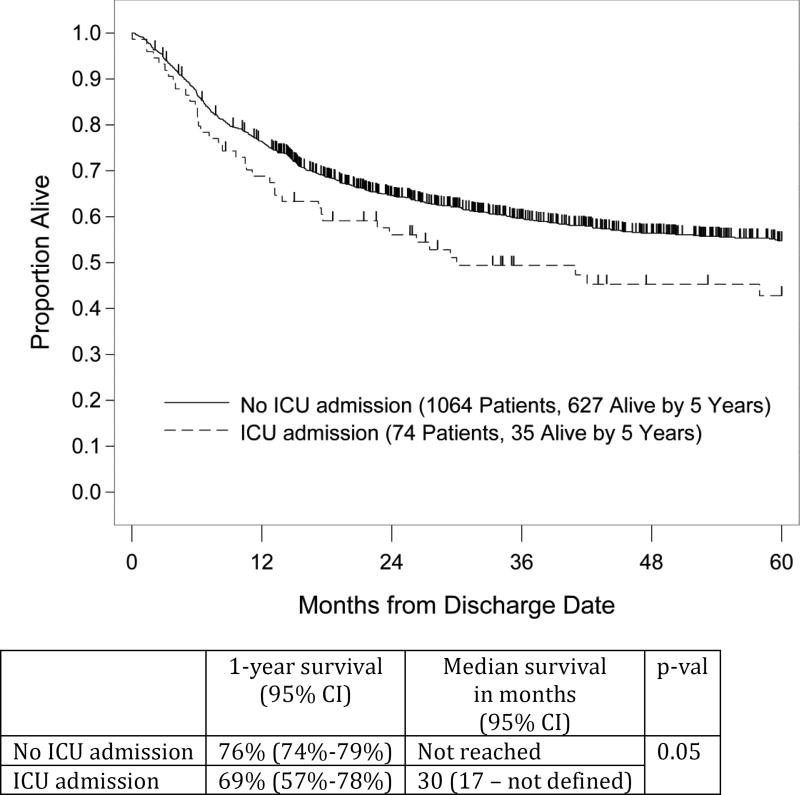
The ICU and hospital survival rates for the entire ICU study cohort were 65% and 46%, respectively. Median OS from ICU admission was 2.6 months with 1- and 5-year OS rates of 33% and 20%, respectively. The Kaplan–Meier curve is shown in Figure 1. Notably, 74 (46%) of the ICU cohort were eventually discharged from the hospital. The Kaplan-Meier curve comparing their post discharge survival with that of the control patients is shown in Figure 2 and demonstrates an approximate 10% reduction at 5 years for the ICU cohort.
Figure 1.
Overall survival entire study group
Median OS: 2.6 months (95% CI: 1.4 months – 4.6 months)
1 year OS: 33% (95% CI: 26%–40%)
5 year OS: 20% (95% CI: 14%–27%)
Figure 2.
Post discharge survival
Clinical factors influencing survival (ICU, hospital, OS)
The following organ support modalities were found to be negatively associated with survival in the ICU and the entire hospitalization: use of vasopressor agents, mechanical ventilation and hemodialysis. At time of ICU transfer, both mechanical ventilation and vasopressor requirement were negatively associated with ICU and hospital survival, while need for renal replacement did not confer a higher risk of death. Peripheral blood (PB)/cord blood (CB) versus BM as the stem cell source portended a higher risk of death in the ICU but not during the hospitalization, a finding limited by the low number (12) of BM transplants.
Although no statistical difference in either ICU or hospital survival was observed for the 16 study cohort patients diagnosed with acute GVHD, the small number of patients prohibits any definitive conclusions. Sepsis, gram positive bacteremia and failure in more than one organ system were negatively associated with both ICU and hospital survival. (Table 2)
Table 2.
Univariate analysis for ICU- and hospital survival
| Alive at ICU Discharge | Alive at Hospital Discharge | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | No (%) | Yes (%) | p | No (%) | Yes (%) | p |
| Stem cells | BM | 1 (8) | 11 (92) | 0.007 | 8 (67) | 4 (33) | 0.07 |
| PB | 40 (33) | 81 (67) | 59 (49) | 62 (51) | |||
| CB | 16 (57) | 12 (43) | 20 (71) | 8 (29) | |||
| TBI | N | 29 (29) | 70 (71) | 0.04 | 49 (49.5) | 50 (50.5) | 0.19 |
| Y | 28 (45) | 34 (55) | 38 (61) | 24 (39) | |||
| Renal replacement | N | 31 (27) | 85 (73) | <0.001 | 50 (43) | 66 (57) | <0.001 |
| Y | 26 (58) | 19 (42) | 37 (82) | 8 (18) | |||
| Vasopressors | N | 10 (12) | 73 (88) | <0.001 | 26 (31) | 57 (69) | <0.001 |
| Y | 47 (60) | 31 (40) | 61 (78) | 17 (22) | |||
| Vasopressors at transfer | N | 35 (30) | 83 (70) | 0.02 | 56 (47.5) | 62 (52.5) | <0.001 |
| Y | 22 (51) | 21 (49) | 31 (72) | 12 (28) | |||
| MV | N | 2 (3) | 57 (97) | <0.001 | 11 (19) | 48 (81) | <0.001 |
| Y | 55 (54) | 47 (46) | 76 (74.5) | 26 (25.5) | |||
| MV at transfer | N | 24 (26) | 68 (74) | 0.005 | 39 (42) | 53 (58) | <0.001 |
| Y | 33 (48) | 36 (52) | 48 (70) | 21 (30) | |||
| Gram positive bacteremia | N | 32 (29) | 77 (71) | 0.02 | 52 (48) | 57 (52) | 0.03 |
| Y | 25 (48) | 27 (52) | 35 (67) | 17 (33) | |||
| Primary organ failure | Circulatory | 5 (13) | 33 (87) | 0.002 | 9 (24) | 29 (76) | <0.001 |
| Respiratory | 48 (44) | 62 (56) | 72 (65.5) | 38 (34.5) | |||
| Other | 4 (31) | 9 (69) | 6 (46) | 7 (54) | |||
| Number of organ failures | 1 | 16 (21) | 59 (79) | 0.002 | 29 (39) | 46 (61) | 0.004 |
| 2 | 25 (47) | 28 (53) | 36 (68) | 17 (32) | |||
| 3+ | 16 (48.5) | 17 (51.5) | 22 (67) | 11 (33) | |||
| Sepsis | N | 28 (28) | 71 (72) | 0.02 | 43 (43) | 56 (57) | <0.001 |
| Y | 29 (47) | 33 (53) | 44 (71) | 18 (29) | |||
| Year group | 2002–2007 | 27 (43.5) | 35 (56.5) | 0.09 | 43 (69) | 19 (31) | 0.002 |
| 2008–2013 | 30 (30.3) | 69 (69.7) | 44 (44) | 55 (56) | |||
| Days post aHSCT to ICU | Median (IQR) | 13 (5 – 33) | 9.5 (5 – 17.5) | 0.13 | 11 (6 – 31) | 8.5 (3 – 18) | 0.03 |
| MPM score | Median (IQR) | 43.3 (26.8 – 57.4) | 30.1 (19.8 – 51.8) | 0.03 | 41.5 (26.3 – 58.3) | 26.5 (17.5 – 46.1) | 0.003 |
| Tbili at trans | Median (IQR) | 1.5 (0.7–2.8) | 0.8 (0.5–1.5) | 0.002 | 1.2 (0.7–2.4) | 0.7 (0.4–1.3) | <0.001 |
| Cr at trans | Median (IQR) | 1.6 (1.2–3.1) | 1.3 (0.9–2.0) | 0.01 | 1.6 (1.2–2.4) | 1.2 (0.9–1.8) | 0.002 |
| # PRBCs in ICU | Median (IQR) | 7 (4–11) | 3 (1–5) | <0.001 | 5 (3–11) | 2.5 (1–4) | <0.001 |
BM=bone marrow, CB=cord blood, Cr = Creatinine, IQR = interquartile range, MPM = Mortality Probability Model-II, MV = mechanical ventilation, PB=peripheral blood,TBI = total body irradiation, Tbili = total bilirubin, trans = ICU-transfer, PRBCs = packed red blood cells
Several continuous variables were tested for their impact on ICU and hospital survival and found to be negatively associated: Time from aHSCT to ICU transfer (for hospital survival only), MPM II score, number of packed red blood cell transfusions, elevated bilirubin and/or creatinine at ICU transfer.(Table 2) Other factors such as age, gender, transplant indication, remission status, TCD, ATG, myeloablative conditioning, matched donor, sibling donor, prior autologous transplant, transplantation on a clinical protocol, and documented bacteremia had no statistically significant impact on ICU or hospital survival in univariate analysis.
After a step-wise selection procedure, the following factors were found to be independently associated with a poor outcome: for ICU survival - vasopressor use, mechanical ventilation and elevated bilirubin, and for hospital survival - vasopressor use, mechanical ventilation and hemodialysis. (Table 3)
Table 3.
Final multivariate models after step-wise selection procedures for ICU- and hospital mortality and overall survival
| Variable | OR* (95% CI) | p | |
|---|---|---|---|
| ICU mortality | Vasopressors (Y vs N) | 4.8 (2.0–11.9) | <0.001 |
| Mechanical ventilation (Y vs N) | 19.0 (3.8–95.5) | <0.001 | |
| Tbili at transfer (per unit increase) | 1.3 (1.0–1.6) | 0.02 | |
| Hospital mortality | Renal replacement (Y vs N) | 2.9 (1.1–7.5) | 0.03 |
| Vasopressors (Y vs N) | 3.2 (1.4–7.2) | 0.007 | |
| Mechanical ventilation (Y vs N) | 6.2 (2.6–15.0) | <0.001 | |
| HR** (95% CI) | |||
| Overall survival | aGVHD | 1.9 (1.1–3.2) | 0.02 |
| Vasopressors (Y vs N) | 2.4 (1.6–3.5) | <0.001 | |
| Mechanical ventilation (Y vs N) | 2.7 (1.7–4.2) | <0.001 | |
| Days post aHSCT to ICU (10 day increase) | 1.20 (1.08–1.32) | <0.001 |
aHSCT=allogeneic stem cell transplant, CI=confidence interval, Tbili=total bilirubin
Odds ratios (OR) are reported for ICU- and hospital mortality.
Hazard ratios (HR) are reported for overall survival
In assessing OS, the following factors were associated with a poor outcome on univariate analysis: acute GVHD (aGVHD), CB as the stem cell source, HLA-mismatch, hemodialysis during ICU stay, vasopressors and mechanical ventilation at both transfer and during ICU stay. In addition, gram positive bacteremia, sepsis, more than one organ system failure, longer time from aHSCT to ICU transfer, higher MPM score, and elevated bilirubin and creatinine were also associated with a negative outcome. (Supplemental tables) After a step-wise selection procedure, the following factors remained significantly associated with shorter survival: aGVHD, vasopressor use, mechanical ventilation, and longer interval between aHSCT and ICU transfer. (Table 3)
Clinical patterns and outcomes before and after 2008
In order to evaluate the impact of change in clinical practice on transfer to the ICU as well as outcomes, the study period was divided into two 6-year blocks, January 2002 to December 2007 (62 patients) and January 2008 to December 2013 (99 patients). There was an increase in median age from 49.5 to 53.5 yrs for the later group (p=0.04), reflective of the shift in age for all aHSCT patients (48 vs. 53 yrs, p<0.001). The proportion of sibling donor aHSCTs, myeloablative conditioning and patients receiving TBI decreased, whereas the proportion of CB transplant recipients increased sharply. (Table 4)
Table 4.
Transplant characteristics pre- and post–2008
| Total cohort (N=161) # (%) |
2002–2007 (N=62) # (%) |
2008–2013 (N=99) # (%) |
p-value | ||
|---|---|---|---|---|---|
| Stem cell source | BM | 12 (8) | 9 (15) | 3 (3) | <0.001 |
| PB | 121 (75) | 50 (81) | 71 (72) | ||
| CB | 28 (17) | 3 (5) | 25 (25) | ||
|
| |||||
| Myeloablative conditioning | 115 (71) | 50 (81) | 65 (66) | 0.05 | |
|
| |||||
| TBI/dose | no TBI | 99 (61.5) | 40 (64.5) | 59 (60) | <0.001 |
| <900 cGy | 22 (14) | 0 | 22 (22) | ||
| ≥900 cGy | 40 (25) | 22 (35.5) | 18 (18) | ||
|
| |||||
| Sibling donor | 44 (27) | 25 (40.3) | 19 (19) | 0.006 | |
CB=cord blood, BM=bone marrow, TBI=total body irradiation, cGy=centiGray
While distribution of transfer diagnoses remained the same for the majority of patients, there was a drop in sepsis (53% vs 29%, p=0.02) along with a decreased median MPM II score (46.7 vs 28.3, p=<0.001) during the later study period. Specifically, the incidence of gram-positive bacteremia after 2007 was lower (20% vs 52%, p=<0.001), but the rate of gram-negative bacteremia remained unchanged over the entire study period. The lower incidence of gram-positive bacteremia in the later years was associated with a change in clinical practice which implemented early prophylactic administration of intravenous vancomycin. Despite these differences, the rates of admission to the ICU were similar for both time periods (15% and 12%, p=0.23). Of note, however, the requirement for organ support changed with a decrease in the use of vasopressors (66% vs 37%, p=<0.001), and mechanical ventilation (77% vs 55%, p=0.004).
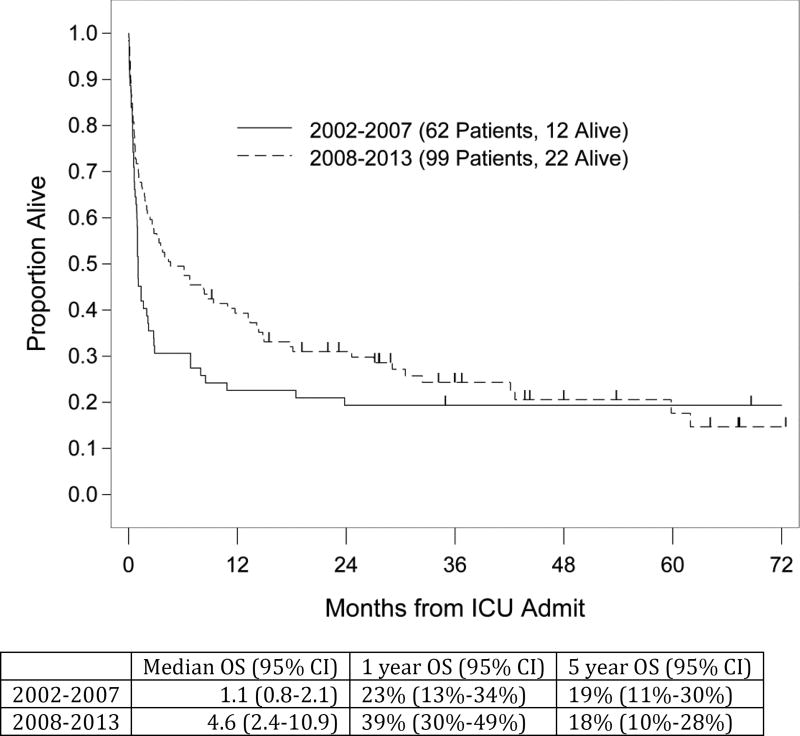
While there was only a trend to higher ICU survival in the later period (69% vs 56%, p=0.09), the hospital survival improved significantly from 30% to 55% (p=0.002). One-year survival rates also showed a trend toward improvement in the latter period with 39% vs. 23%, and LTS remained unchanged. (Figure 3)
Figure 3.
ICU survival pre- and post 2008
Causes of death
We examined causes of early- (within one year post ICU admission) and late death separately. As expected, early deaths were predominantly due to infection, toxicity or GVHD, while beyond one year, relapse and GVHD were the most common causes for the entire group. Early in the years 2002–2007, there were twice as many early deaths due to infection (27/48, 56%) compared to the later period (16/58, 28%). Very few deaths occurred after one year in the early group (2/50, 4%), while in the later period 25% (19/77) of deaths occurred after one year, with most attributable to relapse and GVHD. (Table 5)
Table 5.
Causes of death
| Period | 2002–2007 | 2008–2013 |
|---|---|---|
| #aHSCT | 62 | 99 |
| Dead | 50 | 77 |
| Died < 12 months | 48 | 58 |
| Cause of death | ||
| Infection | 27 | 16 |
| Tox/VOD | 9 | 10 |
| GvHD | 7 | 13 |
| Graft failure | 4 | 4 |
| Relapse | 1 | 6 |
| Other | 0 | 9* |
| Died > 12 months | 2 | 19 |
| Cause of death | ||
| Relapse | 1 | 7 |
| GvHD | 0 | 6 |
| Infection | 0 | 1 |
| Other | 1** | 5*** |
aHSCT=allogeneic stem cell transplants, GVHD=graft vs host disease, Tox= toxicity, VOD=venoocclusive disease
pulmonary failure (3), multiorgan failure (4), unknown(2)
myocardial infacrtion
secondary cancer (3), poor graft function (1), unknown (1)
DISCUSSION
This study presents the outcomes of a large, single-center cohort of aHSCT patients admitted to the ICU during their transplant hospitalization. The stringent criteria imposed in the study produced a homogenous cohort of the most seriously ill aHSCT patients, and eliminated the potential contribution of community-acquired infections which might have been associated with ICU admissions later in the post-transplant course. In addition, we excluded autologous transplant patients whose significantly less compromised status would have biased the outcomes in a more favorable direction [27].
The overall admission rate to the ICU of 13% in this study is at the lower end of the reported spectrum for aHSCT recipients (range 8.6%–40%) [4, 11, 12, 16, 28, 29, 30, 31]. Somewhat surprisingly, there was no significant difference in the ICU admission rates for patients receiving non-myeloablative/RIC vs myeloablative conditioning, 11% vs. 14% (p=0.07), respectively. The ICU survival rate of 65% compares favorably with the 6% to 48% survival rates in previous reports [11, 12, 13, 14, 15, 17, 28], and the 33% 1-year survival rate in this study is also slightly higher than the 16% and 28% range previously reported [11, 13, 15, 17]. Only two prior studies provide data on LTS [14, 15], with 5-year survival ranging from 12% to 17%, compared to the 20% rate in our study. Among the post-discharge ICU survivors, an encouraging 5-year survival rate of 43% was observed compared to the 55% for non-ICU transplant recipients in the same time period at our center (p =0.05, comparing the OS curves). This also is comparable to the 51% 5-year survival rate of critically ill aHSCT recipients reported by Townsend and colleagues.
Consistent with previous reports [5, 8, 11, 12, 13, 17], several factors were independently predictive of death during the ICU admission including mechanical ventilation, vasopressor use, and hyperbilirubinemia. While no association of hyperbilirubinemia with sepsis could be found and the incidence of VOD was very low, the higher ICU mortality rate with liver dysfunction in aHSCT recipients is well reported and most likely a consequence of more advanced multiorgan failure rather than due to a single underlying cause [5, 8, 11].
Multivariate analysis revealed that mechanical ventilation, vasopressors and hemodialysis remained significant predictors for hospital mortality. As hemodynamic instability is often a harbinger of multiorgan failure, a well-established predictor of mortality in aHSCT patients [4, 8, 11, 16], the finding of a requirement for vasopressors and /or an ICU admission diagnosis of sepsis/septic shock predicted a poor outcome as expected. Although renal dysfunction has been identified as a predictor for a higher risk of death in the context of multiorgan failure [31], our study demonstrated that the use of hemodialysis was indeed associated with a worse prognosis in the hospital, but not for OS. As most patients requiring hemodialysis were severely ill with other organ dysfunction, this finding suggests that renal failure is a companion of multiorgan failure but rarely the initial cause of the deterioration [32]. This was confirmed by the observation that a requirement for renal replacement therapy at the time of transfer to the ICU was not associated with hospital mortality.
Several factors were not found to be predictive of outcome. Patient age showed no impact on ICU, hospital, or overall survival. Transplant patients undergo extensive pre-transplant testing to determine their fitness for the procedure, which likely has excluded patients with more severe comorbidities. Factors such as comorbidities and performance status have recently been shown to be more predictive of non-relapse mortality than age. [33, 34] The low incidence of aGVHD observed in patients receiving TCD transplants (~50% of the study cohort), limited the value of aGVHD as a prognostic factor. In contrast to previous reports [11, 35], and acknowledging the limited number of patients who developed aGVHD in this study, it was not a prognostic factor for ICU or hospital mortality, but was associated with poorer OS. Interestingly, despite the use of TCD in a proportion of this patient population, there was no difference in their outcomes compared to patients receiving conventional grafts.
In order to evaluate the impact of changes in clinical patterns over the study period (2002–2013), results were compared for 2002–2007 and 2008–2013. Although the median age of all aHSCT patients in the later period was 5 years older, the ICU transfer rate did not change. The lower MPM II score on ICU admission, decreased use of mechanical ventilation and vasopressors in the latter 6-year period may be due to earlier recognition and intervention for deteriorating patients, a strategy shown to improve outcomes in aHSCT patients [36]. These differences were observed at our center when the number of ICU beds was increased from 12 to 20 in 2008, affording rapid bed availability to aHSCT patients with less severe pathology.
Similarly, there was a dramatic decrease in the prevalence of gram-positive bloodstream infections (BSI) in the period 2008–2013, compared to 2002–2007. This difference can be attributed to the altered practice beginning in 2006 of initiating vancomycin two days prior to transplant or preemptively with the first fever. This was in response to an unexpectedly high rate of Streptococcus viridans-related sepsis and an unusually high mortality of 21% [37] in the earlier period, though a definitive cause was never identified.
While the hospital survival rate was significantly better in the years 2008 and beyond, this did not translate into an increased OS. Nevertheless, there was an encouraging trend toward an improved median (4.6 vs 1.1 months) and one-year survival (39% vs 23%) in the later study period, both parameters that were likely impacted upon by changes in clinical and ICU practice. A higher rate of relapse in the later group is not an outcome that will be impacted upon by such changes, and late deaths from GVHD reflect a change in transplant practice, i.e. greater numbers of unmodified vs. TCD transplants, rather than clinical and ICU practice.
The major strengths of this study are the homogeneous population, selected by the stringent inclusion criteria, as well as the large number of subjects, affording reliable multivariate analyses. The extended time frame for the study allowed consideration of transplantation and practice changes to be factored into the analysis. The exclusion of patients admitted to the ICU after the transplant hospitalization avoided the need to address the impact of community-acquired infections, and the increased heterogeneity of this group.
The limitations of the study are those associated with a single-center, retrospective design. Other limitations include the relatively small number of CB transplant recipients in the study cohort (28/161) - a group known for prolonged and complicated hospitalizations; and the inclusion of 12 patients who were transferred to the ICU for hemodynamic instability due to ATG administration - a group that generally recovered quickly without major sequelae. Additionally, we examined a large number of potential clinical outcome predictors. We realize that this has increased the chance of false positive findings. Replication of these results by other investigators will help to confirm our findings.
In summary, the short- and long-term survival of this cohort of aHSCT patients admitted to the ICU over a 12-year period is comparable to that reported in the literature. Notably, however, there was a significant improvement in hospital survival for those transplanted in the last 6 years. The latter is likely attributable to reduction in lethal infections (due to change in antimicrobial prophylaxis) and improved supportive care including early recognition of impending organ failures, continuous monitoring of toxicities, and greater access to ICU beds. Certainly the character of the patients, i.e., older age; and changes in approach to transplant such as conditioning and alternative donor grafts, will continue to play a significant role in the outcomes described in this study. The overall high mortality rate especially during the ICU admission, necessitates the close collaboration of intensivists and members of the transplant communities who continue to push back the barriers of transplantation.
Supplementary Material
References
- 1.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330(12):827–38. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 2.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89(12):4531–6. [PubMed] [Google Scholar]
- 3.Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol. 2003;21(8) doi: 10.1200/JCO.2003.09.110. 1480- [DOI] [PubMed] [Google Scholar]
- 4.Soubani AO, Kseibi E, Bander JJ, et al. Outcome and prognostic factors of hematopoietic stem cell transplantation recipients admitted to a medical ICU. Chest. 2004;126(5):1604–11. doi: 10.1378/chest.126.5.1604. [DOI] [PubMed] [Google Scholar]
- 5.Price KJ, Thall PF, Kish SK, Shannon VR, Andersson BS. Prognostic indicators for blood and marrow transplant patients admitted to an intensive care unit. Am J Respir Crit Care Med. 1998;158(3):876–84. doi: 10.1164/ajrccm.158.3.9711076. [DOI] [PubMed] [Google Scholar]
- 6.Scales DC, Thiruchelvam D, Kiss A, Sibbald WJ, Redelmeier DA. Intensive care outcomes in bone marrow transplant recipients: a population-based cohort analysis. Crit Care. 2008;12(3):R77. doi: 10.1186/cc6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faber-Langendoen K, Caplan AL, McGlave PB. Survival of adult bone marrow transplant patients receiving mechanical ventilation: a case for restricted use. Bone Marrow Transplant. 1993;12(5):501–7. [PubMed] [Google Scholar]
- 8.Afessa B, Tefferi A, Dunn WF, Litzow MR, Peters SG. Intensive care unit support and Acute Physiology and Chronic Health Evaluation III performance in hematopoietic stem cell transplant recipients. Crit Care Med. 2003;31(6):1715–21. doi: 10.1097/01.CCM.0000065761.51367.2D. [DOI] [PubMed] [Google Scholar]
- 9.Allareddy V, Roy A, Rampa S, et al. Outcomes of stem cell transplant patients with acute respiratory failure requiring mechanical ventilation in the United States. Bone Marrow Transplant. 2014;49(10):1278–86. doi: 10.1038/bmt.2014.130. [DOI] [PubMed] [Google Scholar]
- 10.Galindo-Becerra S, Labastida-Mercado N, Rosales-Padrón J, et al. Outcome of Recipients of Hematopoietic Stem Cell Transplants Who Require Intensive Care Unit Support: A Single Institution Experience. Acta Haematol. 2015;134(2):119–124. doi: 10.1159/000381301. [DOI] [PubMed] [Google Scholar]
- 11.Pène F, Aubron C, Azoulay E, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: a reappraisal of indications for organ failure supports. J Clin Oncol. 2006;24(4):643–9. doi: 10.1200/JCO.2005.03.9073. [DOI] [PubMed] [Google Scholar]
- 12.Neumann F, Lobitz O, Fenk R, et al. The sepsis-related Organ Failure Assessment (SOFA) score is predictive for survival of patients admitted to the intensive care unit following allogeneic blood stem cell transplantation. Ann Hematol. 2008;87(4):299–304. doi: 10.1007/s00277-008-0440-9. [DOI] [PubMed] [Google Scholar]
- 13.Gilli K, Remberger M, Hjelmqvist H, Ringden O, Mattsson J. Sequential Organ Failure Assessment predicts the outcome of SCT recipients admitted to intensive care unit. Bone Marrow Transplant. 2010;45(4):682–8. doi: 10.1038/bmt.2009.220. [DOI] [PubMed] [Google Scholar]
- 14.Mokart D, Granata A, Crocchiolo R, et al. Allogeneic hematopoietic stem cell transplantation after reduced intensity conditioning regimen: Outcomes of patients admitted to intensive care unit. J Crit Care. 2015;30(5):1107–13. doi: 10.1016/j.jcrc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Townsend WM, Holroyd A, Pearce R, et al. Improved intensive care unit survival for critically ill allogeneic haematopoietic stem cell transplant recipients following reduced intensity conditioning. Br J Haematol. 2013;161(4):578–86. doi: 10.1111/bjh.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kew AK, Couban S, Patrick W, Thompson K, White D. Outcome of hematopoietic stem cell transplant recipients admitted to the intensive care unit. Biol Blood Marrow Transplant. 2006;12(3):301–5. doi: 10.1016/j.bbmt.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Benz R, Schanz U, Maggiorini M, Seebach JD, Stussi G. Risk factors for ICU admission and ICU survival after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(1):62–5. doi: 10.1038/bmt.2013.141. [DOI] [PubMed] [Google Scholar]
- 18.Castella X, Artigas A, Bion J, Kari A. A comparison of severity of illness scoring systems for intensive care unit patients: results of a multicenter, multinational study. The European/North American Severity Study Group. Crit Care Med. 1995;23(8):1327–35. doi: 10.1097/00003246-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Giralt S, Ballen K, Rizzo D, et al. Reduced –intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for acute leukaemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet. 1981;2(8242):327–31. doi: 10.1016/s0140-6736(81)90647-4. [DOI] [PubMed] [Google Scholar]
- 21.Tamari R, Chung SS, Papadopoulos EB, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015;21(12):2106–14. doi: 10.1016/j.bbmt.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash RA, Piñeiro LA, Storb R, et al. FK506 in combination with methotrexate for the prevention of graft-versus-host disease after marrow transplantation from matched unrelated donors. Blood. 1996 Nov 1;88(9):3634–41. [PubMed] [Google Scholar]
- 23.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–36. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons; 2000. [Google Scholar]
- 26.Lin DY, Wei LJ, Ying Z. Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 27.Trinkaus MA, Lapinsky SE, Crump M, et al. Predictors of mortality in patients undergoing autologous hematopoietic cell transplantation admitted to the intensive care unit. Bone Marrow Transplant. 2009;43(5):411–5. doi: 10.1038/bmt.2008.336. [DOI] [PubMed] [Google Scholar]
- 28.Kim SW, Kami M, Urahama N, et al. Feasibility of acute physiology and chronic health evaluation (APACHE) II and III score-based screening in patients receiving allogeneic hematopoietic stem-cell transplantation. Transplantation. 2003;75(4):566–70. doi: 10.1097/01.TP.0000045709.72746.44. [DOI] [PubMed] [Google Scholar]
- 29.Jackson SR, Tweeddale MG, Barnett MJ, et al. Admission of bone marrow transplant recipients to the intensive care unit: outcome, survival and prognostic factors. Bone Marrow Transplant. 1998;21(7):697–704. doi: 10.1038/sj.bmt.1701158. [DOI] [PubMed] [Google Scholar]
- 30.Torrecilla C, Cortes JL, Chamarro C, Rubio JJ, Galdos P, Dominguez de Villota E. Prognostic assessment of the acute complications of bone marrow transplantation requiring intensive therapy. Intensive Care Medicine. 1988;14(4):393–8. doi: 10.1007/BF00262895. [DOI] [PubMed] [Google Scholar]
- 31.Huynh TN, Weigt SS, Belperio JA, Territo M, Keane MP. Outcome and prognostic indicators of patients with hematopoietic stem cell transplants admitted to the intensive care unit. J Transplant. 2009;2009:917294. doi: 10.1155/2009/917294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersting S, Koomans HA, Hené RJ, Verdonck LF. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007;39(6):359–65. doi: 10.1038/sj.bmt.1705599. [DOI] [PubMed] [Google Scholar]
- 33.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112(9):1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 34.Corradini P, Zallio F, Mariotti J, et al. Effect of age and previous autologous transplantation on nonrelapse mortality and survival in patients treated with reduced-intensity conditioning and allografting for advanced hematologic malignancies. J Clin Oncol. 2005;23(27):6690–8. doi: 10.1200/JCO.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 35.Lengliné E, Chevret S, Moreau AS, et al. Changes in intensive care for allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015;50(6):840–5. doi: 10.1038/bmt.2015.55. [DOI] [PubMed] [Google Scholar]
- 36.Bokhari SW, Munir T, Memon S, Byrne JL, Russell NH, Beed M. Impact of critical care reconfiguration and track-and-trigger outreach team intervention on outcomes of hematology patients requiring intensive care admission. Ann Hematol. 2010;89(5):505–12. doi: 10.1007/s00277-009-0853-0. [DOI] [PubMed] [Google Scholar]
- 37.Jaffe D, Jakubowski A, Sepkowitz K, et al. Prevention of peritransplantation viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clin Infect Dis. 2004;39(11):1625–32. doi: 10.1086/425612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.