Abstract
Having the correct number of chromosomes is vital for normal development and health. Sex chromosome trisomy (SCT) affects 0.1% of the human population and is associated with infertility. We show that during reprogramming to induced pluripotent stem cells (iPSC), fibroblasts from sterile trisomic XXY and XYY mice lose the extra sex chromosome, by a phenomenon we term trisomy-biased chromosome loss (TCL). Resulting euploid XY iPSCs can be differentiated into the male germ cell lineage and functional sperm that can be used in intracytoplasmic sperm injection to produce chromosomally normal, fertile offspring. Sex chromosome loss is comparatively infrequent during mouse XX and XY iPSC generation. TCL also applies to other chromosomes, generating euploid iPSCs from cells of a Down syndrome mouse model. It can also create euploid iPSCs from human trisomic patient fibroblasts. The findings have relevance to overcoming infertility and other trisomic phenotypes.
The mammalian sex chromosomes have specialized roles in male (XY) and female (XX) germ cell development (1). Sex chromosome abnormalities are the most common genetic cause of human infertility (2). In the SCTs Klinefelter (XXY) and Double Y (XYY) syndrome, spermatogenesis is disrupted by excess X- and Y-genes, respectively (2). XYY men are commonly fertile due to spontaneous loss of the extra sex chromosome (mosaicism). In XXY men, mosaicism is less common. Testicular sperm retrieval has enabled reproduction from some young Klinefelter men, but is less successful in older patients (3, 4). XXY and XYY individuals without XY germ cells are infertile.
To study SCT-infertility, we generated adult XXY and XYY mice carrying the fluorescent reporter transgenes Blimp1-mVenus (BV) and Stella-ECFP (SC) (5) to monitor differentiation of pluripotent stem cells into primordial germ cell-like cells (PGCLCs) (6). XXY males were created by mating a wildtype female to a sex chromosome variant male that produces XY-containing sperm (fig. S1). Generation of XYY mice requires inheritance of a Y chromosome from both parents. We therefore used a paternally inherited wild type Y chromosome, and a maternally inherited Yd1 chromosome that does not express the testis-determinant Sry (fig. S1) (7). As shown previously (8, 9), the spermatogenesis phenotype in both models recapitulated that in SCT men, with arrest at the prospermatogonial stage in XXY mice and at pachynema in XYY mice (fig. S2). Spermatogenesis was normal in euploid XY BVSC transgenic siblings.
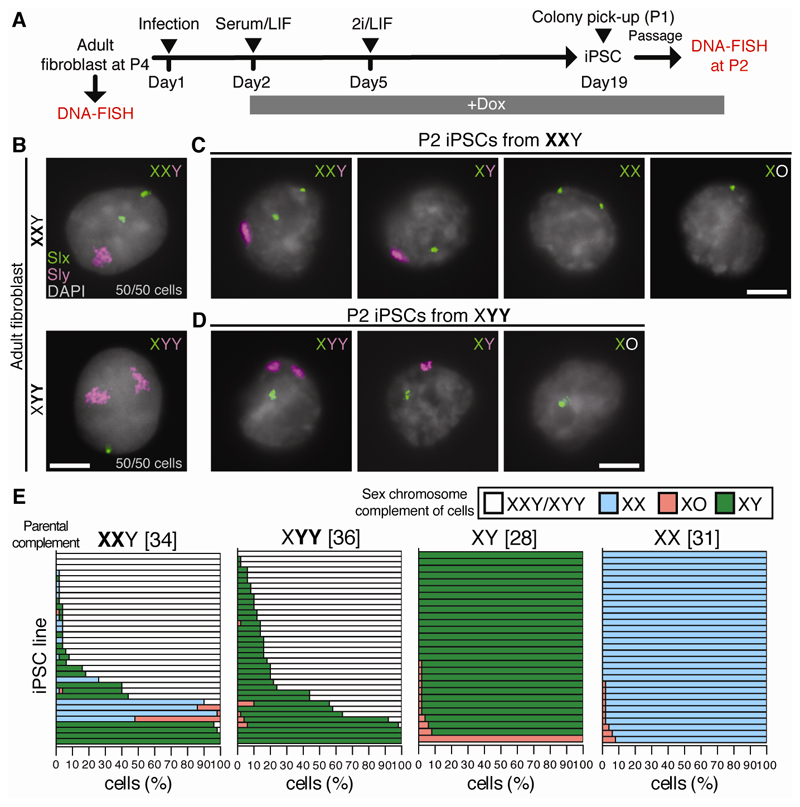
Next, we established fibroblasts from SCT and control XY and XX mice (Fig. 1A). DNA-FISH for the X-gene Slx and Y-gene Sly confirmed that passage 4 (P4) SCT and control fibroblasts had retained their original sex chromosome complements (Fig. 1B, fig. S3A). Fibroblasts were reprogrammed to induced pluripotent stem cells (iPSCs) (10) in a doxycycline (Dox)-inducible manner. DNA-FISH was performed in resulting P2 iPSCs (Fig. 1A).
Fig. 1. Chromosome loss through iPSC reprogramming of SCT fibroblasts.
(A) Experimental scheme to generate XXY, XYY, XY and XX iPSCs. 2i: inhibitors of GSK3ß and Mek1/2. LIF: leukemia inhibitory factor. (B-D) Slx (green) and Sly (magenta) DNA-FISH of (B) fibroblasts and (C, D) P2 iPSCs from XXY and XYY mice (n = 50 cells). Scale bars: 5 µm. (E) P2 iPSC sex chromosome complements. Each bar represents an iPSC line and the percentage of cells exhibiting each complement (n = 50 cells per line). Numbers in brackets show number of iPSC lines examined. Data for two animals combined.
A high proportion of SCT-derived iPSC lines exhibited sex chromosome loss. From XXY mice we observed XY, XX and XO iPSCs (Fig. 1C, 1E). The incidence of loss was similar for the X and Y chromosome (p=0.062, Mann-Whitney test). From XYY mice we observed XY and XO iPSCs (Fig. 1D-E). Y chromosome loss in XYY males occurred at a similar frequency to that observed for the X and Y chromosome combined in XXY males (p=0.089, Mann-Whitney test). We then compared the incidence of sex chromosome loss between SCT-derived and euploid XY- and XX-derived iPSCs. Sex chromosome loss was more common in SCT- than euploid-derived iPSCs (Fig. 1E), irrespective of the cutoff used to define sex chromosome loss (fig. S12D).
Sex chromosome loss could occur during reprogramming of SCT cells, or during iPSC propagation to P2, perhaps conferring a proliferative advantage to resulting euploid cells. Indeed, sex chromosome instability has been observed in pluripotent stem cells (11, 12). To test the latter hypothesis, we analyzed sex chromosome stability between P2 to P6 in iPSCs with highly parental (>90%) complements (fig. S4A). We observed sex chromosome loss in XX and XXY iPSC lines (p<0.01 and 0.05 respectively, Wilcoxon signed-rank test), but not in XY and XYY iPSC lines (p=0.21 and 0.66 respectively, Wilcoxon signed-rank test). However, no iPSC line showed more than a 15% decrease in parental complement (fig. S4B). Furthermore, sex chromosome loss between P2 and P6 was not trisomy-biased (fig. S4B). SCT-derived euploid XY iPSCs also exhibited no proliferative advantage over XXY or XYY iPSCs (fig. S5). Since SCT fibroblasts were also karyotypically stable (Fig. 1B and fig. S3A), chromosome loss is likely induced during iPSC reprogramming and is thus distinct from sex chromosome instability in pluripotent stem cells (11, 12). We refer to the phenomenon as trisomy-biased chromosome loss (TCL).
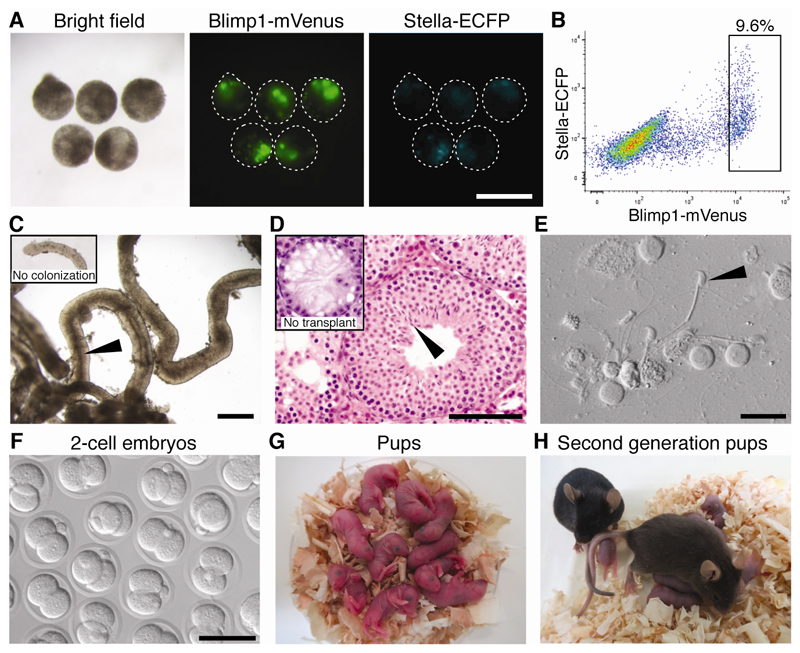
We next determined whether euploid XY iPSCs derived from SCT fibroblasts would form functional sperm. We selected highly euploid (≥80% of cells XY) P6 iPSCs adapted to Dox-free medium (fig. S6). For our XYY experiments, only XY iPSC lines that retained the wild type Y rather than the Yd1 chromosome were used for PGCLC experiments (fig. S7). Karyotyping confirmed that all SCT-derived XY iPSC lines and a control XY iPSC line were euploid (fig. S8). These iPSC lines were differentiated (6) through epiblast-like state to create PGCLC aggregates positive for BV and SC (Fig. 2A). BV-positive PGCLCs (table S1) were isolated by fluorescence-activated cell sorting (FACS; Fig. 2B) and transplanted into germ-cell deficient W/Wv (Kit mutant) testes (13).
Fig. 2. PGCLC derivation and spermatogenesis from SCT-derived euploid iPSCs.
(A) PGCLC aggregates from iPSC line XXY 7-29-1. Green: Blimp1-mVenus. Cyan: Stella-ECFP. (B) FACS of resulting aggregates. Rectangle: population used for transplantation. (C) Tubule from transplanted testis. Dark region (arrowhead) indicates area with spermatids. Inset: area without spermatogenic colonies. (D) Histology of transplanted testis stained with hematoxylin and eosin. Inset: W/Wv testis without transplantation. Arrowhead: elongated spermatids. (E) Sperm (arrowhead) isolated from transplanted testis. (F-G) 2-cell embryos (F), and pups (G) produced by ICSI from XXY-derived sperm. (H) Pups from iPSC line XXY 7-29-1-derived male and female. Scale bars: 400 (A,C), 100 (D,F), 25 (E) µm.
Spermatogenesis in recipients was evaluated 9-10 weeks post-transplantation. Teratomas, which are observed following iPSC-derived PGCLC transplantation (6), were present in 29% of XXY-derived and 50% of XYY-derived transplanted lines (fig. S9). Reconstitution of spermatogenesis, revealed by the presence of spermatogenic colonies (Fig. 2C) and by histology (Fig. 2D) was observed for all XXY- and XYY-derived iPSC lines used (Table 1). Thus, SCT-derived XY iPSCs can differentiate to germ cells in vitro and complete spermatogenesis post-transplantation.
Table 1. Spermatogenesis from transplanted PGCLCs.
| Sex chromosome complement | iPSC line name | No. of testes transplanted | No. of testes dissected † | No. of testes with spermatogenesis | No. of spermatogenic colonies in each testis |
|---|---|---|---|---|---|
| XY from XXY | XXY 7-6 | 10 | 8 | 1 | 1* |
| XXY 7-7-1 | 6 | 6 | 3 | 1,1,1 | |
| XXY 7-7-2 | 2 | 1 | 1 | 13 | |
| XXY 7-8-2 | 6 | 5 | 4 | 3,3,4,8 | |
| XXY 7-15-2 | 4 | 3 | 2 | 7,10 | |
| XXY 7-21-2 | 6 | 5 | 3 | 2,3,15 | |
| XXY 7-29-1 | 6 | 4 | 4 | 2,6,8,16 | |
| XY from XYY § | XYY 5-4 | 4 | 3 | 3 | 1,9,9 |
| XYY 5-5 | 6 | 6 | 2 | 2,10 | |
| XYY 5-8 | 6 | 6 | 3 | 2,2,3 | |
| XYY 7-3 | 6 | 4 | 3 | 5,11,14 | |
| Control XY | XY 6-6 | 6 | 4 | 3 | 1,15,20 |
Some testes were not dissected and were used for histology analyses.
XY iPSC lines with wild type Y chromosome were used for transplantation.
Sperm were very few.
We asked whether sperm created via transplantation could support reproduction. Intracytoplasmic sperm injection (ICSI) using sperm from two XXY- and two XYY-derived XY iPSC lines (Fig. 2E, fig. S10A) generated zygotes that developed into 2-cell embryos in vitro (efficiency 76.7-87.3%, Fig. 2F, fig. S10B, table S2) and grossly normal offspring when transplanted into recipients (efficiency 46.9-59.4%, Fig. 2G, fig. S10C, table S2). PCR genotyping confirmed that the offspring were derived from the transplanted PGCLCs (fig. S10D). Pups from XXY- and XYY-derived iPSC lines showed comparable growth to those derived from control XY iPSCs (fig. S10E). Notably, the XXY- and XYY-derived pups had euploid (XY or XX) complements (fig. S11). Three matured males and three females from each XXY- and XYY-derived iPSC line were mated to each other, and all were fertile (Fig. 2H, fig. S10F). Thus, sperm from SCT-derived XY iPSCs give rise to chromosomally normal, healthy, and fertile offspring.
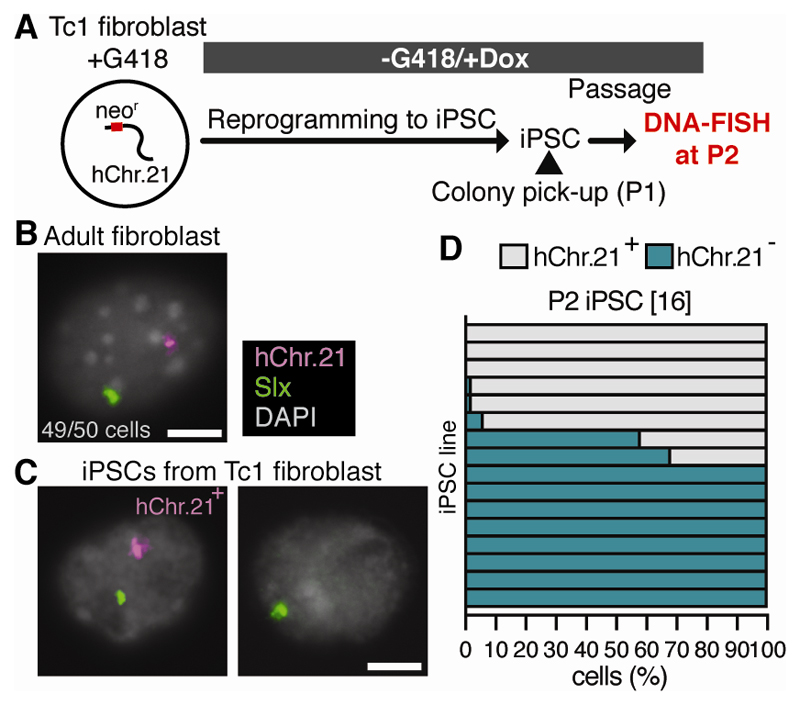
We addressed if TCL is specific to trisomy of sex chromosomes. Since mouse models with trisomy for a complete autosome are not available (14), we repeated our experiments in male Tc1 transchromosomic mice, a Down syndrome model with an accessory human chromosome 21 (hChr.21; 15). Tc1 mice carry a hChr.21-inserted neomycin resistance cassette, selection for which reduces hChr.21 mosaicism (15). We therefore first enriched adult Tc1 fibroblasts for the presence of hChr.21 using the neomycin analog G418 (Fig. 3A). DNA-FISH showed that the vast majority (≥96%) of Tc1 fibroblasts retained hChr.21 (Fig. 3B, fig. S3B). These Tc1 fibroblasts were reprogrammed without G418 selection, and the resulting iPSCs were analyzed at P2. Ten of the 16 iPSC lines generated (62.5%) showed loss of hChr.21 in ≥10% of cells (Fig. 3C-D, fig. S12D). In contrast, following G418 removal, hChr21 was retained in Tc1 fibroblasts cultured for the same period as that used for iPSC reprogramming (18 days), and in P6 iPSC lines that had highly parental (>90% hChr.21 positive) complements at P2 (fig. S12). We conclude that loss of hChr.21 in Tc1 cells is promoted by reprogramming rather than G418 removal, and therefore that TCL also affects an accessory chromosome.
Fig. 3. Accessory chromosome 21 loss during iPSC reprogramming.
(A) Experimental scheme to generate Tc1 iPSCs. (B-C) Slx (green) and hChr.21 (magenta) DNA-FISH of (B) fibroblasts and (C) P2 iPSCs from Tc1 mice (n = 50 cells). Scale bars: 5 µm. (D) P2 iPSC chromosomal complements. Each bar represents an iPSC line and the percentage of cells exhibiting each complement (n = 50 cells per line). Numbers in brackets show number of iPSC lines examined. Data for two animals combined.
We next asked whether TCL occurs in human cells. Instances of chromosome loss have been observed during human trisomic cell culture (16, 17), but its prevalence and relationship to reprogramming has not been systematically analyzed. We selected human Klinefelter syndrome, Down syndrome, and euploid XY and XX fibroblast lines exhibiting minimal mosaicism (fig. S13A, S13D), reprogrammed them, and determined the chromosome complements of resulting iPSC lines. We observed XY and XX iPSCs from Klinefelter syndrome fibroblasts, and euploid iPSCs from Down syndrome fibroblasts (fig. S13, B-C and F-G). Chromosome loss was more common in trisomic than in disomic cells, demonstrating that TCL also occurs during human reprogramming. However, the frequency of highly euploid iPSC lines was lower than that observed in trisomy-derived mouse iPSCs (fig. S13, F-H).
We have shown that TCL produces euploid iPSCs from SCT and autosomal trisomic mice and patients (fig. S12E). In mice, the resulting “corrected” iPSCs can form functional sperm, enabling production of chromosomally euploid offspring from infertile SCT individuals. TCL complements existing iPSC-therapies for chromosome abnormalities (17–21). The mechanisms that cause TCL are unknown. Cellular stresses associated with reprogramming may select against trisomic cells, permitting emergence of euploid cells. We observed less frequent TCL in human than in mouse cells (fig. S12D and S13H). Even if rare, TCL could offer treatments for infertile SCT patients for whom alternative approaches are unsuccessful. However, the clinical use of human germ cells made in vitro should be carefully considered ethically and legally (22–24). Furthermore, complete in vitro spermatogenesis will have to be developed in order to avoid the risk of teratoma formation arising through germ-cell transplantation.
TCL also permits production of female iPSCs from males, offering potential for the genetic dissection of sexual dimorphisms (25). By creating isogenic iPSC lines that differ only with respect to their sex chromosomes, sex differences identified during iPSC disease modelling could be attributed to X or Y chromosomal effects.
Supplementary Material
Acknowledgments
This was supported by the Francis Crick Institute which receives its core funding (FC001193) from Cancer Research UK, UK Medical Research Council, and Wellcome Trust; by European Research Council (CoG 647971), Japan Science and Technology Agency (JPMJER1104), and Japan Society for the Promotion of Science (17H06098). We thank the Francis Crick Institute Biological Research, Light Microscopy, and Experimental Histopathology facilities, M. Sangrithi and I. Okamoto for technical advice, V. Tybulewicz for Tc1 mice, and K. Niakan, R. Lovell-Badge and Turner lab members for comments.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References
- 1.Hughes JF, Page DC. The Biology and Evolution of Mammalian Y Chromosomes. Annu Rev Genet. 2015;49:507–527. doi: 10.1146/annurev-genet-112414-055311. [DOI] [PubMed] [Google Scholar]
- 2.Heard E, Turner J. Function of the sex chromosomes in mammalian fertility. Cold Spring Harb Perspect Biol. 2011;3:a002675. doi: 10.1101/cshperspect.a002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasamy R, et al. Successful Fertility Treatment for Klinefelter’s Syndrome. J Urol. 2009;182:1108–1113. doi: 10.1016/j.juro.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Greco E, et al. Birth of 16 healthy children after ICSI in cases of nonmosaic Klinefelter syndrome. Hum Reprod. 2013;28:1155–1160. doi: 10.1093/humrep/det046. [DOI] [PubMed] [Google Scholar]
- 5.Ohinata Y, Sano M, Shigeta M, Yamanaka K, Saitou M. A comprehensive, non-invasive visualization of primordial germ cell development in mice by the Prdm1-mVenus and Dppa3-ECFP double transgenic reporter. Reproduction. 2008;136:503–14. doi: 10.1530/REP-08-0053. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–32. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 7.Capel B, et al. Deletion of Y chromosome sequences located outside the testis determining region can cause XY female sex reversal. Nat Genet. 1993;5:301–307. doi: 10.1038/ng1193-301. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PA, et al. Germ cell loss in the XXY male mouse: altered X-chromosome dosage affects prenatal development. Mol Reprod Dev. 1998;49:101–11. doi: 10.1002/(SICI)1098-2795(199802)49:2<101::AID-MRD1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Mahadevaiah SK, Evans EP, Burgoyne PS. An analysis of meiotic impairment and of sex chromosome associations throughout meiosis in XYY mice. Cytogenet Cell Genet. 2000;89:29–37. doi: 10.1159/000015585. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Eggan K, et al. Male and female mice derived from the same embryonic stem cell clone by tetraploid embryo complementation. Nat Biotechnol. 2002;20:455–459. doi: 10.1038/nbt0502-455. [DOI] [PubMed] [Google Scholar]
- 12.Robertson EJ, Evans MJ, Kaufman MH. X-chromosome instability in pluripotential stem cell lines derived from parthenogenetic embryos. J Embryol Exp Morphol. 1983;74:297–309. [PubMed] [Google Scholar]
- 13.Mintz B, Russell ES. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957;134:207–237. doi: 10.1002/jez.1401340202. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard O, Wiseman FK, Ruparelia A, Tybulewicz VLJ, Fisher EMC. Mouse models of aneuploidy. Sci World J. 2012;2012:214078. doi: 10.1100/2012/214078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Doherty A, et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science. 2005;309:2033–7. doi: 10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs JA, et al. Integration-free induced pluripotent stem cells model genetic and neural developmental features of down syndrome etiology. Stem Cells. 2013;31:467–78. doi: 10.1002/stem.1297. [DOI] [PubMed] [Google Scholar]
- 17.Maclean GA, et al. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc Natl Acad Sci U S A. 2012;109:17567–72. doi: 10.1073/pnas.1215468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li LBB, et al. Trisomy Correction in Down Syndrome Induced Pluripotent Stem Cells. Cell Stem Cell. 2012;11:615–619. doi: 10.1016/j.stem.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, et al. Translating dosage compensation to trisomy 21. Nature. 2013;500:296–300. doi: 10.1038/nature12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bershteyn M, et al. Cell-autonomous correction of ring chromosomes in human induced pluripotent stem cells. Nature. 2014;507:99–103. doi: 10.1038/nature12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amano T, et al. Correction of Down syndrome and Edwards syndrome aneuploidies in human cell cultures. DNA Res. 2015;22:331–342. doi: 10.1093/dnares/dsv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irie N, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160:253–268. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki K, et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell. 2015;17:178–94. doi: 10.1016/j.stem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Ishii T, Pera RAR, Greely HT. Ethical and legal issues arising in research on inducing human germ cells from pluripotent stem cells. Cell Stem Cell. 2013;13:145–8. doi: 10.1016/j.stem.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Arnold AP, Chen X, Itoh Y. What a difference an X or Y makes: Sex chromosomes, gene dose, and epigenetics in sexual differentiation. Handb Exp Pharmacol. 2012;214:67–88. doi: 10.1007/978-3-642-30726-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- 27.Hunt PA, Eicher EM. Fertile male mice with three sex chromosomes: evidence that infertility in XYY male mice is an effect of two Y chromosomes. Chromosoma. 1991;100:293–9. doi: 10.1007/BF00360527. [DOI] [PubMed] [Google Scholar]
- 28.Carey BW, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci U S A. 2009;106:157–62. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Hulst C, Parvanova I, Tomoiaga D, Sapar ML, Feinstein P. Fast quantitative real-time pcr-based screening for common chromosomal aneuploidies in mouse embryonic stem cells. Stem Cell Reports. 2013;1:350–359. doi: 10.1016/j.stemcr.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Karyotyping Mouse Cells. Cold Spring Harb Protoc. 2008 doi: 10.1101/pdb.prot4706. [DOI] [PubMed] [Google Scholar]
- 31.Nakaki F, et al. Induction of mouse germ-cell fate by transcription factors in vitro. Nature. 2013;501:222–6. doi: 10.1038/nature12417. [DOI] [PubMed] [Google Scholar]
- 32.Kanatsu-Shinohara M, et al. Allogeneic offspring produced by male germ line stem cell transplantation into infertile mouse testis. Biol Reprod. 2003;68:167–73. doi: 10.1095/biolreprod.102.008516. [DOI] [PubMed] [Google Scholar]
- 33.Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids. Development. 1995;121:2397–2405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]
- 34.Brambrink T, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–9. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.