Abstract
Adeno-associated viral (AAV) vectors are one of the most promising gene delivery systems to the central nervous system. We now report, that AAV1 can be used to express transgenes trans-neuronally in neurons distant from the injection site. Specifically, intracortical injection of a bicistronic AAV1 vector trans-neuronally transduced spinal neurons as shown by fluorescence microscopy, the presence of AAV genome and AAV transcript in the contralateral spinal cord. Prior pyramidotomy abolished spinal transduction, confirming anterograde axonal transport of AAV1 in the corticospinal tract. These observations demonstrate the potential of bicistronic AAV1 for trans-neuronal expression of therapeutic transgenes in neurological disorders or reporter genes in connectivity studies.
Introduction
One of the most complex challenges facing systems neuroscience is the mapping of neuronal pathways in the intact and injured mammalian central nervous system (CNS). Furthermore, increasing the distribution of therapeutic genes within the injured or diseased CNS and developing the ability to assess neuronal plasticity after a therapeutic intervention are key goals in regenerative medicine.
Existing methods for anterograde, trans-neuronal delivery of therapeutic transgenes or tract-tracing include the use of live herpes simplex virus or dual viral-vector mediated WGA-Cre systems1, 2, which are complex and challenging to employ. By comparison, recombinant-deficient adeno-associated viral (AAV) vectors are a leading, simple, safe gene delivery system for the CNS which can undergo anterograde axonal transport in vivo3–11. Pseudotyping an AAV genome with the capsid from a different AAV serotype can generate vectors with different cellular tropisms as well as different axonal transport properties, which can improve the efficiency and pattern of transduction to specific regions of the CNS11–14. Evidence for trans-neuronal transduction exists, but has been confounded by the possibility of vector diffusion and the presence of reciprocal axonal connections between injection site and distal sites of transduction.
Anterograde axonal transport of AAV1, 2 and 5 has been previously demonstrated by detecting AAV vector genome in the spinal cord following injection into the brain4, 7. However the presence and cellular location of the protein product expressed by the AAV vector was either not investigated7 or found not to be increased4. Using AAV vector serotypes 1, 2, 6 and 9, several independent studies have observed transduced neurons in brain regions distal to the AAV injection site, suggesting that these vectors can undergo anterograde axonal transport to projection sites where the AAV vectors are released to transduce local neurons. However, the close proximity of the projection sites to the injection site and the existence of multiple reciprocal connections between brain regions limit what definitive conclusions can be drawn5, 6, 9–11. Other studies report the presence of a growth factor protein in neurons at sites distal to an AAV injection; however this may be due to axonal transport, secretion and re-uptake of the growth factor protein rather than the AAV vector8, 15, 16.
We previously reported AAV1 as the optimal serotype for transducing corticospinal neurons following cortical injection17, 18. The corticospinal tract (CST) is a major descending motor tract originating from the corticospinal neurons in layer V of the motor cortex (Fig.1a)19. The CST axons project through the medullary pyramids in the ventral brainstem, where the majority of fibers decussate to the contralateral side. In the rat, the bulk of corticospinal axons run in the dorsal medial spinal cord, with minor components running in the dorso-lateral and the ventral medial columns20. CST axon collaterals arborise predominantly in intermediate spinal laminae, however terminations are also found in dorsal and ventral laminae (Fig.1a)21, 22.
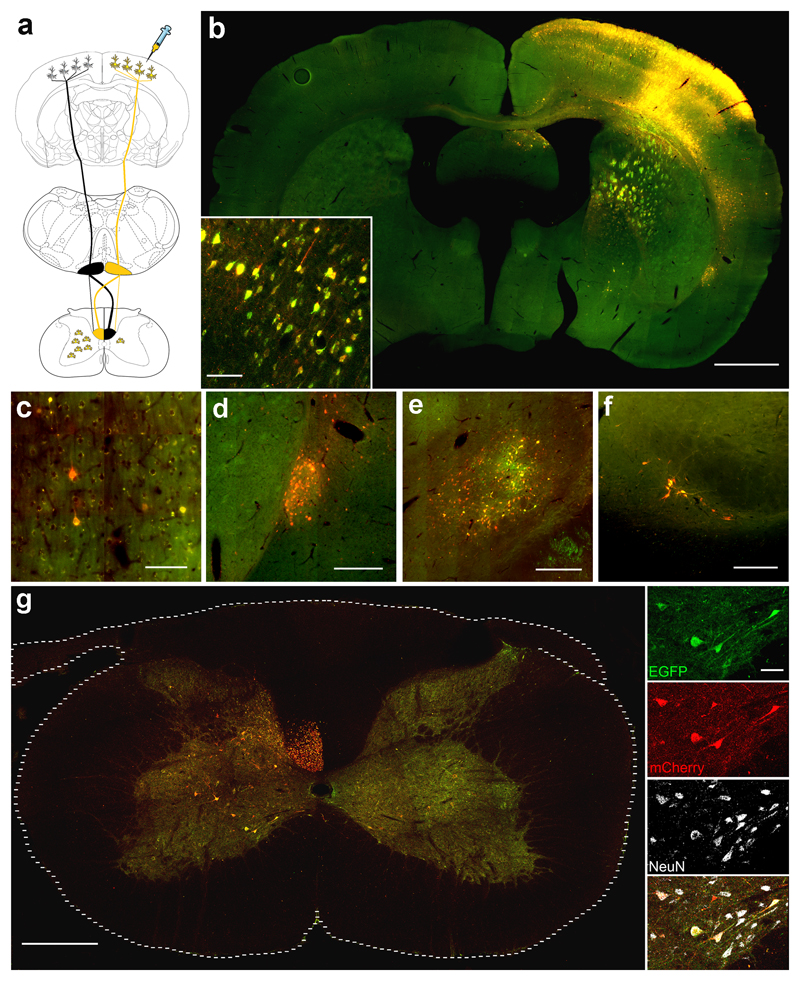
Figure 1. Transduced neurons expressing mCherry and eGFP are detected in the contralateral spinal cord following intracortical AAV injection.
(a) Schematic representation of the rat CST: black represents the non-transduced CST and yellow represents the transduced CST. (b) Transduction of the injected cortex, including corticospinal neurons. Scale bar: 1000 µm. Inset shows transduced cortical neurons. Scale bar: 70 µm. Transduced neurons were also observed in regions of the brain that receive direct connections from the cortex including (c) contralateral cortex, Scale bar: 70 µm (d) claustrum nucleus, Scale bar: 250 µm (e) Thalamic regions, Scale bar: 250 µm (f) substantia nigra, Scale bar: 250 µm (g) Numerous mCherry and eGFP positive axons are observed in the contralateral dorsomedial CST. Spinal neurons positive for mCherry and eGFP are observed in the contralateral grey matter. Scale bar: 250 µm. Inset shows higher-magnification images with NeuN staining. Scale bar: 30 µm.
Here, we demonstrate trans-neuronal transduction of second-order spinal neurons following cortical injection and anterograde axonal transport of a bicistronic AAV1 vector in the corticospinal tract. We made this AAV1-mCherry-2A-EGFP vector as a control vector in another experiment where we were testing whether certain regeneration associated genes (RAG) could enhance axon regeneration (AAV1-RAG-2A-EGFP) with EGFP being used as a reporter so we could identify transduced neurons. The AAV1 was injected intracortically. While analysing the tissue from this study we noticed the transduction of neurons in the contralateral spinal cord and therefore decided to investigate this serendipitous observation further.
Results
Intracortical delivery of AAV1-mCherry-2A-eGFP resulted in anterograde axonal transport and trans-neuronal transduction of spinal neurons in the contralateral spinal cord, which was abolished by prior pyramidotomy
Six weeks after unilateral injection of the bicistronic AAV1-mCherry-2A-eGFP vector into the right sensorimotor cortex, we observed extensive transduction of the injected cortex including the corticospinal neurons in layer V as well as neurons in layers 2/3 (Fig.1b). mCherry and eGFP positive fiber bundles projecting to the pyramids were observed in the striatum, the internal capsule and cerebral peduncles (Fig.1b). Transgene-positive Transcallosal fibers were observed projecting to the contralateral cortex (Fig.1b). We observed mCherry and eGFP positive neurons in brain regions that receive direct connections from the cortex suggesting AAV anterograde axonal transport and trans-neuronal transduction. Transduced brain regions include the contralateral cortex (Fig.1c), claustrum (Fig.1d)23, thalamic regions (Fig.1e)24 and substantia nigra (Fig.1f)25.
In the spinal cord we observed numerous mCherry and eGFP positive CST axons in the dorsal columns of the left (contralateral) spinal cord (Fig.1c). We also observed many transduced NeuN-positive spinal neurons expressing both mCherry and eGFP in the contralateral grey matter, with occasional transduced spinal neurons also in the right (ipsilateral) grey matter (Fig.1c). These results are consistent with the trajectory of the rat CST whose major component decussates at the medullary pyramids and runs in the contralateral spinal cord, with a minor component of uncrossed axons running in the ipsilateral spinal cord (Fig.1a)21, 26. Importantly, when the CST was unilaterally lesioned by performing a pyramidotomy27 one week prior to ipsilateral cortical AAV1 injection, the transduced axons and spinal neurons were no longer observed in the spinal cord.
Intracortical injection of AAV1-mCherry-2A-eGFP resulted in increased levels of the vector genome DNA and mRNA transcript in the contralateral spinal cord, which was abolished by prior pyramidotomy
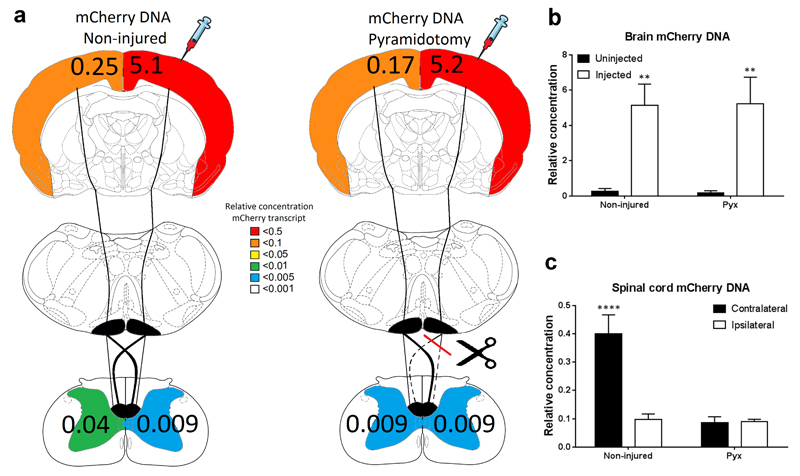
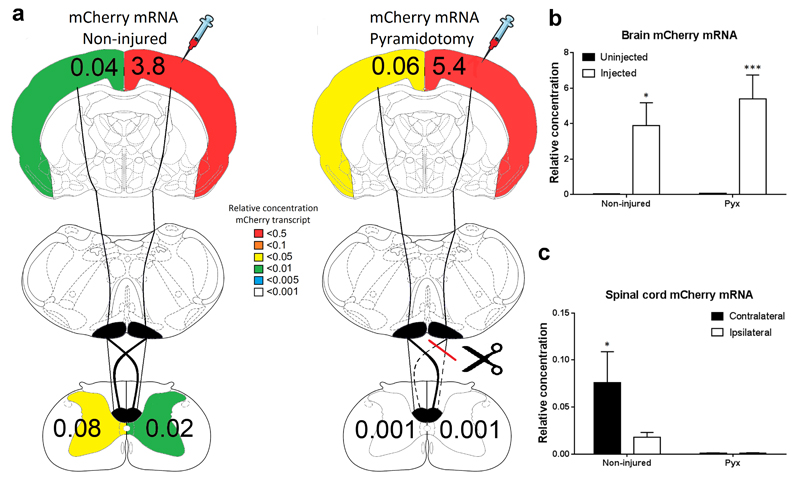
Following AAV1 injection into the right cortex, the amount of AAV genome (mCherry DNA) or transcript (mCherry mRNA) was analysed in each cortical hemisphere using qPCR and qRT-PCR. Significantly higher levels of mCherry DNA (n=6/group, Two-Way ANOVA, brain hemisphere p<0.0001, Fig.2a, b) and mCherry mRNA (n=6/group, Two-Way ANOVA, brain hemisphere p<0.0001, Fig.3a, b) were detected in the injected cortical hemisphere, compared to the un-injected cortical hemisphere. There was no difference in the amount of mCherry DNA or mRNA detected in each hemisphere between the un-injured and pyramidotomised animals (n=6/group Two-Way ANOVA group p>0.05, Fig.2a, b and Fig.3a, b). Low levels of AAV genome and transcript were detected in the un-injected cortex which is likely due to axonal transport of vector particles between cortical hemispheres, mCherry and GFP-positive colossal fibers and transduced cortical neurons in the contralateral cortex were observed to support this (Fig.1a, c).
Figure 2. AAV genome (mCherry DNA) was detected in the spinal cord after cortical AAV1 injection, which was reduced by a prior pyramidotomy.
(a) Schematic representation of the rat CST and a heatmap showing the amount of mCherry DNA detected in each hemisphere’s cortex and each half of the spinal cord of uninjured or pyramidotomised rats. Numbers represent the average concentration of mCherry DNA relative to GAPDH. (b) Quantification of the relative level of mCherry DNA in the left (uninjected) and right (injected) cortex of uninjured or pyramidotomised rats. A significantly higher level of mCherry DNA was detected in the injected cortex and no difference between uninjured and pyramidotomised animals was observed. (c) Quantification of the relative level of mCherry DNA in the contralateral and ipsilateral spinal cord of uninjured or pyramidotomised rats. A significantly increased level of mCherry DNA was detected in the contralateral spinal cord of uninjured animals, which was significantly reduced by a prior pyramidotomy.
Figure 3. Significantly higher levels of AAV transcript (mCherry mRNA) were detected in the contralateral spinal cord after cortical AAV1 injection, which was abolished by a prior pyramidotomy.
(a) Schematic representation of the rat CST and a heatmap showing amount of mCherry mRNA detected in each hemisphere’s cortex and each half of the spinal cord of uninjured or pyramidotomised rats. Numbers represent the average concentration of mCherry mRNA relative to GAPDH. (b) Quantification of the relative level of mCherry mRNA in the left (uninjected) and right (injected) cortex of uninjured or pyramidotomised rats. A significantly higher level of mCherry mRNA was detected in the injected cortex and no difference between uninjured and pyramidotomised animals was observed. (c) Quantification of the relative level of mCherry mRNA in the contralateral and ipsilateral spinal cord of uninjured or pyramidotomised rats. A significantly increased level of mCherry mRNA was detected in the contralateral spinal cord of uninjured animals, which was abolished by a prior pyramidotomy.
Analysis of each half of the cervical spinal cord confirmed that mCherry DNA (n=6/group, Two-Way ANOVA, post-hoc Fisher's LSD p<0.0001, Fig.2a, c) and mRNA (n=6/group, Two-Way ANOVA, post-hoc Fisher's LSD p<0.02, Fig.2a, c) was significantly increased in the contralateral spinal cord compared to the ipsilateral. Low levels of mCherry DNA and mRNA was also detected on the ipsilateral side, which is consistent with the decussation pattern of the CST axons. When the CST was unilaterally lesioned one week prior to AAV1 injection, significantly lower levels of mCherry DNA (n=6/group Two-Way ANOVA, group p<0.001, Fig.2a, c) and mRNA (n=6/group Two-Way ANOVA, group p=0.01 Fig.3a, c) were detected in the spinal cord compared to the uninjured animals.
Discussion
In this study, we demonstrate the trans-neuronal transduction of spinal neurons in the contralateral spinal cord following cortical injection and anterograde axonal transport of a bicistronic AAV1 vector. Specifically, we observed the expression of mCherry and eGFP reporter proteins in second-order spinal neurons and the detection of AAV vector genome and transcript in the contralateral spinal cord following intracortical AAV1 injection, which were both abolished by prior pyramidotomy.
Two previous studies have detected AAV1 genome in the spinal cord following brain injections4, 7. Following injection of an AAV1 vector encoding vascular endothelial growth factor (VEGF) into the motor cortex and internal capsule Bucher at al detected VGEF RNA and protein throughout the injected hemisphere of the feline brain. Anterograde transport of the AAV1 vectors into the spinal cord was confirmed by the presence of VEGF DNA and mRNA throughout the spinal cord. However, despite this, no increase in VEGF protein level was detected in the spinal cord4. The authors suggest that the vector genome may be confined to spinal axons where it is unable to be translated; this apparent lack of distal trans-neuronal transduction and protein expression in the spinal cord is at odds with our findings. This may be due to differences in AAV titer, injection location or volume; although we can’t rule out that it could be due to a property of our bicistronic AAV1 vector which is not shared with other AAV1 vectors. In a separate study by Ciron et al, AAV1 vector genome was detected throughout the spinal cord following injection into the putamen and internal capsule of the non-human primate brain. Within the spinal cord, higher levels of the AAV vector genome were primarily detected in the contralateral spinal cord, which is consistent with our findings. However the level of expression or cellular location of the AAV protein product; human α-iduronidase (hIDUA) was not investigated7.
Others have reported the presence of a transgene protein product at sites distant from an AAV injection within the brain, but in these studies the vectors expressed growth-factor proteins and transport, secretion and re-uptake of the protein itself cannot be ruled out8, 15, 16.
We observed neuronal transduction in brain regions that receive direct connections from the site of AAV injection. These observations are consistent with previous studies that have reported anterograde axonal transport of various AAV serotypes and trans-neuronal transduction within the brain 5, 6, 9–11. However, due the presence of multiple reciprocal connections between brain regions, retrograde axonal transport of the AAV vector cannot be completely excluded, and neither can vector diffusion from the injection site. In contrast, we also provide evidence of spinal neuron transduction in a pattern consistent with the termination of the corticospinal axons and which was abolished following a prior pyramidotomy, confirming that this bicistronic AAV1 vector can undergo trans-neuronal transduction following anterograde axonal transport.
Our observations provide strong evidence of transneuronal transduction for a number of reasons: 1) The mCherry and eGFP reporter proteins are not secreted. 2) The pattern of transduction in the spinal cord follows the decussation pattern of CST axons. 3) The near elimination of vector DNA, mRNA and protein in the spinal cord following pyramidotomy provide compelling evidence that the AAV1 vector trans-neuronally transduces spinal neurons after anterograde transport in the CST axons. 4) Spinal neurons have no direct connections to the contralateral cortex eliminating the possibility of retrograde transport from the cortical injection site. 5) Diffusion in the cerebrospinal fluid or blood would lead to similar numbers of transduced spinal neurons on both sides of the spinal cord and would not be affected by pyramidotomy.
AAV1 may only trans-neuronally transduce neurons that express α2,3 and α2,6 N-linked sialic acids on their cell surface28, highlighting the importance of identifying the optimal AAV vector serotype for the transduction of the target neuronal population. Prior over-expression of these sialic acids or development of new capsids should enable AAVs to be used for trans-neuronal transduction of additional pathways and neuronal populations.
In conclusion, we report that this bicistronic AAV1 vector provides a novel method of investigating spinal circuitries, for assessing plasticity and increasing the distribution of transgenes within the CNS. Diseases which affect specific neuronal circuits such as Motor neuron disease, Parkinson’s disease, stroke and spinal cord injury may benefit from trans-neuronal transfer of therapeutic transgenes.
Materials and Methods
Bicistronic AAV-2A plasmid
The psubCMV-2A-WPRE plasmid was a kind gift from Dr. Hansruedi Bueler, University of Kentucky. The AAV-mCherry-2A-EGFP-WPRE plasmid was generated by amplifying mCherry and EGFP using PCR, followed by ligation into the psubCMV-2A-WPRE plasmid, see 18 for detailed methods on the cloning procedure.
AAV vector production
The bicistronic AAV1-mCherry-2A-eGFP vector uses a Foot and Mouth Virus 2A sequence to express two separate fluorescent proteins from a single promoter by causing ribosomal skipping during protein translation. AAV1-mCherry-2A-eGFP vector was generated by the Miami Project Viral Vector Core using fast protein liquid chromatography based purification and pseudotyped with AAV capsid serotype 1; the vector titre was 2.9x10^13 GC/ml. A separate batch of AAV1-mCherry-2A-eGFP vector was also produced by UPenn viral vector core for independent verification; the vector titre was 1.3x10^13 GC/ml.
Animals
Experiments were performed on 24 adult female Lister hooded rats weighing between 200-250g (Harlan, Blackthorn, UK). All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by the local veterinarian and ethical committee.
Intracortical injections
Rats were anaesthetised with an intraperitoneal injection of 0.5 mg/kg Domitor (medetomidine hydrochloride) and 100 mg/kg Vetelar (ketamine hydrochloride), and body temperature was maintained at 37°C. This procedure has been previously described in detail 18. Briefly, the head was placed into a stereotaxic frame (World Precision Instruments, FL, USA) and seven holes were drilled with a micro-drill (Power Performance, UK) using coordinates reported in a microstimulation study29 relative to Bregma, defined as anterior–posterior (AP), medial–lateral (ML): 1st +3.5mm ML, -0.5 mm AP, 2nd +3.5mm ML, +0.5mm AP, 3rd +3.5mm ML, +2mm AP, 4th +2.5mm ML, +1.5mm AP, 5th +2.5mm ML, +0.5mm AP, 6th +1.5mm ML, +1mm AP, 7th +2mm ML, +3.5mm AP. A total of 3.5 ul (0.5 µl / hole) of the AAV1 vector was injected at a depth of 1.5 mm into the cortex, at a rate of 150 nl/min. The skin was then sutured, 1 mg/kg Antisedan (atipamezole hydrochloride) was administered subcutaneously and the rats were placed in an incubator at 37°C. Once awake, 5 mg/kg Carprofen was administered for postoperative analgesia.
Pyramidotomy surgery
To determine whether trans-neuronal transport occurred in the corticospinal tract, six rats received pyramidotomy one week prior to intracortical injection of AAV. Using our routine methods for pyramidotomy27, rats were anesthetised with 2% isoflurane in O2 and their body temperature was maintained at 37°C. A midline incision was made along the throat, the trachea was displaced to one side and the muscle was blunt dissected to expose the basioccipital skull. A craniotomy of the occipital bone was performed to allow for access to the medullary pyramids. The right pyramid was cut using Vannas microscissors medially up to the basilar artery. The wound was closed with 3-0 sutures and the rats were placed in an incubator at 32°C. Once fully awake, 5 mg/kg Carprofen was administered for postoperative analgesia.
Histology
Six weeks post AAV injection, twelve rats (six uninjured, six pyramidotomised) were terminally anaesthetised by intraperitoneal injection of 400 mg/kg sodium pentobarbital and transcardially perfused with phosphate-buffered saline (PBS) (pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS. Brains and spinal cords were dissected, post-fixed in 4% PFA for 4 hours and cryoprotected in 30% sucrose (Sigma, Gillingham, UK). Brains were embedded in 10% porcine gelatin (Sigma), cut rostro-caudally into 50 µm free floating coronal sections using a vibratome (Leica, Peterborough, UK), mounted and coverslipped with Mowiol. Cervical spinal cords were embedded in OCT and frozen with liquid nitrogen. Spinal cords were cut transversely into 30 µm sections using a cryostat and mounted immediately on glass slides.
Immunohistochemistry and Imaging
Spinal cord sections were washed with 0.3% Triton-X-100 in PBS (PBST) and stained with primary antibody against NeuN (mouse monoclonal, 1:500, Abcam, Cambridge, UK) diluted in PBST with 10% normal goat serum for 24h at room temperature. The sections were then washed with PBS and incubated with secondary antibody (1:1000, goat anti-mouse DyLight 650, Invitrogen, Paisley, UK) diluted in PBST for 2h at room temperature. Sections were then washed with PBS and coverslipped with Mowiol. Mosaic images were captured using a Zeiss Apotome fluorescent microscope (Carl Zeiss, Welwyn Garden City, UK).
Tissue preparation for PCR analysis
Six weeks post AAV injection, twelve rats (six uninjured, six pyramidotomised) were terminally anaethetised with sodium pentobarbital and transcardially perfused with PBS. The cerebral cortex around the injection site and the cervical spinal cord were dissected and the left and right hemispheres or sides were separated by cutting sagittally along the midline. The tissue was then snap frozen in liquid nitrogen and stored at -80°C.
DNA and mRNA extraction
Genomic DNA was extracted using a DNeasy kit (Qiagen, Manchester, UK) which included RNase treatment (Qiagen). For RNA extraction tissue samples were homogenised in Trizol (Invitrogen) and total RNA extracted using a combination of a phenol-chloroform extraction and an RNeasy kit (Qiagen) which included DNase treatment (Qiagen). For cDNA synthesis 400 ng RNA was reverse transcribed using the Superscript II kit (Invitrogen) according to manufacturer’s protocol.
Quantitative PCR
Quantitative PCR (qPCR) was performed using the Roche LightCycler 480 II and SYBR Green master mix (Roche, Welwyn Garden City, UK). Samples underwent an initial denaturation at 95°C for 10 mins. Samples were amplified for 40 cycles (60°C for 10 sec, 72°C for 20 sec, 95°C for 10 sec) and then a final extension at 72°C for 1 min. Forward and reverse primers for GAPDH and mCherry were designed using NCBI Primer-Blast software. Primer sequences were: GAPDH forward 5′-ATG-GGA-AGC-TGG-TCA-TCA-AC-3′ and reverse 5′-CCA-CAG-TCT-TCT-GAG-TGG-CA-3′. mCherry forward 5′-GCG-CCT-ACA-ACG-TCA-ACA-TC-3′ and reverse 5′-GCG-TTC-GTA-CTG-TTC-CAC-GA-3′. Transcript levels were measured and normalised against GAPDH. Two technical repeats were performed per animal. A no-template negative control was included and gel electrophoresis of the PCR products along with melt-curve analysis confirmed primer specificity, PCR products of the expected size, and the absence of primer-dimer.
Statistical analysis
Data was analysed with the appropriate parametric test using Prism 6, Graphpad. PCR data was analysed with two-way ANOVA followed by Fisher's LSD test (Least Squares Difference). Results are expressed as mean ± Standard Error of Mean (SEM).
Acknowledgements
This work was funded by the European Research Council under the EU's Seventh Framework Programme (FP/2007-2013) Grant Agreement n. 309731, by the Henry Smith Charity and by a Nathalie Rose Barr PhD studentship from The International Spinal Research Trust which included funding from the Rosetrees Trust. Thanks to Hansruedi Bueler (University of Kentucky, KY, USA) for the psubCMV-2A-WPRE plasmid. We thank the Penn Vector Core at the Gene Therapy Program of the University of Pennsylvania for production of AAVs.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18(6):617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Current gene therapy. 2005;5(3):333–338. doi: 10.2174/1566523054064995. [DOI] [PubMed] [Google Scholar]
- 4.Bucher T, Colle MA, Wakeling E, Dubreil L, Fyfe J, Briot-Nivard D, et al. scAAV9 intracisternal delivery results in efficient gene transfer to the central nervous system of a feline model of motor neuron disease. Human gene therapy. 2013;24(7):670–682. doi: 10.1089/hum.2012.218. [DOI] [PubMed] [Google Scholar]
- 5.Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH. Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Human gene therapy. 2014;25(8):705–720. doi: 10.1089/hum.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27(37):9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciron C, Cressant A, Roux F, Raoul S, Cherel Y, Hantraye P, et al. Human alpha-iduronidase gene transfer mediated by adeno-associated virus types 1, 2, and 5 in the brain of nonhuman primates: vector diffusion and biodistribution. Human gene therapy. 2009;20(4):350–360. doi: 10.1089/hum.2008.155. [DOI] [PubMed] [Google Scholar]
- 8.Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16(6):1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadaczek P, Mirek H, Bringas J, Cunningham J, Bankiewicz K. Basic fibroblast growth factor enhances transduction, distribution, and axonal transport of adeno-associated virus type 2 vector in rat brain. Human gene therapy. 2004;15(5):469–479. doi: 10.1089/10430340460745793. [DOI] [PubMed] [Google Scholar]
- 10.Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20(3):348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Human gene therapy. 2005;16(7):781–791. doi: 10.1089/hum.2005.16.781. [DOI] [PubMed] [Google Scholar]
- 13.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13(3):528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. Journal of virology. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foust KD, Flotte TR, Reier PJ, Mandel RJ. Recombinant adeno-associated virus-mediated global anterograde delivery of glial cell line-derived neurotrophic factor to the spinal cord: comparison of rubrospinal and corticospinal tracts in the rat. Human gene therapy. 2008;19(1):71–82. doi: 10.1089/hum.2007.104. [DOI] [PubMed] [Google Scholar]
- 16.Ciesielska A, Mittermeyer G, Hadaczek P, Kells AP, Forsayeth J, Bankiewicz KS. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol Ther. 2011;19(5):922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson TH, Verhaagen J, Yanez-Munoz RJ, Moon LD. Corticospinal tract transduction: a comparison of seven adeno-associated viral vector serotypes and a non-integrating lentiviral vector. Gene Ther. 2012;19(1):49–60. doi: 10.1038/gt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutson TH, Kathe C, Menezes SC, Rooney MC, Bueler H, Moon LD. The use of an adeno-associated viral vector for efficient bicistronic expression of two genes in the central nervous system. Methods in molecular biology (Clifton, N.J. 2014;1162:189–207. doi: 10.1007/978-1-4939-0777-9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemon RN. Descending pathways in motor control. Annual review of neuroscience. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 20.Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. The Journal of comparative neurology. 1997;386(2):293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Raineteau O, Fouad K, Bareyre FM, Schwab ME. Reorganization of descending motor tracts in the rat spinal cord. The European journal of neuroscience. 2002;16(9):1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- 22.Liang FY, Moret V, Wiesendanger M, Rouiller EM. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. The Journal of comparative neurology. 1991;311(3):356–366. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- 23.Smith JB, Alloway KD. Functional specificity of claustrum connections in the rat: interhemispheric communication between specific parts of motor cortex. J Neurosci. 2010;30(50):16832–16844. doi: 10.1523/JNEUROSCI.4438-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs F, Usrey WM. Emerging views of corticothalamic function. Curr Opin Neurobiol. 2008;18(4):403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naito A, Kita H. The cortico-pallidal projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res. 1994;653(1–2):251–257. doi: 10.1016/0006-8993(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 26.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nature medicine. 2005;11(12):1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 27.Kathe C, Hutson TH, Chen Q, Shine HD, McMahon SB, Moon LDF. Unilateral Pyramidotomy of the Corticospinal Tract in Rats for Assessment of Neuroplasticity-inducing Therapies. J Vis Exp. 2014;(94):51843. doi: 10.3791/51843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. Journal of virology. 2006;80(18):9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396(1):77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]