Summary
Stroke is the dominant cause of sensorimotor disability that primarily affects the elderly. We now show that neuroplasticity and functional recovery after stroke is constrained by inhibitory chondroitin sulphates. In two blinded, randomised pre-clinical trials, degradation of chondroitin sulphate using chondroitinase ABC reactivated neuroplasticity and promoted sensorimotor recovery after stroke in elderly rats. Three days after stroke, chondroitinase ABC was microinjected into the cervical spinal cord to induce localized plasticity of forelimb sensorimotor spinal circuitry. Chondroitinase ABC effectively removed chondroitin sulphate from the extracellular matrix and perineuronal nets. Three different tests of sensorimotor function showed that chondroitinase ABC promoted recovery of forelimb function. Anterograde and retrograde tracing showed that chondroitinase ABC also induced sprouting of the contralesional corticospinal tract in the aged treated hemicord. Chondroitinase ABC did not neuroprotect the peri-infarct region. Excitingly, we show for the first time delayed chondroitinase ABC treatment promotes neuroanatomical and functional recovery after focal ischemic stroke in an elderly nervous system.
Keywords: stroke, chondroitin sulphate proteoglycans, plasticity, aging, perineuronal nets
Introduction
Stroke is the largest cause of long-term sensorimotor disability (Di Carlo 2009), with nearly 90% of incidents occurring in individuals aged >65 years (Truelsen et al., 2006). The elderly population are more likely to become disabled by stroke and they have a reduced capacity to recover from these disabilities compared to younger stroke survivors (Kelly-Hayes et al., 2003). However, the majority of experimental research is still conducted on young animals, despite recommendations by the Stroke Therapy Academic Industry Roundtable and other stroke committees urging for the use of aged animals in preclinical studies when modelling the clinical disorder (Macleod et al., 2009;Saver et al., 2009).
Many human stroke survivors have small infarcts (Brott et al., 1989) and the location of these focal strokes predicts the type of functional deficit, thus it is important to model both these aspects in an elderly system. The most common functional deficit following stroke are motor impairments of the contralateral upper limb with more than 80% of subjects experiencing this acutely (Cramer et al., 1997) and 60% chronically (Dobkin 2004). Additionally, reports also indicate up to 94% of human stroke survivors experience sensory deficits and disturbed performance of motor tasks requiring somatosensory information (Carey et al., 1993). Structural imaging of human stroke subjects demonstrates highly significant correlations between the extent of these sensorimotor impairments and focal ischemic damage to the corticospinal tract (CST) (Lindenberg et al., 2010;Zhu et al., 2010). This indicates that CST integrity plays a critical role in functional outcome and that localized ischemic damage to this pathway is an important goal in animal models of focal stroke. Focal ischemic lesions can be produced using endothelin-1 which occludes arteries and restricts regional blood flow to produce localized and dose dependent ischemic injury in various brain regions in young animals (Adkins et al., 2004;Windle et al., 2006). Importantly, we have previously shown that focal ischemic damage to the CST using endothelin-1 produces sustained sensorimotor deficits similar to those observed in human stroke survivors, great reproducibility and low mortality rates in aged animals (Soleman et al., 2010). We now show that this model provides a powerful method for evaluating therapies that promote plasticity, which is vital as there is currently no drug treatment available beyond the acute stage of stroke.
Following injury to the adult central nervous system (CNS), undamaged neurons can undergo plastic responses and neuroanatomical reorganisation to replace damaged synaptic connections (Carmichael 2003;Dancause et al., 2005;Hsu and Jones 2006). However, the extent of reorganisation remains constrained by many intrinsic and extrinsic factors that limit neuronal growth. Age-related changes are also known to alter neuroplasticity and the physiological responses after ischemic injury (Badan et al., 2003;Ward 2005;Li et al., 2010) which can influence the effects of some drugs (Fisher and Ratan 2003). Thus, this reveals age as an important factor when assessing potential therapeutics for neural repair. Various drug interventions that enhance events of structural plasticity have been shown to cause functional recovery after CNS injury (Chen et al., 2002;Wiessner et al., 2003;Lee et al., 2004;Clarkson et al., 2010). Of particular interest is chondroitinase ABC (ChABC) which degrades inhibitory chondroitin sulphate proteoglycans (CSPGs) present in the extracellular matrix. We and others have shown that degradation of chondroitin sulphate with ChABC reduces the neurite-inhibitory environment, reactivates plasticity and promotes functional recovery after different central and peripheral nervous system injuries (Moon et al., 2001;Bradbury et al., 2002;Barritt et al., 2006;Massey et al., 2006;Pizzorusso et al., 2006;Galtrey et al., 2007;Cafferty et al., 2008;Garcia-Alias et al., 2009;Alilain et al., 2011). However, to date the effects of ChABC following stroke have yet to be explored.
Unlike most conventional stroke therapies that typically target the brain or the entire neuraxis, we administered ChABC into the denervated cervical spinal cord to induce localized plasticity of spinal circuitry. Here, we tested the efficacy of this intraspinal ChABC delivered 3 days after focal ischemic stroke on neuroplasticity and functional recovery from sensorimotor deficits in aged animals. Our results demonstrate that delayed intraspinal ChABC is able to alter the composition of diffuse CSPGs and CSPG-rich perineuronal nets (PNNs) present in the aged spinal cord to promote axonal reorganisation and behavioural recovery after stroke. These findings constitute evidence that ChABC promotes plasticity of the uninjured CNS after injury and propose ChABC as a novel therapeutic candidate in ischemic stroke for aged individuals.
Material and methods
Subjects
Forty aged male and female Long Evans rats (304 – 595g; 16 – 19 months of age) were housed 2-3 to a cage in standard laboratory conditions. Experiments were performed in accordance with guidelines from the Stroke Therapy Academic Industry Roundtable and others (Fisher et al., 2005;Macleod et al., 2009;Saver et al., 2009) and our findings were reported in accordance with the Animals in Research: Reporting In Vivo Experiments guidelines (Kilkenny et al., 2010). Surgeries, behavioural testing and analysis were performed with investigators blinded to treatment groups.
Experimental design
We investigated whether delayed intraspinal administration of ChABC would promote functional recovery after unilateral stroke in aged (>16 month old) rats. Anterograde tracing was used to assess sprouting of uninjured corticospinal tract (CST) collaterals into areas of partial CST-denervation in the spinal cord.
In our second study, early behavioural improvements observed in the first study following delayed intraspinal administration of ChABC were further investigated. Retrograde tracing was used to further assess sprouting of uninjured CST collaterals and any neuroprotective effect on surviving corticospinal neurons (CSNs). Experimental designs and groups are outlined in Supplementary Fig 1. All surgeries were performed using a randomised block design and the experimenters were blinded to treatment groups during behavioural and histological assessment. Penicillinase (P-ase) served as a control as it is an enzyme shown not to degrade CSPGs (Pizzorusso et al., 2002;Moon et al., 2001).
Surgery
All procedures were in accordance with guidelines from the UK Home Office and Animals (Scientific Procedures) Act of 1986. Animals were anesthetized with isoflurane (4% in O2 for induction) and maintained at 1.5–2% in O2 delivered via a facemask. Body temperature was monitored via a rectal thermometer and maintained at approximately 36 °C with a heating pad.
Stroke lesions
Prior to surgery, rats were allocated to an experimental group in a counterbalanced fashion to ensure no difference in group mean preoperative performance of the dominant forepaw on the staircase test. Unilateral lesions were performed in the hemisphere contralateral to the dominant forelimb (Supplementary Fig 3), as determined by staircase behavioural test. Stroke lesions (n = 32) were performed as previously described (Soleman et al., 2010) using a randomised block design. Briefly, animals were transferred to a stereotaxic frame (David Kopf Instruments, USA) where a midline incision was made, the sensorimotor cortex was then exposed via craniotomy and the dura mater was incised. Since skull thickness varied amongst aged rats, a craniotomy was performed to enable accurate depth placement of endothelin-1 intracortical injections. Four 2 μl injections of endothelin-1 (200 pmol/μl; 0.5 μg/μl dissolved in sterile saline; CalBioChem) were delivered via a glass micropipette connected to a syringe (Hamilton). The first 1 μl was administered at a depth of 1 mm from the brain surface and the subsequent 1 μl applied to the surface of the cortex at four co-ordinates. Prior to suturing, the animal was left undisturbed for 5 mins. Modifying previous work (Soleman et al., 2010), the skull fragment was then replaced and sealed using bone wax. Sham-operated (n = 8) rats received all procedures up to, but not including, craniotomy. Animals were administered buprenorphine (0.01 mg/kg, subcutaneously) for postoperative pain relief after recovering from anaesthesia. Our method of inducing stroke with endothelin-1 is advantageous for evaluating regenerative stroke therapies for four reasons: 1) our model produces ischemic lesions that model small focal human strokes rather than larger “malignant” strokes that tend to be fatal in humans (Carmichael 2005) ; 2) our model targets specific neuronal circuits that are typically affected after stroke including the CST pathway that originates in sensorimotor cortex; 3) our stroke model has statistically powerful reproducibility (Soleman et al., 2010) and involves only low mortality rates in aged animals; 4) our model causes sustained sensorimotor deficits which are the most common neurological symptoms of human stroke.
Spinal delivery of ChABC
Animals received two unilateral spinal injections of either ChABC (10 U/ml; Seikagaku) or the control enzyme penicillinase (P-ase; same µg/µl of protein; Sigma) delivered into the partially CST-denervated side of the C5 and C8 spinal cord. ChABC was delivered intraspinally as previously described (Wong et al., 2006;Galtrey et al., 2007). Briefly, a partial laminectomy was performed to expose the cervical spinal cord at level C5 and C8. At each site, 1 µl injections of either ChABC or P-ase were performed using a glass micropipette connected to a 10 µl syringe (Hamilton) and delivered at a rate of 0.25 μl/min. The micropipette was positioned 1 mm lateral from the midline and lowered 1.5 mm below the spinal cord surface; this was left in situ for 2 mins before being withdrawn. To assess the extent of chondroitin sulphate degradation after ChABC treatment rats were perfused one week after injections (n=2). In our two main experiments, animals received unilateral intraspinal injections three days post-stroke injury. The number of rats per treatment group was based on sample size calculations from previous work (Soleman et al., 2010), which showed that a minimum of 8 rats per treatment group would be required to identify a treatment which caused a 50% improvement on the staircase test (α=0.05; power>0.80).
Anterograde tracing
In the first experiment, seven weeks post-stroke, animals were given biotinylated dextran amine (BDA; 10%; 10,000 MW, Invitrogen) unilaterally into the uninjured sensorimotor cortex to visualize uninjured CST axons. Animals were placed in a sterotaxic frame and six burr holes were made into the skull at the following coordinates (defined as anterioposterior (AP), mediolateral (ML): 1) AP: +1 mm, ML: 1.5 mm; 2) AP: +0.5 mm, ML: 2.5 mm; 3) AP: +1.5 mm, ML: 2.5 mm; 4) AP: +0.5 mm, ML: 3.5 mm; 5) AP: +2.0 mm, ML: 3.5 mm; 6) AP: - 0.5 mm, ML: 3.5 mm, relative to bregma. At each site, 0.5 μl injections of BDA were delivered using a glass micropipette attached to a Hamilton syringe inserted 2 mm from the skull surface and delivered at a rate of 0.25 μl/min. Animals were subsequently left for 2 weeks before being perfused.
Retrograde tracing
In the second experiment, five weeks post-stroke, animals were given unilateral intraspinal injections of the retrograde fluorescent tracer Fast Blue (2% in PBS; Sigma) into the denervated side of the spinal cord and at areas of ChABC-mediated digestion. The spinal cord (C6 to C8) was exposed as described above. Each animal had five injections of Fast Blue (200 nl/site) spaced at approximately 1 mm apart along the spinal cord. A glass micropipette was positioned 1 mm lateral and 1.8mm below the spinal cord surface. Animals were left for 10 days before being perfused. Location of the injections was verified post-mortem. Animals that failed to have consistent unilateral tracing mostly confined to the spinal gray matter were excluded from analysis (ChABC group, n = 3; P-ase group, n = 3).
Behavioural testing
Tests previously found to be effective in assessing sensory and motor deficits were included (Soleman et al., 2010). Rats were given 4 weeks of daily training on the staircase test to identify forepaw preference. All behavioural testing was carried out by an experimenter blinded to surgery and treatment groups. Baseline values were recorded 3 days before surgery on all behavioural tasks.
Assessment of fine motor function
The staircase test was used to assess reaching performance; this provides a sensitive measure of skilled forepaw motor function (Montoya et al., 1991). The staircase apparatus (Campden Instruments Ltd., UK) consists of a chamber with a central platform for the rat to climb onto and a set of seven steps is located on either side. Three sucrose pellets (45 mg, Research Diets Inc, New Brunswick, NJ) were placed in the well on each step and may be retrieved by the rat reaching down either side of the platform. The number of pellets retrieved (maximum of 21 pellets per side) and the maximum step reached using each forepaw was recorded during each 15min trial. Scoring the number of pellets retrieved indicates successful grasp and retrieval (fine motor function), whilst the maximum step reached assesses the lowest step a pellet is displaced regardless of successful grasping (gross limb control). Each weekly session consisted of two trials; mean scores per rat per week were calculated. The minimum criterion to be included in the study was the retrieval of 11 pellets using their dominant paw during preoperative testing.
Assessment of forelimb asymmetry
The cylinder test was used to assess asymmetries in forelimb use for postural support during rearing (Schallert et al., 2000) within a transparent 20 cm diameter and 30 cm high cylinder. An angled mirror was placed behind the cylinder to allow movements to be recorded when the animal turned away from the camera. During exploration, rats rear against the vertical surface of the cylinder. The first forelimb to touch the wall was scored as an independent placement for that forelimb. Subsequent placement of the other forelimb against the wall to maintain balance was scored as “both.” If both forelimbs were simultaneously placed against the wall during rearing this was scored as “both.” Lateral movements along the wall using both forelimbs alternately were also scored as “both.” Scores were obtained from a total number of 10 full rears to control for differences in rearing between animals. Once scores had been acquired, forelimb asymmetry was calculated using the formula: 100 × (ipsilateral forelimb use + 1/2 bilateral forelimb use)/total forelimb use observations (Hsu and Jones 2005).
Assessment of somatosensory function
The magnitude of somatosensory asymmetry and sensorimotor impairments in forepaw function after stroke was assessed using the bilateral tactile stimulation test (Schallert et al., 1982;Schallert et al., 2000). For each trial, round adhesive patches (13 mm diameter, Ryman) were applied to the plantar surface of both forepaws and the animal was returned to its homecage. Two times were recorded for both forepaws: (1) contact and (2) remove; where “contact” represents the time taken for the animal to notice the adhesive patch on its forepaw and bring it to its mouth, and “remove” represents the time taken for the animal to remove the adhesive patch from its forepaw. To determine whether the rats showed bias for their affected or less-affected forelimbs, the order and side of label removal was recorded. This was repeated four times per session until a >75% preference had been found; if this was not the case a fifth trial was conducted. The magnitude of asymmetry was established using the seven levels of stimulus pairs on both forepaws as previously described (Figure 2C) (Schallert and Whishaw 1984;Schallert et al., 2000). During this phase, to determine the extent of ipsilateral response bias, the size of the stimulus was progressively increased on the affected forepaw and decreased on the less-affected forepaw by an equal amount (14.1 mm2), until the rat removed the stimulus on the affected forepaw first (reversal of original bias). This reversal represents the magnitude of asymmetry, where the higher the score indicates the greater the degree of somatosensory impairment.
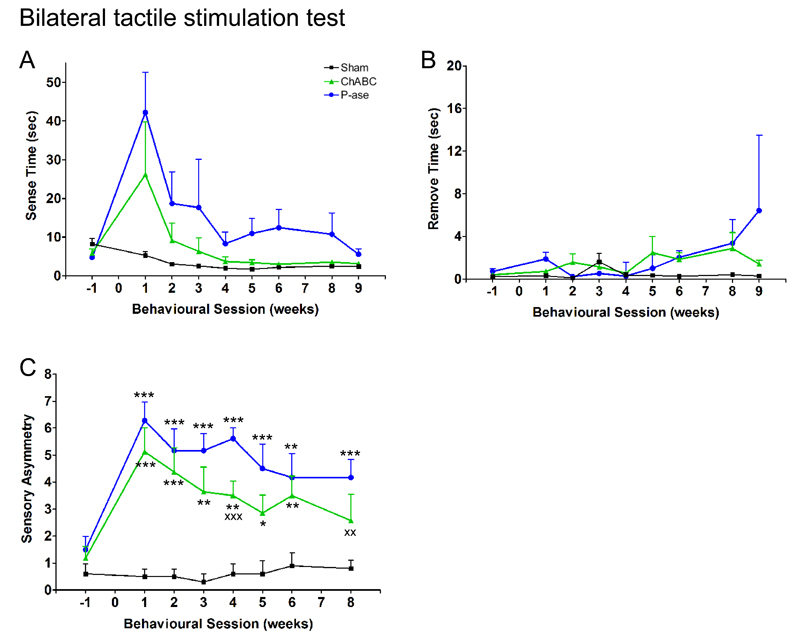
Figure 2. Delayed ChABC promotes forelimb sensory recovery following stroke in aged rats.
The bilateral tactile stimulation test assesses sensory function using adhesive sticky patches placed on the rats’ forepaws (A). Stroke increased the time animals took to sense (B) and remove (C) these adhesive patches placed on their affected forepaw, and both treatment groups showed a similar extent of recovery [sense: F(2,17) = 1.57, P = 0.24; remove: F(2,17) = 0.63, P = 0.55]. (D, E) The magnitude of sensory asymmetry (tactile extinction) was determined using the seven pairs of sensory stimuli [see text]. (D) Stimulus size was progressively increased on the affected forepaw and decreased on the less-affected forepaw by an equal amount, until the rat removed the stimulus on the affected forepaw first. This reversal represents the magnitude of asymmetry. (E) Testing begins at level 3 (black box) and asymmetry scores indicate the severity of sensory asymmetry (e.g. severe impairment = 7). (F) Stroke increased the magnitude of sensory asymmetry (tactile extinction) in both groups. P-ase-treated animals remained impaired whereas animals treated with ChABC showed significant improvements in detecting the sensory stimulus on their affected forepaw [group differences F(2,17) = 12.2, P = 0.001]. Results are presented as mean ± SEM and were analysed using RM ANCOVA with post hoc Least Squares Difference tests. Significance is denoted as: * P < 0.05, ** P < 0.01, *** P < 0.001 vs. sham animals (n = 5) and X P < 0.05, XX P < 0.01, XXX P < 0.001 vs. treatment groups (n = 9 per group).
Histology
Animals were terminally anesthetized with sodium pentobarbital (80 mg/kg; intraperitoneally) and perfused transcardially with heparinized saline followed by 4% paraformaldehyde. Brains and spinal cords were post fixed for 2 h at 4 °C then transferred to 30% sucrose in PBS for 2 days. The brain and spinal cord were separately embedded in 10% gelatin and blocks were post fixed in 4% paraformaldehyde for 24 h. Free-floating serial sections were then cut using a freezing stage microtome (Kryomat; Leitz, Germany). Ten series of rostral to caudal tissue sections were collected in 24 well plates containing PBS (with 0.1% sodium azide) and stored at 4 °C.
Immunohistochemistry
All sections used were transverse C4-C8 spinal cord sections (40 μm). Chondroitin-4-sulphate: To confirm CSPG digestion, sections were incubated in mouse anti-C-4-S (1:1000; MP Biomedicals) and goat anti-mouse Alexa 488 (1:500; Invitrogen). Wisteria floribunda agglutinin: To assess degradation of CSPG-rich PNNs, sections were incubated in biotin-conjugated W. floribunda agglutinin (15 μg/ml; Sigma) and Extra-Avidin Tetramethyl Rhodamine Isothiocyanate (1:1000; Sigma). BDA: To assess fibre sprouting from the uninjured CST, sections were incubated in hydrogen peroxide (0.3%), ABC reagent (1:400; Vector Laboratories), biotinyl tyramide (1:75; PerkinElmer Life Sciences) and Extra-Avidin Fluorescein Isothiocyanate (1:1000; Sigma). All sections were coverslipped with Fluorsave mounting medium.
Histological analysis
Images were captured using a Carl Zeiss AxioImager Z1 microscope with Z-stack intervals. All images were re-coded during analysis so the investigator was blinded to treatment groups.
Analysis of BDA labelled axons
Transverse spinal cord sections in the treated area (C6 – C8) were selected. To ensure consistent tracing within animals, the number of BDA-labelled axons present in the dorsal column were quantified and averaged from ten sections per animal. In the denervated gray matter of these sections, sprouting BDA-labelled axons were quantified. To reduce between animal tracing variations, the axon index was calculated as previously described (Zheng et al., 2005;Lee et al., 2010). For this, the number of BDA-labelled axons present in the denervated gray matter was normalised against the total number of labelled axons present in the dorsal column. The distance (µm) of each BDA-labelled axon along the medio-lateral axis from the midline was determined from the central canal.
Analysis of Fast Blue labelled CSNs
Coronal brain sections from regions +3.5 mm to -1 mm relative to bregma were selected. The total number of retrogradely labelled CSNs present in both hemispheres was quantified in twelve sections per animal. In the injured hemisphere, cell area (µm2) was assessed in all CSNs from two cortical 500 µm regions in the ipsilesional hemisphere: 1) the area directly around the lesion cavity (Supplementary Fig 2A’), 2) the area neighbouring the injury site (Supplementary Fig 2A’’). For the latter, as no visible cavity was present we used the following approach: in sections that had lesion cavities, the distance from the lesion centre to the midline interhemispheric fissure was measured and applied to the sections with no visible lesion. A region 500 µm from this calculated lesion midline was applied on either side and cells within this area were also measured. Cell size distributions were then tabulated.
Quantification of infarct volume
Serial coronal sections from +3.5 mm to −2.5 mm relative to bregma were captured using a light microscope with a high resolution digital camera (MiniVID Digital Eyepiece Camera., LW Scientific). Infarct size was calculated as previously described (Soleman et al., 2010). For each section, the total area of the ipsilesional hemisphere was subtracted from the area of the contralesional hemisphere. Hemisphere area measurements did not include necrotic tissue, cysts or cavities, or ventricles. Volume of injury (mm3) was calculated as the sum of the area from each section, multiplied by the distance between sections (Buchan et al., 1992). Before the end of the study, one animal died due to age-related pituitary tumours and is thus absent from histological analysis (ChABC, n = 1).
Statistical analysis
Behavioural data were analysed using repeated measures analysis of covariance (RM ANCOVA), using preoperative performances as covariates. Pairwise group comparisons were performed using two-tailed Least Squares Difference post hoc tests. Infarct volumes were compared using two-tailed t-tests. The number of Fast Blue labelled cells and BDA axon counts were compared between groups using the Kruskal-Wallis and Mann Whitney tests. Cell size distributions between groups were analysed using repeated measures analysis of variance (RM ANOVA) and the Kolmogorov-Smirnov test. Data is presented as mean ± standard error of the mean (SEM) and asterisks indicate significance as follows: *P ≤0.05; **P ≤0.01; ***P ≤0.001.
Results
Delayed ChABC promotes forelimb motor recovery following stroke in aged rats
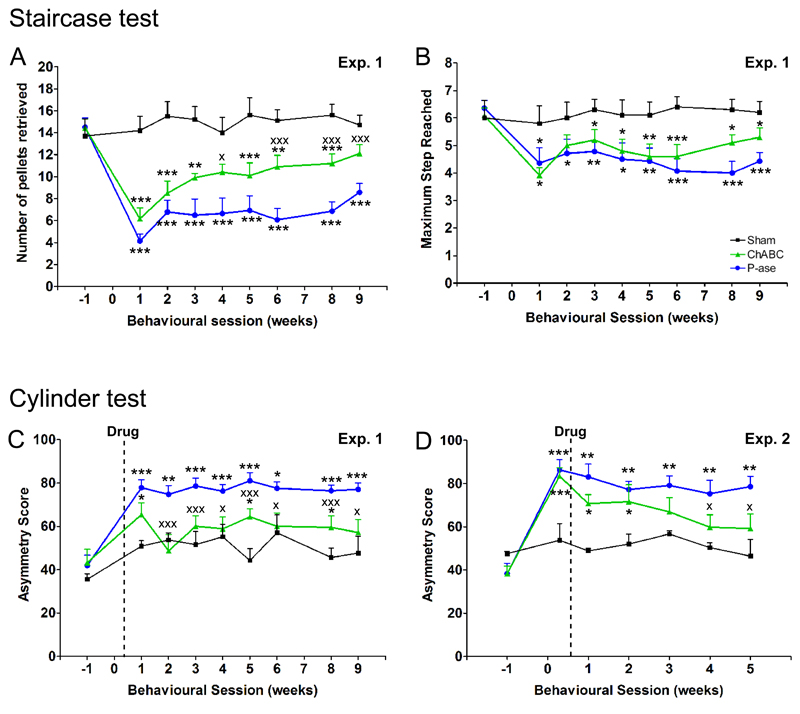
To assess motor function, dexterity of the affected forepaw was examined using the staircase test (Fig 1A; Supplementary Movie 1). Here, two parameters were measured: the number of pellets retrieved (skilled motor function) and maximum step from which pellets were retrieved or displaced (unskilled limb control). Concerning the number of pellets retrieved, one week after stroke all animals showed a marked reduction in their ability to retrieve pellets with their affected forepaw (sham vs. either stroke group, P <0.001), with no difference between treatment groups at this time-point (ChABC vs. P-ase, P = 0.19; Fig 1B). Compared to pre-operative levels, P-ase-treated animals were able to recover to a maximum of 59%: in comparison, ChABC-treated animals were more successful at retrieving pellets (P = 0.007) and were able to recover to a maximum of 85%. Concerning the maximum step, stroke groups remained impaired relative to sham animals (ChABC, P = 0.01; P-ase, P <0.001; Fig 1C), with no difference between treatment groups (ChABC vs. P-ase, P = 0.24). Thus, our data shows that ChABC treatment is capable of significantly improving forepaw dexterity to aid successful pellet retrieval, while unskilled limb movements were unaffected by treatment demonstrating some spontaneous recovery.
Figure 1. Delayed ChABC promotes motor recovery following stroke in aged rats.
The staircase test assesses forepaw dexterity; (A) a photograph and Supplementary Movie 1 show a rat performing this task. (B) After stroke, rats treated with ChABC retrieved more pellets with their affected forelimb than rats treated with P-ase [overall effect F(2,13) = 34.6, P = 0.0001; interaction of group by time F(14,91) = 1.88, P = 0.04], confirming that ChABC promotes improvements in dexterity. (C) There was no difference in the maximum step reached by either treatment groups of rats, although they were both impaired relative to shams [group differences F(2,13) = 9.5, P = 0.003]. (D) The cylinder test assesses forelimb use for postural support during rearing. (E) Following stroke, rats exhibited an increase in asymmetry score indicating preferential use of the less-affected forelimb for weight support. P-ase-treated animals remained impaired whereas animals treated with ChABC significantly recovered [group differences F(2,17) = 13.2, P = 0.0001; Supplementary Movie 2 and 3]. (F) In a second experiment, testing at 2 days confirmed both treatment groups possessed similar behavioural deficits before drug intervention. As previously observed, P-ase-treated animals remained impaired whereas ChABC-treated animals significantly recovered [group differences F(2,11) = 6.1, P = 0.02]. Results are presented as mean ± SEM and were analysed using RM ANCOVA with post hoc Least Squares Difference tests. Significance is denoted as: * P < 0.05, ** P < 0.01, *** P < 0.001 vs. sham animals (Experiment 1, n = 5; Experiment 2, n = 3) and X P < 0.05, XX P < 0.01, XXX P < 0.001 vs. treatment groups (Experiment 1, n = 9 per group; Experiment 2, n = 7 per group).
To further assess forelimb motor function, we employed the cylinder test. This measures forelimb use for postural support during rearing (Fig 1D). Sham animals demonstrated approximately equal use of forelimbs during postural support (asymmetry score ≈ 50). One week after stroke, animals exhibited an increase in asymmetry score indicating preferential use of their less-affected forelimb for support during rearing (sham vs. P-ase, P = 0.002; sham vs. ChABC, P = 0.08; Fig 1E). Sustained functional deficits were apparent in the P-ase-treated animals, with little recovery occurring beyond the first week of stroke (week 1 vs. 9, P = 0.87; Supplementary Movie 2). In contrast, ChABC-treatment significantly reduced the functional deficits compared to P-ase animals (P = 0.002), indicating use of their affected forelimb for postural support. By week 9 post-stroke, there was no significant difference between ChABC-treated and sham animals (P = 0.17; Supplementary Movie 3).
Interestingly, our data showed a rapid effect of ChABC as animals had smaller functional deficits by the first week of behavioural testing (four days after treatment) compared to the P-ase group (P = 0.06). To confirm the immediate effects of ChABC were not due to baseline differences from the lesion, a second experimental study was repeated with a behavioural testing time point at 2 days post-stroke (1 day before drug intervention). Importantly, both groups prior to treatment showed an equal extent of functional impairment (ChABC vs. P-ase, P = 0.68; Fig 1F) indicating both stroke groups possessed equivalent stroke lesions and behavioural deficits. After treatment, P-ase-treated rats remained persistently impaired (day 2 vs. week 5; P = 0.36). Interestingly, ChABC-treated rats exhibited immediate behavioural recovery in the first week (day 2 vs. week 1; P = 0.06) and improved until the end of the study (day 2 vs. week 5; P = 0.005). By week 5, ChABC-treated animals were significantly different to P-ase-treated animals (P = 0.04) and no different to sham animals (P = 0.23). Together these two studies show ChABC is able to promote use of the affected forelimb during postural support and reduces forelimb deficits following cerebral ischemia in aged rats.
Delayed ChABC promotes forelimb sensory recovery following stroke in aged rats
To assess forepaw impairments and asymmetries in somatosensory function, we used the bilateral tactile stimulation test. A small adhesive patch is stuck to the plantar surface of each forepaw and the time taken to contact and remove both stimuli with their mouth is recorded (Fig 2A). Sham animals rapidly contacted and removed both stimuli. Stroke rats showed impairments in both sensing and removing the adhesive patch from their affected forepaw (Fig 2B, C). However, there was no effect of drug treatment as animals displayed a similar extent of recovery in contact (ChABC vs. P-ase, P = 0.19; Fig 2B) and removal time (ChABC vs. P-ase, P = 0.71; Fig 2C).
We also assessed recovery from tactile extinction, a phenomenon manifested in many stroke patients who fail to detect a touch stimulus on their affected hand once it has been applied simultaneously on both hands (Rose et al., 1994). We obtained sensory asymmetry scores using pairs of sensory stimuli (Schallert et al., 2000) (Fig 2D, E). After stroke, all animals had high asymmetry scores, indicating that animals neglected the larger stimulus on their affected forepaw and preferentially removed the smaller stimulus from their less-affected forepaw first. P-ase-treated animals remained persistently impaired relative to sham animals (P <0.001; Fig 2F). In comparison, ChABC induced significant recovery from asymmetrical sensory impairments with animals able to detect smaller stimuli on their affected forepaw (ChABC vs. P-ase, P = 0.04). In conclusion, whilst ChABC had no effect on sensory response times, it overcame somatosensory neglect of the affected forepaw following stroke. This is important as tactile extinction was shown to be the single most important predictor of functional outcome (Rose et al., 1994), thus improving this ability to detect and process sensory data should enable improvements in motor function.
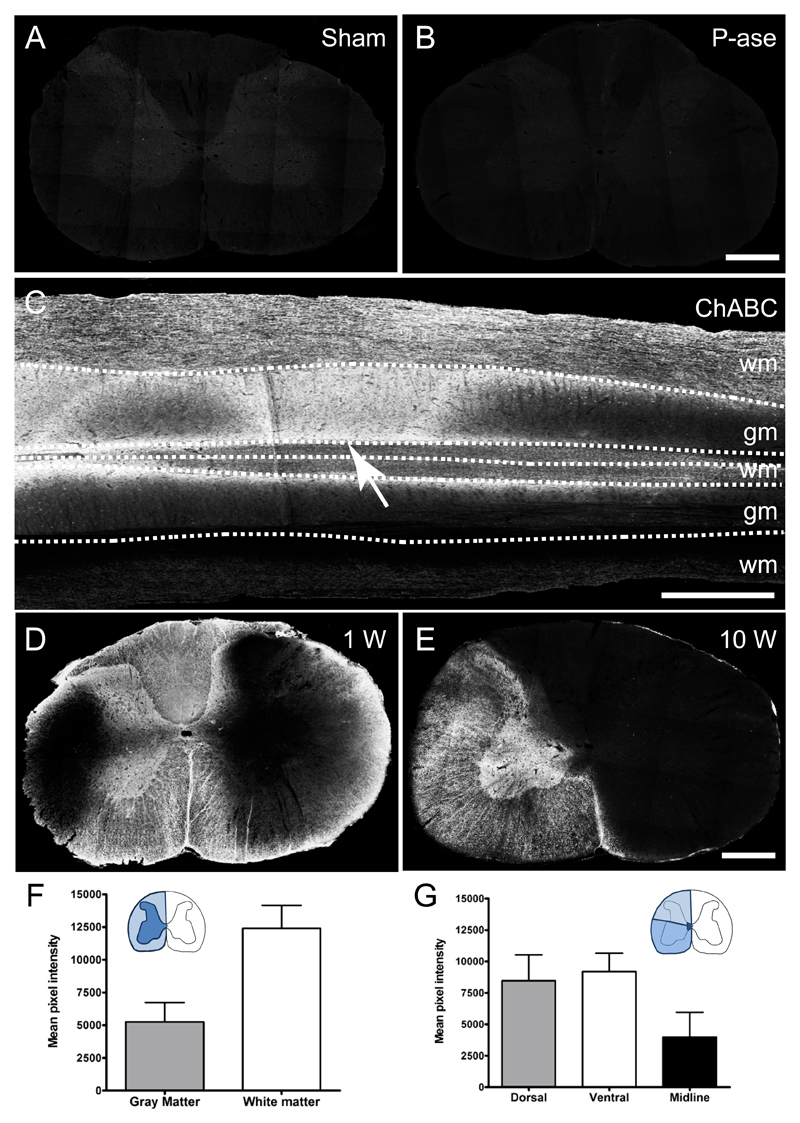
ChABC injections effectively degrade diffuse CSPGs present in the aged spinal cord
Unilateral intraspinal injections of ChABC were carried out at C5 and C8 to encourage localized plasticity and midline crossing of the intact CST into ChABC-treated areas of the aged cervical spinal cord. We examined the pattern of CSPG digestion using an antibody that recognises the chondroitin-4-sulphate stub epitope on CSPG core proteins following chondroitin sulphate removal. As expected, no immunoreactivity for chondroitin-4-sulphate was detected in sham controls or P-ase treated animals (Fig 3A, B). In a pilot study, we assessed the extent and spread of CSPG degradation. One week following ChABC injections, extensive and predominantly unilateral chondroitin-4-sulphate immunoreactivity spanned segments C4 to T1 (Fig 3C, D). Even ten weeks after ChABC intraspinal injections, intense immunoreactivity was still present in the cervical cord, confirming ChABC effectively degraded CSPGs and that its effects are long lasting (Fig 3E). Quantification of chondroitin-4-sulphate immunoreactivity revealed intense digestion in both the spinal cord gray and white matter, (Fig 3F) and spanning dorsal, ventral and midline regions of the spinal cord (Fig 3G). The greatest degree of CSPG digestion was observed in the ventral horn and funiculus. Thus, these results indicate that intraspinal ChABC injections were effective in degrading growth inhibitory CSPGs.
Figure 3. Intraspinal Chondroitinase ABC injections degrade matrix CSPGs.
(a-e) Immunolabelling using the 2B6 antibody that recognises the chondroitin-4-sulphate stub region of CSPGs after degradation of chondroitin sulphate by ChABC. Chondroitin-4-sulphate immunoreactivity in the C6 region of the spinal cord was absent in sham (A) and penicillinase-treated (B) rats. (C) In ChABC-treated animals, after one week there was an intense immunoreactivity surrounding the injection site (indicated by arrow) revealing extensive CSPG digestion from C6 – T1 in horizontal sections of the spinal cord. Transverse sections demonstrate unilateral CS degradation throughout the C5 spinal cord after one week (D) and 10 weeks post-ChABC injections (E). (F, G) Quantification revealed intense degradation in both the gray and white matter (F), as well as throughout dorsal, ventral and midline areas (G). Scale bar: 500 μm (A, B, D, E), 1 mm (C). Results are presented as mean ± SEM, n = 9.
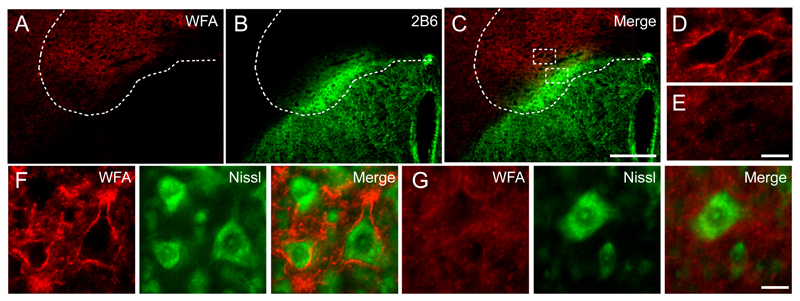
ChABC injections degrade perineuronal nets present in the aged spinal cord
Previous studies have demonstrated that digestion of CSPGs in PNNs with ChABC can reactivate plasticity (Pizzorusso et al., 2002;Massey et al., 2006), leading to the hypothesis that PNNs are involved in the control of plasticity in the CNS (Carulli et al., 2010). We examined W. floribunda agglutinin reactivity, a marker of CSPG-rich PNNs, one week following ChABC delivery. Here, we observed that ChABC was able to reduce W. floribunda agglutinin reactivity (Fig 4A), and this effect was only present in areas of CSPG degradation (Fig 4B, C). Intact PNNs surrounding motoneurons in the ventral horn were observed outside areas of ChABC-mediated digestion (Fig 4D, F) and were completely absent within degraded areas (Fig 4E, G). Thus, our results confirm that intraspinal ChABC delivery not only alters the composition of the diffuse extracellular matrix but also alters the condensed matrix in these CSPG-rich PNNs that are found around neurons present in the spinal cord, particularly around motoneurons.
Figure 4. Intraspinal Chondroitinase ABC injections degrade perineuronal nets.
Transverse C5 spinal cord sections were stained with W. floribunda agglutinin (red) to label CSPGs present in perineuronal nets (PNNs). (A) One week following intraspinal ChABC injections, W. floribunda agglutinin reactivity was reduced. (B) Chondroitin-4-sulphate immunolabelling (green) revealed PNNs were only reduced in areas of CSPG degradation (C). (D, E) Dashed boxes denote the area of high power magnification highlighting PNNs present in undigested areas (D) and absent in areas of CSPG digestion (E). (F, G) PNNs were also observed encasing Nissl-stained (green) motoneurons in the ventral horn in undigested regions (F) and appeared absent around motoneurons in digested regions (G). Scale bar: 250 μm (A-C), 20 μm (D-G).
Delayed ChABC promotes collateral sprouting of the uninjured corticospinal tract in the aged spinal cord
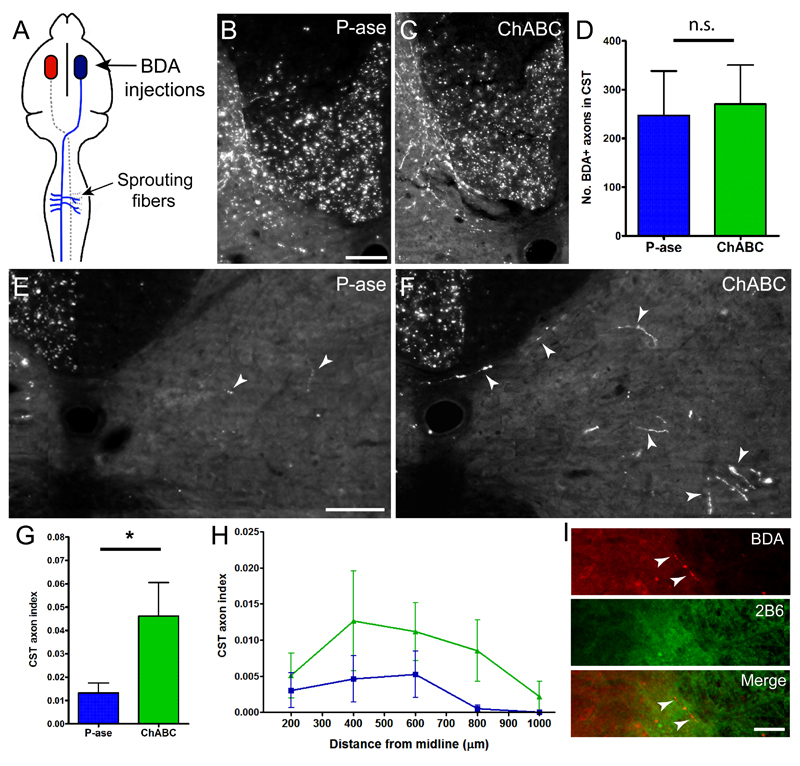
In our first experiment, BDA was unilaterally injected into the contralesional hemisphere to anterogradely label intact CST axons and collaterals (Fig 5A). Immunofluorescent visualization of BDA was used to assess axonal sprouting into ChABC-treated areas of the spinal cord gray matter. To control for variations in BDA labelling amongst individual animals, axonal counts in the denervated gray matter were normalized against the total number of BDA labelled CST axons present in the dorsal column (Zheng et al., 2005). No group differences in BDA labelling were observed (P = 0.77; Fig 5B-D), confirming similar tracing efficiency. P-ase-treated animals possessed few BDA-labelled CST collaterals in the gray matter of the treated side (Fig 5E). In contrast, ChABC-treatment significantly increased CST collateral sprouting on the treated side (P = 0.02; Fig 5F, G). Furthermore, the distance of uninjured CST collaterals sprouting from the midline revealed a strong trend for ChABC to encourage more axons to sprout at longer distances compared to P-ase [F(1,6) = 4.85, P = 0.07; Fig 5H]. In ChABC-treated animals, it was also observed that some BDA-positive axons were sprouting within areas of CSPG degradation (Fig 5I).
Figure 5. ChABC induces cervical collateral sprouting from the intact CST following stroke.
(A) Schematic illustrating that BDA injections were administered into the uninjured hemisphere to label projections from the intact CST. (B, C) Photomicrographs of BDA-positive fibres present in the dorsal column of the cervical spinal cord in P-ase-treated animals (B) and ChABC-treated animals (C). (D) Quantification of these fibres revealed no significant difference between treatments groups, confirming similar tracing efficiency between animals. (E, F) Photomicrographs of BDA-positive axons projecting to the denervated side of the spinal cord in P-ase-treated animals (E) and ChABC-treated animals (F). Dashed lines indicate the spinal cord midline and arrow-heads highlight axons present on the treated side of the spinal cord. (G) Quantification revealed that animals treated with ChABC showed a significant increase in the number of BDA-labelled axons present on the treated side compared to P-ase treated animals. (H) Mediolateral spatial distribution of BDA-positive fibres throughout the treated side of the spinal cord in ChABC-treated animals compared to P-ase-treated animals. (I) Example of axons (red) in areas of CSPG digestion (green). Results are presented as mean ± SEM and were analysed using Kruskal-Wallis and Mann Whitney tests (D, G) or RM ANOVA (H). Significance is denoted as: * P < 0.05 between treatment groups (n = 4 per group). Scale bars: 100 µm (B, C, E, F).
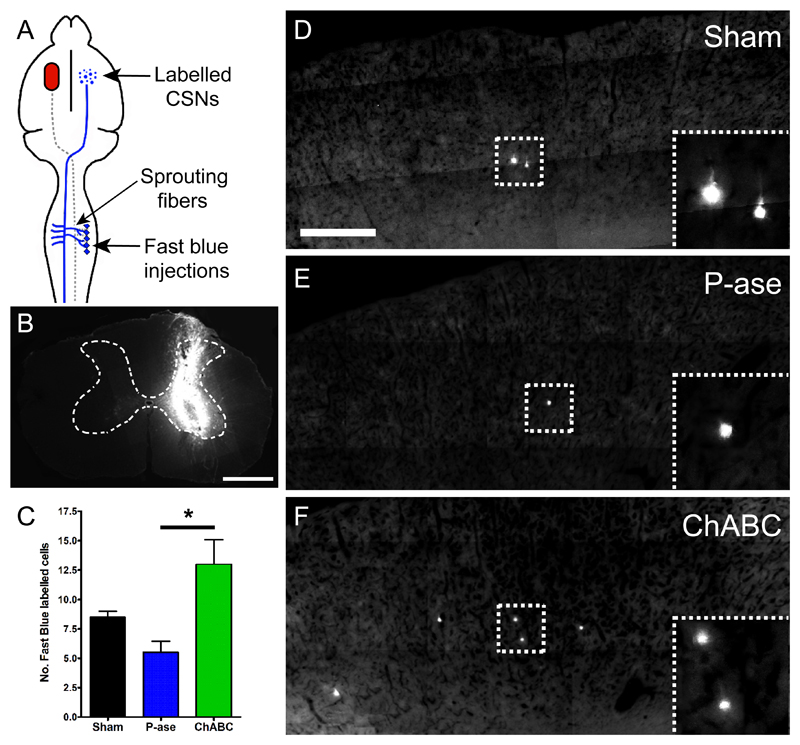
In our second experiment, Fast Blue was used to further assess CST collateral sprouting by retrogradely labelling CSNs of the uninjured CST (Fig 6A): Fast Blue was carefully and unilaterally injected into ChABC-treated areas of the cervical gray matter (Fig 6B). We quantified the number of CSNs present in the intact sensorimotor cortex that projected collaterals into these ChABC-treated areas. Labelled CSNs were primarily located rostral to Bregma, particularly from the primary motor (M1) and somatosensory forelimb (S1FL) cortical regions. Sham and P-ase treated animals displayed similar numbers of Fast Blue-labelled CSNs (P = 0.13; Fig 6C-E). However, ChABC-treated animals possessed significantly greater numbers of labelled CSNs in the sensorimotor cortex (Fig 6C, F) compared to P-ase-treated animals (p=0.03). Together these results demonstrate that ChABC is able to induce plasticity and cause axonal collateral sprouting from the intact corticospinal tract into ChABC-treated areas of the spinal cord in aged rats.
Figure 6. ChABC increases the number of CSNs projecting to areas of denervation following stroke.
(A) Schematic illustrating the five unilateral Fast Blue injection sites which retrogradely labelled CSNs in the uninjured hemisphere that sprouted uninjured CST projections into areas of ChABC-mediated digestion. (B) Transverse C5 spinal cord sections confirmed that the Fast Blue injections were unilateral and within the gray matter of the spinal cord. (C) Quantification of the number of labelled CSNs present in the uninjured sensorimotor cortex revealed that ChABC-treatment increased the number of CSNs projecting to the denervated side of the spinal cord. (D-F) Photomicrographs of the sensorimotor cortex (Bregma +0.5 mm) showing the number of Fast Blue-labelled CSNs in sham (D), P-ase-treated (E) and ChABC-treated animals (F). Arrow-heads highlight Fast Blue-labelled CSNs in sensorimotor cortex. Results are presented as mean ± SEM and were analysed using Kruskal-Wallis and Mann Whitney tests. Significance is denoted as: * P < 0.05 between treatment groups (n = 4 per group). Scale bar: 1 mm (B), 500 µm (D-F).
Delayed ChABC does not promote neuroprotection after stroke in aged rats
We and others previously reported that surviving CSNs in the ipsilesional hemisphere following stroke display a degree of cell atrophy (Enright et al., 2007;Soleman et al., 2010). As ChABC has been shown to reverse atrophy of CSNs after spinal cord injury (Carter et al., 2008), we analysed the soma sizes of CSNs retrogradely-labelled with Fast Blue at 5 weeks post-injury (Supplementary Fig 2A). At 7 weeks post-stroke, cell atrophy was evident in P-ase-treated animals compared to sham controls, particularly for cell sizes between 150 and 300 µm2 (Supplementary Fig 2B, C, E). Following ChABC treatment, CSNs appeared to possess slightly larger-looking somata (Supplementary Fig 2D), with cell size distribution analysis revealing a trend towards significance between treatment groups for cells between 150-300 µm2 (P = 0.07). However, overall drug treatment had no significant effect on cell size distribution [F(2,6) = 0.73, P = 0.52] and cumulative frequency (P = 0.3; Supplementary Fig 2E, F). There was also no difference in the total number of surviving Fast Blue-labelled CSNs between treatment groups (P = 0.29; Supplementary Fig 2G), showing that delayed ChABC does not prevent CSN cell death in the injured hemisphere.
Additionally, histological analysis of the brain at 10 weeks after stroke revealed the extent of ischemic damage in lesioned aged rats (Supplementary Fig 3A, B). Affected areas primarily included the primary motor cortex (M1) with varying amounts of the secondary motor cortex (M2) and primary somatosensory cortex (S1, particularly the forelimb and hindlimb regions). Infarct volume analysis revealed no differences between drug treatment groups (P = 0.1; Supplementary Fig 3C), confirming both groups had similar extents of ischemic injury. Thus, our results suggest that intraspinal delivery of ChABC does not reverse CSN atrophy associated with ischemic stroke or affect infarct size and therefore we attribute recovery to plasticity of the CST and other intraspinal circuits.
Discussion
Ischemic stroke induces sustained sensorimotor impairments and limited spontaneous behavioural recovery. This was supported in our study where control-treated animals showed little improvement in behavioural function after stroke, consistent with our previous work (Soleman et al., 2010). Importantly, we show for the first time that intraspinal delivery of ChABC initiated 3 days post-stroke in an aged CNS is able to significantly reduce sensorimotor impairments of the affected forelimb, including fine motor function, postural weight support and somatosensory function. Our results extend previous work showing ChABC promotes behavioural recovery after different neurological injuries (Bradbury et al., 2002;Pizzorusso et al., 2006;Galtrey et al., 2007;Cafferty et al., 2008;Garcia-Alias et al., 2009;Alilain et al., 2011). Approximately 16 million people worldwide suffer from stroke every year (Strong et al., 2007) and there is still no restorative drug treatment beyond the acute stage of stroke. Our study proposes ChABC as a potential treatment to promote neural repair after stroke that is clinically attractive as it also counteracts existing problems of treatment time-windows and age as ChABC was administered 3 days post-stroke in an elderly CNS system.
Human and animals studies have shown that the adult CNS is capable of some functional reorganisation which is associated with the limited spontaneous recovery observed following stroke (Cramer 2008). Two main mechanisms considered for reorganizational and plastic changes after stroke are: (1) formation of new neuronal circuitry and (2) unmasking/strengthening of existing pathways. In the present study, ChABC was administered into the denervated side of the spinal cord to encourage localized plasticity of spinal circuitry and limit the extent of ChABC-mediated digestion throughout the neuraxis, thus minimising effects on other brain regions. As delivery of ChABC was spatially restricted this suggests recovery was possibly a consequence of enhanced plasticity within local spinal circuitry. It is likely that ChABC stimulated anatomical changes in a number of spinal pathways that may have contributed to the observed functional improvements. However, here we anatomically traced and primarily focused on the intact corticospinal tract, a major pathway known to modulate both sensory and motor function. Our results demonstrate that ChABC is able to enhance plastic changes and axonal sprouting of the intact CST from the contralesional hemisphere. This is consistent with other stroke studies that have enhanced anatomical sprouting of uninjured spinal pathways from the contralesional hemisphere and consequently improved recovery of function (Chen et al., 2002;Papadopoulos et al., 2002;Lee et al., 2004;Markus et al., 2005). Other human and animal studies have also shown that the contralesional hemisphere is involved in recovery following stroke, including changes in dendritic arborisation (Jones and Schallert 1992;Biernaskie and Corbett 2001;Uryu et al., 2001) and enhanced cortical activation (Dijkhuizen et al., 2003;Schaechter and Perdue 2008). It has been reported that stroke induces re-organization in two regions of the cortex: an area immediately adjacent to the infarct core that has a substantial increase in CSPGs, and a more distant area from the infarct that has reduced levels of CSPG and a loss of PNNs that contributes to anatomical plasticity (Carmichael et al., 2005). Whether further digestion of CSPGs in these peri-infarct regions enhances local plasticity and functional recovery has yet to be determined, but may reveal the potential for local as well as distant ChABC treatment in stroke.
Additionally, the rapid recovery of function may be attributed to the unmasking of physiologically silent synapses (Carmel et al., 2010) which may be recruited after alterations in neural activity following treatment. Recently, it has been shown that intraspinal injections of CSPG acutely depresses axonal conduction in a dose-dependent manner (Hunanyan et al., 2010) and intraspinal ChABC injections prevent the decline in axonal conduction in intact fibres after spinal cord injury (Hunanyan et al., 2010). This demonstrates an inhibitory action of CSPGs on axonal conduction and that their removal using ChABC can enhance neural activity. Thus, it may also be possible that ChABC enhanced conduction and synaptic transmission in the present study.
Another mechanism for both rapid and progressive recovery may be the removal of PNNs. In the adult spinal cord, CSPGs are distributed diffusely in both the gray and white matter extracellular matrix (Tang et al., 2003) and are also highly condensed into structures known as PNNs that surround specific neuronal cell bodies, such as the motoneurons (Takahashi-Iwanaga et al., 1998). The time of the appearance of PNNs is closely correlated with the termination of plasticity and the end of the critical period in development (Pizzorusso et al., 2002). Developmental attenuation of PNNs extends this critical period (Carulli et al., 2010) and digestion of CSPG-rich PNNs in the adult visual cortex using ChABC is known to reactivate plasticity (Pizzorusso et al., 2002), confirming that PNNs control plasticity in the CNS. A recent study has also shown that removal of PNNs around phrenic motor neurons using ChABC promotes rapid functional recovery of diaphragmatic function after spinal cord injury one week after treatment (Alilain et al., 2011). We have shown that microinjections of ChABC directly into the spinal cord produces rapid, localised and long-lasting CSPG digestion throughout both the gray and white matter, consistent with previous studies (Galtrey et al., 2007;Cafferty et al., 2008;Garcia-Alias et al., 2009). We also found ChABC effectively removed both diffuse CSPGs in the cervical spinal cord and CSPG-rich PNNs present around motoneurons. Our data indicate that removal of PNNs may be a probable mechanism through which ChABC rapidly reactivates plasticity and recovery of function.
In summary, our work is the first to demonstrate that ChABC can promote recovery of sensorimotor forelimb function after stroke and plasticity in the uninjured CNS. Excitingly, we show that recovery and neuroplasticity occurs in an elderly CNS, even when treatment is delayed by three days, and proposes ChABC as a novel therapeutic candidate in ischemic stroke for aged individuals.
Supplementary Material
Acknowledgments
We thank Gary Fulcher for assistance during behavioural testing. This work was supported by the Medical Research Council (grant number G0600998), by a Research Councils UK Academic Fellowship and by the British Pharmacological Society (BPS)’s Integrative Pharmacology Fund.
Abbreviations
- BDA
biotinylated dextran amine
- ChABC
Chondroitinase ABC
- CSPGs
chondroitin sulphate proteoglycans
- CSNs
corticospinal neurons
- CST
corticospinal tract
- P-ase
penicillinase
- PNNs
perineuronal nets
References
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, et al. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brott T, Marler JR, Olinger CP, Adams HP, Jr, Tomsick T, Barsan WG, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke. 1989;20:871–875. doi: 10.1161/01.str.20.7.871. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Slivka A, Xue D. The effect of the NMDA receptor antagonist MK-801 on cerebral blood flow and infarct volume in experimental focal stroke. Brain Res. 1992;574:171–177. doi: 10.1016/0006-8993(92)90814-p. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, et al. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28:11998–12009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil. 1993;74:602–611. doi: 10.1016/0003-9993(93)90158-7. [DOI] [PubMed] [Google Scholar]
- Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci. 2008;28:14107–14120. doi: 10.1523/JNEUROSCI.2217-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Schaechter JD, Kaplan JD, Finklestein SP. Computerized measurement of motor performance after stroke. Stroke. 1997;28:2162–2168. doi: 10.1161/01.str.28.11.2162. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo A. Human and economic burden of stroke. Age Ageing. 2009;38:4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright LE, Zhang S, Murphy TH. Fine mapping of the spatial relationship between acute ischemia and dendritic structure indicates selective vulnerability of layer V neuron dendritic tufts within single neurons in vivo. J Cereb Blood Flow Metab. 2007;27:1185–1200. doi: 10.1038/sj.jcbfm.9600428. [DOI] [PubMed] [Google Scholar]
- Fisher M, Albers GW, Donnan GA, Furlan AJ, Grotta JC, Kidwell CS, et al. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- Fisher M, Ratan R. New perspectives on developing acute stroke therapy. Ann Neurol. 2003;53:10–20. doi: 10.1002/ana.10407. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Asher RA, Nothias F, Fawcett JW. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain. 2007;130:926–939. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci. 2005;22:2069–2080. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Hunanyan AS, Garcia-Alias G, Alessi V, Levine JM, Fawcett JW, Mendell LM, et al. Role of chondroitin sulfate proteoglycans in axonal conduction in Mammalian spinal cord. J Neurosci. 2010;30:7761–7769. doi: 10.1523/JNEUROSCI.4659-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1:94–99. doi: 10.4103/0976-500X.72351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- Markus TM, Tsai SY, Bollnow MR, Farrer RG, O'Brien TE, Kindler-Baumann DR, et al. Recovery and brain reorganization after stroke in adult and aged rats. Ann Neurol. 2005;58:950–953. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L, Bakal DA, Fung TS, Farn P, Weaver LE. Tactile extinction and functional status after stroke. A preliminary investigation. Stroke. 1994;25:1973–1976. doi: 10.1161/01.str.25.10.1973. [DOI] [PubMed] [Google Scholar]
- Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex. 2008;18:638–647. doi: 10.1093/cercor/bhm096. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- Soleman S, Yip P, Leasure JL, Moon L. Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Exp Neurol. 2010;222:13–24. doi: 10.1016/j.expneurol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H, Murakami T, Abe K. Three-dimensional microanatomy of perineuronal proteoglycan nets enveloping motor neurons in the rat spinal cord. J Neurocytol. 1998;27:817–827. doi: 10.1023/a:1006955414939. [DOI] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–598. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- Uryu K, MacKenzie L, Chesselet MF. Ultrastructural evidence for differential axonal sprouting in the striatum after thermocoagulatory and aspiration lesions of the cerebral cortex in adult rats. Neuroscience. 2001;105:307–316. doi: 10.1016/s0306-4522(01)00203-2. [DOI] [PubMed] [Google Scholar]
- Ward NS. Plasticity and the functional reorganization of the human brain. Int J Psychophysiol. 2005;58:158–161. doi: 10.1016/j.ijpsycho.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M, et al. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Wong LF, Yip PK, Battaglia A, Grist J, Corcoran J, Maden M, et al. Retinoic acid receptor beta2 promotes functional regeneration of sensory axons in the spinal cord. Nat Neurosci. 2006;9:243–250. doi: 10.1038/nn1622. [DOI] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.