Abstract
Overactivation of the mammalian target of rapamycin (mTOR) has been implicated in the pathogenesis of syndromic forms of autism spectrum disorders (ASDs), such as tuberous sclerosis complex, neurofibromatosis 1, and fragile X syndrome. Administration of mTORC1 (mTOR complex 1) inhibitors (e.g. rapamycin) in syndromic mouse models of ASDs improved behavior, cognition, and neuropathology. However, since only a minority of ASDs are due to the effects of single genes (~10%), there is a need to explore inhibition of mTOR activity in mouse models that may be more relevant to the majority of nonsyndromic presentations, such as the genetically inbred BTBR T+Itpr3tf/J (BTBR) mouse model of ASDs. BTBR mice have social impairment and exhibit increased stereotypic behavior. In prior work, d-cycloserine, a partial glycineB site agonist that targets the N-methyl-d-aspartate (NMDA) receptor, was shown to improve sociability in both Balb/c and BTBR mouse models of ASDs. Importantly, NMDA receptor activation regulates mTOR signaling activity. The current study investigated the ability of rapamycin (10 mg/kg, i.p. × four days), an mTORC1 inhibitor, to improve sociability and stereotypic behavior in BTBR mice. Using a standard paradigm to assess mouse social behavior, rapamycin improved several measures of sociability in the BTBR mouse, suggesting that mTOR overactivation represents a therapeutic target that mediates or contributes to impaired sociability in the BTBR mouse model of ASDs. Interestingly, there was no effect of rapamycin on stereotypic behaviors in this mouse model.
Keywords: Rapamycin, NMDA receptor, mTOR signaling, Sociability, BTBR mice
1. Introduction
The BTBR T+Itpr3tf/J (BTBR) inbred mouse strain models impaired sociability and stereotypic behavior displayed by persons with autism spectrum disorders (ASDs) (McFarlane et al., 2008; Silverman et al., 2010; Wöhr et al., 2011; Yang et al., 2007). Recent data support a role for the mammalian target of rapamycin (mTOR) signaling pathway in the pathogenesis of impaired sociability in ASD (Crino, 2011). The mTOR signaling pathway is expressed in brain and plays a major role in brain development by regulating neuronal cell proliferation, synaptogenesis and development of dendrites and axons (Ehninger and Silva, 2011; Ehninger, 2013; Garelick and Kennedy, 2011; Sunnen et al., 2011). mTOR is a serine/threonine kinase that complexes with other proteins to form the mTOR complex 1 (mTORC1), which regulates cellular energy metabolism, cell growth and proliferation, autophagy, and affects protein synthesis and translation through downstream targets such as ribosomal protein p70S6 kinase 1 (S6K1), ribosomal S6 protein (S6), and eukaryotic translation initiation factor 4E (eIF4E) (Ehninger, 2013; Talos et al., 2012). The activity of mTORC1 is negatively regulated by the heterodimeric tuberous sclerosis complexes 1 and 2 (TSC1/2). Essentially, signaling along either PI3K/AKT or Ras/ERK leads to dissociation of the TSC1/2 complex and disinhibition of Rheb (Ras homologue enriched in brain), which, in turn, leads to activation of mTOR (Chong et al., 2012; Ehninger, 2013; Talos et al., 2012). Overactivation of mTOR is a common point of convergence in the pathogenesis of several syndromic forms of ASD. For instance, increased levels of mTOR activity are found in transgenic mice with absent expression of TSC1 in cerebellar Purkinje cells; these mutant mice show deficits of social interaction, impairments of spatial memory and cerebellar abnormalities (Tsai et al., 2012). Importantly, postnatal chronic treatment with rapamycin (6 mg/kg) improved sociability, spatial working memory, and increased the number of cerebellar Purkinje cells to levels observed in the wild type controls (Tsai et al., 2012).
NMDA receptor activation is an important regulator of mTOR signaling activity. Specifically, sustained NMDA receptor activation leads to the rapid internalization of two isoforms of the cationic amino acid transporter, resulting in diminished arginine transport into cortical neurons (Huang et al., 2007). Intraneuronal concentrations of arginine are detected by ‘nutrient sensors’ that influence mTORC1 activity (Huang et al., 2007). Thus, when intraneuronal concentrations of arginine are low, mTORC1 activity is dampened, resulting in diminished rates and amounts of protein synthesis (Huang et al., 2007). Further, NMDA receptor activation regulates the duration of signaling by the phosphorylated form of extracellular signal regulated kinase1/2 (ERK1/2), an important driver of mTORC1 activity. Specifically, NMDA receptor activation leads to the calcium ion-dependent activation of calcineurin, an important phosphatase that cleaves phosphate from and, thereby, activates ‘STriatal Enriched protein tyrosine Phosphatase (STEP)’ (Fitzpatrick and Lombroso, 2011; Paul and Connor, 2010; Paul et al., 2003). STEP is an important phosphatase enriched within anatomic brain areas that serve as important nodes within circuits necessary for sociability and cognition, including frontal cortex and hippocampus (Fitzpatrick and Lombroso, 2011). As noted above, the phosphorylated form of ERK1/2 is an important driver of mTOR signaling; NMDA receptor activation leads to dephosphorylation of ERK1/2 by STEP, which may dampen mTOR signaling activity.
Unlike transgenic knockout mouse models, whose impaired sociability may be causally related to diminished or absent expression of single major genes, the Balb/c and BTBR mouse models are particularly interesting models of ASD because their impaired sociability may reflect subtle epistatic interactions within a network of related genes, many of which may be normal polymorphisms (Cuscó et al., 2009; Levitt and Campbell, 2009). Syndromic forms of ASD due to the effects of single major genes (e.g. tuberous sclerosis, neurofibromatosis and fragile X syndrome) occur much less commonly than nonsydromic presentations. The genetic mechanisms contributing to or underlying the impaired sociability of Balb/c and BTBR mice may more closely resemble what occurs in most presentations of ASDs (Burket et al., 2013; Cuscó et al., 2009; Veenstra-Vanderweele et al., 2004). The current study investigated the ability of rapamycin (10 mg/kg, i.p.), an mTORC1 inhibitor, to improve sociability and stereotypic behavior in BTBR mice.
2. Methods
2.1. Animals
Experimentally-naïve, 4-week old male, outbred Swiss Webster (Charles River Laboratories, Wilmington, MA) and genetically inbred BTBR T+Itpr3tf/J (BTBR) test mice (Jackson Laboratories, Bar Harbor, ME) were housed 2 per cage, in hanging clear Plexiglas cages with free access to food and water, and maintained on a 12 h light/dark cycle. The stimulus mice were 4-week old male ICR mice (Charles River Laboratories, Wilmington, MA), housed 4 per cage. Housing conditions were adopted from prior literature (Sankoorikal et al., 2006). Mice were individually weighed prior to drug administration and up to 20 mice were tested in each condition (group sizes are shown in the bars of each of the figures). All animal procedures were approved by the Eastern Virginia Medical School Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Drugs
Rapamycin (LC Laboratories, Woburn, MA) was dissolved in 10% DMSO/PBS solution and stored in aliquots at −20 °C until the day of the experiment. Rapamycin (10 mg/kg or vehicle) was injected intraperitoneally (i.p.) in a volume of 0.01 ml/g of body weight on four consecutive days; sociability was tested 60 min after the last administration of rapamycin. The dose of rapamycin (10 mg/kg, i.p.) was selected based on literature describing behavioral effects of its administration to mouse models of tuberous sclerosis complex (Meikle et al., 2008; Sato et al., 2012; Tsai et al., 2012), and depression (Cleary et al., 2008).
2.3. Apparatus
The three-compartment testing apparatus consisted of a black Plexiglas rectangular box (52.07 cm × 25.40 cm × 22.86 cm), without a top or bottom. The center compartment was slightly smaller (12.07 cm × 25.40 cm) than the two end compartments that were of equal size (19.05 cm × 25.40 cm). Inverted wire cups (Galaxy Cup, Kitchen Plus) were placed in each side of the end compartments during sessions I and II (discussed below) and housed the stimulus mouse. 500 ml glass bottles were placed on top of the inverted wire cups to prevent climbing during testing. After each test mouse was studied in the sociability paradigm, the apparatus and wire cups were thoroughly cleaned with Quatricide PV solution, as required by Eastern Virginia Medical School’s Institutional Animal Care and Use Committee.
2.4. Sociability procedure
The laboratory adopted an established mouse behavioral procedure for the quantitative assessment of sociability (Brodkin, 2007; Burket et al., 2013, 2010; Crawley, 2007, 2004; Deutsch et al., 2012, 2011; Jacome et al., 2011a; Sankoorikal et al., 2006). Briefly, in the first session, a test mouse is placed in the middle compartment and allowed to acclimate to the sociability apparatus for 5 min. In the second 5-min session, a stimulus mouse is enclosed in an inverted wire cup in the side designated as the social compartment, and an empty inverted wire cup is placed in the side designated as the nonsocial compartment. The side designated for the location of the enclosed stimulus mouse is randomly assigned in a counterbalanced fashion throughout the experiment. In the third 5-min session, the stimulus mouse is released from the inverted wire cup, and the test and stimulus mice are allowed to interact freely with each other. All sessions are conducted in dim lighting and videotaped using a Sony HDR-CX560V HD Video Camera (Sony Corp., Tokyo, Japan) for future viewing and data collection.
The amount of time test mice spend in the social and nonsocial compartments, the amount of time test mice explore (sniff) within a 2-cm vicinity of the social and nonsocial inverted cups and their transitions between compartments are measured in the second 5-min session. The following measures of sociability, stereotypic behaviors and transitions between compartments are reliably obtained in the third 5-min session of free interaction between test and stimulus mice and analyzed in this report: discrete episodes of social approach; discrete episodes of social avoidance; discrete episodes of anogenital sniffing; discrete episodes of social pursuit; amount of time engaged in social pursuit; discrete episodes of rearing; discrete episodes of self-grooming; amount of time engaged in self-grooming; and number of transitions between compartments (Burket et al., 2013; Deutsch et al., 2012; Jacome et al., 2011b). Social approach is defined as a discrete episode of initiation of sniffing the social stimulus mouse by the test mouse within at least a 2-cm vicinity of each other. Social avoidance is a nonsocial response of the test mouse defined as a discrete episode of freezing, withdrawing, or turning its head away while within a 2-cm vicinity of the socially salient stimulus mouse. Anogenital sniffing is a social behavior displayed by the test mouse defined as a discrete episode of sniffing the anogenital area of the stimulus mouse. A discrete episode of social pursuit is defined as the test mouse following or chasing the socially salient stimulus mouse while they are within a 2-cm vicinity of each other; in addition to discrete episodes of social pursuit, the amount of time in seconds the test and stimulus mice remained within a 2-cm vicinity of each other after each discrete episode of pursuit was also measured (Burket et al., 2013; Deutsch et al., 2012; Jacome et al., 2011b). Rearing is defined as a discrete episode of raising forelimbs and standing on hindlimbs. Grooming is defined as the number of discrete episodes the test mouse is engaged in licking and rubbing of fur with forelimbs during the 5-min period of free social interaction; it was also measured by the amount of time in seconds the test mice spent engaged in this behavior (i.e., third 5-min session). A transition between compartments is defined as the number of times all four extremities cross between compartments, measured in all three sessions.
2.5. Statistics
A two-way ANOVA was used to examine effects of strain (BTBR versus Swiss Webster), treatment condition (i.e., rapamycin versus vehicle), and their interaction on the number of transitions between compartments; discrete episodes of social approach; discrete episodes of social avoidance; discrete episodes of anogenital sniffing; discrete episodes of social pursuit; amount of time engaged in social pursuit; discrete episodes of rearing; discrete episodes of self-grooming; and amount of time engaged in self-grooming. When ANOVA was significant, post hoc comparisons were performed with the Tukey–Kramer multiple comparison test. Paired t-tests were used to determine effects of rapamycin on the salience of the enclosed social stimulus mouse for BTBR and Swiss Webster mice. Specifically, for both the vehicle and rapamycin treatment conditions, within strain comparisons were made with respect to time spent in the compartment containing the enclosed social stimulus mouse and time spent exploring (i.e., sniffing) the inverted cup containing the social stimulus mouse. Outliers more than 2 standard deviations above or below the mean of individual outcome measures were removed from analysis; group sizes ranged from 17 to 20 mice.
3. Results
3.1. Effects on transitions between compartments
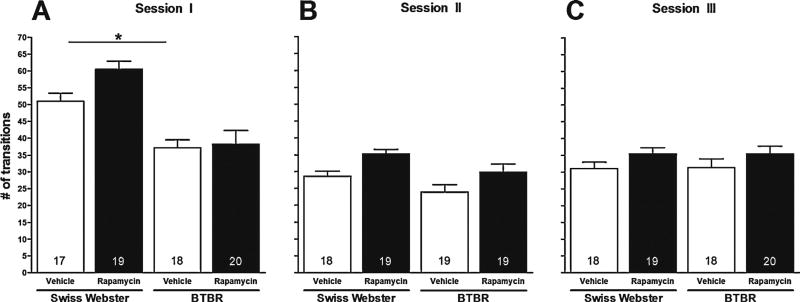
Analyses explored effects of strain, treatment condition and their interaction on this measure during acclimation in the absence of a stimulus mouse (session I), and in the presence of an enclosed (session II) and freely moving (session III) stimulus mouse. In session I, rapamycin and vehicle-treated BTBR and comparator Swiss Webster mice were allowed to acclimate to the sociability apparatus for 5 min (Fig. 1, Panel A). A two-way ANOVA showed significant main effects for strain (i.e., BTBR versus Swiss Webster; F[1, 73] = 36.39, p < 0.0001) on number of transitions between compartments (Fig. 1, Panel A). Post hoc comparisons with the Tukey–Kramer multiple comparison test confirmed that saline-treated BTBR mice made fewer transitions between compartments than saline-treated Swiss Webster mice (p < 0.05).
Fig. 1.
Effect of rapamycin on transitions between compartments. Bars represent means ± SEM of the number of transitions made between compartments by 4-week-old male Swiss Webster and BTBR mice during acclimation (A), in the presence of an enclosed 4-week-old male ICR stimulus mouse (B) and when test and stimulus mice were allowed to interact freely (C) 60 min after treatment with rapamycin (10 mg/kg, i.p.) or vehicle. *p < 0.05 significance of post hoc comparisons using the Tukey–Kramer multiple comparison test. Numbers in bars represent group sizes.
In session II, when stimulus mice were enclosed in an inverted cup, a two-way ANOVA showed a significant main effect for strain (i.e., BTBR versus Swiss Webster; F[1, 74] = 6.83, p < 0.05) and treatment condition (i.e., rapamycin versus vehicle; F[1, 74] = 10.57, p < 0.01) on number of transitions between compartments (Fig. 1, Panel B). Post hoc comparisons with the Tukey–Kramer multiple comparison test showed that treatment of BTBR and Swiss Webster mice with rapamycin had no significant effect on number of transitions between compartments (p > 0.05).
In session III, when test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed no significant main effects on number of transitions between compartments (Fig. 1, Panel C).
3.2. Effects on the salience of the social stimulus mouse (session II)
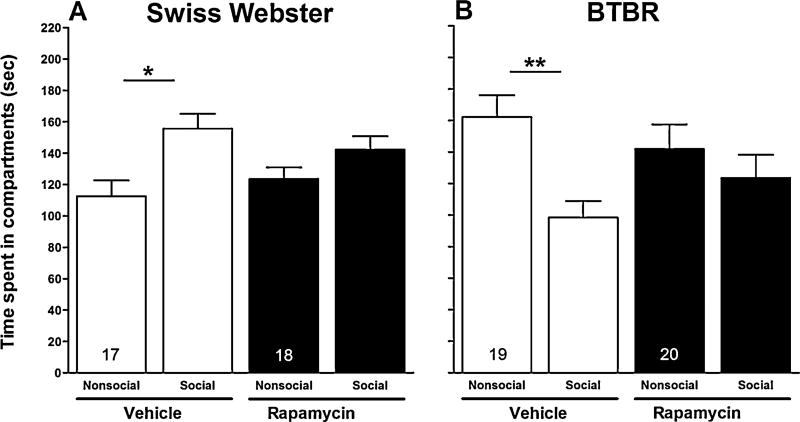
Paired t-tests were used to determine effects of rapamycin on the salience of the enclosed social stimulus mouse for BTBR and Swiss Webster mice. For both the vehicle and rapamycin treatment conditions, within-strain comparisons were made with respect to time spent in the compartment containing the enclosed social stimulus mouse and time spent exploring (i.e., sniffing) the enclosed social stimulus mouse. As expected, vehicle-treated Swiss Webster mice spent more time in the compartment containing the enclosed social stimulus mouse (155.65 ± 9.4 [SEM]) than the compartment containing the empty inverted cup (112.59 ± 10.1 [SEM]; p < 0.05) (Fig. 2, panel A), whereas the vehicle-treated BTBR mice spent significantly less time in the compartment containing the enclosed social stimulus mouse (98.42 ± 10.5 [SEM]) than the compartment containing the empty inverted cup (162.21 ± 13.8 [SEM]; p < 0.01) (Fig. 2, panel B). These data suggest that the social stimulus mouse lacked social salience for the 4-week old BTBR mice. Treatment with rapamycin had no significant effect on the amount of time either Swiss Webster or BTBR mice spent in the compartment containing the enclosed social stimulus mouse.
Fig. 2.
Effect of rapamycin on time spent in social and nonsocial compartments in session II. Bars represent means ± SEM of time spent (s) in the social and nonsocial compartments 60 min after treatment with rapamycin (10 mg/kg, i.p.) or vehicle in Swiss Webster (A) and BTBR (B) mice. *p < 0.05 and **p < 0.01 compare time spent in social and nonsocial compartments within groups. Numbers in bars represent group sizes.
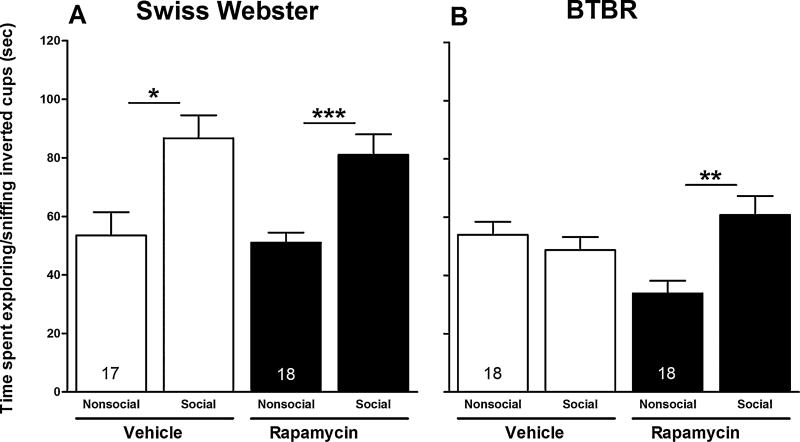
Further, as expected, vehicle-treated Swiss Webster mice spent significantly more time exploring (sniffing) the enclosed social stimulus mouse (86.71 ± 7.8 [SEM]) than the empty inverted cup (53.47 ± 7.9 [SEM]; p < 0.05) (Fig. 3, panel A), whereas vehicle-treated BTBR mice showed no preference for exploring (sniffing) the enclosed social stimulus mouse over the empty inverted cup (Fig. 3, panel B). However, treatment of BTBR mice with rapamycin resulted in significantly increased social salience of the enclosed social stimulus mice for this mouse strain (60.78 ± 6.5 [SEM]), compared to the empty inverted cup (33.83 ± 4.4 [SEM]; p < 0.01) (Fig. 3, panel B).
Fig. 3.
Effect of rapamycin on time spent exploring/sniffing social and nonsocial inverted cups in session II. Bars represent means ± SEM of time spent exploring (sniffing) the social and nonsocial inverted cups 60 min after treatment with rapamycin (10 mg/kg, i.p.) or vehicle in Swiss Webster (A) and BTBR (B) mice. *p < 0.05, **p < 0.01 and ***p < 0.001 compare time spent exploring (sniffing) social and nonsocial inverted cups within groups. Numbers in bars represent group sizes.
3.3. Effects on measures of sociability and stereotypic behavior during free interaction between test and stimulus mice (session III)
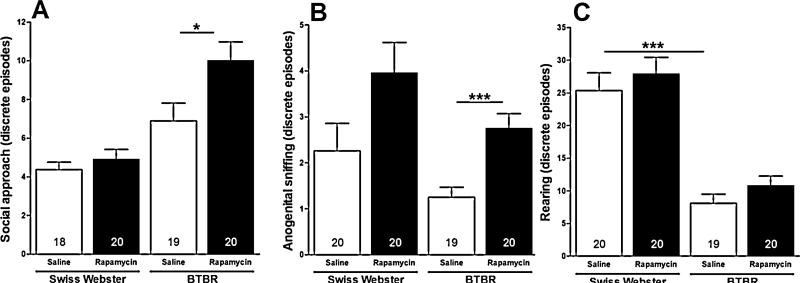
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined social approach during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed significant main effects for strain (i.e., BTBR versus Swiss Webster; F[1, 78] = 26.29, p < 0.0001) and treatment condition (i.e., rapamycin versus vehicle; F[1, 78] = 5.95; p < 0.05) on discrete episodes of social approach made by the test mice (Fig. 4A). Post hoc comparisons with the Tukey–Kramer multiple comparison test showed that treatment of BTBR mice with rapamycin (10 mg/kg, i.p.) increased their discrete episodes of social approach, relative to vehicle-treated BTBR mice (p < 0.05).
Fig. 4.
Effect of rapamycin on measures of sociability and stereotypic behavior in session III. Bars represent means ± SEM of the number of discrete episodes of social approach (A), discrete episodes of anogenital sniffing (B), and discrete episodes of rearing (C) made by 4-week-old male BTBR and Swiss Webster mice when test and stimulus mice were allowed to interact freely. Mice were treated with rapamycin (10 mg/kg, i.p.) or vehicle 60 min before testing in the sociability apparatus. *p < 0.05 and ***p < 0.001 significance of post hoc comparisons using the Tukey–Kramer multiple comparison test. Numbers in bars represent group sizes.
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined anogenital sniffing during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for treatment condition (F[1, 78] = 17.06; p < 0.001) on number of discrete episodes of anogenital sniffing (Fig. 4B). Post hoc comparisons with the Tukey–Kramer multiple comparison test showed that treatment of BTBR mice with rapamycin increased their discrete episodes of anogenital sniffing, relative to saline-treated BTBR mice (p < 0.001).
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined rearing during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for strain (F[1, 78] = 67.77; p < 0.0001) on number of discrete episodes of rearing (Fig. 4C). Post hoc comparisons with the Tukey–Kramer multiple comparison test showed that vehicle-treated BTBR mice displayed significantly fewer discrete episodes of rearing, compared to vehicle-treated Swiss Webster mice (p < 0.001).
Two-way ANOVA showed no significant main effects on the following measures of sociability and stereotypic behaviors obtained in session III: discrete episodes of social avoidance; discrete episodes of social pursuit; amount of time engaged in social pursuit; discrete episodes of self-grooming; and amount of time engaged in self-grooming (data not shown).
4. Discussion
As seen in the data on locomotor activity in sessions II and III, the impaired sociability of the BTBR mouse strain is not an epiphenomenon of a relative reduction of this strain’s locomotor activity. Importantly, measures of sociability obtained in the standard three-compartment apparatus are dependent on locomotor activity, and reduced locomotor activity can confound interpretation of sociability data (Benson et al., 2013). Specifically, in comparison to the Swiss Webster strain, the stimulus mouse lacked social salience for the BTBR strain, as evidenced by the fact BTBR mice do not spend more time in the compartment containing the enclosed stimulus mouse and more time exploring (sniffing) the enclosed stimulus mouse, relative to the empty inverted cup. Thus, the BTBR mouse is a good model of impaired sociability (i.e., decreased motivation to explore conspecifics). Rapamycin, an inhibitor of the mTORC1 complex significantly increased the amount of time the BTBR mouse spent engaged in active exploration (sniffing) of the enclosed ICR stimulus mouse. Further, consistent with this prosocial effect of rapamycin on the BTBR mouse strain observed in session II, it significantly increased the numbers of discrete episodes of social approach and anogenital sniffing made by BTBR mice when they were allowed to interact freely with the ICR stimulus mouse in session III. Interestingly, although the BTBR mouse displays impaired sociability on several objective outcome measures, this mouse strain did not manifest more intensive stereotypic behaviors (i.e., rearing and self-grooming) during its free interaction with the ICR stimulus mouse in session III, compared to the Swiss Webster strain. We measure stereotypic behaviors emerging during free interaction in session III because their emergence during social interactions in patients with ASDs can be disruptive in social settings (Campbell et al., 1990; Goldman et al., 2009). These latter data on stereotypic behaviors in session III suggest that the symptom domains of impaired sociability and stereotypic behaviors may be dissociable and mediated by different neural circuits (Burket et al., 2013; Jacome et al., 2011b). Importantly, rapamycin did not worsen stereotypic behaviors in the BTBR mouse, supporting development of anti-mTOR pharmacotherapeutic strategies for ASD; an ideal medication for ASDs should not worsen stereotypic behaviors while improving sociability and vice versa.
The data contribute to an emerging literature supporting translational exploration of pharmacological strategies for mTOR inhibition in both syndromic and nonsyndromic forms of ASDs (Crino, 2011; Ehninger, 2013; Meikle et al., 2008; Sharma et al., 2010; Spilman et al., 2010; Talos et al., 2012; Tsai et al., 2012). Most clearly, this strategy appears effective for the syndromic forms of ASDs associated with overactivation of mTORC1, such as tuberous sclerosis complex (Ehninger and Silva, 2011; Ehninger, 2013; Meikle et al., 2007, 2008; Tsai et al., 2012). However, there are concerns about the effects of chronic administration of selective mTOR inhibitors (e.g., immunosuppression), which may be required in chronic neurodevelopmental disorders, such as syndromic and nonsyndromic forms of ASDs. Thus, there is interest in less-toxic alternative strategies for chronic dampening of mTOR activity, including NMDA receptor activation (Fitzpatrick and Lombroso, 2011; Paul and Connor, 2010; Paul et al., 2003). Alternatively, rapamycin may have a therapeutic role in a novel treatment paradigm characterized by its as-needed administration prior to exposure to predictable social stressors. Moreover, development of less-toxic analogs of rapamycin and low-doses of dual inhibitors of the Ras and PI3K signaling pathways may be more suitable for chronic dosing paradigms in patients with ASDs.
In any event, the current results encourage further exploration of strategies to inhibit mTOR signaling activity for the treatment of ASDs and are consistent with a role for dysregulated mTOR signaling in the pathogenesis of impaired sociability, a major symptom domain of ASDs.
Acknowledgments
The authors acknowledge the support they received from the Office of the Dean of Eastern Virginia Medical School, a Research Enhancement Grant from Eastern Virginia Medical School, and a grant from the Commonwealth Health Research Board of the Commonwealth of Virginia.
Footnotes
Conflict of interest
The authors have no financial conflicts of interest to disclose.
References
- Benson AD, Burket JA, Deutsch SI. Balb/c mice treated with d-cycloserine arouse increased social interest in conspecifics. Brain Res. Bull. 2013;99C:95–99. doi: 10.1016/j.brainresbull.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav. Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Burket JA, Herndon AL, Deutsch SI. Locomotor activity of the genetically inbred Balb/c mouse strain is suppressed by a socially salient stimulus. Brain Res. Bull. 2010;83:255–256. doi: 10.1016/j.brainresbull.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. d-Cycloserine improves sociability in the BTBR T+Itpr3tf/J mouse model of autism spectrum disorders with altered Ras/Raf/ERK1/2 signaling. Brain Res. Bull. 2013;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Locascio JJ, Choroco MC, Spencer EK, Malone RP, Kafantaris V, Overall JE. Stereotypies and tardive dyskinesia: abnormal movements in autistic children. Psychopharmacol. Bull. 1990;26:260–266. [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog. Neurobiol. 2012;99:128–148. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary C, Linde JAS, Hiscock KM, Hadas I, Belmaker RH, Agam G, Flaisher-Grinberg S, Einat H. Antidepressive-like effects of rapamycin in animal models, implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res. Bull. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol. Med. 2011;17:734–742. doi: 10.1016/j.molmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Cuscó I, Medrano A, Gener B, Vilardell M, Gallastegui F, Villa O, González E, Rodríguez-Santiago B, Vilella E, Del Campo M, Pérez-Jurado LA. Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Hum. Mol. Genet. 2009;18:1795–1804. doi: 10.1093/hmg/ddp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Urbano MR, Herndon AL, Winebarger EE. Impaired sociability of the Balb/c mouse, an animal model of autism spectrum disorders, is attenuated by NMDA receptor agonist interventions: clinical implications. In: Mohammadi M-R, editor. A Comprehensive Book on Autism Spectrum Disorders. InTech. 2011. http://dx.doi.org/10.5772/18613, Available from: http://www.intechopen.com/books/a-comprehensive-book-on-autism-spectrum-disorders/impaired-sociability-of-the-balb-c-mouse-an-animal-model-of-autism-spectrum-disorders-is-attenuated-
- Deutsch SI, Pepe GJ, Burket JA, Winebarger EE, Herndon AL, Benson AD. d-Cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res. 2012;1439:96–107. doi: 10.1016/j.brainres.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Ehninger D. From genes to cognition in tuberous sclerosis: implications for mTOR inhibitor-based treatment approaches. Neuropharmacology. 2013;68:97–105. doi: 10.1016/j.neuropharm.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ. Rapamycin for treating tuberous sclerosis and autism spectrum disorders. Trends Mol. Med. 2011;17:78–87. doi: 10.1016/j.molmed.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Lombroso PJ. The role of striatal-enriched protein tyrosine phosphatase (STEP) in cognition. Front. Neuroanat. 2011;5:47. doi: 10.3389/fnana.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick MG, Kennedy BK. TOR on the brain. Exp. Gerontol. 2011;46:155–163. doi: 10.1016/j.exger.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Wang C, Salgado MW, Greene PE, Kim M, Rapin I. Motor stereotypies in children with autism and other developmental disorders. Dev. Med. Child Neurol. 2009;51:30–38. doi: 10.1111/j.1469-8749.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J. Neurosci. 2007;27:449–458. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Cannon WR, Deutsch SI. d-serine improves dimensions of the sociability deficit of the genetically-inbred Balb/c mouse strain. Brain Res. Bull. 2011a;84:12–16. doi: 10.1016/j.brainresbull.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. Genetically inbred Balb/c mice differ from outbred Swiss Webster mice on discrete measures of sociability: relevance to a genetic mouse model of autism spectrum disorders. Autism Res. 2011b;4:393–400. doi: 10.1002/aur.218. [DOI] [PubMed] [Google Scholar]
- Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J. Clin. Invest. 2009;119:747–754. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J. Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B–NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J. Neurochem. 2010;114:1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat. Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat. Commun. 2012;3(1292) doi: 10.1038/ncomms2295. http://dx.doi.org/10.1038/ncomms2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS ONE. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annu. Rev. Genomics Hum. Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int. J. Dev. Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]