Abstract
The genetically inbred BTBR T+ Itpr3tf/J (BTBR) mouse is a proposed model of autism spectrum disorders (ASDs). Similar to several syndromic forms of ASDs, mTOR activity may be enhanced in this mouse strain as a result of increased Ras signaling. Recently, d-cycloserine, a partial glycineB site agonist that targets the NMDA receptor, was shown to improve the sociability of the Balb/c mouse strain, another proposed genetically inbred model of ASDs. NMDA receptor activation is an important regulator of mTOR signaling activity. Given the ability of d-cycloserine to improve the sociability of the Balb/c mouse strain and the regulatory role of the NMDA receptor in mTOR signaling, we wondered if d-cycloserine would improve the impaired sociability of the BTBR mouse strain. d-Cycloserine (320 mg/kg, ip) improved measures of sociability in a standard sociability paradigm and spontaneous grooming that emerged during social interaction with an ICR stimulus mouse in the BTBR strain; however, similar effects were observed in the Swiss Webster comparator strain, raising questions about their strain-selectivity. Importantly, the profile of d-cycloserine's effects on both measures of sociability and stereotypies is consistent with that of a desired medication for ASDs; specifically, a desired medication would not improve sociability at the expense of worsening stereotypic behaviors or vice versa.
Keywords: d-Cycloserine, NMDA receptor, Sociability, Stereotypies, BTBR mice
1. Introduction
The genetically inbred BTBR T+ Itpr3tf/J (BTBR) mouse displays impaired sociability (i.e., decreased social interaction with con-specifics) and is a proposed model of autism spectrum disorders (ASDs) (McFarlane et al., 2008; Scattoni et al., 2011, 2008; Silverman et al., 2010; Wöhr et al., 2011). The activity of the mammalian target of rapamycin (mTOR) complex I (mTORC1) is regulated by Ras and PI3K signaling pathways and this signaling intermediate is located at a point of convergence of these two intracellular signaling pathways; mTOR is a serine/threonine kinase that controls translation of mRNA in the periphery of neurons as part of mTORC1, among its other functions (Cleary et al., 2008; Crino, 2011; Talos et al., 2012). Interestingly, several syndromic forms of ASDs due to the effects of single major genes (e.g., tuberous sclerosis complex and fragile X syndrome) are associated with overactivation of mTOR signaling (Ehninger and Silva, 2011; Sahin, 2012; Sharma et al., 2010). Importantly, the BTBR mouse strain has upregu-lated Ras/Raf/ERK1/2 signaling, which governs activity of mTORC1 (Yang et al., 2011; Zou et al., 2011). Specifically, relative to the B6 comparator strain, BTBR mice showed increased expression of immunoreactive Ras protein in frontal cortex and cerebellum (Zou et al., 2011). Moreover, the relative expression of immunoreactive phosphorylated Raf kinases was increased in the frontal cortex (i.e., A-Raf, B-Raf, and C-Raf) and cerebellum (i.e., C-Raf) of the BTBR mouse strain, relative to the B6 strain, whereas the immunoreactive total protein content of the Raf kinases did not differ between the strains. Further, immunoreactive content of phosphorylated forms of mitogen activated protein kinase kinases (MEK1/2) and extra-cellular signal-regulated kinase (ERK1/2) were increased in frontal cortex of BTBR mice, relative to the B6 strain; again, strains did not differ in their expression of total immunoreactive protein content of MEK1/2 and ERK1/2. These data are consistent with upregulated Ras/Raf/ERK1/2 signaling in the BTBR mouse model of ASDs, which is an important driver of mTORC1 activity (Zou et al., 2011).
In prior work, d-cycloserine, a partial glycineB site agonist that targets the N-methyl-d-aspartate (NMDA) receptor, was shown to improve the sociability of the Balb/c mouse strain, another proposed genetic inbred model of ASDs (Deutsch et al., 2012, 2011a, b; Jacome et al., 2011b). The Balb/c mouse strain is known to have functional alteration of its endogenous tone of NMDA receptor-mediated neurotransmission; specifically, this strain is more sensitive to antagonism of electrically precipitated seizures and elicitation of irregular episodes of intense jumping behavior, termed “popping,” and circling behavior by MK-801 (dizocilpine), a noncompetitive NMDA receptor antagonist, than other inbred and outbred comparator strains (Burket et al., 2010a; Deutsch et al., 1998, 1997). The NMDA receptor contributes to regulation of sociability and the activity of the mTOR signaling pathway (Cuscó et al., 2009; Deutsch et al., 2011a, b; Halene et al., 2009; Huang et al, 2007; Labrie et al., 2008; Paul et al., 2003). For example, transgenic mice with diminished expression of the NR1 NMDA receptor subunit or reduced affinity of this subunit for the obligatory glycine co-agonist show impaired sociability in standard paradigms (Halene et al., 2009; Labrie et al., 2008). Also, NMDA activation regulates mTOR signaling activity by causing rapid internalization of the cationic amino acid transporters responsible for arginine transport into the neuron, and affecting the duration of ERK1/2 signaling activity (Huang et al., 2007; Paul et al., 2003). Intracellular levels of arginine are detected by a nutrient sensor that influences mTORC1 activity (Huang et al., 2007); also, NMDA activation leads to dephosphorylation of ERK1/2, which terminates its activation of mTORC1 (Paul et al, 2003). Given the impaired sociability of the BTBR strain and the regulatory role of NMDA activation in both sociability and mTOR signaling in mice, we wondered if d-cycloserine would affect the sociability of the BTBR strain, a strain with known alterations of Ras/Raf/ERK1/2 signaling activity (Yang et al., 2011; Zou et al., 2011). The ability of d-cycloserine to improve the sociability of the BTBR mouse strain would lend further support to exploration of targeted NMDA receptor agonist interventions for the treatment of ASDs, whose possible mechanism(s) of prosocial effects could include an effect on mTOR signaling activity.
2. Methods
2.1. Animals
Experimentally naïve, 4-week-old male, outbred Swiss Webster (Charles River Laboratories, Wilmington, MA) and genetically inbred BTBR T+ Itpr3tf/J (BTBR) test mice (Jackson Laboratories, Bar Harbor, ME) were housed 2 per cage, in hanging clear Plexiglas cages with free access to food and water, and maintained on a 12 h light/dark cycle. The stimulus mice were 4-week-old male ICR mice (Charles River Laboratories, Wilmington, MA), housed 4 per cage. Housing conditions were adopted from prior literature (Sankoorikal et al., 2006). Mice were individually weighed prior to drug administration and up to 20 mice were tested in each condition (group sizes are shown in the bars of each of the figures). All animal procedures were approved by the Eastern Virginia Medical School Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Drugs
d-Cycloserine (Sigma–Aldrich Co.; St. Louis, MO) was dissolved in 0.9% saline and prepared each day of the experiment. d-Cycloserine (320 mg/kg or saline) was injected intraperitoneally in a volume of 0.01 ml/g of body weight 20 min prior to testing sociability. As we previously reported, the dose of d-cycloserine (320 mg/kg, ip) was selected based on earlier dose-response data, its reliable demonstration of prosocial effects in 4- and 8-week-old male Balb/c mice on a large variety of sociability outcome measures, and literature pertaining to its half-life, clearance from plasma and uptake into brain (Deutsch et al., 2012, 2011a, b; Wlaź et al., 1994).
2.3. Apparatus
The three-compartment testing apparatus consisted of a black Plexiglas rectangular box (52.07 cm × 25.40 cm × 22.86 cm), without a top or bottom. The center compartment was slightly smaller (12.07 cm × 25.40 cm) than the two end compartments that were of equal size (19.05 cm × 25.40 cm). Inverted wire cups (Galaxy Cup, Kitchen Plus, http://www.kitchen-plus.com) were placed in each side of the end compartments during sessions I and II (discussed below) and housed the stimulus mouse. 500 ml glass bottles were placed on top of the inverted wire cups to prevent climbing during testing. After each test mouse was studied in the sociability paradigm, the apparatus and wire cups were thoroughly cleaned with Quatricide PV solution, as required by Eastern Virginia Medical School's Institutional Animal Care and Use Committee.
2.4. Sociability procedure
The laboratory adopted an established mouse behavioral procedure for the quantitative assessment of sociability (Brodkin, 2007; Crawley, 2007, 2004; Deutsch et al., 2012, 2011a, b; Jacome et al., 2011a; Sankoorikal et al., 2006). Briefly, in the first session, a test mouse is placed in the middle compartment and allowed to acclimate to the sociability apparatus for 5 min. In the second 5-min session, a stimulus mouse is enclosed in an inverted wire cup in the side designated as the social compartment, and an empty inverted wire cup is placed in the side designated as the nonsocial compartment. The side designated for the location of the enclosed stimulus mouse is randomly assigned in a counterbalanced fashion throughout the experiment. In the third 5-min session, the stimulus mouse is released from the inverted wire cup, and the test and stimulus mice are allowed to interact freely with each other. All sessions are conducted in dim lighting and videotaped using a Sony HDR-CX560V HD Video Camera (Sony Corp., Tokyo, Japan) for future viewing and data collection.
The amount of time test mice spend in the social and nonsocial compartments, the amount of time test mice explore (sniffing) within a 2-cm vicinity of the social and nonsocial inverted cups and their transitions between compartments are measured in the second 5-min session. The following measures of sociability, stereotypic behaviors and transitions between compartments are reliably obtained in the third 5-min session of free interaction between test and stimulus mice and analyzed in this report: discrete episodes of social approach; amount of time engaged in social approach; discrete episodes of social avoidance; discrete episodes of anogenital sniffing; discrete episodes of social pursuit; amount of time engaged in social pursuit; discrete episodes of rearing; discrete episodes of self-grooming; amount of time engaged in self-grooming; and number of transitions between compartments (Jacome et al., 2011c). Social approach is defined as a discrete episode of initiation of sniffing the social stimulus mouse by the test mouse within at least a 2-cm vicinity of each other; social approach was also measured by the amount of time in seconds the test and stimulus mice interacted within a 2-cm vicinity of each other after each discrete episode of social approach. Social avoidance is a nonsocial response of the test mouse defined as a discrete episode of freezing, withdrawing, or turning its head away while within a 2-cm vicinity of the socially salient stimulus mouse. Anogenital sniffing is a social behavior displayed by the test mouse defined as a discrete episode of sniffing the anogenital area of the stimulus mouse within a 2-cm vicinity. A discrete episode of social pursuit is defined as the test mouse following or chasing the socially salient stimulus mouse while they are within a 2-cm vicinity of each other; in addition to discrete episodes of social pursuit, the amount of time in seconds the test and stimulus mice remained within a 2-cm vicinity of each other during each discrete episode of pursuit was also measured (Jacome et al., 2011c) Rearing is defined as a discrete episode of raising forelimbs and standing on hindlimbs. Grooming is defined as the number of discrete episodes the test mouse is engaged in licking and rubbing of fur with fore-limbs during the 5-min period of free social interaction; it was also measured by the amount of time in seconds the test mice spent engaged in this behavior (i.e., third 5-min session). A transition between compartments is defined as the number of times all four extremities cross between compartments, measured in all three sessions.
2.5. Statistics
A two-way ANOVA was used to examine effects of strain (BTBR vs. Swiss Webster), treatment condition (i.e., d-cycloserine vs. saline), and their interaction on the number of transitions between compartments; discrete episodes of social approach; amount of time engaged in social approach; discrete episodes of social avoidance; discrete episodes of anogenital sniffing; discrete episodes of social pursuit; amount of time engaged in social pursuit; discrete episodes of rearing; discrete episodes of self-grooming; and amount of time engaged in self-grooming. When ANOVA was significant, exploratory Fisher's LSD Multiple Comparison Tests were applied in post hoc comparisons, where appropriate. Thereafter, when Fisher's LSD tests were significant, more conservative Tukey–Kramer Multiple Comparison Tests were also conducted. Paired t-tests were used to determine effects of d-cycloserine on the salience of the enclosed social stimulus mouse for BTBR and Swiss Webster mice. Specifically, for both the saline and d-cycloserine treatment conditions, within strain comparisons were made with respect to time spent in the compartment containing the enclosed social stimulus mouse and time spent exploring (i.e., sniffing) the inverted cup containing the social stimulus mouse.
3. Results
3.1. Effects on transitions between compartments
Analyses explored effects of strain, treatment condition and their interaction on this measure during acclimation in the absence of a stimulus mouse (session I), and in the presence of an enclosed (session II) and freely moving (session III) stimulus mouse. In session I, d-cycloserine and saline-treated BTBR and comparator Swiss Webster mice were allowed to acclimate to the sociability apparatus for 5 min (Fig. 1, panel A). A two-way ANOVA showed significant main effects for strain (i.e., BTBR versus Swiss Webster; F[1,77] =32.56, p < 0.0001) and treatment condition (i.e., d-cycloserine versus saline; F[1,77] = 21.67, p < 0.0001) on number of transitions between compartments (Fig. 1, panel A). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that saline-treated BTBR mice (31.0 ± 3.41 [SEM]) made fewer transitions between compartments than saline-treated Swiss Webster mice (50.05 ± 3.33 [SEM]) (p < 0.001). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their number of transitions between compartments (46.65 ± 2.60 [SEM] and 64.55 ± 3.55 [SEM], respectively), relative to saline-treated BTBR (p < 0.01) and Swiss Webster mice (p < 0.01), respectively. The number of transitions between compartments did not differ between d-cycloserine-treated BTBR mice and saline-treated Swiss Webster mice (p >0.05). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that saline-treated BTBR mice made fewer transitions between compartments than saline-treated Swiss Webster mice (p < 0.001). Post hoc comparisons with the Tukey–Kramer Multiple Comparison Test also confirmed that treatment of BTBR mice with d-cycloserine (320 mg/kg, ip) increased their number of transitions between compartments, relative to saline-treated BTBR mice (p < 0.01). Moreover, using the Tukey–Kramer Multiple Comparison Test, the number of transitions between compartments did not differ between d-cycloserine-treated BTBR and saline-treated Swiss Webster mice (p >0.05). The data show that, irrespective of strain and in the absence of a salient stimulus mouse, d-cycloserine increases transitions between compartments. For session I only, in the absence of the enclosed or freely moving social stimulus mouse, transitions between compartments may serve as a surrogate measure of locomotor activity.
Fig. 1.
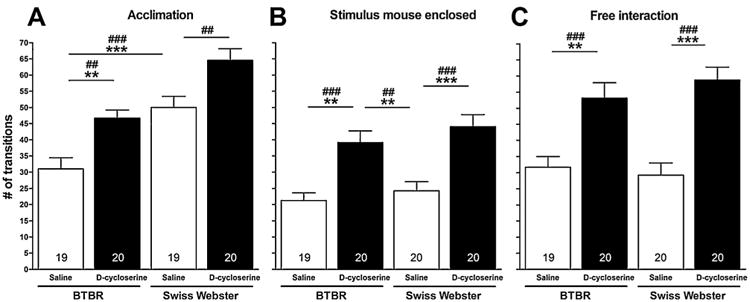
Effect of d-cycloserine on transitions between compartments. Bars represent means ± SEM of the number of transitions made between compartments by 4-week-old male BTBR and Swiss-Webster mice during acclimation (A), in the presence of an enclosed 4-week-old male ICR stimulus mouse (B) and when test and stimulus mice were allowed to interact freely (C) 20 min after treatment with d-cycloserine (320 mg/kg, ip) or saline. ##p < 0.01 and ###p < 0.001 significance of post hoc comparisons using the Fisher's LSD Multiple Comparison Test. **p < 0.01 and ***p < 0.001 significance of post hoc comparisons using the Tukey–Kramer Multiple Comparison Test. Numbers in bars represent group sizes.
In session II, when stimulus mice were enclosed in an inverted cup, a two-way ANOVA showed a significant main effect for treatment condition (i.e., d-cycloserine versus saline; F[1,78] = 34.89, p < 0.0001) on number of transitions between compartments (Fig. 1, panel B). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their number of transitions between compartments (39.15 ± 3.57 [SEM] and 44.0 ± 3.73 [SEM], respectively), relative to saline-treated BTBR and Swiss Webster mice (21.26 ± 2.39 [SEM] and 24.25 ± 2.78 [SEM]), respectively (p < 0.001). d-Cycloserine-treated BTBR mice made a significantly greater number of transitions between compartments in session II than saline-treated Swiss Webster mice, as shown with both the Fisher's LSD Multiple Comparison Test (p < 0.01) and the Tukey–Kramer Multiple Comparison Test (p < 0.01). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their number of transitions between compartments, relative to saline-treated BTBR (p < 0.01) and Swiss Webster mice (p < 0.001), respectively. Again, irrespective of strain, d-cycloserine increased transitions between compartments of BTBR and Swiss Webster mice in the presence of an enclosed salient social stimulus mouse.
In session III, when test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for treatment condition (i.e., d-cycloserine versus saline; F[1,78] = 40.33; p < 0.0001) on number of transitions between compartments (Fig. 1, panel C). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their number of transitions between compartments (53.15 ± 4.78 [SEM] and 58.75 ± 4.01 [SEM], respectively), relative to saline-treated BTBR and Swiss Webster mice (31.68 ± 3.29 [SEM] and 29.25 ± 3.75 [SEM]), respectively (p < 0.001). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their number of transitions between compartments, relative to saline-treated BTBR (p < 0.01) and Swiss Webster mice (p < 0.001), respectively. Thus, consistent with the data obtained in session III, d-cycloserine increased transitions between compartments of BTBR and Swiss Webster mice when they freely interacted with the salient social stimulus mouse.
3.2. Effects on the salience of the social stimulus mouse (session II)
Differences in time spent by test mice (i.e., BTBR and Swiss Webster) in the compartment containing an enclosed social stimulus mouse, compared to time spent in the compartment containing the empty inverted cup (Fig. 2) and differences in time spent exploring (i.e., sniffing) an enclosed social stimulus mouse, compared to time spent exploring (i.e., sniffing) the empty inverted cup (Fig. 3) are measures of the salience of the social stimulus mouse. Paired t-tests were used to determine effects of d-cycloserine on the salience of the enclosed social stimulus mouse for BTBR and Swiss Webster mice. For both the saline and d-cycloserine treatment conditions, within-strain comparisons were made with respect to time spent in the compartment containing the enclosed social stimulus mouse and time spent exploring (i.e., sniffing) the enclosed social stimulus mouse. Time spent in the social versus nonsocial compartment (Fig. 2, panel A) and time spent exploring (i.e., sniffing) the enclosed social stimulus mouse versus the empty inverted cup (Fig. 3, panel A) did not differ for the saline-treated BTBR mice (p > 0.05). These data suggest that the social stimulus mouse lacked social salience for the 4-week-old BTBR mice. However, as expected, saline-treated Swiss Webster mice spent significantly more time in the compartment containing the enclosed social stimulus mouse than the compartment containing the empty inverted cup (t = −5.119; p < 0.0001; Fig. 2, panel B) and spent more time exploring (i.e., sniffing) the enclosed social stimulus mouse than the empty inverted cup (t= −3.944; p < 0.01; Fig. 3, panel B). Importantly, treatment of BTBR mice with d-cycloserine resulted in their spending significantly more time in the compartment containing the enclosed social stimulus mouse (t = −2.597; p < 0.01; Fig. 2, panel A) and exploring (i.e., sniffing) the enclosed social stimulus mouse (t = -4.044; p < 0.001; Fig. 3, panel A). These data suggest that d-cycloserine increased the salience of the social stimulus mouse for the BTBR strain. Not surprisingly, d-cycloserine-treated Swiss Webster mice spent significantly more time in the compartment containing an enclosed social stimulus mouse (t = −5.991; p < 0.0001; Fig. 2, panel B) and significantly more time exploring (i.e., sniffing) the enclosed social stimulus mouse (t = −5.811; p < 0.0001; Fig. 3, panel B).
Fig. 2.
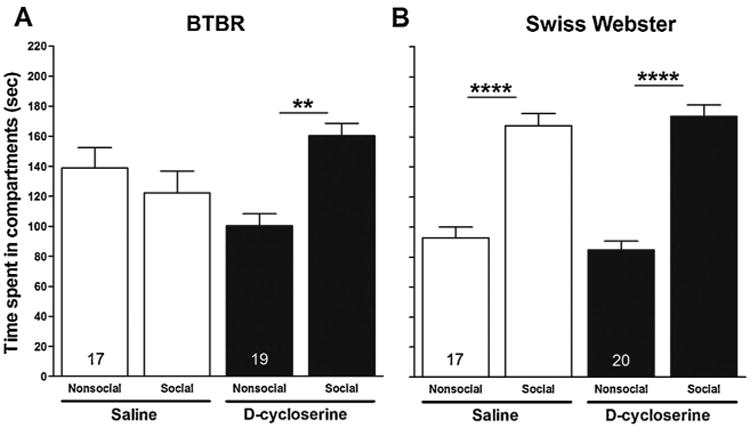
Effect of d-cycloserine on time spent in social and nonsocial compartments in session II. Bars represent means ± SEM of time spent (s) in the social and nonsocial compartments 20 min after treatment with d-cycloserine (320 mg/kg, ip) or saline in BTBR(A) and Swiss-Webster (B) mice. **p<0.01 and ****p< 0.0001 compare time spent in social and nonsocial compartments within groups. Numbers in bars represent group sizes.
Fig. 3.
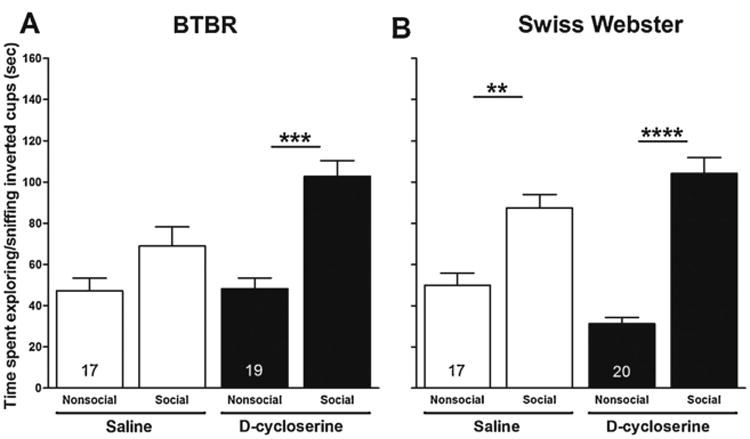
Effect of d-cycloserine on time spent exploring/sniffing social and nonsocial inverted cups in session II. Bars represent means ± SEM of time spent exploring (sniffing) the social and nonsocial inverted cups 20 min after treatment with d-cycloserine(320 mg/kg, ip) or saline in BTBR (A) and Swiss-Webster (B) mice. **p< 0.01, ***p< 0.001 and ****p < 0.0001 compare time spent exploring (sniffing) social and nonsocial inverted cups within groups. Numbers in bars represent group sizes.
3.3. Effects on measures of sociability during free interaction between test and stimulus mice (session III)
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined social approach during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for treatment condition (i.e., d-cycloserine versus saline; F[1,76] = 28.55; p < 0.0001) on discrete episodes of social approach made by the test mice (Fig. 4A). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their discrete episodes of social approach (17.35 ± 2.07 [SEM] and 15.75 ± 1.50 [SEM], respectively), relative to saline-treated BTBR and saline-treated Swiss Webster mice (8.11 ± 1.27 [SEM], p < 0.001 and 8.32±1.13 [SEM], p < 0.01), respectively. Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased their discrete episodes of social approach, relative to saline-treated BTBR (p < 0.001) and Swiss Webster (p < 0.01) mice. Very importantly, although saline-treated BTBR and saline-treated Swiss Webster mice did not differ in terms of their number of discrete episodes of social approach; the “quality” of the observed interaction between these two strains of test mice and stimulus mice differed. Unfortunately, the “quality” of the interaction was not rated in this experiment.
Fig. 4.
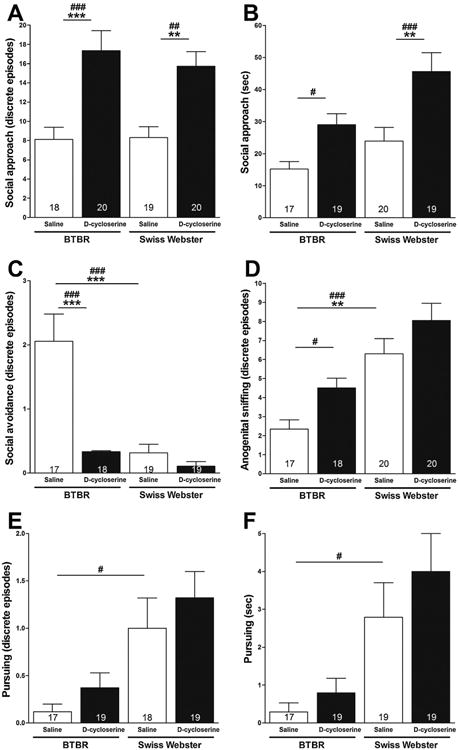
Effect of d-cycloserine on dimensions of sociability in session III. Bars represent means ± SEM of the number of discrete episodes of social approach (A), amount of time spent approaching (B), discrete episodes of social avoidance (C), discrete episodes of anogenital sniffing (D), discrete episodes of pursuit (E), and time spent pursuing (F) made by 4-week-old male BTBR and Swiss-Webster mice toward a 4-week-old male ICR stimulus mouse when test and stimulus mice were allowed to interact freely 20 min after treatment with d-cycloserine (320 mg/kg, ip) or saline. #p < 0.05, ##p < 0.01, ###p < 0.001 significance of post hoc comparisons using Fisher's LSD Multiple Comparison Test.**p < 0.01 and ***p < 0.001 significance of post hoc comparisons using the Tukey–Kramer Multiple Comparison Test. Numbers in bars represent group sizes.
Effects of strain, treatment condition and their interaction on the amount of time test mice spent engaged with the stimulus mouse after a discrete episode of social approach during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed significant main effects for strain (F[1,74] =8.77, p < 0.01) and treatment condition (F[1,74] = 17.37, p < 0.001) on the amount of time (sec) spent engaged after a discrete episode of social approach (Fig. 4B). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR and Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased the amount of time (s) test and stimulus mice remained engaged after each discrete episode of social approach (29.05 ± 3.39 [SEM] and 45.63 ± 5.86 [SEM], respectively), relative to saline-treated BTBR and saline-treated Swiss Webster mice (15.18±2.37 [SEM], p < 0.05 and 23.90 ± 4.29 [SEM], p < 0.001), respectively. Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that treatment of Swiss Webster mice with d-cycloserine (320 mg/kg, ip) increased the amount of time (s) they remained engaged with the stimulus mouse after each discrete episode of approach, relative to saline-treated Swiss Webster (p < 0.01) mice.
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined social avoidance during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed significant main effects for strain (F[1,72] = 19.42; p< 0.0001), treatment condition (F[1,72] = 18.74; p < 0.0001), and their interaction (F[1,72] = 11.47; p < 0.01) on discrete episodes of social avoidance made by the test mice (Fig. 4C). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that saline-treated BTBR mice (2.06 ± 0.42 [SEM]) made a significantly greater number of discrete episodes of social avoidance than saline-treated Swiss Webster mice (0.32±0.13 [SEM]) (p < 0.001). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test also showed that treatment of BTBR mice with d-cycloserine (320 mg/kg, ip) decreased their discrete episodes of social avoidance (0.33 ± 0.14 [SEM]), relative to saline-treated BTBR(p < 0.001). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that saline-treated BTBR mice made a significantly greater number of discrete episodes of social avoidance than saline-treated Swiss Webster mice (p < 0.001). The Tukey–Kramer Multiple Comparison Test also confirmed that treatment of BTBR mice with d-cycloserine (320 mg/kg, ip) decreased their discrete episodes of social avoidance, relative to saline-treated BTBR mice (p < 0.001).
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined anogenital sniffing during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed significant main effects for strain (F[1,74] = 27.15; p < 0.0001) and treatment condition (F[1,74] = 7.34; p < 0.01) on number of discrete episodes of anogenital sniffing (Fig. 4D). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that saline-treated BTBR mice (2.35 ± 0.47 [SEM]) made fewer discrete episodes of anogenital sniffing compared to saline-treated Swiss Webster mice (6.30 ± 0.79 [SEM]), when test and stimulus mice interacted freely (p < 0.001). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR mice with d-cycloserine increased their discrete episodes of anogenital sniffing (4.50 ± 0.52 [SEM]), relative to saline-treated BTBR mice (p < 0.05). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that saline-treated BTBR mice made fewer discrete episodes of anogenital sniffing compared to saline-treated Swiss Webster mice, when test and stimulus mice interacted freely (p < 0.01).
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined pursuit during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for strain (F[1,72] = 15.40, p < 0.001), on discrete episodes of pursuit (Fig. 4E). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that saline-treated BTBR mice (0.12±0.08 [SEM]) made fewer discrete episodes of pursuit when test and stimulus mice interacted freely than saline-treated Swiss Webster mice (1.00 ± 0.32 [SEM]) (p < 0.05).
Effects of strain, treatment condition and their interaction on the amount of time spent pursuing during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for strain (F[1,73] = 15.11, p < 0.001), on the amount of time spent pursuing (Fig. 4F). Post hoc comparisons with the Fisher's Multiple Comparison Test showed that saline-treated BTBR mice (0.29 ± 0.24 [SEM]) spent less time pursuing when test and stimulus mice interacted freely than saline-treated Swiss Webster mice (2.79±0.91 [SEM]) (p < 0.05).
3.4. Effects on stereotypic behaviors emerging spontaneously during free interaction between test and stimulus mice (session III)
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined rearing during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed significant main effects for strain (F[1,73] = 40.82, p < 0.0001), treatment condition (F[1,73] = 7.97, p < 0.01) and their interaction (F[1,73] =4.77, p < 0.05) on discrete episodes of rearing (Fig. 5A). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that saline-treated BTBR mice made fewer discrete episodes of rearing when test and stimulus mice interacted freely (6.71 ± 1.30 [SEM]) than saline-treated Swiss Webster mice (21.84 ± 2.23 [SEM]) (p < 0.001). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of Swiss Webster mice with d-cycloserine reduced their number of discrete episodes of rearing (13.0 ± 2.02 [SEM]), relative to saline-treated Swiss Webster mice (p < 0.001). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that saline-treated BTBR mice made fewer discrete episodes of rearing when test and stimulus mice interacted freely than saline-treated Swiss Webster mice (p < 0.001). Post hoc comparisons with the Tukey–Kramer Multiple Comparison Test also confirmed that treatment of Swiss Webster mice with d-cycloserine reduced their number of discrete episodes of rearing, relative to saline-treated Swiss Webster mice (p < 0.01).
Fig. 5.
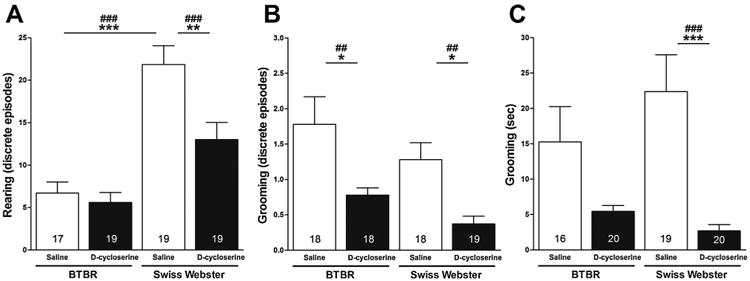
Effect of d-cycloserine on stereotypic behaviors in session III. Bars represent means ± SEM of the number of discrete episodes of rearing (A), discrete episodes of grooming (B), and time spent grooming (C) made by 4-week-old male BTBR and Swiss-Webster mice, 20 min after treatment with d-cycloserine (320 mg/kg, ip) or saline, when test and stimulus mice were allowed to interact freely. ##p < 0.01 and ###p < 0.001 significance of post hoc comparisons using Fisher's LSD Multiple Comparison Test. *p<0.05,**p < 0.01 and ***p < 0.001 significance of post hoc comparisons using the Tukey–Kramer Multiple Comparison Test. Numbers in bars represent group sizes.
Effects of strain, treatment condition and their interaction on discrete episodes of operationally defined grooming during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for treatment condition (F[1,72] = 15.88, p < 0.001) on discrete episodes of grooming (Fig. 5B). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of BTBR mice with d-cycloserine reduced their number of discrete episodes of grooming when test and stimulus mice interacted freely (0.78 ± 0.10 [SEM]), relative to saline-treated BTBR mice (1.78±0.39 [SEM]) (p < 0.01). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test also showed that treatment of Swiss Webster mice with d-cycloserine reduced their number of discrete episodes of grooming (0.37 ± 0.11 [SEM]), relative to saline-treated Swiss Webster mice (1.28 ± 0.24 [SEM]) (p < 0.01). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that treatment of BTBR mice with d-cycloserine reduced their number of discrete episodes of grooming when test and stimulus mice interacted freely, relative to saline-treated BTBR mice (p < 0.05). Post hoc comparisons with the Tukey–Kramer Multiple Comparison Test also confirmed that treatment of Swiss Webster mice with d-cycloserine reduced their number of discrete episodes of grooming, relative to saline-treated Swiss Webster mice (p < 0.05).
Effects of strain, treatment condition and their interaction on the amount of time spent grooming during session III were analyzed. When test (i.e., BTBR and Swiss Webster) and stimulus mice were allowed to interact freely, a two-way ANOVA showed a significant main effect for treatment condition (F[1,74] = 18.07, p < 0.001) on the amount of time (s) spent grooming (Fig. 5C). Post hoc comparisons with the Fisher's LSD Multiple Comparison Test showed that treatment of Swiss Webster mice with d-cycloserine reduced their time spent grooming (2.70±0.86 [SEM]), relative to saline-treated Swiss Webster mice (22.37 ± 5.21 [SEM]) (p < 0.001). Post hoc comparisons with the more conservative Tukey–Kramer Multiple Comparison Test confirmed that treatment of Swiss Webster mice with d-cycloserine reduced the amount of time spent grooming, relative to saline-treated Swiss Webster mice (p < 0.001).
4. Discussion
Similar to the genetically inbred Balb/c mouse strain, the genetically inbred BTBR mouse is a proposed model of ASDs (Brodkin, 2007; Deutsch et al., 2011a, b; Jacome et al., 2011c; McFarlane et al., 2008; Sankoorikal et al., 2006; Scattoni et al., 2011, 2008; Silverman et al., 2010; Wöhr et al., 2011; Yang et al., 2007). Interestingly, unlike the Balb/c mouse strain, the number of transitions between compartments made by the BTBR strain was not significantly suppressed in the presence of an enclosed or freely moving ICR stimulus mouse (Burket et al., 2010b; Deutsch et al., 2012). Thus, unlike the Balb/c strain, suppression of transitions between compartments in the presence of a stimulus mouse cannot be used with the BTBR strain as a reliable outcome measure of its impaired sociability (Burket et al., 2010b; Deutsch et al., 2012). However, similar to our earlier findings with the Balb/c strain, d-cycloserine increased transitions between compartments independently of strain in the absence or presence of an enclosed or freely moving stimulus mouse (Deutsch et al., 2012).
The data in session II confirmed that the enclosed male ICR stimulus mouse lacked significant social salience for the BTBR test strain. Specifically, unlike the comparator Swiss Webster strain, the saline-treated BTBR mouse did not spend more time in the compartment containing the enclosed stimulus mouse or more time exploring the enclosed stimulus mouse, relative to time spent in the nonsocial compartment or time spent exploring the empty inverted cup, respectively. However, consistent with the earlier findings with the Balb/c strain, d-cycloserine (320 mg/kg, ip) increased the salience of the stimulus mouse for the BTBR strain; these latter prosocial effects of d-cycloserine are not likely to be simply epiphenomena of d-cycloserine's effect on locomotor activity (Deutsch et al., 2012). d-Cycloserine also improved measures of sociability in the BTBR strain in session III, including discrete episodes of social approach, time spent engaged in social approach behavior, discrete episodes of social avoidance, and discrete episodes of anogenital sniffing. However, similar to our earlier findings, positive prosocial effects of d-cycloserine were observed in the Swiss Webster comparator strain. Interestingly, although BTBR mice showed deficient social pursuit as measured by both discrete episodes and time spent engaged in pursuit behaviors, there were no prosocial effects of d-cycloserine observed on these sociability outcome measures. Thus, dimensions of sociability may differ in their sensitivity to prosocial effects of d-cycloserine (Deutsch et al., 2012).
The comparator Swiss Webster strain showed more intense rearing than the BTBR strain and the two test strains did not differ with respect to the intensity of their grooming behavior, which were the two representative repetitive, stereotypic behaviors measured in this study. We were interested in measuring repetitive, stereotypic behaviors in the test mice that emerged spontaneously during their free interaction with stimulus mice and the effects of d-cycloserine on these behaviors because stereotypies observed spontaneously during social interactions can be disabling symptoms in patients with ASDs (Campbell et al., 1990; Goldman et al., 2009). Importantly, consistent with our earlier findings, d-cycloserine diminished the intensity of these stereotypic behaviors in the Swiss Webster comparator strain. d-Cycloserine also reduced discrete episodes of grooming in the BTBR strain. These data suggest that severity of impaired sociability and intensity of stereotypic behaviors may occur independently of each other (i.e., the BTBR strain with greater impairment of sociability showed less intense rearing than the Swiss Webster strain). Also, very importantly, a viable prosocial pharmacotherapeutic strategy should not worsen stereotypic behaviors at the expense of improving sociability; d-cycloserine fulfilled this latter requirement in the Balb/c and BTBR strains.
NMDA receptors are complex ligand-gated ion channels, whose channel opening requires the binding of glutamate and an obligatory co-agonist (i.e., d-serine or glycine). NMDA receptors are a heterogeneous group that differ in terms of their subunit compositions and regional localizations. Recently, the distinctions between synaptic and extrasynaptic localizations have assumed increased importance and relevance to the understanding of mechanisms underlying conditioned fear, extinction, long-term potentiation, long-term depression, neuroprotection and neurotoxicity (Haller et al, 2011; Langton and Richardson, 2010; Papouin et al, 2012; Vizi et al., 2013). d-Serine is the preferred co-agonist for synaptic NMDA receptors, whose subunit composition contains primarily GluN2A, whereas glycine is the preferred co-agonist for extrasynaptic receptors, whose subunits contain primarily GluN2B (Papouin et al., 2012; Vizi et al., 2013). Moreover, d-serine and glycine co-agonists may influence the subunit composition of functional NMDA receptors inserted into the synaptic membrane (Papouin et al., 2012; Vizi et al., 2013). Importantly, modulatory effects of exogenous lig-ands acting at the glycineB site may only be observable when this site is not saturated with either endogenous d-serine or glycine itself. Future studies should clarify whether synaptic or extrasynaptic NMDA receptors mediate prosocial effects of d-cycloserine in the BTBR and Balb/c mouse models of ASDs. For example, experiments to evaluate whether prosocial effects of d-cycloserine are mediated by extrasynaptic GluN2B-containing receptors could involve exploration of their blockade by Ro25-6981, a selective antagonist of extrasynaptic GluN2B containing receptors, or saturating levels of extrasynaptic glycine.
Similar to several syndromic forms of ASD, the BTBR mouse strain has evidence of heightened activity of Ras signaling, which may contribute to mTOR overactivity and the impaired sociability of this strain (Zou et al., 2011). d-Cycloserine's prosocial effects in the BTBR strain could be related to NMDA activation, which controls mTOR activity via regulating the cationic transport of arginine into neurons and the duration of signaling by the phosphorylated form of ERK 1/2 (Huang et al., 2007). mTOR signaling activity is regulated by nutrient sensors that detect intracellular concentrations of arginine; NMDA activation leads to rapid internalization of cationic amino acid transporters embedded in the neuron's surface membrane, resulting in lowered arginine levels and diminished mTOR signaling activity (Huang et al, 2007). Further, NMDA activation leads to a calcineurin-dependent dephosphorylation and activation of the STriatal Enriched protein tyrosine Phosphatase (STEP), an enzyme enriched in several brain areas implicated in the pathogenesis of ASDs (e.g., cerebral cortex, hippocampus and amygdala) that shortens the duration of phosphorylated ERK1/2 signaling; duration of ERK1/2 signaling is a key regulator of mTOR activity (Fitzpatrick and Lombroso, 2011; Paul and Connor, 2010; Paul et al., 2003). In any event, pathological overactivity of mTOR seems to be a converging mechanism in several neurodevelopmental disorders that are due to the effects of single major genes and associated with high prevalence of comorbid ASDs (e.g., tuberous sclerosis complex, fragile X syndrome, neurofibromatosis 1, and PTEN deficiency) (Crino, 2011; Cuscó et al., 2009; Ehninger and Silva, 2011; Sahin, 2012; Sharma et al, 2010; Talos et al., 2012; Tsai et al., 2012; Zhou et al., 2009). Importantly, in several of the mouse models of these syndromic forms of ASDs, pharmacotherapeutic strategies targeting mTOR overactivation (e.g., rapamycin) improved measures of sociability, cognition and histopathology in postnatal mice (Cleary et al., 2008; Ehninger and Silva, 2011; Talos et al., 2012; Tsai et al., 2012). Thus, there is the possibility that pharmacotherapeutic strategies that target the metabolic defects associated with increased mTOR signaling activity may have promise in translational clinical trials. Conceivably, d-cycloserine's prosocial therapeutic actions in the BTBR and, possibly, Balb/c mouse strains relate to this regulatory effect on mTOR activity.
Acknowledgments
This study was supported by a Research Enhancement Grant from Eastern Virginia Medical School (SID and AHT, principal investigators).
Footnotes
Conflict of interest: The authors declare that they have no competing financial interests.
References
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behavioural Brain Research. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Burket JA, Cannon WR, Jacome LF, Deutsch SI. MK-801 a noncompetitive NMDA receptor antagonist, elicits circling behavior in the genetically inbred Balb/c mouse strain. Brain Research Bulletin. 2010a;83:337–339. doi: 10.1016/j.brainresbull.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Burket JA, Herndon AL, Deutsch SI. Locomotor activity of the genetically inbred Balb/c mouse strain is suppressed by a socially salient stimulus. Brain Research Bulletin. 2010b;83:255–256. doi: 10.1016/j.brainresbull.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Campbell M, Locascio JJ, Choroco MC, Spencer EK, Malone RP, Kafantaris V, Overall JE. Stereotypies and tardive dyskinesia: abnormal movements in autistic children. Psychopharmacology Bulletin. 1990;26:260–266. [PubMed] [Google Scholar]
- Cleary C, Linde JAS, Hiscock KM, Hadas I, Belmaker RH, Agam G, Flaisher-Grinberg S, Einat H. Antidepressive-like effects of rapamycin in animal models Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Research Bulletin. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathology. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. mTOR: A pathogenic signaling pathway in developmental brain malformations. Trends in Molecular Medicine. 2011;17:734–742. doi: 10.1016/j.molmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Cuscó I, Medrano A, Gener B, Vilardell M, Gallastegui F, Villa O, González E, Rodríguez-Santiago B, Vilella E, Del Campo M, Pérez-Jurado LA. Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Human Molecular Genetics. 2009;18:1795–1804. doi: 10.1093/hmg/ddp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Jacome LF, Cannon WR, Herndon AL. d-Cycloserine improves the impaired sociability of the Balb/c mouse. Brain Research Bulletin. 2011a;84:8–11. doi: 10.1016/j.brainresbull.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Urbano MR, Herndon AL, Winebarger EE. Impaired Sociability of the Balb/c Mouse, an Animal Model of Autism Spectrum Disorders, is Attenuated by NMDA Receptor Agonist Interventions: Clinical Implications. In: Mohammad-Reza M, editor. A Comprehensive Book on Autism Spectrum Disorders. InTech; 2011. [Google Scholar]
- Deutsch SI, Mastropaolo J, Powell DG, Rosse RB, Bachus SE. Inbred mouse strains differ in their sensitivity to an antiseizure effect of MK-801. Clinical Neuropharmacology. 1998;21:255–257. [PubMed] [Google Scholar]
- Deutsch SI, Pepe GJ, Burket JA, Winebarger EE, Herndon AL, Benson AD. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Research. 2012;1439:96–107. doi: 10.1016/j.brainres.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Paul SM, Riggs RL, Mastropaolo J. Inbred mouse strains differ in sensitivity to popping behavior elicited by MK-801. Pharmacology Biochemistry and Behavior. 1997;57:315–317. doi: 10.1016/s0091-3057(96)00347-4. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ. Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends in Molecular Medicine. 2011;17:78–87. doi: 10.1016/j.molmed.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Lombroso PJ. The role of striatal-enriched protein tyrosine phosphatase (STEP) in cognition. Frontiers in Neuroanatomy. 2011;5:47. doi: 10.3389/fnana.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Wang C, Salgado MW, Greene PE, Kim M, Rapin I. Motor stereotypies in children with autism and other developmental disorders. Developmental Medicine and Child Neurology. 2009;51:30–38. doi: 10.1111/j.1469-8749.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, Blendy JA, Dow HC, Brodkin ES, Schneider F, Gur RC, Siegel SJ. Assessment of NMDA receptor NR1 subunit hypofunction in miceasa model for schizophrenia. Genes, Brain and Behavior. 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Nagy R, Toth M, Pelczer KG, Mikics E. NR2B subunit-specific NMDA antagonist Ro25-6981 inhibits the expression of conditioned fear: a comparison with the NMDA antagonist MK-801 and fluoxetine. Behavioural Pharmacology. 2011;22:113–121. doi: 10.1097/FBP.0b013e328343d7b2. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The Cationic Amino Acid Transporters CAT1 and CAT3 Mediate NMDA Receptor Activation-Dependent Changes in Elaboration of Neuronal Processes via the Mammalian Target of Rapamycin mTOR Pathway. Journal of Neuroscience. 2007;27:449–458. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Cannon WR, Deutsch SI. D-serine improves dimensions of the sociability deficit of the genetically-inbred Balb/c mouse strain. Brain Research Bulletin. 2011a;84:12–16. doi: 10.1016/j.brainresbull.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. D-Cycloserine enhances social exploration in the Balb/c mouse. Brain Research Bulletin. 2011b;85:141–144. doi: 10.1016/j.brainresbull.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. Genetically inbred Balb/c mice differ from outbred Swiss Webster mice on discrete measures of sociability: relevance to a genetic mouse model of autism spectrum disorders. Autism Research. 2011c;4:393–400. doi: 10.1002/aur.218. [DOI] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berlin) 2008;200:217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Langton JM, Richardson R. The effect of d-cycloserine on immediate vs delayed extinction of learned fear. Learning & Memory. 2010;17:547–551. doi: 10.1101/lm.1927310. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain and Behavior. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SHR. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. Journal of Neurochemistry. 2010;114:1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nature Neuroscience. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Sahin M. Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Current Opinion in Neurobiology. 2012;22:895–901. doi: 10.1016/j.conb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biological Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes, Brain and Behavior. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. Journal of Neuroscience. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc 1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Kisfali M, Lőrincz T. Role ofnonsynapticGluN2B-containing NMDA receptors in excitotoxicity Evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects. Brain Research Bulletin. 2013;93:32–38. doi: 10.1016/j.brainresbull.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Wlaź P, Baran H, Löscher W. Effect of the glycine/NMDA receptor partial agonist d-cycloserine, on seizure threshold and some pharmacodynamic effects of MK-801 in mice. European Journal of Pharmacology. 1994;257:217–225. doi: 10.1016/0014-2999(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes, Brain and Behavior. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Sheikh AM, Malik M, Wen G, Zou H, Brown WT, Li X. Upregulation of Ras/Raf/ERK1/2 signaling and ERK5 in the brain of autistic subjects. Genes, Brain and Behavior. 2011;10:834–843. doi: 10.1111/j.1601-183X.2011.00723.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6 J mothers. International Journal of Developmental Neuroscience. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. Journal of Neuroscience. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Yu Y, Sheikh AM, Malik M, Yang K, Wen G, Chadman KK, Brown WT, Li X. Association of upregulated Ras/Raf/ERK1/2 signaling with autism. Genes, Brain and Behavior. 2011;10:615–624. doi: 10.1111/j.1601-183X.2011.00702.x. [DOI] [PubMed] [Google Scholar]


