Abstract
Type 1 diabetes mellitus (T1DM) results from autoimmune destruction of pancreatic β-cells after an asymptomatic period over years. Insulitis activates antigen presenting cells, which trigger activating CD4+ helper-T cells, releasing chemokines/cytokines. Cytokines activate CD8+ cytotoxic –T cells, which lead to β-cell destruction. Apoptosis pathway consists of extrinsic (receptor-mediated) and intrinsic (mitochondria-driven) pathway. Extrinsic pathway includes Fas pathway to CD4+-CD8+ interaction, whereas intrinsic pathway includes mitochondria-driven pathway at a balance between anti-apoptotic B-cell lymphoma (Bcl)-2 and Bcl-xL and pro-apoptotic Bad, Bid, and Bik proteins. Activated cleaved caspse-3 is the converging point between extrinsic and intrinsic pathway. Apoptosis takes place only when pro-apoptotic proteins exceed anti-apoptotic proteins. Since the concordance rate of T1DM in identical twins is about 50%, environmental factors are involved in the development of T1DM, opening a door to find means to detect and prevent further development of autoimmune β-cell destruction for a therapeutic application.
KEY WORDS: Apoptosis, autoimmunity, β-cells, Bcl family, caspases, cytokines, insulitis, Type 1 diabetes
INTRODUCTION
Type 1 diabetes mellitus (T1DM) results from severe insulin deficiency by loss of insulin-producing β-islet cells, and it develops mostly in young and accounts for 5-10% of the diabetic subjects [1,2]. T1DM develops as the consequence of progressive β-cell destruction by autoimmune processes, after an asymptomatic period over several years [2]. Major histocompatibility complex (MHC) Class II are expressed at the surface of antigen presenting cells [APCs] (e.g., dendritic cells, macrophages, and B-lymphocytes) including Human Leukocyte Antigen - antigen D Related (HLA-DR), HLA-DQ, and HLA-DP, and some specific combination of alleles for DQA1, DQB1, and DRB1 genes (DRB1*03 and DRB1*04) significantly increase the development of T1DM [2-5]. The earliest signs of autoimmunity against β-cells are often detectable months or years of clinical T1DM, and the most common autoantibodies in pre-diabetic subjects are directly against glutamic acid decarboxylase-65 (GAD65), tyrosine phosphatase-like protein IA2, and insulin [6]. Up to 90% of newly diagnosed T1DM subjects have autoantibodies to one or more of these antigens [6], and autoimmunity detection rates increase to 98% when combining the detection of these three antibodies plus antibodies against the newly discovered β-cell autoantigen ZnT8 [7,8]. In identical twins, the concordance rate is below 50% for T1DM as compared to a higher concordance rate for T2DM, suggesting that environmental or non-genetic factors contribute to T1DM as in the case of T2DM; the latter has the concordance rate of about 90% in the identical twins [9]. Epidemiological data suggest that autoimmune process is triggered early in life, which may indicate that the pool of self-reactive naive T-cells stays in the control of the immune system for several years, and some antibody positive-subjects never develop insulitis nor proceed to overt diabetes [10]. The environmental factors include viral infection, including that of Coxsackievirus B (CVB), rubella, mumps, rotavirus and cytomegalovirus [11], as well as toxins, dietary factors during infancy, vaccination and others [12,13]. T1DM follows a slow-progressing autoimmune process before presenting typical clinical symptoms through a cascade of complicated immunological sequences, as reported by Pirot et al. [3]. Autoimmune destruction of pancreatic β-cells:
-
(A)
After the early stages of insulitis, activated local APCs recruit and activate CD4+ helper T-cells via migration to the pancreatic lymph nodes to present β-cell antigen and release chemokines/cytokines (Figure 1A).
-
(B)
CD4+ helper T-cells, in turn, stimulate APC secretion of cytokines and nitric oxide (Figure 1B).
-
(C)
Cytokines induce secretion of chemokines by endothelial cells, which enhance the recruitment of immune cells into the islets and activate CD8+ cytotoxic T-cells together with cytokines (Figure 1C).
-
(D)
β-cells also secrete chemokines in response to viral infection or cytokines, enhancing the recruitment and activation of immune cells. Activated CD8+ cells in turn induce β-cell apoptosis (Figure 1D).
-
(E)
Fas pathway: Fas (CD 95), a member of tumor necrosis factor (TNF) superfamily, is activated via binding of Fas L (CD 178) and Fas and Fas L are detected, respectively, at the surface of β-cells and T-cells infiltrating islets [3]. Once activated, Fas trimerizes and recruits the Fas-associated death domain (FADD), which recruits pro-caspase-8 leading to its activation by autocleavage. Activated caspase-8 subsequently cleaves the effector caspase-3 or activates the BH3 protein Bid (Figure 1E).
-
(F)
The perforin/granzyme system: Perforin and granzyme are contained in granules inside CD8+ T-cells. Perforin is involved in pore formation across the membranes via Ca2+ dependent mechanisms. The pore enables the entry of serine protease granzyme inside the cell, causing the cleavage and activation of several targets, such as effector caspase-3 and the BH3 protein Bid (Figure 1F).
-
(G)
Interleukin-1β (IL-1β) activates nuclear factor κB (NF-κB) and the kinases protein kinase C (PKC), p38, and c-Jun N-terminal kinase (JNK). IL-1β binding to its receptor induces the formation of multiprotein complex at its cytoplasmic domain, including IL-1R accessory protein (IL-1R AcP), Toll interacting protein (Tollip), Myeloid differentiation primary response gene 88 (MyD88) and IL-1 receptor-associated kinase (IRAK) family, namely, IRAK-1 and IRAK-4 (Figure 1G).
-
(H)
TNF-α activates caspase-8, NF-kβ, and the mitogen-activated protein kinase (MAPK) p38 and JNK. Activation of TNF receptor 1 (TNF-R1) upon TNF-α binding leads to its trimerization and recruitment of adaptor protein TNF-associated death domain protein (TRADD), which in turn recruits tumor necrosis factor receptor-associated factor-2 (TRAF-2) and serine-threonine kinase Rip. TRAF-2 activates NF-kB and MAPK pathways. TNF-α phosphorylates and activates p38 and JNK. Caspase-8 activates effector caspase-3 in the same way in the Fas pathway (Figure 1H).
-
(I)
Interferon ϒ (IFN-ϒ) activates signal transducer and activator of transcription 1 (Stat-1) and extracellular signal-regulated kinase (ERK). IFN-ϒ binding to its receptor induces its oligomerization and cytoplasmic recruitment of two members of Janus kinase (Jak) family, Jak1 and Jak2 [3], which recruit Stat-1 and trigger its activation by phosphorylation. Stat-1 then homodimerizes and migrates to nucleus where it regulates expression of Interferon-Gamma-Activated Sequence (GAS) elements such as caspases, Fas, and inducible nitric oxide synthase (iNOS) (Figure 1I) [3]. In this theory, activated caspase-8 cleaves and activates effector caspase-3 and BH3 protein Bid in the (E) Fas pathway and (H) TNF-α pathway and in part in (I) the IFN-ϒ pathway.
FIGURE 1.
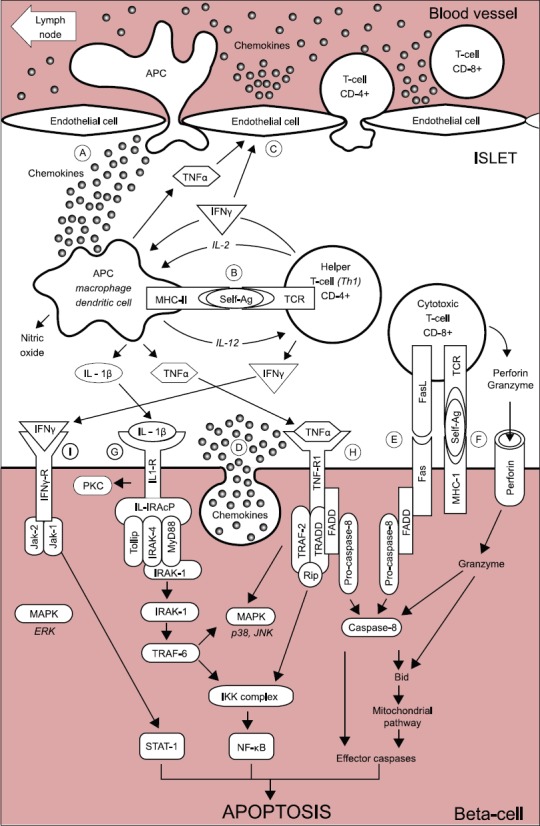
Schematic representation of the auto-immune attack to the beta-cells in Type 1 diabetes mellitus. (A) At the early stages of insulitis, activated local antigen presenting cells (APC) recruit and activate CD4+ helper T-cells via migration to the pancreatic lymph node, presentation of beta-cells antigens, and release of chemokines/cytokines. (B) CD4+ helper T-cells, in turn, stimulate APC secretion of cytokines and nitric oxide. (C) Cytokines induce the secretion of chemokines by endothelial cells, which enhance the recruitment of immune cells into the islets and, together with cytokines, activate CD8+ cytotoxic T-cells. (D) The beta-cell themselves also secrete chemokines in response to viral infection, or cytokines, further enhancing the recruitment and activation of immune cells. Activated CD8+ cytotoxic T-cells, in turn, induce beta-cell apoptosis via (E) the Fas pathway and (F) the granzyme/perforin system. Cytokines also bind to receptors at the surface of beta-cells: (G) Interleukin-1β (IL-1β) activates nuclear factor κB (NF-κB) and the kinases protein kinase C (PKC), p38, and c-Jun N-terminal kinase (JNK). (H) Tumor necrosis factor α (TNF α) activates caspase-8, NF-κB and the mitogen-activated protein kinase (MAPK) p38 and JNK. (I) Interferon ϒ (IFNϒ) activates Stat-1 and the extracellular signal–regulated kinase (ERK). From reference 3 - Pirot P, Cardozo AK, Eizirk DL. Arq Bras Endocrinol Metab 2008;52(2):156-165.
Extrinsic and intrinsic apoptosis pathway. There are two main apoptosis pathways, the extrinsic (receptor-mediated) and the intrinsic (mitochondrial-driven). Above described theory refers mostly to the extrinsic pathway and is not distinctively clarified into extrinsic or intrinsic pathway, respectively. Each pathway will be described below despite some redundancy between the above and below description on apoptosis.
It is the extrinsic pathway that is activated upon ligation of the cell surface death receptors(s), which in turn activate(s) a downstream effector mechanism orchestrated by the caspase family of cysteine proteases (Figure 2) [14]. The prototype example of death signaling via the extrinsic pathway is the Fas death receptor, which instigates assembly of the death-inducing signaling complex (DISC), a multi-protein complex comprising the cytoplasmic aspects of the Fas receptor, the adaptor protein FADD and procaspase-8 (Figure 2) [14]. Caspase-3 is the converging point of the apoptotic pathway (Figure 2) [14] and its peptide inhibitors have been shown to prevent islet apoptosis and improve islet graft function [15,16]. Apoptosis induced by ligation of cell surface receptors like Fas or TNF receptors, represents a pathway controlled by caspases [17,18]. Ligand binding of the receptor causes assembly of series of proteins of DISC, which then activates the apical caspase, procaspase-8 [19]. The resulting events proceed in a cascade where caspase-8 induces activation of caspase-3 [18]. Apoptosis manifests in two major execution programs, downstream of the death signal - the caspase pathway [20], and upstream of irreversible cellular damage where reside the Bcl family members, which are proteins with both pro-apoptotic and anti-apoptotic properties, playing a pivotal role in the life and death of cells (Figure 2) [14]. Anti-apoptotic members of the Bcl family, including B-cell lymphoma-2 (Bcl-2) and Bcl-xL, blunt intrinsic death signaling by blocking the recruitment of pro-apoptotic members to the mitochondria [21]. The cumulative data support the notion that high glucose might modulate the balance of the pro-apoptotic caspase family and anti-apoptotic Bcl proteins toward apoptosis, thus leading to β-cell death [22]. One of immunocytochemical markers for apoptosis is cleaved caspase-3. The caspase-3 protein is a member of the cysteine-aspartic acid protease (caspase) family and plays a central role in the execution-phase of cell apoptosis [23]. Caspases exist as zymogens in soluble cytoplasm, endoplasmic reticulum, mitochondrial membrane space, and nuclear matrix [17]. Caspases are inactive proenzymes that undergo proteolytic processing at conserved aspartic residues to produce two subunits, large mass of ~20 kDa and small mass of ~10 kDa, that dimerize to form the active enzyme [17]. This activated enzyme cleaves and activates caspase-6 and -7, and the protein itself is processed and activated by caspases-8, -9 and -10 [24]. One of these cleaved caspases is present on activated caspase-3, a ubiquitously distributed caspase, which is the main effector caspase of the apoptotic cascade within cells [24-28]. Caspase-3 is active over a broad pH range that is more basic than the pH range for many other executioner caspases, indicating that caspase-3 will be fully active under normal and apoptotic cell condition [24]. Caspase-3 is activated in both, the extrinsic and intrinsic apoptosis pathways. In the extrinsic pathway, caspase-3 plays a dominant role by activating the caspase cascade of apoptotic pathway. In the intrinsic pathway, cytochrome C from the mitochondria works in combination with caspase-9, apoptosis-activating factor (Apaf-1), and ATP to process procaspase-3 [25,26]. The commercially available polyclonal anti-cleaved caspase-3 detects endogenous levels of the large (17/19 kDa) cleaved caspase-3 resulting from cleavage adjacent to Asp 175 and does not recognize the full length or other cleaved caspases [29], and its immunopositive staining is specifically in the islet cell nucleus alone [30,31]. Recently, an involvement of caspase-3 in both T1DM and T2DM was implicated. In T1DM, Fas (CD 95)-Fas L (CD 178) may be critical for β-cell destruction, as apoptosis in β-cell clone expressing the human Fas is mediated by elevated caspase-3-like activity in tissue culture [32], and the frequency of β-cell apoptosis in T2DM pancreatic tissues from autopsy is increased when using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) [33]. We performed cleaved caspase-3 immunocytochemical staining in pancreas samples of 8 T1DM cases compared with 8 controls, to study the converging point of the extrinsic and intrinsic pathway [14]. T1DM islets showed higher amounts of cleaved caspase-3 positive cells at 16% of the total islet cells, with large and small islets positive at 14% and 17%, respectively, at 3.3, 3.6, and 2.4 times that of the corresponding control values (Table 1) [34]. The T1DM islets were a mixture of small and large, regenerating islets consisting of non-β-cells of α-, δ- and pancreatic polypeptide (PP)-cells, with moderately increased caspase-3 positive cells (Figure 3A-E - right column and Table 1) [34]. These increased caspase-3 positive islet cells in T1DM pancreas may correspond to a more accelerated apoptosis cascade than in T2DM islets, before entirely exhausting the β-cell mass by apoptosis [34]. The pancreas was obtained at autopsy from T1DM subjects of ages 18-75, and the immunocytochemical presence of cleaved caspase-3 suggests that cleaved caspase-3 mediated apoptosis was in the ongoing process even at the subjects’ death, with a delicate balance of β-cell apoptosis and regeneration. These data also support that β-cell apoptosis and regeneration process are progressive from the preclinical stage to the end-stage of T1DM [34]. Among generally smaller islets in T1DM pancreas (Case 6), which consisted of + insulin cells (3-24 %) and more glucagon cells (50%), there were 13% caspase-3 positive cells, at 3 times that of the controls (Figure 4A-D) [34]. The majority of T1DM islets still retained weaker but insulin-positive β-cells (Figure 3A, right column) [34]. There were rare but occasional large islets (Case 8) among the major smaller islets from older patients; the former appeared to be regenerating large islets consisting of no insulin cells (+/-, <1%) (Figure 4E), but major islet cells were α- and PP-cells (a combined 100%) (Figure 4F and G) [34], with normal percentage of cleaved caspase-3 positive cells (about 4%) (Figure 4H) [34]. These large islets corresponded to the end-stage islets with completed accelerated apoptosis after long-standing, exhausted insulin secretion. The number of cleaved caspase-3 positive islet cells was higher in T1DM islets than in T2DM islets [34,35].
FIGURE 2.
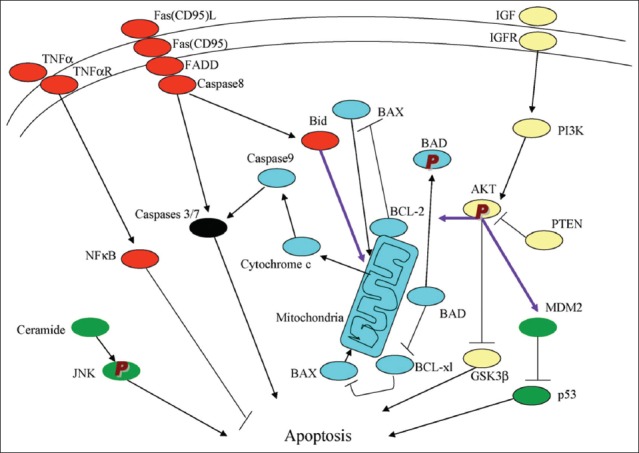
There are the extrinsic (receptor-mediated, red) and intrinsic (mitochondria-driven, blue) apoptosis pathways as opposed to the survival proteins such as the PI3K/Akt signaling circuitry (yellow). Other signaling loops (green) and executioner caspase (black), are activated in both extrinsic and intrinsic pathways; inter-talk between pathways (arrows); phosphorylation (P). From reference 14 - Lee, SC, Pervaiz, S. Int J Biochem Cell Biol 2007;39(3):497-504.
TABLE 1.
Cleaved caspase-3 immunostaining in islets (total 30 islets for each case) from 8 Type 1 diabetes mellitus (T1DM) subjects compared to 8 control subjects. From reference 34 - Tomita T, Islets 2010; 2 (1):24-29, with permission
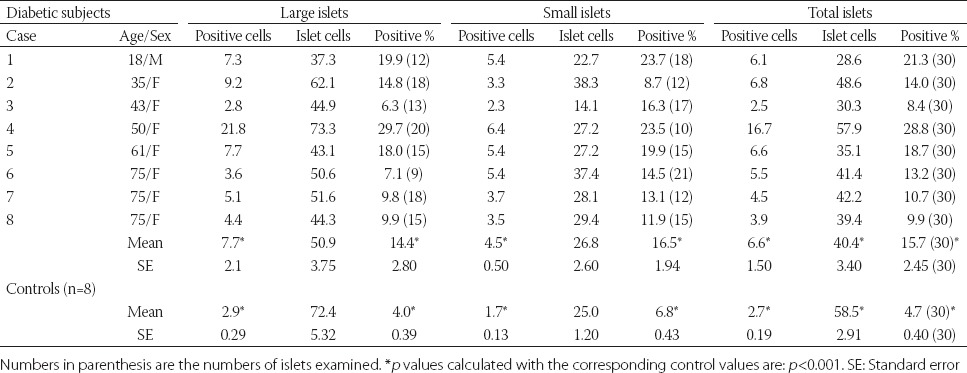
FIGURE 3.
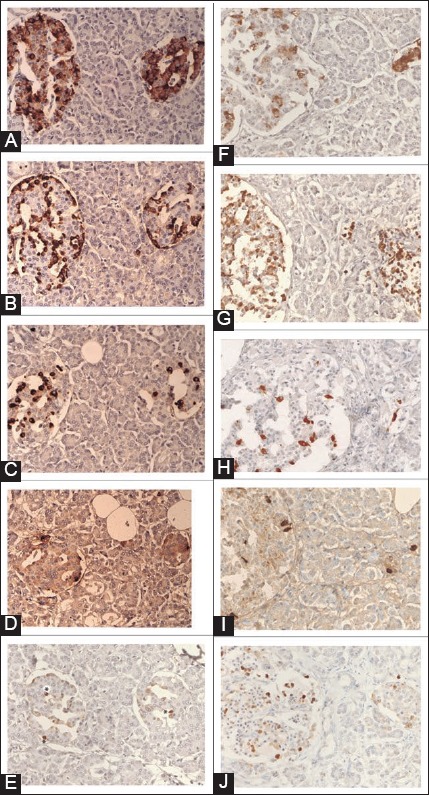
Normal islets (A-E). Normal islets consist of major insulin cells (80%), next major glucagon cells (10%) and minor somatostatin (>7%) and pancreatic polypeptide (PP)-cells (>5%). Cleaved caspase-3 immunostaining reveals scattered positive dense nuclear staining with less adjacent cytoplasmic staining, the latter appears to be an extension from the dense nuclear staining. Large islet: Left islet, Small islet: Right islet, *dense nuclear staining. (A) Insulin, (B) Glucagon, (C) Somatostatin, (D) PP, (E) Cleaved caspase-3 immunostaining. Insulin cell-less and glucagon cell-rich type 1 diabetic islet. Case 5 (F-J). Insulin cells are less (+: 3–24%) and weakly stained as compared to glucagon-cells (80%) with small cytoplasm, which are the major cells with relatively increased somatostain and normal PP-cells. Cleaved caspase-3 immunostaining is mostly in the nucleus and approximately matches with location of insulin cells. (F) Insulin, (G) Glucagon, (H) Somatostatin, (I) PP, (J) Cleaved caspase-3 immunostaining. From reference 34 - Tomita T. Islets 2010;2(1):24-29, with permission.
FIGURE 4.
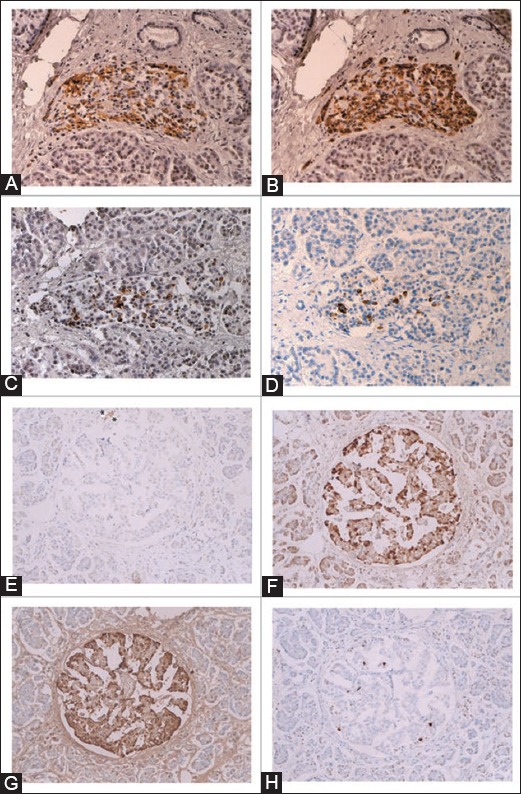
Insulin cell-poor islets (A-D) and insulin cell-absent islet (E-H) from Type 1 diabetes mellitus subjects. The islets from Case 6 (A-D) are generally small consisting of insulin-cell poor (25%), glucagon-cell rich (75%) and somatostatin-cell rich (10%) with increased cleaved caspase-3 positive cells (13%). The large islet from Case 8 (E-H) has practically no insulin-cells (<1%) and consists of major glucagon and pancreatic polypeptide (PP)-cells (a combined 100%) with increased somatostatin-cells (10%) and a normal number of cleaved caspase-3 positive cells (4%). *Residual insulin-cells, (A and E): Insulin, (B and F): Glucagon, (C): Somatostatin, (G): PP, (D and H): Cleaved caspase-3 immuno-stained. From reference 34 - Tomita T. Islets 2010; 2(1):24-29, with permission.
The intrinsic pathway evolves in the mitochondria responding to a deleterious effect of chronic hyperglycemia on β-cells [14]. Meier et al. [36] studied T1DM islets using cleaved caspase-3 and TUNEL immunofluorescence. Cleaved caspase-3 revealed 5.8% for T1DM islets compared to 2.7% for control islets, at twice as much for T1DM of the control islets [36]. Our data on cleaved caspase-3 positive cells were 15.7% for T1DM islets and 4.7% for control islets, at 3 times more caspase-3 positive cells in T1DM than in control islets [34]. Butler’s group reported TUNEL immunofluorescence at 0.2% for T1DM islets as compared to 0% for control islets [37]. Observing immunofluorescence photomicrographs, the positive staining was not clear and was hazy for both, cleaved caspase-3 and TUNEL, including positive staining in the perinuclear cytoplasm [36,37]; not as clear as our cleaved caspase-3 immunoperoxidase staining [33,34]. Another study using TUNEL reported 0.5% positive cells for normal β-cells [38]. Immunopositive cleaved caspase-3 is present in apoptotic cells and ongoing apoptotic cells, which may explain a higher percentage of apoptotic cells revealed by cleaved caspase-3 immunoperoxidase staining than TUNEL staining [24]. Thus, cleaved caspase-3 immunoperoxidase staining recognizes protein levels better than TUNEL, the latter recognizes DNA levels. The Bcl-2 family governs mitochondrial outer membrane permeabilization and can be either pro-apoptotic (Bad, Bid, Bik, and Bak) or anti-apoptotic (Bcl-2, Bcl-xL, and Bcl-w), and there is a total of 25 genes known in the Bcl-2 family [39]. Bcl-2 family exerts pro- and anti-apoptotic effects by activation or inactivation of an inner mitochondrial permeability transition pore, which is involved in the regulation of matrix Ca2+, pH and voltage. Bcl-2 family proteins can induce apoptosis by the pro-apoptotic members or inhibit apoptosis by the anti-apoptotic members, through the release of cytochrome C into the cytosol, which activates caspase-9 and -3, leading to apoptosis [40]. The members of the Bcl-2 family share one or more of the four characteristic domains (Bcl-2 homology [BH]1, BH2, BH3, and BH4) and the anti-apoptotic Bcl-2 and Bcl-xL contain all four BH domains [41]. All pro-apoptotic proteins contain a BH3 domain necessary for dimerization with other proteins of Bcl-2 family (Bax and Bak). The BH3-only of the Bcl-2 family of proteins contains only a single BH3-domain and plays a key role in promoting apoptosis. The BH3-only family members are Bim, Bid, and Bad [41].
To study glucose toxicity as a deleterious effect of chronic hyperglycemia on β-cells in vitro, Federici et al. [22] cultured 400 isolated human islets per batch for 5 days in a low glucose (5.5 mmol/L) and a high glucose medium (16.6 mmol/L), for studying possible high glucose effects on Bcl-2 family gene expression by reverse transcription polymerase chain reaction [22].
-
(1)
Bcl-2 was highly expressed in both, low and high glucose media, and the expression did not change between the high and low glucose conditions (Figure 5A) [22].
-
(2)
However, Bcl-xL was reduced by 45% in the high glucose cultured islets compared with that of the low glucose cultured islets, supporting that a reduction in Bcl-xL gene expression was due to the high glucose exposure (Figure 5B) [22].
-
(3)
Bad, Bid, and Bik were expressed in the low glucose medium at low levels. Bad gene expression was greatly increased with the high glucose medium and Bad RNA level increased 80% as well, compared with that of the low level glucose medium (Figure 5C) [22]. Bid gene expression was markedly increased in the high glucose medium and so was Bik gene expression (Figure 5D and E) [22]. Thus, anti-apoptotic Bcl-2 was unaffected by the high glucose but pro-apoptotic genes, Bad, Bid and Bik markedly increased in the high glucose cultured islets (Figure 5A-D) [22]. These data support that chronic high glucose incubation in vitro modulates the balance of pro- and anti-apoptotic Bcl proteins toward apoptosis, thus leading to eventual β-cell death [22]. Using MIN6N8 cells, which are SV40-transformed insulinoma cells derived from non-obese diabetic (NOD) mice, Kim et al. [42] extensively studied the effects of chronic hyperglycemia in the tissue culture. When MIN6N8 cells were cultured in different concentrations of glucose for varying time periods, a high glucose (33.3 mmol/L) induced marked genomic DNA fragmentation in a time- and dose-dependent manner and caused a significant increase of TUNEL-positive cells compared to low glucose (5.5 mmol/L) cultured cells, as well as concomitant cleavage of poly (adenosine diphosphate-ribose) polymerase (PARP) similar to caspase-3 cleavage (Figure 6A) [42]. Pretreatment with a specific caspase-3 inhibitor (z-DEVD-CHO) completely reduced the high glucose-induced PARP cleavage (Figure 6C) and apoptosis [42]. Culturing with high glucose significantly increased Fas and P53 expression, whereas it decreased Bcl-2 and Bcl-xL expression (Figure 6B) [42]. A significant mitochondrial release of cytochrome C into cytosol was observed after 2 days (Figure 6C) [42]. Translocation of Bax was confirmed by immunostaining (Figure 6D); Bax interacted with voltage-dependent anion channel [VDAC] (Figure 6E) [42] and Bax oligomerization substantially increased during 2 days in the high glucose medium (Figure 6F) [42]. Thus, chronic exposure to a high glucose increased Bax oligomerization, cytochrome C release and caspase-3 activation, leading to β-cell apoptosis [42]. Glucose toxicity on β-cell apoptosis was studied with chronic exposure to a high glucose, which markedly reduced glucokinase (GCK, hexokinase IV) expression in a time- and dose-dependent manner but guanosine triphosphate (GTP) cyclohydrolase (GCH) expression was unaffected [43]. Immunocytochemistry on tissue culture cells showed that glucose reduces GCK expression dose-dependently, decreasing by 40% in a high glucose medium. Chronic exposure to a high glucose for 4 days abolished to stimulate insulin content and inhibited ATP production [44]. The main molecular studies of apoptotic Bcl proteins had been performed in artificially forced overexpression experiments in vitro [44-46]. Caspases are activated in a hierarchical order, in which initiator caspases (caspase-8 and -10) function to cleave effector caspases (caspase-3 and -7), the latter in turn degrade intercellular protein substrates and lead to the classical morphological changes of apoptosis (Figure 2) [14]. Extracellular events present during the inflammatory response through the release of cytokines, including IFN-d, IL-1β and IFN-ϒ, can initiate apoptosis by infiltrating leukocytes or by direct cytotoxic T-cell engagement [14]. These intrinsic cues function via surface molecules in the death receptor pathway, where specific ligand-receptor binding such as TNF-TNF receptor binding and Fas-Fas L binding leads to receptor clustering, adaptor molecule recruitment, and formation of DISC (Figure 2) [14]. Caspase-8 associates with DISC complex, where it is activated and released, and it further leads to effector activation for caspase-3 [38]. Intracellular cues such as DNA damage, hypoxia, nutrient deprivation, or reactive oxygen species (ROS) function via the mitochondrial pathway, which is tightly modulated by the Bcl-2 proteins (Figure 6) [6,42]. In healthy cells, pro-apoptotic Bcl-2 proteins (Bim, Bid, Bad, Bax, and Bak) are present in the mature form, while anti-apoptotic Bcl-2 proteins (Bcl-2 and Bcl-xL) are constitutively active and reside in the outer membrane of mitochondria (Figure 6) [6,42]. Following an intrinsic cue, proapoptotic Bcl proteins become activated and translocate to the mitochondria, where they bind to inactivate anti-apoptotic Bcl-2 proteins or form pores in the mitochondrial membrane, which facilitates the release of cytochrome C into the cytosol (Figure 2) [14]. When cytochrome C accumulates in the cytosol, it forms a complex with procaspase-9 and Apaf-1 to assemble the “apoptosome”, which in turn activates caspase-3 (Figure 2) [14]. Both intrinsic and extrinsic signaling cascades converge at the point of caspase-3 activation, which is often considered as the “point of no return” in apoptosis [14,47]. Apoptosis can occur only when the concentration of pro-apoptotic Bcl proteins exceeds that of anti-apoptotic proteins at the mitochondrial membrane of the intrinsic pathway [22]. Using transgenic mice, Danial et al. found that glucose-induced changes in the mitochondrial membrane potential were significantly reduced in Bad-/- β-cells, and the average (Ca2+) I response to 11 mM glucose was significantly lower in Bad-/- β-cells [48,49]. An intact BH3 domain is required for glucose-stimulated insulin secretion by its binding to Bcl-2 and Bcl-xL. Treatment with BAD stabilized α-helix of Bcl-2 domain A (SAHBA) restored the secretion defect in Bad-/- islets, underscoring the sequence specificity of the BAD SAHB effect [49]. They further identified GCK as a novel and direct physiological target of the BAD BH3 domain in β-cells and that phosphorylation within the BH3 domain drives the metabolic functionality of BAS and severs as a physiological switch of its apoptotic and metabolic effects [49]. The early event of β-cell dysfunction includes endoplasmic reticulum stress and oxidative stress [50], leading to β-cell exhaustion and eventual apoptosis. Thus, the therapeutic application of BAD SAHBA and other BAD SAHBs that activates glucose-stimulated insulin secretion, but does not affect the survival function of Bcl-xL, may serve as a prototype therapeutic application in diabetes and islet transplantation [49].
FIGURE 5.
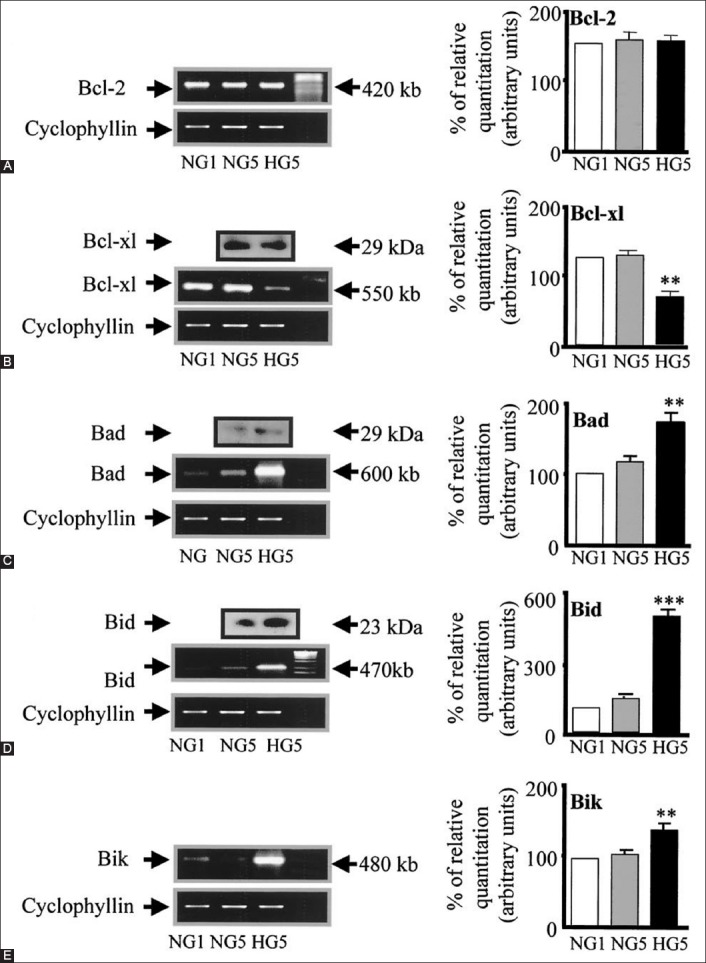
Bcl family gene regulation in human islets cultured in high versus normal glucose. Expression of Bcl-2, Bcl-xl, Bad, Bid and Bik mRNA was detected by reverse transcription polymerase chain reaction and quantified by FluorImager analysis of ethidium bromide signal. In each experiment, band densities were normalized against cyclophyllin, and the result are expressed as mRNA level to NG1 control islets (NG1 = 100%). (A) Bcl-2, (B) Bcl-xl (HG5 versus [vs.] NG5, **p<0.01), (C) Bad (HG5 vs. NG5, **p<0.01), (D) Bid (HG5 vs. NG5, ***p<0.001), (E) Bik (HG5 vs. NG5, **p<0.01). From reference 22 - Federici M, Hrivbal M, Perego L, Ranalli M, Caradonna Z, Perego C, et al. Diabetes 2001;50(6):1290-1301. © 2001 by the American Diabetes Association Diabetes 2001 Jun; 50(6):1290-1301 Reprinted with permission from the American Diabetes Association.
FIGURE 6.
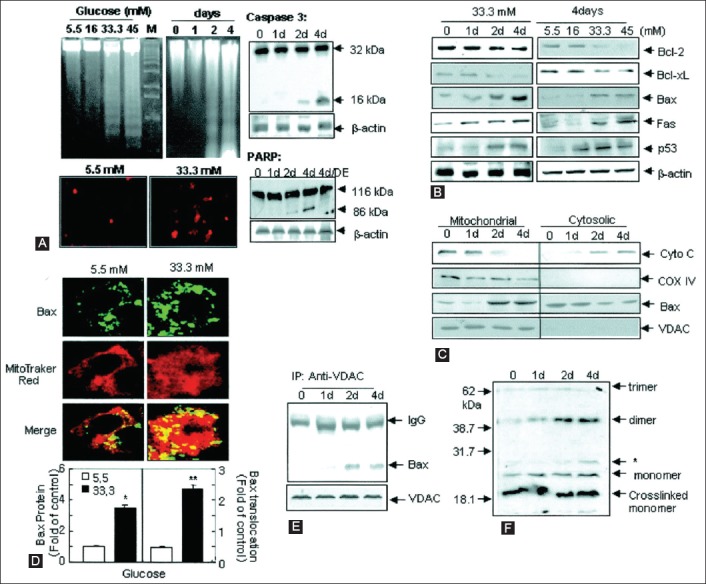
Effects of high glucose on apoptosis in MIN6N8 cells. The MIN6N8 cells were treated with different glucose concentrations (5.5-45 mM) for the indicated times. (A) DNA fragmentation (upper left) and transferase-mediated 2’-deoxyuridine 5’-triphosphate nick-end labeling (TUNEL) assay (lower left) were carried out. The cleavage of caspase-3 (upper right) and poly (adenosine diphosphate-ribose) polymerase (PARP) (lower left) was analyzed. (B) Expression on apoptotic proteins. (C) Release of cytochrome (Cyto) C and Bax translocation. The blots were reprobed with antibodies to cytochrome C oxidase (COX) IV and voltage-dependent anion channels (VDAC). (D) Bax immunocytochemistry. Fluorescent microscopic images for Bax (green), MitoTracker CMXRos (red) and the final merged images (localization of Bax at mitochondria) are shown (upper). Fold of cells exhibiting punctuate Bax and percentage of Bax colocalization with mitochondria was determined by counting -20-100 cells for each condition (lower). Results represent the average ± standard error (SE) from three independent experiments (*p<0.05, **p<0.01). (E) Interaction of Bax with VDAC. (F) Bax oligomerization (*nonspecific bands). All data are representative of three independent experiments. From reference 42 - Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, et al. Diabetes 2005;54(9):2602-2611. © 2005 by the American Diabetes Association Diabetes 2005 Sep; 54(9): 2602-2611 Reprinted with permission from the American Diabetes Association.
CONCLUSION
As mentioned so far, there are a milliard of biological processes for β-cells distraction by an autoimmune reaction in T1DM, while β-cells are replaced by non-β-cells at a delicate balance of islet cell apoptosis and regeneration in a long time frame. Since other environmental factors are involved in the development of T1DM in addition to genetic factors, there are many ways to theoretically and eventually prevent or retard autoimmune reactions in the complicated processes of T1DM development. Stem cells may be programed to restore insulin secretion or the existing non-functioning β-cells in T1DM islets can be activated to produce and secrete more insulin. It is also evident that an adequate glucose homeostasis in T1DM is a prudent way to protect the remaining β-cells in T1DM subjects against hyperglycemia-induced β-cell apoptosis. There is a future hope for therapeutic application to slow or even prevent β-cell apoptosis pathways in T1DM at the molecular level by clever biomedical engineering.
DECLARATION OF INTERESTS
The author declares no conflict of interests.
REFERENCES
- [1].Daneman D. Type 1 diabetes. Lancet. 2006;367(9513):847–58. doi: 10.1016/S0140-6736(06)68341-4. https://doi.org/10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- [2].Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12(6):580–91. doi: 10.1097/01.mjt.0000178767.67857.63. https://doi.org/10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- [3].Pirot P, Cardozo AK, Eizirik DL. Mediators and mechanisms of pancreatic beta-cell death in Type 1 diabetes. Arq Bras Endocrinol Metabol. 2008;52(2):156–65. doi: 10.1590/s0004-27302008000200003. https://doi.org/10.1590/S0004-27302008000200003. [DOI] [PubMed] [Google Scholar]
- [4].Jahromi MM, Eisenbarth GS. Cellular and molecular pathogenesis of Type 1A diabetes. Cell Mol Life Sci. 2007;64(7-8):865–72. doi: 10.1007/s00018-007-6469-4. https://doi.org/10.1007/s00018-007-6469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in Type 1 and Type 2 diabetes: Many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. https://doi.org/10.2337/diabetes.54asuppl_2.S97. [DOI] [PubMed] [Google Scholar]
- [6].Kim MS, Polychronakos C. Immunogenetics of Type 1 diabetes. Horm Res. 2005;64(4):180–8. doi: 10.1159/000089190. https://doi.org/10.1159/000089190. [DOI] [PubMed] [Google Scholar]
- [7].Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–64. doi: 10.1038/ng2068. https://doi.org/10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Turner D. The human leucocyte antigen (HLA) system. Vox Sang. 2004;87(Suppl 1):87–90. doi: 10.1111/j.1741-6892.2004.00438.x. https://doi.org/10.1111/j.1741-6892.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- [9].Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. https://doi.org/10.2337/diabetes.54asuppl_2.S52. [DOI] [PubMed] [Google Scholar]
- [10].Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human Type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(43):17040–5. doi: 10.1073/pnas.0705894104. https://doi.org/10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: Pros and cons. Diabetes. 2008;57(11):2863–71. doi: 10.2337/db07-1023. https://doi.org/10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].In’t Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56(9):2400–4. doi: 10.2337/db07-0416. https://doi.org/10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- [13].Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of Type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–36. doi: 10.2337/diabetes.54.suppl_2.s125. https://doi.org/10.2337/diabetes.54asuppl_2.S125. [DOI] [PubMed] [Google Scholar]
- [14].Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39(3):497–504. doi: 10.1016/j.biocel.2006.09.007. https://doi.org/10.1016/j.biocel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [15].Nakano M, Matsumoto I, Sawada T, Ansite J, Oberbroeckling J, Zhang HJ, et al. Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas. 2004;29(2):104–9. doi: 10.1097/00006676-200408000-00004. https://doi.org/10.1097/00006676-200408000-00004. [DOI] [PubMed] [Google Scholar]
- [16].Cheng G, Zhu L, Mahato RI. Caspase-3 gene silencing for inhibiting apoptosis in insulinoma cells and human islets. Mol Pharm. 2008;5(6):1093–102. doi: 10.1021/mp800093f. https://doi.org/10.1021/mp800093f. [DOI] [PubMed] [Google Scholar]
- [17].Nicholson DW, Thornberry NA. Caspases: Killer proteases. Trends Biochem Sci. 1997;22(8):299–306. doi: 10.1016/s0968-0004(97)01085-2. https://doi.org/10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- [18].Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50(Suppl 1):S44–7. doi: 10.2337/diabetes.50.2007.s44. https://doi.org/10.2337/diabetes.50.2007aS44. [DOI] [PubMed] [Google Scholar]
- [19].Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10(5):545–51. doi: 10.1016/s0952-7915(98)80222-7. https://doi.org/10.1016/S0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- [20].Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. https://doi.org/10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- [21].Green DR. Apoptotic pathways: Ten minutes to dead. Cell. 2005;121(5):671–4. doi: 10.1016/j.cell.2005.05.019. https://doi.org/10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- [22].Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: A potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–301. doi: 10.2337/diabetes.50.6.1290. https://doi.org/10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- [23].Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87(2):171. doi: 10.1016/s0092-8674(00)81334-3. https://doi.org/10.1016/S0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- [24].Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem. 1997;272(41):25719–23. doi: 10.1074/jbc.272.41.25719. https://doi.org/10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- [25].Katunuma N, Matsui A, Le QT, Utsumi K, Salvesen G, Ohashi A. Novel procaspase-3 activating cascade mediated by lysoapoptases and its biological significances in apoptosis. Adv Enzyme Regul. 2001;41:237–50. doi: 10.1016/s0065-2571(00)00018-2. https://doi.org/10.1016/S0065-2571(00)00018-2. [DOI] [PubMed] [Google Scholar]
- [26].Li P, Nijhawn D, Wang X. Mitochondrial activation of apoptosis. Cell. 2004;116(Suppl 21):57–9. doi: 10.1016/s0092-8674(04)00031-5. https://doi.org/10.1016/S0092-8674(04)00031-5. [DOI] [PubMed] [Google Scholar]
- [27].Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998;187(4):587–600. doi: 10.1084/jem.187.4.587. https://doi.org/10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81(5):801–9. doi: 10.1016/0092-8674(95)90541-3. https://doi.org/10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- [29].Cell Signaling Technology. Cleaved Caspase-3 (Asp 175) Antibody, Cat No. 9661. Vol. 9661. Beverly, MA: Cell Signaling Technology; 2006. [Google Scholar]
- [30].Tomita T. Caspase-3 immunocytochemical staining for pancreatic islets and pancreatic endocrine tumors. Hum Pathol. 2009;40(7):1050–2. doi: 10.1016/j.humpath.2009.02.010. https://doi.org/10.1016/j.humpath.2009.02.010. [DOI] [PubMed] [Google Scholar]
- [31].Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: Immunohistochemistry using antibodies to cleaved caspase3. J Histochem Cytochem. 2002;50(4):449–54. doi: 10.1177/002215540205000401. https://doi.org/10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- [32].Martin SJ, Green DR. Protease activation during apoptosis: Death by a thousand cuts? Cell. 1995;82(3):349–52. doi: 10.1016/0092-8674(95)90422-0. https://doi.org/10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- [33].Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with Type 2 diabetes. Diabetes. 2003;52(1):102–10. doi: 10.2337/diabetes.52.1.102. https://doi.org/10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- [34].Tomita T. Immunocytochemical localization of cleaved caspase-3 in pancreatic islets from Type 1 diabetic subjects. Islets. 2010;2(1):24–9. doi: 10.4161/isl.2.1.10041. https://doi.org/10.4161/isl.2.1.10041. [DOI] [PubMed] [Google Scholar]
- [35].Tomita T. Immunocytochemical localisation of caspase-3 in pancreatic islets from Type 2 diabetic subjects. Pathology. 2010;42(5):432–7. doi: 10.3109/00313025.2010.493863. https://doi.org/10.3109/00313025.2010.493863. [DOI] [PubMed] [Google Scholar]
- [36].Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing Type 1 diabetes: Indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–8. doi: 10.1007/s00125-005-1949-2. https://doi.org/10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- [37].Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset Type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50(11):2323–31. doi: 10.1007/s00125-007-0794-x. https://doi.org/10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- [38].Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307(5708):380–4. doi: 10.1126/science.1104345. https://doi.org/10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- [39].Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281(5381):1322–6. doi: 10.1126/science.281.5381.1322. https://doi.org/10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- [40].Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16(17):2265–82. doi: 10.1038/sj.onc.1201989. https://doi.org/10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- [41].Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644(2-3):83–94. doi: 10.1016/j.bbamcr.2003.08.012. https://doi.org/10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- [42].Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, et al. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: Downregulation of glucokinase in pancreatic beta-cells. Diabetes. 2005;54(9):2602–11. doi: 10.2337/diabetes.54.9.2602. https://doi.org/10.2337/diabetes.54.9.2602. [DOI] [PubMed] [Google Scholar]
- [43].Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: From mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol. 2004;31(3):119–28. doi: 10.1111/j.1440-1681.2004.03975.x. https://doi.org/10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- [44].Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. doi: 10.1038/35037710. https://doi.org/10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- [45].Ou D, Wang X, Metzger DL, James RF, Pozzilli P, Plesner A, et al. Synergetic inhibition of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human pancreatic beta cells by Bcl-2 and X-linked inhibitor of apoptosis. Hum Immunol. 2005;66(3):274–84. doi: 10.1016/j.humimm.2004.12.002. https://doi.org/10.1016/j.humimm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [46].Saldeen J. Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology. 2000;141(6):2003–10. doi: 10.1210/endo.141.6.7523. https://doi.org/10.1210/en.141.6.2003. https://doi.org/10.1210/endo.141.6.7523. [DOI] [PubMed] [Google Scholar]
- [47].Hui H, Dotta F, Di Mario U, Perfetti R. Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol. 2004;200(2):177–200. doi: 10.1002/jcp.20021. https://doi.org/10.1002/jcp.20021. [DOI] [PubMed] [Google Scholar]
- [48].Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424(6951):952–6. doi: 10.1038/nature01825. https://doi.org/10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- [49].Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14(2):144–53. doi: 10.1038/nm1717. https://doi.org/10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern Med. 2003;42(1):7–14. doi: 10.2169/internalmedicine.42.7. https://doi.org/10.2169/internalmedicine.42.7. [DOI] [PubMed] [Google Scholar]


