Abstract
Perinatal hypoxia-ischemia is a specific and important pathological event in neonatal care practice. The data on relationship between the concentrations of cytokines in blood and cerebrospinal fluid (CSF) and perinatal brain injury are scarce. The aim of this study is to evaluate changes in interleukin (IL-1β, IL-6, and IL-18) and tumor necrosis factor alpha (TNF-α) levels in newborns with perinatal hypoxia (PNH). CSF and serum samples of 35 term and near-term (35-40 weeks) newborns with PNH, at the age of 3-96 hours, were analyzed using enzyme-linked immunosorbent assay. Control group consisted of 25 non-asphyxic/non-hypoxic infants of the same age sampled for clinically suspected perinatal meningitis, but proven negative and healthy otherwise. The cytokine values in CSF and serum samples were determined in relation to initial hypoxic-ischemic encephalopathy (HIE) staged according the Sarnat/Sarnat method, and compared with neurological outcome at 12 months of age estimated using Amiel-Tison procedure. The concentrations of IL-6 and TNF-α in serum of PNH patients were significantly higher compared to control group (p = 0.0407 and p = 0.023, respectively). No significant difference between average values of cytokines in relation to the stage of HIE was observed. Significantly higher levels of IL-6 and IL-18 corresponded to a mildly abnormal neurological outcome, while higher levels of IL-6 and TNF-α corresponded to a severely abnormal neurological outcome, at 12 months of age. Elevated serum levels of IL-6 and TNF-α better corresponded with hypoxia/ischemia compared to CSF values, within 96 hours of birth. Also, higher serum levels of IL-6, TNF-α, and IL-18 corresponded better with abnormal neurological outcome at 12 months of age, compared to CSF values.
KEY WORDS: Neonates, cytokines, perinatal hypoxia, hypoxic-ischemic encephalopathy, neurological outcome, IL-1β, IL-6, IL-18, TNF-α
INTRODUCTION
Cytokines are cell signaling proteins involved in regulation of defense, growth, and repair mechanisms. Perinatal hypoxic-ischemic insults often result in acute and subacute brain damage and long-term neurological disabilities. Produced at the time of injury, the cytokine function depends on multiple factors. In addition, cytokines can have pro- or anti-inflammatory and neuroprotective or neurodestructive effects, depending on their state and concentration [1]. Cytokines have been studied in various animal and human pathological conditions during the past two decades [2-5]. However, the findings obtained with animal models and in specific human conditions cannot be completely transposed to complex neonatal conditions.
The role of cytokines in brain damage is context specific. Thus, understanding the role of cytokines at multiple levels during brain damage, is important. These include: Fetal and neonatal inflammatory responses at systemic (blood) and local (brain) levels, level and characteristics of the insult, and timing of the insult during brain development, i.e. different development stages in term and preterm infants [6]. In general, the functions of cytokines are pleiotropic, so the overall effects can be pro- and anti-inflammatory. Despite a number of studies, the consequences of neonatal hypoxic injury in humans are still controversial. However, it is accepted today that in neonatal brain a hypoxic-ischemic insult stimulates a cascade of proinflammatory cytokines that directly or indirectly induce the process of brain injury. According to the initial suggestions, proinflammatory cytokines, such as TNF-α, can affect the development of the brain and blood-brain barrier (BBB), and are responsible for early and late brain damage [7]. Proinflammatory cytokines TNF-α, IL-1, and IL-6 are studied frequently in newborn populations. Most of the studies found elevated levels of these biomarkers in hypoxic newborns compared to control groups [8-12]. On contrary, only a few studies addressed the function of IL-18 in the context of perinatal brain injury [13,14]. Based on animal experiments, it can be inferred that IL-18 has a role in the development of white matter damage (WMD) in the immature brain [13].
Multiple factors affect cytokine levels in the perinatal period, including: The cellular origin and concentration of cytokines, evolution of injury, and response to therapy. All of these factors can negatively affect measuring cytokines in the sensitive perinatal period. In this study, we measured cytokine levels in blood and cerebrospinal fluid (CSF) samples of patients with perinatal hypoxia (PNH) to investigate the relationship between systemic (blood) and brain (CSF) cytokines and the association between the cytokine profiles and early and late neurological sequelae.
MATERIALS AND METHODS
Patients
A prospective cohort analysis of cytokine profiles was carried out in neonates treated for PNH and receiving care at the Department of Neonatology and Intensive Care of Clinic for Child Diseases, University Hospital Mostar between June 2006 and June 2010. The approval for this study was granted by the University of Mostar (N: 01-478-a/05) and written parental consent was obtained for all patients.
Neonates who met at least three of the following eligibility criteria for perinatal hypoxia/asphyxia (adopted from the statement of the American Academy of Pediatrics [15] and adapted for local practice) were included in the study: 1) Birth distress recorded on cardiotocography (more than one hour of serious disorders, deceleration or bradycardia longer than half an hour); 2) Early passage of thick meconium; 3) Blood pH less than 7.20 within the first hour after birth; 4) The need for neonatal resuscitation with positive pressure ventilation for more than two minutes; 5) Apgar score ≤5 in the fifth minute of life; 6) Abnormal neurological status in the first 72 hours of life (abnormal muscle tone, reflexes, and consciousness); and 7) Multi-system perinatal failure. Neonates positive for infection, those with congenital malformations or metabolic disorders, and cases with artificial hemorrhage (traumatic hemorrhage at lumbar puncture) were excluded.
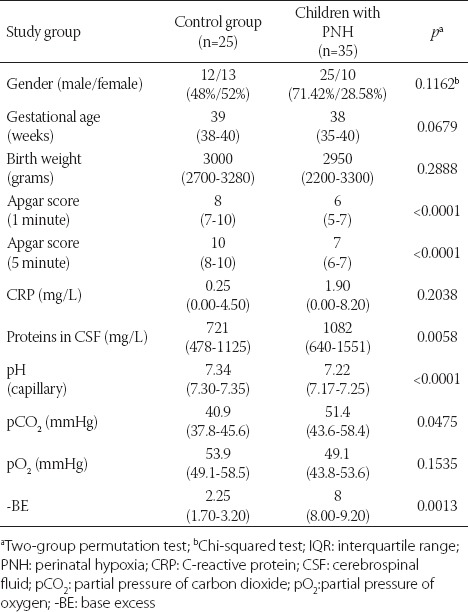
The control group consisted of infants who, being suspected of perinatal meningitis, underwent lumbar puncture and hematological evaluation, but for whom the test results were, hematologically and clinically, negative. The patients with PNH and control group did not differ significantly in body weight, gender, gestational age, and serum C-reactive protein (CRP) levels. However, the two groups differed significantly with regard to the Apgar score in the 1st and 5th minute of life, blood pH, partial pressure of carbon dioxide (pCO2), and base excess (-BE). Partial pressure of oxygen (pO2) did not differ significantly between the two groups, because PNH patients were artificially ventilated immediately after the birth.
Blood and CSF samples
Blood samples were collected at the age of 3-96 hours (average 37.5), and CSF samples were collected at the age of 5-96 hours (average 39.2). Both types of samples were centrifuged for three minutes at 14,000 rpm. The supernatants were stored at -20°C in the Chemical Laboratory of the Medical Faculty in Mostar, and then at -70°C at the Department of Physiology, School of Medicine, University of Zagreb, until analysis. Collection of CSF and serum samples from PNH patients was difficult in some cases due to the vulnerable condition of the infants, and some of these samples were quantitatively insufficient for determination of all cytokines. Out of 35 patients with clinical condition of PNH, 25 (71.4%) CSF and 27 (77.1%) serum samples were sufficient for detection of IL-6. In 11/35 (31.4%) CSF and 7/35 (20%) serum samples IL-1β was measured. In 12/35 (34.3%) CSF and serum samples IL-18 was measured. TNF-α was determined in 15/35 (42.3%) CSF and 16/35 (45.7%) serum samples.
Cytokine detection
Enzyme-linked immunosorbent assay (ELISA) was used for measuring cytokine levels in serum and CSF samples. Quantikine kits (R&D Systems, Minneapolis, USA) were used for detection of IL-6 and IL-1β, with minimum detectable concentrations below 0.70 pg/mL and 1 pg/mL, respectively. IL-18 was measured with sandwich ELISA kit (Diaclone Research, Bensancon, France) with minimum detectable concentrations below 45 pg/mL. TNF-α was also measured using specific ELISA kit (R&D Systems Minneapolis, MN, USA). The absorbance was analyzed using Dynatech MR5000 optical microplate reader (Dynatech, Billinghurst, Sussex, UK) with minimum detectable levels below 5.5 pg/mL.
Hypoxic-ischemic encephalopathy (HIE) and neurological outcome assessmentAll the patients with asphyxia/hypoxia were clinically observed during the neonatal and infant periods. In the acute phase of the illness, the level of HIE was estimated by the method of Sarnat/Sarnat [16] and classified as follows: 1 - mild HIE (1st Stage; excitation, exaggerated reflexes, frequent convulsions); 2 - moderate HIE (2nd Stage; lethargy, diminished reflexes, convulsions +/-); 3 - severe HIE (3rd Stage; stupor/coma, absence of reflexes, convulsions +).
Neuropediatricians and physiotherapists assessed neurological outcomes at the ages of 12 and 18 months using the Amiel-Tison method [17], and the patients were classified into three groups: 1 - normal outcome; 2 - mild motor impairment (abnormal muscle tonus); and 3 - adverse outcome (cerebral palsy or death). During the neonatal period, ultrasound examination of the brain was performed with 3.75, 5, and 6 MHz probes on a mobile Ultrasound Toshiba instrument (Japan, 1998, SSA-220 A model). A follow-up ultrasound was performed every week during the neonatal period, and after that once a month until the end of the first year of life. Cerebral ultrasound examination was performed with five standard sagittal and coronal views. According to the cranial sonography, brain involvement was classified as one of the following: 1 - normal; 2 - cerebral edema; 3 - hyperechogenic; 4 - intraventricular hemorrhage (IVH) [grades I-IV]; and 5 - ventriculomegaly [18].
Statistical analysis
Measured data are presented as medians and interquartile ranges (IQR), except gender data which are given as numbers and percentages. Cytokine values were not normally distributed, so we used non-parametric tests (Kruskal–Wallis H and two-sample permutation test) to compare PNH and control group, as well as to compare the cytokine levels at different stages of HIE and in relation to neurological outcomes [19]. Gender data were analyzed using χ2 test. All tests were two-tailed and results were considered statistically significant at p ≤ 0.05. Data analysis was performed using Statistica for Windows (version 7 StatSoft, Inc. STATISTICA Data Analysis Software System: http://www.statsoft.com) and PAST data analysis software (version 3.12 https://folk.uio.no/ohammer/past).
RESULTS
Basic perinatal data for PNH (n = 35) and control (n = 25) group are given in Table 1. In summary, the differences in Apgar score in the 1st and 5th minute, blood pH, -BE, and CSF proteins were observed between the two groups.
TABLE 1.
Basic perinatal data on newborns. Gender data are presented as numbers and percentages. Measured data are presented as medians and IQR
Infants with PNH were classified as having mild (n = 24), moderate (n = 9), and severe HIE (n = 2). Two infants (Stage 3 HIE) died at the age of three days due to multiorgan failure. At the end of the first week, 30/35 infants had abnormal ultrasound findings. Twelve of thirty infants (40%) had thalamus hyperechogenicity; 9/30 (30%) had diffuse cerebral edema; 4/30 (13.3%) had Grade 1 IVH and hyperechogenicity; 2/30 infants had Grade 3 IVH; and 3/30 infants had ventriculomegaly.
Of the 35 infants, 33 infants (94.3%) were followed for 12 and 18 months and their neurological outcomes was assessed according to the Amiel-Tison method. Among the 33 surviving infants, 6 (18.2%) had an abnormal motor outcome (4 with spastic tetraparesis, 2 with spastic paraparesis), 15 (45.4%) infants had mild motor impairment, and 12 (36.4%) had a normal motor outcome.
Significantly higher concentrations of IL-6 were found in serum samples of PNH patients compared with the control group (p = 0.04). No significant difference was observed in the concentration of IL-6 in CSF samples between PNH and control group (Table 2, Figure 1).
TABLE 2.
Median values and IQR of cytokines in CSF and serum samples of PNH patients and control group
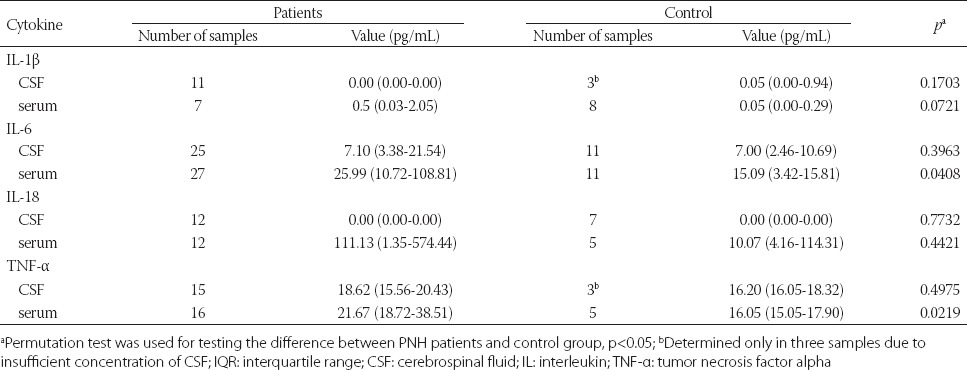
FIGURE 1.
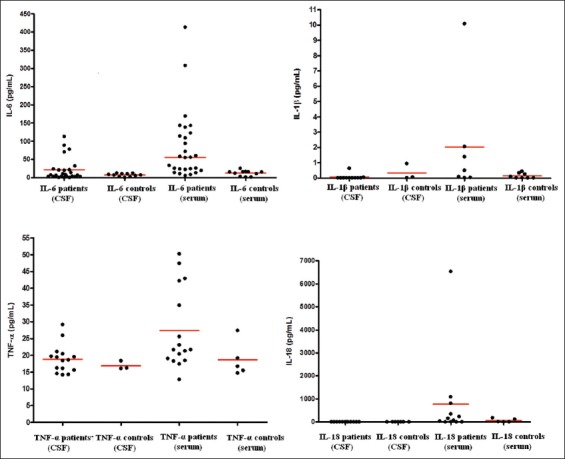
Levels of IL-6, IL-1β, TNF-α, and IL-18 in CSF and serum samples of PNH patients and control group. Individual values are plotted (in pg/mL) and horizontal bars indicate medians. The difference was statistically significant between patients and controls for IL-6 and TNF-α serum values (Permutation test; p<0.05). IL: interleukin; TNF-α: tumor necrosis factor alpha; CSF: cerebrospinal fluid; PNH: perinatal hypoxia.
In the CSF samples of PNH and control groups, IL-1β could not be measured. In the serum samples of both groups, higher levels of IL-1β were detected in PNH compared with the control group, but the difference was not statistically significant (Table 2, Figure 1).
The level of TNF-α was significantly higher in the serum samples of PNH patients compared with the control group (p = 0.02). However, no significant difference was observed in the level of TNF-α in the CSF samples, between the two groups.
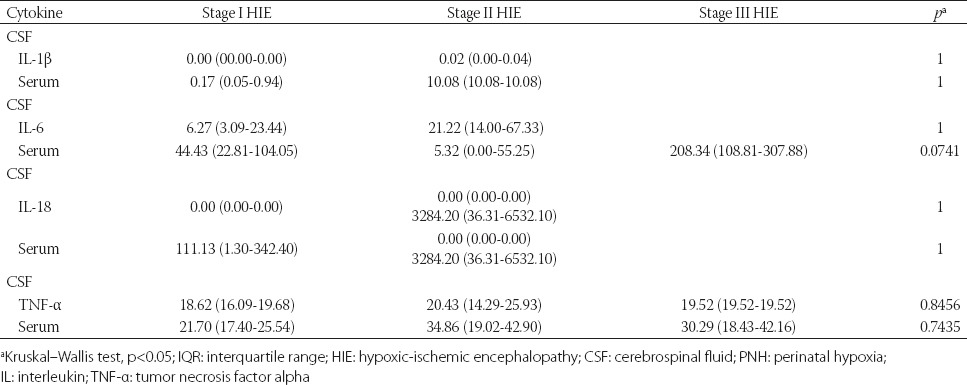
The level of IL-18 could not be measured in 12 CSF samples in PNH group and in 7 CSF samples in the control group. Higher levels of IL-18 were observed in the serum samples of PNH patients (n = 12) compared to the control group (n = 5); however, the difference was not statistically significant (Table 2). The cytokine (IL-1β, IL-6, TNF-α, and IL-18) values are presented in Figure 1. Although no significant difference was observed between the cytokine concentrations and HIE stage, higher values of IL-6 were observed in the serum samples of infants with Stage III HIE. In addition, higher serum concentrations of IL-18 and TNF-α were observed in children with Stage II HIE, but the differences were not statistically significant (Table 3).
TABLE 3.
Median values (pg/mL) and IQR of cytokines in CSF and serum samples of PNH newborns in relation to the stages of HIE, estimated with Sarnat/Sarnat method [16]
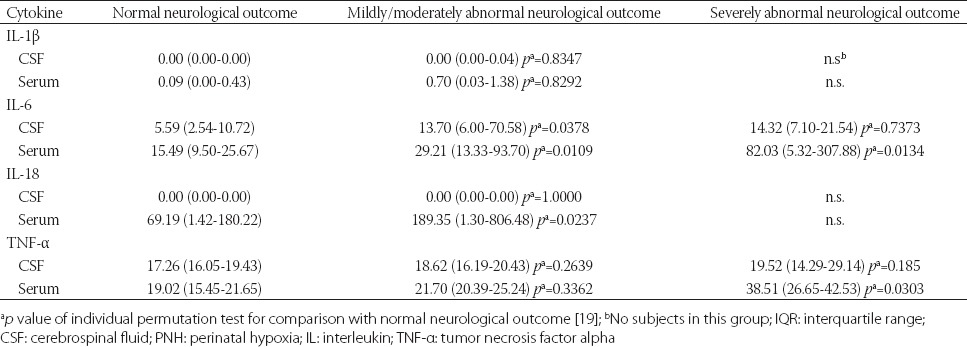
A statistically significant correlation was observed between median IL-6 values of CSF (p = 0.03) and serum samples (p = 0.01), median IL-18 values of serum samples (p = 0.02) and moderately abnormal neurological outcome, at 12 months of age. Also, statistically significant higher serum levels of IL-6 (p = 0.01) and TNF-α (p = 0.03) were observed in children with poor neurological outcome (Table 4).
TABLE 4.
Median values (pg/mL) and IQR of cytokines in CSF and serum samples of children with PNH in relation to neurological outcome at 12 months of age (estimated with the Amiel-Tison method) [17]
DISCUSSION
Clinically, newborn hypoxia is a very complex condition with a number of interfering factors. Consequently, the conclusions with regard to this condition can be context specific [20-23]. In this study we investigated the values of several cytokines from blood and CSF samples in PNH newborns in the first four days (96 hours) after the injury and in the control group. IL-6 was the most frequently observed cytokine, both in the serum (27/35) and CSF (25/35) samples. However, statistically significant differences were observed only for serum values of IL-6 and TNF-α between PNH and control group. No statistically significant differences were observed in CSF cytokine values between PNH and control group (Table 2).
It is not clear whether cytokines are produced in central nervous system (CNS) cells after injury, or do they come from systemic compartment. Following PNH, a newborn reacts systemically with multisystem involvement [24]. It is assumed that cytokines play a similar role in serum and cerebrospinal fluid [23,25,26]; however, the simultaneous cytokine expression in both compartments in PNH is still not sufficiently investigated [10-12]. Multiorgan dysfunction in post-asphyxial syndrome can cause a higher increase of cytokines in systemic (serum) compartment compared with brain compartment (CSF) [27,28]. A significant increase of IL-6 in the serum samples of PNH group compared with control group, observed in our study, is in agreement with this observation. Similar results have been found in other studies [29-31]. Moreover, some studies showed significantly higher values of IL-6 in CSF samples of children with asphyxia, but the serum samples were not tested in those studies [9,11,12,29]. These results indicate a direct production of IL-6 in injured tissue, i.e., brain. In another study, higher values of IL-6 were found in both, CSF and serum samples after a cerebral insult, with CSF IL-6 concentrations being higher compared to the serum concentrations [32]. Sampling time is considered an important factor in the assessment of cytokine values after the injury, due to the short half-life of cytokines [27]. In this study, we investigated cytokine levels only at one time point, and these levels could have a decreasing trend in the period after hypoxia. In addition, therapy procedures, such as hypothermia therapy, can influence cytokine levels [33].
IL-1β and TNF-α are the best characterized early response cytokines and are often expressed concurrently [34-36]. In this study, IL-1β was not detected neither in serum nor in CSF samples of PNH patients. Because our samples were collected 24 hours after the injury, this could be a reason why measurable IL-1β levels were not detected. Sävman et.al. [29] showed similar results in their study. On the other hand, some other studies demonstrated increased levels of IL-1β in serum samples of children with PNH, but these studies did not analyze CSF samples [31,37]. IL-1β and TNF-α can affect the progression of injury, as well as the process of healing, by stimulating the synthesis of other cytokines. This, however, depends on a wide range of interactive factors in the early stage of recovery after brain injury [38].
Other research on the levels of TNF-α in PNH samples showed heterogeneous results. While some studies found significantly higher levels of TNF-α in CSF samples, other studies demonstrated higher TNF-α levels in serum samples of hypoxic newborns [11,12]. In addition, some studies did not find increased levels of TNF-α after hypoxic injury [31,38]. In their study, Aly et al. confirmed increased levels of TNF-α in both, serum and CSF samples [12]. Significantly higher values of TNF-α in the PNH serum samples (but not in the CSF samples) in our study, confirm that this type of cytokine can be found in the blood circulation 30 hours after a hypoxic injury. TNF-α (together with IL-1β) is the earliest response cytokine in brain injury, however, the neurotoxic and neuroprotective functions of TNF-α are still not clear [2,9,11,29,39].
IL-18 is anti-inflammatory and immune stimulating cytokine. IL-18 has a crucial role in mediating neuroinflammation and neurodegeneration in the CNS in pathological conditions [40]. Our analysis confirmed increased levels of IL-18 in the serum of patients with PNH (median 111.13 pg/mL), compared to control group (median 10.7 pg/mL). We assume that the observed difference is not statistically significant due to the small sample size. In the study of Minagawa et al. [41], increased levels of IL-18 in umbilical blood samples of newborns correlated with the white matter damage and cerebral palsy in these patients [41]. In another study, increased concentrations of IL-18 in CSF samples of premature newborns with posthemorrhagic hydrocephalus correlated with the level of posthemorrhagic hydrocephalus and cystic white matter damage (cWMD) [42]. At the moment, we cannot explain why IL-18 was detected only in our serum, and not CSF samples.
It is not clear in what way and to what extent the BBB regulates the passage of IL-18 between the two compartments. It is also not clear to what extent brain immaturity or pathological conditions can influence or change the permeability of BBB to cytokines. The transport through BBB is probably different for different types of cytokines [43-45]. Although the transport of cytokines from serum to CSF and brain interstitium and from CSF to serum is described, the exact mechanism is still not clear [46,47].
Even though slightly higher values of IL-6 in the CSF, and IL-1β, IL-18 and TNF-α in the serum samples were observed in the children with Stage II HIE, in general, we did not observe significantly higher concentrations of cytokines in relation to different stages of hypoxia. This may be due to the small sample size but also owing to the fact that the majority of our patients had mild hypoxia. Several other studies compared the levels of cytokines with different stages of HIE [9,11,12,29,30], confirming the role of cytokines as biomarkers of early hypoxic injury. Further studies are required for a more comprehensive conclusion on the correlation between elevated concentrations of cytokines and the degree of hypoxic-ischemic injury to the brain and whole organism.
Our analysis confirms the results of previous studies on the association between elevated levels of cytokines and poor neurological outcomes [8,11,29-31,37]. We found a significantly higher concentration of IL-6 in the CSF and serum samples, and IL-18 in the serum samples of PNH infants with moderately abnormal neurological outcome. Also, we observed significantly higher IL-6 and TNF-α serum levels in the hypoxic newborns in relation to a severely abnormal neurological outcome at 12 months of age. These results are consistent with previous findings that cytokine levels are elevated in children with an abnormal neurological outcome at 12 months of age [8,37]. Overall, according to our results, elevated serum levels of IL-6, TNF-α, and IL-18 better corresponded to an abnormal neurological outcome at 12 months of age compared to CSF cytokine levels. To better understand the cytokine production in perinatal hypoxia and the effects on HIE and late neurological outcomes, further clinical studies are required in both compartments in a human model.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
We greatly appreciate the laboratory assistance of Dr Ljiljana Poljak, and Dr Gordana Horvat, at the Department of Physiology, School of Medicine, University of Zagreb.
The research was supported by Grant of Federal Ministry of Education and Science, Federation of Bosnia and Herzegovina (N0: 04-39-4893/5) in 2005/6 and by Grant from Ministry of Sciences, Higher Education and Sports of Republic of Croatia (No: 219-0000000-0328).
REFERENCES
- [1].Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, et al. Production of cytokines following brain injury: Beneficial and deleterious for the damaged tissue. Mol Psychiatr. 1997;2(2):133–6. doi: 10.1038/sj.mp.4000227. https://doi.org/10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- [2].Hagberg H, Gilland E, Bona E, Hanson LA, Hahin-Zoric M, Blennow M, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr Res. 1996;40(4):603–9. doi: 10.1203/00006450-199610000-00015. https://doi.org/10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- [3].Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26(6):1093–100. doi: 10.1161/01.str.26.6.1093. https://doi.org/10.1161/01.STR.26.6.1093. [DOI] [PubMed] [Google Scholar]
- [4].Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53(4):600–7. doi: 10.1203/01.PDR.0000056802.22454.AB. https://doi.org/10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- [5].Miller LC, Isa S, LoPreste G, Schaller JG, Dinarello CA. Neonatal interleukin-1β, interleukin-6 and tumor necrosis factor: Cord blood levels and cellular production. J Pediatr. 1990;117(6):961–5. doi: 10.1016/s0022-3476(05)80145-3. https://doi.org/10.1016/S0022-3476(05)80145-3. [DOI] [PubMed] [Google Scholar]
- [6].Dammann O, O’Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35(4):643–63. doi: 10.1016/j.clp.2008.07.011. https://doi.org/10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Megyeri P, Abrahám CS, Temesvári P, Kovács J, Vas T, Speer CP. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett. 1992;148(1-2):137–40. doi: 10.1016/0304-3940(92)90823-p. https://doi.org/10.1016/0304-3940(92)90823-P. [DOI] [PubMed] [Google Scholar]
- [8].Martin-Ancel A, García-Alix A, Pascual-Salcedo D, Cabañas F, Valcarce M, Quero J. Interleukin-6 in the cerebrospinal fluid after perinatal asphyxia is related to early and late neurological manifestations. Pediatrics. 1997;100(5):789–94. doi: 10.1542/peds.100.5.789. https://doi.org/10.1542/peds.100.5.789. [DOI] [PubMed] [Google Scholar]
- [9].Oygur N, Sonmez O, Saka O, Yegin O. Predictive value of plasma and cerebrospinal fluid tumor necrosis factor-alpha and interleukin-1 beta concentrations on outcome of full term infants with hypoxic-ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F190–3. doi: 10.1136/fn.79.3.f190. https://doi.org/10.1136/fn.79.3.F190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines and brain injury in term infants. Pediatrics. 2002;110(4):673–80. doi: 10.1542/peds.110.4.673. https://doi.org/10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- [11].Silveira RC, Procianoy RS. Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J Pediatr. 2003;143(5):625–9. doi: 10.1067/S0022-3476(03)00531-6. https://doi.org/10.1067/S0022-3476(03)00531-6. [DOI] [PubMed] [Google Scholar]
- [12].Aly H, Khashaba MT, El-Ayouty M, El-Sayed O, Hasanein BM. IL-1beta IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28(3):178–82. doi: 10.1016/j.braindev.2005.06.006. https://doi.org/10.1016/j.braindev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [13].Hedtjärn M, Mallard C, Arvidsson P, Hagberg H. White matter injury in the immature brain: Role of interleukin-18. Neurosci Lett. 2005;373(1):16–20. doi: 10.1016/j.neulet.2004.09.062. http://dx.doi.org/10.1016/j.neulet.2004.09.062. [DOI] [PubMed] [Google Scholar]
- [14].Hedtjärn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22(14):5910–9. doi: 10.1523/JNEUROSCI.22-14-05910.2002. https://dx.doi.org/20026587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Relationship between perinatal factors and neurologic outcome. In: Poland RL, Freeman RK, editors. Guidelines for perinatal care. 3rd ed. USA, IL: Elk Grove Village AAP; 1992. pp. 221–4. [Google Scholar]
- [16].Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. DOI: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- [17].Amiel-Tison C, Ellison P. Birth asphyxia in the fullterm newborn: Early assessment and outcome. Dev Med Child Neurol. 1986;28(5):671–82. doi: 10.1111/j.1469-8749.1986.tb03914.x. https://dx.doi.org/10.1111/j.1469-8749.1986atb03914.x. [DOI] [PubMed] [Google Scholar]
- [18].Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34. doi: 10.1016/s0022-3476(78)80282-0. https://doi.org/10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- [19].Good PI. Analyzing the large number of variables in biomedical and satellite imagery. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2011. https://doi.org/10.1002/9780470937273.ch2. [DOI] [PubMed] [Google Scholar]
- [20].Farber JL, Chien KR, Mittnacht S., Jr Myocardial ischemia: The pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981;102(2):271–81. [PMC free article] [PubMed] [Google Scholar]
- [21].Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41(5):599–606. doi: 10.1203/00006450-199705000-00001. https://doi.org/10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- [22].Johnston MV, Trescher WH, Ishida A, Nakajima W, Zipursky A. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49(6):735–41. doi: 10.1203/00006450-200106000-00003. https://doi.org/10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- [23].Vanucci RC. Experimental biology of cerebral hypoxia-ischemia: Relation to perinatal brain damage. Pediatr Res. 1990;27(4 Pt 1):317–26. doi: 10.1203/00006450-199004000-00001. https://doi.org/10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- [24].Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F152–5. doi: 10.1136/adc.2002.023093. https://dx.doi.org/10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clark WM. Cytokines and reperfusion injury. Neurology. 1997;49(5 Suppl 4):S10–4. doi: 10.1212/wnl.49.5_suppl_4.s10. https://doi.org/10.1212/WNL.49.5_Suppl_4S10. [DOI] [PubMed] [Google Scholar]
- [26].Cohen MC, Cohen S. Cytokine function: A study in biologic diversity. Am J Clin Pathol. 1996;105(5):589–98. doi: 10.1093/ajcp/105.5.589. https://doi.org/10.1093/ajcp/105.5.589. [DOI] [PubMed] [Google Scholar]
- [27].Hill A, Volpe JJ. Pathogenesis and management of hypoxic-ischemic encephalopathy in the term newborn. Neurol Clin. 1985;3(1):31–46. [PubMed] [Google Scholar]
- [28].Dammann D, Leviton A. Brain damage in preterm newborns: Biological response modification as a strategy to reduce disabilities. J Pediatr. 2000;136(4):433–8. doi: 10.1016/s0022-3476(00)90004-0. https://doi.org/10.1016/S0022-3476(00)90004-0. [DOI] [PubMed] [Google Scholar]
- [29].Sävman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res. 1998;43(6):746–51. doi: 10.1203/00006450-199806000-00006. https://dx.doi.org/10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- [30].Boskabadi H, Afshari JT, Ghayour-Mobarhan M, Maamouri G, Shakeri MT, Sahebkar A, et al. Association between serum interleukin-6 levels and severity of perinatal asphyxia. Asian Biomed. 2010;4(1):79–85. [Google Scholar]
- [31].Fotopoulos S, Pavlou K, Skouteli H, Papassotiriou I, Lipsou N, Xanthou M. Early markers of brain damage in premature low-birth-weight neonates who suffered from perinatal asphyxia and/or infection. Biol Neonate. 2001;79(3-4):213–8. doi: 10.1159/000047094. https://doi.org/10.1159/000047094. [DOI] [PubMed] [Google Scholar]
- [32].Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995;26(8):1393–8. doi: 10.1161/01.str.26.8.1393. https://doi.org/10.1161/01.STR.26.8.1393. [DOI] [PubMed] [Google Scholar]
- [33].Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cerebr Blood Flow Metab. 2012;32(10):1888–96. doi: 10.1038/jcbfm.2012.83. https://doi.org/10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochem Int. 1997;30(4-5):375–83. doi: 10.1016/s0197-0186(96)00072-1. https://doi.org/10.1016/S0197-0186(96)00072-1. [DOI] [PubMed] [Google Scholar]
- [35].Herx LM, Rivest S, Yong VW. Central nervous system initiated inflammation and neurotrophism in trauma IL-1βis required for the production of ciliary neurotrophic factor. J Immunol. 2000;165(4):2232–9. doi: 10.4049/jimmunol.165.4.2232. https://doi.org/10.4049/jimmunol.165.4.2232. [DOI] [PubMed] [Google Scholar]
- [36].Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF-αpromotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116–22. doi: 10.1038/nn738. https://doi.org/10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- [37].Foster-Barber A, Dickens B, Ferriero DM. Human perinatal asphyxia: Correlation of neonatal cytokines with MRI and outcome. Dev Neurosci. 2001;23(3):213–8. doi: 10.1159/000046146. https://doi.org/10.1159/000046146. [DOI] [PubMed] [Google Scholar]
- [38].Yamasaki Y, Shozuhara H, Onodera H, Kogure K. Blocking of interleukin-1 activity is beneficial approach to ischemia brain edema formation. Acta Neurochir Suppl (Wien) 1994;60:300–2. doi: 10.1007/978-3-7091-9334-1_80. https://doi.org/10.1007/978-3-7091-9334-1_80. [DOI] [PubMed] [Google Scholar]
- [39].Gubits RM, Burke RE, Casey-McIntosh G, Bandele A, Munell F. Immediate early gene induction after neonatal hypoxia-ischemia. Brain Res Mol Brain Res. 1993;18(3):228–38. doi: 10.1016/0169-328x(93)90194-t. https://doi.org/10.1016/0169-328X(93)90194-T. [DOI] [PubMed] [Google Scholar]
- [40].Felderhoff –Mueser U, Schmidt OI, Oberholzer A, Bührer C, Stahel PF. IL-18: A key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005;28(9):487–93. doi: 10.1016/j.tins.2005.06.008. https://dx.doi.org/10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [41].Minagawa K, Tsuji Y, Ueda H, Koyama K, Tanizawa K, Okamura H, et al. Possible correlation between high levels of IL-18 in the cord blood of pre-term infants and neonatal development of periventricular leukomalacia and cerebral palsy. Cytokine. 2002;17(3):164–70. doi: 10.1006/cyto.2001.0988. https://doi.org/10.1006/cyto.2001.0988. [DOI] [PubMed] [Google Scholar]
- [42].Schmitz T, Heep A, Groenendaal F, Hüseman D, Kie S, Bartmann P, et al. Interleukin-1β, interleukin-18, and interferon-γexpression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus - markers of white matter damage? Pediatr Res. 2007;61(6):722–6. doi: 10.1203/pdr.0b013e31805341f1. http://dx.doi.org/10.1203/pdr.0b013e31805341f1. [DOI] [PubMed] [Google Scholar]
- [43].Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS. 2011;8(1):3. doi: 10.1186/2045-8118-8-3. https://doi.org/10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. https://doi.org/10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- [45].Chen X, Threlked SW, Cummings EE, Juan I, Makeyev O, Besio WG, et al. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience. 2012;226:89–100. doi: 10.1016/j.neuroscience.2012.08.043. https://doi.org/10.1016/j.neuroscience.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57(2):282–6. doi: 10.1203/01.PDR.0000148286.53572.95. https://doi.org/10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- [47].Banks WA. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr Pharm Des. 2005;11(8):973–84. doi: 10.2174/1381612053381684. https://doi.org/10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]


