Abstract
The newly proposed nomenclature and diagnostic criteria for encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC), the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), could improve the consistency and accuracy of diagnosing this entity. Diagnosis of NIFTP requires evaluation of the complete tumor border or capsule. The presence of tumor invasion in follicular thyroid neoplasms with papillary-like nuclear features has been recently discussed by many authors. In this study, we examined the predictive value and association of follicular morphological characteristics with the tumor invasion. In addition, we analyzed the association between tumor encapsulation and molecular profile in EFVPTC/NIFTP cases. A total of 106 cases of FVPTC were included in the study. The tumors were grouped based on the presence of tumor capsule and characteristics of tumor border, as 1) completely encapsulated tumors without invasion, 2) encapsulated tumors with invasion, 3) infiltrative tumors without a capsule. Clinicopathological features, histomorphological features [nuclear criteria, minor diagnostic features, follicles oriented perpendicular to tumor border/capsule (FOPBC)] and molecular alterations in BRAF, NRAS, and KRAS genes were evaluated. FOPBC were significantly more frequently seen in encapsulated tumors with invasion (p = 0.008). The nuclear features were not associated with the presence of encapsulation and characteristics of tumor border. BRAF mutation was more frequent in infiltrative tumors, while NRAS mutation was more frequent in encapsulated tumors, but the results were not statistically significant (p = 0.917). In conclusion, FOPBC histomorphological feature may be associated with tumor invasion in EFVPTC/NIFTP. Additionally, BRAF/KRAS/NRAS mutation analysis may prevent inadequate treatment in these patients.
KEY WORDS: Noninvasive follicular thyroid neoplasm with papillary-like nuclear features, NIFTP, EFVPTC, histomorphological features, follicular variant of papillary thyroid carcinoma, FVPTC, NRAS, BRAF, mutation
INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer. The incidence of PTC has increased in the past few decades due to improved detection and diagnosis of subclinical thyroid cancers as well as environmental factors [1-3]. Several histological variants of PTC have been described in the literature, considering different criteria such as tumor size, architecture (follicular, macrofollicular, cribriform-morular, solid, and micropapillary), cellular characteristics (tall cell, columnar, oncocytic, clear cell, and hobnail), presence of tumor capsule (encapsulated and nonencapsulated), additional tumor components, stromal features, or a combination of these criteria [4-6]. In 1960, follicular variant of papillary thyroid carcinoma (FVPTC) was introduced [7,8], and became widely recognized by the late 1970s [9]. One of the characteristics of FVPTC is follicular neoplasm composed of thyroid epithelial cells with nuclear features of papillary carcinoma [9].
FVPTC can be classified into three subtypes according to the presence of tumor capsule and invasive growth patterns: Encapsulated FVPTC (EFVPTC), nonencapsulated infiltrative FVPTC (IFVPTC), and nonencapsulated diffuse or multinodular FVPTC [10]. The third subtype is uncommon and has an aggressive clinical course. The diagnosis of EFVPTC is challenging; in the absence of invasion, it is based on nuclear features. If nuclear features of papillary carcinoma are not well developed and diffusely distributed it is difficult to make differential diagnosis between EFVPTC and follicular adenoma (FA). Due to the lack of well-established criteria for FVPTC diagnosis in such borderline cases, a marked interobserver variation has been demonstrated. In addition, previous studies showed that, in noninvasive EFVPTC, the lesions are predominantly neoplasms rather than hyperplastic lesions [3,11].
Increased surveillance of thyroid nodules and improvements in detection methods have resulted in a significant increase in the reported incidence of PTC. Moreover, a large number of these nodules were diagnosed as FVPTC, particularly EFVPTC [7,3,12-14].
Although classified as PTC, EFVPTC has a number of overlapping features with other benign or malignant follicular-patterned lesions (i.e., FA or follicular carcinoma [FC]) [15,16,3]. In addition, FVPTC often has an indolent clinical course in contrast to conventional PTC (CPTC) and other, more aggressive, variants such as tall cell variant (TCV) of PTC [1,17-19].
Different molecular alterations are detected in different variants of PTC. For example, RET translocation and BRAFV600E mutations are associated with CPTC, while RAS (H, N, or KRAS) and PAX8/PPARγ mutations are frequent in follicular-pattern tumors, including FA, FC, and EFVPTC [20-22].
Despite a number of biological differences, the same treatment is often used for patients with EFVPTC and those with CPTC, which includes removing the rest of the thyroid (completion thyroidectomy) or adjuvant radioactive iodine (RAI) therapy [23]. Nevertheless, it is suggested that patients with noninvasive EFVPTC will not likely benefit from immediate completion thyroidectomy and RAI therapy [3].
To improve diagnostic accuracy and treatment options for PTC patients, recently, a new nomenclature has been developed for the lesions classified as noninvasive EFVPTC [3]. The nomenclature includes the main histopathological features of these lesions, such as the absence of invasion, follicular growth pattern, nuclear features of PTC, and clonal origin indicating that the lesion is a neoplasm. In addition, a new term was proposed for this type of PTC “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” [3].
The presence of vascular or capsular invasion in EFVPTC/NIFTP has been recently discussed by many authors, especially in tumors larger than 10 mm [3,7]. In this study, we examined the predictive value and association of follicular morphological characteristics with the tumor invasion. In addition, we analyzed the association between tumor encapsulation and molecular profile in EFVPTC/NIFTP cases.
MATERIALS AND METHODS
Patient selection
We reviewed the medical reports of patients who presented at the Department of Pathology, Faculty of Medicine of Trakya University, between August 2007 and August 2014. The study protocol was approved by the local Ethics Committee of the Hospital of Trakya University (Edirne). Patients selected for the study had either been diagnosed with FVPTC or had undergone a lobectomy or total thyroidectomy with or without central neck lymph node dissection. The exclusion criteria were: Diagnosis of a different variant of thyroid carcinoma including diffuse or multinodular FVPTC, the presence of >1% of “true” papillae, and partial sampling of tumor specimens during the re-evaluation of the slides. A total of 106 patients who matched the inclusion criteria were included in the study. Data on age at the time of diagnosis, sex, RAI therapy, and clinical follow-up (recurrence of disease, re-operation, and retreatment with RAI) were obtained from the records of the Department of Clinical Endocrinology and Metabolism Diseases, Department of Nuclear Medicine and the Department of General Surgery, Trakya University.
The BRAF mutation status of 106 patients and NRAS/KRAS mutation status of 38 patients were obtained from the records at the Department of Medical Genetics and Laboratory of Molecular Pathology, Department of Pathology, Trakya University. Data on the number and size of tumors were retrieved from the hospital database. Sections were obtained from paraffin-embedded blocks of tumor tissue and stained with hematoxylin and eosin [H&E] (≥6 sections per nodule). The H&E slides were re-evaluated by one pathologist who was blinded to the diagnosis, clinical, and prognostic data (NC). In multifocal tumors, histopathological features were evaluated based on the largest tumor focus.
Clinicopathological criteria
The following diagnostic criteria were used for FVPTC [3]:
Predominantly follicular architecture (≤1% “true” papillae);
Nuclear features of PTC:,
Nuclear score of 2-3 according to alterations in nuclear size and shape;
Nuclear size and shape (nuclear enlargement, overlapping, and elongation);
Nuclear membrane irregularities (irregular nuclear contours, nuclear grooves, and intranuclear pseudoinclusions);
-
Chromatin characteristics (chromatin clearing/glassy nuclei and peripheral margination of the chromatin).
The exclusion criteria for FVPTC were [3]:
Necrosis;
Solid/trabecular/cribriform growth patterns, tall cell, or columnar cell features;
More than 3 mitoses per 10 high-power fields (HPFs).
FVPTCs completely surrounded by a fibrous capsule or clearly demarcated from the surrounding thyroid tissue were reclassified as EFVPTC/NIFTP. Encapsulated tumors with capsular invasion (CI) or lymphovascular invasion (LVI) and tumors with irregular margins or structures penetrating into the adjacent thyroid tissue were classified as IFVPTC.
All tumor samples were re-evaluated based on the above criteria and classified into three groups according to the presence of tumor capsule, tumor border configuration, and the presence of LVI or CI:
Group 1 - Encapsulated tumors without LVI or CI and tumors with regular border (Figure 1A and B).
Group 2 - Encapsulated tumors with LVI or CI (Figure 1C and D).
Group 3 - Infiltrative tumors without a capsule (truly infiltrative).
FIGURE 1.
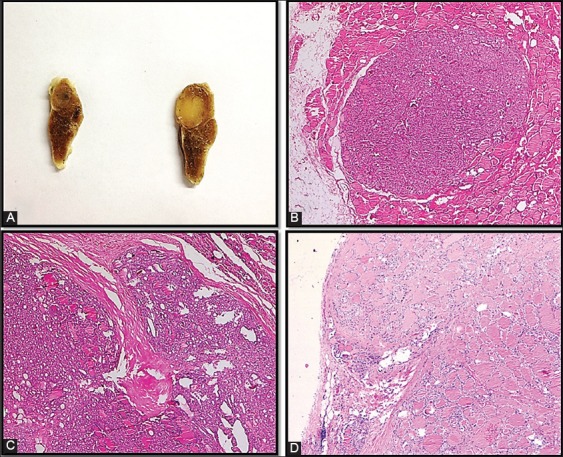
(A) Gross appearance of encapsulated tumor; (B) encapsulated tumor well demarcated from the surrounding thyroid (Nikon Eclipse 80i, hematoxylin and eosin (H&E) ×200); (C-D) capsular invasion (Nikon Eclipse 80i, H&E ×40).
Follicles oriented perpendicular to the tumor border/capsule (FOPBC) were defined as elongated follicles (at least one follicle per HPS with length twice as large as that of the neighboring follicle) aligned perpendicular to the tumor border or capsule without pushing or penetrating the tumor border or capsule (Figure 2A and B). Intratumoral fibrosis was characterized by the presence of fibrous foci not associated with hemorrhage or cholesterol clefts. Nuclear scoring was performed according the criteria proposed by Nikiforov et al. [3] (Figure 2C).
FIGURE 2.
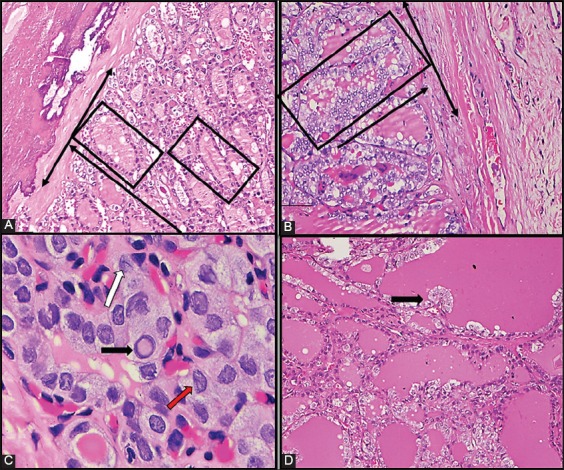
(A-B) Follicles oriented perpendicular to the tumor capsule [marked with lines] (Nikon Eclipse 80i, H&E ×200); (C) membrane irregularities: Accurate intranuclear pseudoinclusions (black arrow), nuclear groove (white arrow), irregular contour [red arrow] (Nikon Eclipse 80i, H&E, immersion objective); (D) abortive papillae formation [black arrow] (Nikon Eclipse 80i, H&E ×100).
In tumors ≤10 mm in size, the whole tumor area was sampled for histopathological examination, while in tumors >10 mm the surface of tumor capsule and adjacent thyroid tissue were sampled. CI was defined as tumor surrounded by a thin fibrous capsule extending to the adjacent thyroid tissue [24]. LVI was defined as tumor cells invading through a vessel wall and the presence of thrombus adherent to intravascular tumor [25,26]. The H&E slides (at least 6 slides per nodule, but deeper serial sections were evaluated when necessary) of the tumor samples were reviewed for the presence of minor diagnostic features [abortive papillae (Figure 2D), multinucleated giant cells (MGCs), and intratumoral fibrosis] FOPBC, CI (for tumors with a fibrous capsule), and LVI.
The clinical features considered in the statistical analysis were age at the time of diagnosis (<45 years and ≥45 years) and sex (male or female). Histopathological features included nuclear score, presence or absence of CI (for tumors with a fibrous capsule), LVI, minor diagnostic features (MGCs, intratumoral fibrosis, abortive papillae), FOPBC, and clinicopathological features including tumor size (≤10 mm and >10 mm), tumor focality (unifocal, multifocal), tumor laterality (unilateral, bilateral), extrathyroidal extension (ETE) [absent, present], and lymph node metastasis (LNM) [absent, present].
BRAF and K/NRAS mutation analysis
For BRAF analysis, tumor tissue containing at least 70% of tumor cells was isolated from the sections of 106 patients and for KRAS and NRAS analysis the tissue samples were isolated from 38 patients. DNA purification was performed using DNA isolation kits for DNA samples from formalin-fixed, paraffin-embedded tissue sections (QIAampÒ DNA FFPE Tissue Kit, EZ1Ò DNA Tissue Kit, PAXgeneÒ Tissue Containers, and PAXgene Tissue DNA Kit, Qiagen, Germany). Following polymerase chain reaction (PCR), pyrosequencing analysis was performed on a PyroMark Q24 device (Qiagen, Germany), using Seq Primer BRAF 600 or Seq Primer BRAF 464–469 (Qiagen, Germany) for BRAF, KRAS Pyro Kit (Qiagen, Germany) for KRAS (Codon 12, 13, and 61), and NRAS Pyro Kit (Qiagen, Germany) for NRAS (Codon 12, 13, and 61).
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics for Windows Version 20.0. (IBM Corp., Armonk, NY, USA). Chi-squared test, Pearson correlation coefficient, Yates’ correction, or Fisher’s exact test were used to compare the clinicopathological, molecular, nuclear, minor diagnostic features and FOPBC with the presence of capsule and tumor border configuration. Kruskal–Wallis H test was used for comparing numerical data (age, nuclear scores, and the number of tumor foci) with the presence of capsule and tumor border configuration. A p value <0.05 was considered statistically significant.
RESULTS
Clinicopathological features of patients in the study group
The clinicopathological features of the patients are summarized in Table S1. The mean age of the patients was 50.03 ± 11.2 years. The number of patients aged <45 years was 33 (31.1%), while the number of patients ≥45 years old was 73 (68.9%). Out of the 106 patients, 94 (88.7%) were female and 12 (11.3%) were male. When the tumors were grouped according to the presence of a capsule and capsular or vascular invasion, the number and percentage of patients in each group was as follows: 64 patients (60.3%) in group 1 (encapsulated/well-circumscribed tumors without LVI or CI); 17 patients (16%) in group 2 (encapsulated tumors with LVI or CI); and 25 patients (23.6%) in group 3 (infiltrative tumors without a capsule).
TABLE S1.
Clinicopathological features of patients in the study group.
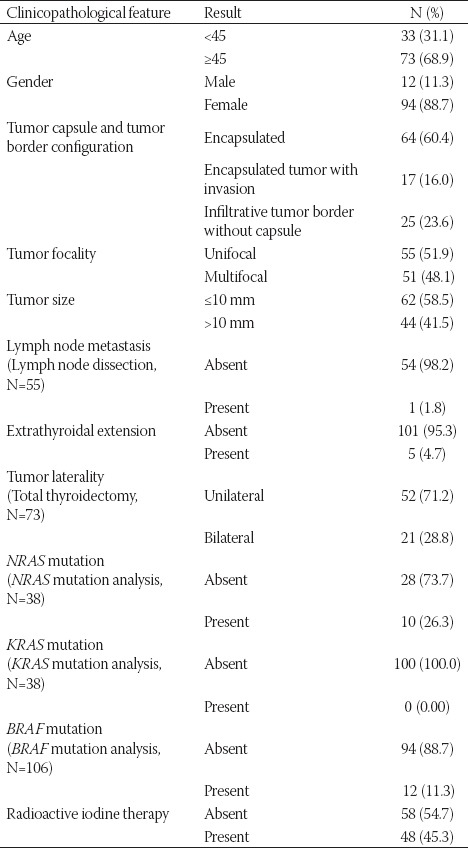
When the patients were grouped according to the tumor size, 62 (58.5%) patients had microcarcinoma, and the tumor size was >10 mm in 44 (41.5%) cases. Multifocality was identified in 51 (48.1%) cases. The tumor was limited to the thyroid in 101 (95.3%) patients, while ETE was present in 5 (4.7%) patients. LNM was observed in 1 (1.8%) patient out of 55 patients who had lymph node dissection. Tumor was present in bilateral thyroid lobes in 21 (28.8%) patients out of 73 who underwent total thyroidectomy. The mean follow-up period for all patients was 16.3 ± 13.3 months. RAI therapy was given to 48/106 (45.3%) patients. None of the patients had distant metastasis or recurrence. All patients were alive at the time of conducting this study.
BRAF mutation was detected in 12/106 (11.3%) cases. NRAS mutation (Q61R) was present in 10/38 (26.3%) patients. None of the 38 patients had KRAS mutation.
Clinicopathological characteristics in relation to the presence of tumor capsule and tumor border configuration
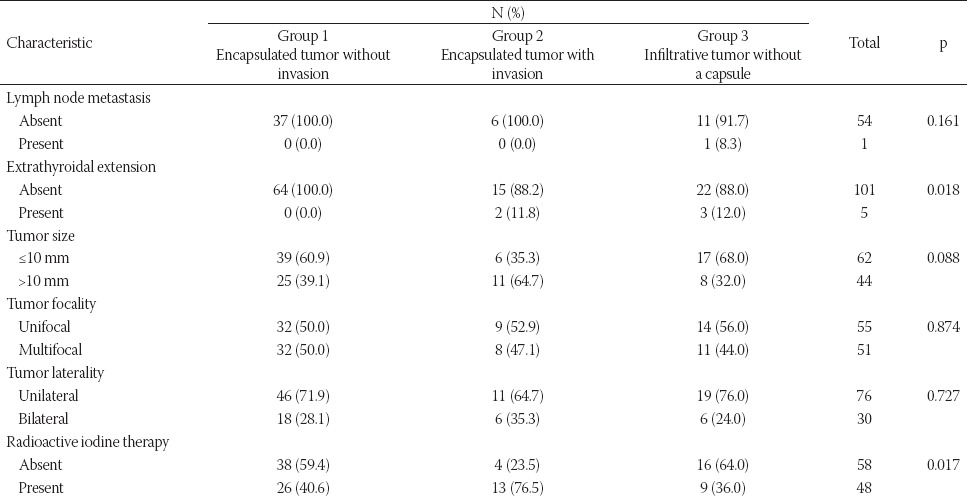
A comparison of clinicopathological features with the presence of tumor capsule and tumor border configuration is presented in Table 1. ETE was not observed in encapsulated tumors without CI or LVI (p = 0.018). RAI therapy was more commonly indicated in encapsulated tumors with CI or LVI compared to encapsulated without CI or LVI or infiltrative tumors without a capsule (p = 0.017). Both ETE and RAI therapy were significantly related to the presence of tumor capsule and tumor border configuration. Encapsulated tumors with CI or LVI were >10 mm in size more frequently than encapsulated without CI or LVI or infiltrative tumors without a capsule, but this result was not significant.
TABLE 1.
Clinicopathological characteristics in relation to the presence of tumor capsule and tumor border configuration
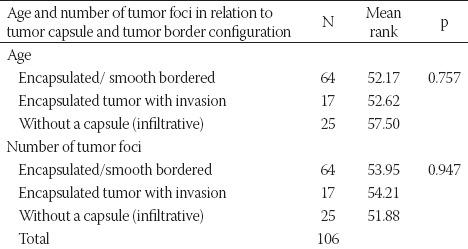
No significant relationship was observed between the number of tumor foci, patient age, the presence of tumor capsule, and tumor border configuration (Table S2).
TABLE S2.
Patient age and number of tumor foci in relation to tumor capsule and tumor border configuration
Nuclear features in relation to the presence of tumor capsule and tumor border configuration
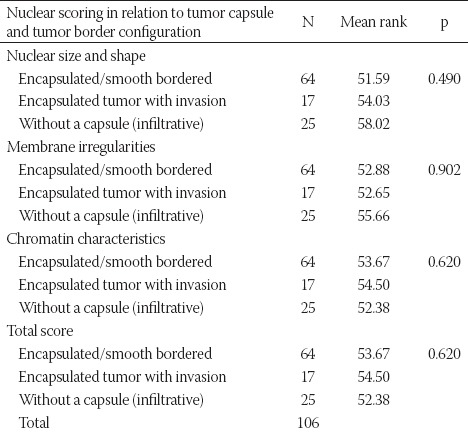
The nuclear size and shape characteristics (nuclear enlargement, elongation, and overlapping) and nuclear membrane irregularities (irregular nuclear contours, nuclear grooves, and intranuclear pseudoinclusions) were scored separately and as a group. The chromatin characteristics were scored only as a group due to the possibility of subjective evaluation. No significant relationship was observed between the nuclear features and nuclear score and the presence of tumor capsule and tumor border configuration (Table 2, Table S3).
TABLE 2.
Nuclear features in relation to the presence of tumor capsule and tumor border configuration
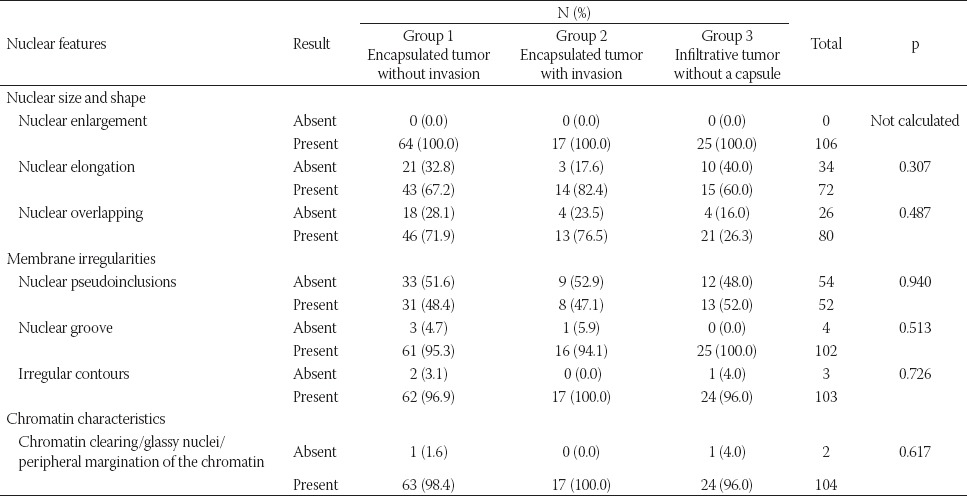
TABLE S3.
Nuclear scoring in relation to tumor capsule and tumor border configuration
Minor diagnostic features and FOPBC in relation to the presence of tumor capsule and tumor border configuration
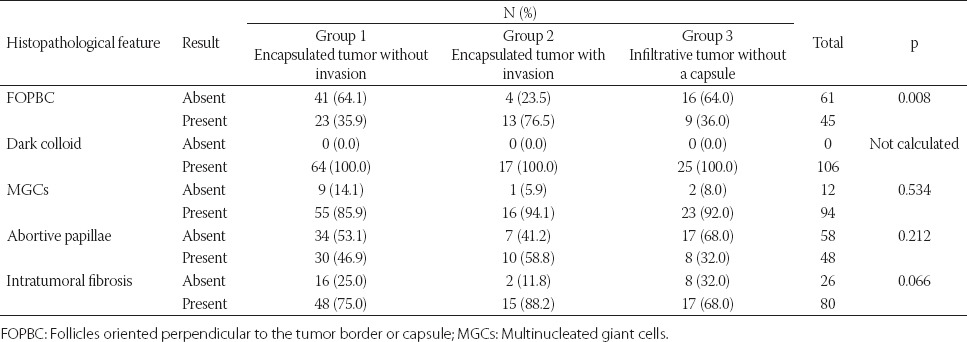
A comparison of minor diagnostic features and FOPBC with the presence of tumor capsule and tumor border configuration is presented in Table 3. The presence of FOPBC was significantly associated with the presence of tumor capsule and tumor border configuration (p = 0.008). FOPBC feature was more common in encapsulated tumors with CI or LVI compared to encapsulated tumors without CI or LVI and infiltrative tumors. Intratumoral fibrosis and abortive papillae were more common in encapsulated tumors with CI or LVI compared to encapsulated tumors without CI or LVI and infiltrative tumors, but this result was not statistically significant. Other features, including dark colloid and MGCs, were not related to the presence of tumor capsule and tumor border configuration.
TABLE 3.
Minor diagnostic features and FOPBC in relation to the presence of tumor capsule and tumor border configuration
Molecular features in relation to the presence of tumor capsule and tumor border configuration
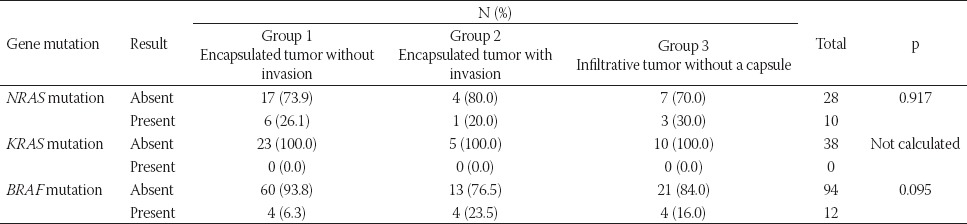
There was no significant relationship between KRAS/NRAS/BRAF mutations and the presence of tumor capsule and tumor border configuration (Table 4). All tumors with NRAS mutation had the mutation on the codon 61 (Q61R) and most of these tumors were encapsulated tumors without CI or LVI. BRAF mutation was more common in encapsulated tumors with LVI or CI (p = 0.095). KRAS mutation was not detected in our group.
TABLE 4.
Molecular features in relation to the presence of tumor capsule and tumor border configuration
DISCUSSION
Although the proposed new nomenclature for EFVPTC/NIFTP [3] may end the argument about the nuclear features of EFVPTC, the presence of invasion in FVPTC, especially in EFVPTC/NIFTP, has been recently discussed by many authors. In this study, we examined the predictive value and association of follicular morphological characteristics and molecular features with the tumor invasion in EFVPTC/NIFTP cases. Our results may be summarized as follows. First, NIFTP has an indolent behaviour due to the absence of ETE, and RAI therapy is not routinely used in these cases. Second, FOPBC may indicate CI or LVI in encapsulated tumors. Third, the presence of NRAS mutation and absence of BRAF mutation may help to exclude LVI and CI in NIFTP. Finally, the nuclear features are not associated with the presence of tumor capsule and tumor border configuration in EFVPTC/NIFTP. In summary, EFVPTC/NIFTP are often indolent tumors bearing NRAS rather than BRAF mutations. The presence of FOPBC in these tumors may be a warning sign of LVI or CI development and it can help rule out the diagnosis of IFVPTC.
The newly proposed nomenclature for EFVPTC/NIFTP, especially the simplified criteria for nuclear features, [3] could greatly assist in the routine diagnosis of this lesion. According to Nikiforov et al. [3], a tumor without CI or LVI, with follicular growth pattern, and nuclear features of PTC should be categorized as NIFTP. Nevertheless, the most accurate criteria for predicting invasion of EFVPTC/NIFTP are still being discussed, particularly in larger tumors. For example, it is still not clear how many sections or how many samples is necessary for the accurate diagnosis. Our results indicate that specific histomorphological features of EFVPTC could be associated with CI or LVI. For instance, FOPBC, frequently observed in follicular-patterned lesions and defined as elongated follicle(s) oriented perpendicular to the tumor border or capsule (without protrusion or penetration to the tumor capsule/border), was significantly associated with the presence of tumor capsule and tumor border configuration. Specifically, we observed FOPBC feature more frequently in encapsulated tumors with invasion compared to encapsulated tumors without invasion or infiltrative tumors without a capsule. We also investigated the association between minor diagnostic criteria for PTC and the presence of tumor capsule and tumor border configuration. Although a statistically significant relationship was not observed, abortive papillae and intratumoral fibrosis were more common in encapsulated tumors with invasion compared with encapsulated tumors without invasion or infiltrative tumors without a capsule. Together, these three features (abortive papillae, intratumoral fibrosis, and FOPBC) may indicate the presence of CI or LVI in encapsulated tumors. Even though we could not present the numerical data due to the low number of cases, we identified CI on deeper sections of the tumor in several patients. These findings suggest that perpendicular orientation of the follicles to the tumor border or capsule may indicate forthcoming or accompanying but not yet demonstrated invasion and may require additional sampling and serial sectioning. While the newly proposed nomenclature was originally developed with tumors >10 mm in size [3], in this study, we applied those criteria to FVPTC of any size. However, a limitation of our study is the small sample size and larger studies are necessary to confirm the prognostic value of specific histomorphological changes, especially FOPBC, in determining invasion of FVPTC.
Ganly et al. [7] compared the clinicopathological features of EFVPTC (subdivided into noninvasive EFVPTC [NIEFVPTC] and invasive EFVPTC [IEFVPTC]) to encapsulated follicular carcinoma (EFC) and FA. They concluded that capsular or vascular invasion rather than nuclear features determines the clinical outcome in encapsulated follicular tumors. Also, they found that the IEFVPTCs were significantly larger than NIEFVPTCs [7].
Infiltrative tumor border has been suggested as an indicator of ETE and LNM [10,7], and this was also confirmed in the study of Nikiforov et al. [3]. Our results indicated that LVI or CI was more common in encapsulated tumors larger than 10 mm, whereas encapsulated tumors without LVI or CI and tumors with irregular borders and without a fibrous capsule were ≤10 mm in size. Also, ETE was not observed in encapsulated tumors without invasion. Furthermore, nuclear features, according to the criteria adopted from the study of Nikiforov et al. [3] and scored individually or as a group, were not significantly associated with the presence of tumor capsule and tumor border configuration in our sample group. Blanchard et al. [27] reported multifocality, angiolymphatic invasion, absence of tumor capsule, and tumor involvement of perithyroid tissue as risk factors predictive of LNM; the rate of LNM was 40.7% in their group [27]. In addition, Walts et al. [28] reported LNM in 21/48 cases (43.8%). Moreover, a low rate (4%) of LNM was also showed in IFVPTC as well as the absence of LNM in EFVPTC [7]. In our study, the rate of LNM was 1.8% (1/55) and this patient had infiltrative tumor border, multifocal tumors (ETE was observed in the largest multifocal tumor), FOPBC, and intratumoral fibrosis; the tumor size was >10 mm. The molecular analyses of the largest multifocal tumor revealed no mutation of KRAS, NRAS, or BRAF gene. This result is in agreement with the previous studies.
Higher rates of BRAF compared to RAS mutations have been reported in IFVPTCs, suggesting that these lesions have a molecular profile similar to that of CVPTC. On contrary, EFVPTC, namely NIFTP, demonstrates mutations that are associated with follicular adenoma/carcinoma group (a high RAS mutation rate) [29-31]. Howitt et al. [32] found that partially encapsulated (PE) and well circumscribed (WC) tumors have similar mutations as EFVPTCs, i.e., frequent RAS and no BRAF mutations. In the present study, BRAF mutation analyses was performed in all cases (106), while KRAS/NRAS mutations were analyzed in 38/106 cases. Despite the lack of the significant relationship between molecular alterations and the presence of tumor capsule and tumor border configuration, we observed Q61R NRAS mutation more frequently in encapsulated tumors without invasion, while BRAF mutation, especially BRAFV600E (data not shown), was more common in encapsulated tumors with CI or LVI and tumors with infiltrative tumor borders. This result is in agreement with previous studies [3,32]. Unexpectedly, we detected BRAFV600 mutation in 6.3% (4/64) of encapsulated tumors without invasion (BRAFV600E versus BRAFV600K, 2/4 versus 2/4). Cho et al. [33] regrouped non-invasive and invasive EFVPTCs based on the complete absence of papillae or presence of ≤1% papillae and investigated the frequency of LNM and mutational profile in 152 cases. In the tumors with the presence of 1% papillae, the rate of LNM was 3% and 9% in non-invasive and invasive EFVPTCs respectively, while the rate of BRAFV600E mutation was 10% in non-invasive and 4% in invasive tumors. In the absence of papillary structures, the BRAF mutation was not observed, but central lymph node micrometastasis was present in 3% of non-invasive EFVPTCs. The authors suggested that non-invasive EFVPTCs should not be treated as a benign neoplasm due to the possibility of lymph node micrometastasis. In addition, according to them, EFVPTCs should not be diagnosed in the presence of papillary structures [33].
In conclusion, our results support the classification of EFVPTC as NIFTP, a tumor that frequently may have an indolent course. In addition to molecular data, histomorphological features such as the presence of FOPBC might indicate the development of invasion in EFVPTC. Furthermore, BRAF/KRAS/NRAS mutation analysis may help reduce both overtreatment and inadequate treatment in EFVPTC/NIFTP and IFVPTC patients.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016;101(1):264–74. doi: 10.1210/jc.2015-2917. https://doi.org/10.1210/jc.2015-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rossi ED, Bizzarro T, Martini M, Capodimonti S, Sarti D, Cenci T, et al. The evaluation of miRNAs on thyroid FNAC: The promising role of miR-375 in follicular neoplasms. Endocrine. 2016;54(3):723–32. doi: 10.1007/s12020-016-0866-0. https://doi.org/10.1007/s12020-016-0866-0. [DOI] [PubMed] [Google Scholar]
- [3].Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–9. doi: 10.1001/jamaoncol.2016.0386. https://doi.org/10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sak SD. Variants of papillary thyroid carcinoma: Multiple faces of a familiar tumor. Turk Patoloji Derg. 2015;31(Suppl 1):34–47. doi: 10.5146/tjpath.2015.01313. https://doi.org/10.5146/tjpath.2015.01313. [DOI] [PubMed] [Google Scholar]
- [5].Taconet S, Bosq J, Hartl D, Schlumberger M, Leboulleux S, Scoazec JY, et al. Composite mucoepidermoid carcinoma and columnar cell variant of papillary carcinoma of the thyroid: A case report and review of the literature. Int J Surg Pathol. 2016;24(4):336–40. doi: 10.1177/1066896915626281. https://doi.org/10.1177/1066896915626281. [DOI] [PubMed] [Google Scholar]
- [6].Kakudo K, Bai Y, Liu Z, Li Y, Ito Y, Ozaki T. Classification of thyroid follicular cell tumors: With special reference to borderline lesions. Endocr J. 2012;59(1):1–12. doi: 10.1507/endocrj.ej11-0184. https://doi.org/10.1507/endocrj.EJ11-0184. [DOI] [PubMed] [Google Scholar]
- [7].Ganly I, Wang L, Tuttle RM, Katabi N, Ceballos GA, Harach HR, et al. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: Further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol. 2015;46(5):657–64. doi: 10.1016/j.humpath.2015.01.010. https://doi.org/10.1016/j.humpath.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lindsay S. Carcinoma of the thyroid gland. A clinical and pathologic study of 293 patients at the University of California Hospital. Springfield, IL: Charles C. Thomas; 1960. [Google Scholar]
- [9].Chem KT, Rosai J. Follicular variant of thyroid papillary carcinoma: A clinicopathologic study of six cases. Am J Surg Pathol. 1977;1(2):123–30. doi: 10.1097/00000478-197706000-00003. https://doi.org/10.1097/00000478-197706000-00003. [DOI] [PubMed] [Google Scholar]
- [10].Liu L, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, et al. Follicular variant of papillary thyroid carcinoma: A clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–64. doi: 10.1002/cncr.22138. https://doi.org/10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- [11].Elsheikh TM, Asa SL, Chan JK, DeLellis RA, Heffess CS, LiVolsi VA, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008;130(5):736–44. doi: 10.1309/AJCPKP2QUVN4RCCP. https://doi.org/10.1309/AJCPKP2QUVN4RCCP. [DOI] [PubMed] [Google Scholar]
- [12].Ertek S, Yılmaz NC, Cicero AF, Vurupalmaz Ö, Demiröz AS, Erdoğan G. Increasing diagnosis of thyroid papillary carcinoma follicular variant in south-east Anatolian region: Comparison of characteristics of classical papillary and follicular variant thyroid cancers. Endocr Pathol. 2012;23(3):157–60. doi: 10.1007/s12022-012-9216-9. https://doi.org/10.1007/s12022-012-9216-9. [DOI] [PubMed] [Google Scholar]
- [13].Yu XM, Schneider DF, Leverson G, Chen H, Sippel RS. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: A population-based study of 10,740 cases. Thyroid. 2013;23(10):1263–8. doi: 10.1089/thy.2012.0453. https://doi.org/10.1089/thy.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ito Y, Hirokawa M, Uruno T, Kihara M, Higashiyama T, Takamura Y, et al. Prevalence and biological behaviour of variants of papillary thyroid carcinoma: Experience at a single institute. Pathology. 2008;40(6):617–22. doi: 10.1080/00313020802320630. https://doi.org/10.1080/00313020802320630. [DOI] [PubMed] [Google Scholar]
- [15].Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H, Asa SL, et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90. doi: 10.1016/j.cell.2014.09.050. https://doi.org/10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA, Jr, Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276–85. doi: 10.1210/jc.2013-2503. https://doi.org/10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol. 2016;29(7):698–707. doi: 10.1038/modpathol.2016.65. https://doi.org/10.1038/modpathol.2016.65. [DOI] [PubMed] [Google Scholar]
- [18].Kakudo K, Bai Y, Liu Z, Ozaki T. Encapsulated papillary thyroid carcinoma, follicular variant: A misnomer. Pathol Int. 2012;62(3):155–60. doi: 10.1111/j.1440-1827.2011.02773.x. https://doi.org/10.1111/j.1440-1827.2011.02773.x. [DOI] [PubMed] [Google Scholar]
- [19].Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: A clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid. 2009;19(2):119–27. doi: 10.1089/thy.2008.0303. https://doi.org/10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- [20].Yip L, Nikiforova MN, Yoo JY, McCoy KL, Stang MT, Armstrong MJ, et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: A study of 1510 patients. Ann Surg. 2015;262(3):519–55. doi: 10.1097/SLA.0000000000001420. https://doi.org/10.1097/SLA.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120(1):71–7. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. https://doi.org/10.1309/ND8D9LAJTRCTG6QD. [DOI] [PubMed] [Google Scholar]
- [22].Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–9. doi: 10.1210/jc.2005-0987. https://doi.org/10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- [23].Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. https://doi.org/10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bullock M. Protocol for the examination of specimens from patients with carcinomas in the thyroid gland 2016 based on AJCC/UICC TNM. 7t ed. College of American Pathologists (CAP); 2016. [Google Scholar]
- [25].Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24(12):1545–52. doi: 10.1038/modpathol.2011.119. https://doi.org/10.1038/modpathol.2011.119. [DOI] [PubMed] [Google Scholar]
- [26].Sezer A, Celik M, Yilmaz Bulbul B, Can N, Tastekin E, Ayturk S, et al. Relationship between lymphovascular invasion and clinicopathological features of papillary thyroid carcinoma. Bosn J Basic Med Sci. 2017;17(2):144–51. doi: 10.17305/bjbms.2017.1924. https://doi.org/10.17305/bjbms.2017.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Blanchard C, Brient C, Volteau C, Sebag F, Roy M, Drui D, et al. Factors predictive of lymph node metastasis in the follicular variant of papillary thyroid carcinoma. Br J Surg. 2013;100(10):1312–7. doi: 10.1002/bjs.9210. https://doi.org/10.1002/bjs.9210. [DOI] [PubMed] [Google Scholar]
- [28].Walts AE, Mirocha JM, Bose S. Follicular variant of papillary thyroid carcinoma (FVPTC): Histological features, BRAF V600E mutation, and lymph node status. J Cancer Res Clin Oncol. 2015;141(10):1749–56. doi: 10.1007/s00432-015-1939-9. https://doi.org/10.1007/s00432-015-1939-9. [DOI] [PubMed] [Google Scholar]
- [29].Xu B, Ghossein R. Encapsulated thyroid carcinoma of follicular cell origin. Endocr Pathol. 2015;26(3):191–9. doi: 10.1007/s12022-015-9376-5. https://doi.org/10.1007/s12022-015-9376-5. [DOI] [PubMed] [Google Scholar]
- [30].McFadden DG, Dias-Santagata D, Sadow PM, Lynch KD, Lubitz C, Donovan SE, et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2014;99(11):E2457–62. doi: 10.1210/jc.2014-2611. https://doi.org/10.1210/jc.2014-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191–1200. doi: 10.1038/modpathol.2010.112. https://doi.org/10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Howitt BE, Jia Y, Sholl LM, Barletta JA. Molecular alterations in partially-encapsulated or well-circumscribed follicular variant of papillary thyroid carcinoma. Thyroid. 2013;23(10):1256–62. doi: 10.1089/thy.2013.0018. https://doi.org/10.1089/thy.2013.0018. [DOI] [PubMed] [Google Scholar]
- [33].Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: The impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017;30(6):810–25. doi: 10.1038/modpathol.2017.9. https://doi.org/10.1038/modpathol.2017.9. [DOI] [PubMed] [Google Scholar]


