Abstract
Monocyte locomotion inhibitory factor (MLIF) is an oligopeptide with anti-inflammatory properties. The carboxyl-terminal end group Cys-Asn-Ser serves as the pharmacophore of MLIF. The aim of this study was to investigate the neuroprotective effects of two new synthetic analogs, Arg-Cys-Asn-Ser and D-Cys-Asn-Ser, on focal cerebral ischemia, which were designed and synthesized to increase the penetrability and enzymatic stability of Cys-Asn-Ser. Ninety-one male Sprague-Dawley rats were randomly divided into six groups: I - Sham; II - Ischemia-reperfusion (I/R); III - Nimodipine; IV - Cys-Asn-Ser; V - D-Cys-Asn-Ser; and VI - Arg-Cys-Asn-Ser. The rats in groups II-VI were subjected to middle cerebral artery occlusion. After 24 hours of reperfusion, the neurological deficit, cerebral infarct volume, and levels of the pro-inflammatory factors interleukin-1β (IL-1β) and tumor necrosis factor-alpha in brain tissue homogenates were assessed. Compared with the sham group, the mean neurological deficit scores were significantly higher in groups II-VI (p ≤ 0.019 for all). The mean infarct volumes were significantly higher in I/R and Cys-Asn-Ser groups compared with the sham group (both p ≤ 0.046). The mean IL-1β level was significantly lower in D-Cys-Asn-Ser and Arg-Cys-Asn-Ser groups compared with I/R group (both p ≤ 0.046). In conclusion, the results showed that Arg-Cys-Asn-Ser and D-Cys-Asn-Ser have the potential for protective effects against focal cerebral ischemia injury.
KEY WORDS: Monocyte locomotion inhibitory factor, inflammatory response, neuroprotection, cerebral ischemia/reperfusion, middle cerebral artery occlusion, rat model, interleukin-1β, tumor necrosis factor-alpha
INTRODUCTION
In cerebral ischemia, various pathogenic factors play a role in increased morbidity and mortality. Although various mechanisms are involved in the pathogenesis of ischemia, it is becoming accepted that excessive inflammation is a critical factor that leads to ischemic brain injury [1]. A marked inflammatory reaction initiated by the expression of cytokines, adhesion molecules, and other inflammatory mediators occurs in cerebral ischemia and neurodegenerative diseases [2]. Other studies have found that the concentration of interleukin-6 (IL-6) and C-reactive protein increases during the 1st week following stroke [1]. It has been speculated that the reduced capability to biosynthesize catecholamines in the brain, after acute ischemic stroke, may be attributable to the inflammatory response. In addition, the inflammatory response may be involved in mediating neurotoxic effects [3]. Both mild and substantial cerebral hypoxia-ischemia influence cerebral microglial/macrophage activation (ED1), pro-inflammatory cytokines (tumor necrosis factor-alpha [TNF-α]), and nitrosative stress (nitrotyrosine), resulting in permanent brain damage [4]. The blocking of various factors involved in the inflammatory cascade reduces damage in experimental ischemia [5-7]. This has been found for both, endogenous and synthesized factors. With regard to endogenous factors, neuroprotective effects have been reported for apelin-13, a ligand for G protein-coupled receptor AP [8], and maresin-1, which is derived from macrophages [9]. With respect to synthesized factors, neuroprotective effects have been observed for FTY720, a sphingosine-1-phosphate receptor modulator [10], LMT497, a seco-nucleoside [11], and WIB801C, an extract of the Chinese caterpillar fungus Cordyceps militaris [12]. Thus, targeting inflammation and monitoring the injury processes with imaging or biomarkers may help guide treatment of patients with acute ischemic stroke [4,13].
Monocyte locomotion inhibitory factor (MLIF) is an anti-inflammatory oligopeptide produced by Entamoeba histolytica [14]. E. histolytica infection may result in colitis and amoebic liver abscess (ALA) formation, but <1% of 50 million patients worldwide with intestinal diseases develop an ALA [15]. An ALA is an unusual inflammatory process in which early intense acute inflammation is followed by an unexpected rare mononuclear cell inflammatory reaction [16]. This suggests that pro-inflammatory and anti-inflammatory factors may both be released by E. histolytica inhibition of leukocyte migration [16]. MLIF may play a role in the unexpected, diminished, late inflammatory reaction found in advanced invasive amebiasis. This diminished reaction results in regeneration of the affected organs without scarring (restitutio ad integrum), following successful treatment of this disease [17]. MLIF is a heat-stable oligopeptide that was isolated by ultra-filtration, gel-sieve chromatography, and high performance liquid chromatography (HPLC) from axenic culture of E. histolytica. It appears that the neuroprotective effect of MLIF involves the ribosomal protein eukaryotic translation elongation factor 1A [18]. Its primary structure is Met-Gln-Cys-Asn-Ser; the last three amino acids (Cys-Asn-Ser carboxyl-terminal end group) are shown to be the pharmacophore of MLIF [19]. The electronic structure and physicochemical features of Cys-Asn-Ser have been characterized [20]. MLIF has the potential to be used therapeutically against inflammatory disease [14,16,17,19]. In our laboratory, two truncated derivatives of MLIF, Tyr-Cys-Asn-Ser and His-Cys-Asn-Ser, have been shown to profoundly attenuate focal-ischemia-induced inflammatory responses and oxidative damage at the cellular and molecular levels in a middle cerebral artery occlusion (MCAO) rat model [21]. Therefore, we believe that MLIF and its analogs represent a promising new strategy for the treatment of ischemia-reperfusion (I/R).
The aim of this study was to investigate the effects of two new derivatives of MLIF, Arg-Cys-Asn-Ser and D-Cys-Asn-Ser, on focal cerebral ischemia-perfusion injuries, that were designed and synthesized to enhance the blood-brain barrier (BBB) penetrability and enzymatic stability of Cys-Asn-Ser [19,20,22].
MATERIALS AND METHODS
Reagents
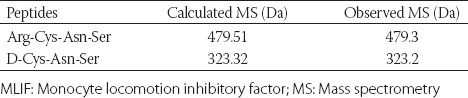
In our laboratory, the tripeptides Cys-Asn-Ser and D-Cys-Asn-Ser and tetrapeptide Arg-Cys-Asn-Ser were synthesized using solid-phase synthesis [23]. The molecular weights are listed in Table 1. The purity of the compounds was determined using reverse phase HPLC (purity >98%) and the chromatographic column, and running protocol was briefly summarized as follows: (i) Chromatographic column: Waters Delta-PakC18 column semi-preparative column (19 mm × 250 mm); (ii) HPLC running procedure: Detection wavelength: 220 nm; mobile phase flow rate: 8 ml/min; sample volume: 40 mg for each; mobile phase E: 0.1% trifluoroacetic acid (TFA)/water; mobile phase F: 0.1% TFA/acetonitrile; linear gradient: 0-30 minutes; ratio of mobile phase F: 5-100%. The effluent was collected at the absorption peak and aliquoted (the thickness of collected effluent was kept to <2 cm to avoid lyophilization). Then the effluent was lyophilized, and the lyophilized powder was harvested to determine the molecular weight by mass spectrometry (MS). The target peptide was collected and weighed to calculate yield rate. The substitution value of Wang resin was 0.49 mmol/g, and the theoretical yield was calculated as follows: Theoretical yield of the polypeptide = substitution value of Wang resin × resin weight (g) × molecular weight of polypeptide. Primary structures of tripeptides were verified using MS and amino acid analysis. Poly-lys and matrigel were obtained from Sigma-Aldrich, Shanghai, China. TNF-α and IL-1β kits were purchased from ShengGong Biological Technology, Shanghai, China. All other chemicals used were of the highest quality available.
TABLE 1.
Molecular weights of the MLIF analogs
A Chinese patent for the biosynthesis of the tripeptides Cys-Asn-Ser and D-Cys-Asn-Ser was obtained (patent number: CN200810211610.0).
Animal models
All animal experiments were carried out according to the Guide for the Care and Use of Laboratory Animals and approved by the Committee of Experimental Animals of Lanzhou University. Ninety-one adult male Sprague-Dawley (SD) rats weighing 250-280 g were randomly divided into six groups: I - Sham group (n = 11), no MCAO; II - I/R group (n = 16), ischemia and reperfusion; III - Nimodipine treatment group (n = 16); IV - Cys-Asn-Ser treatment group (n = 16); V - D-Cys-Asn-Ser treatment group (n = 16); VI - Arg-Cys-Asn-Ser treatment group (n = 16). The rats in groups II-VI were subjected to MCAO. In Groups IV-VI, the peptides were injected into the rats’ tail veins three times at the dose of 1 mg/kg (30 minutes before cerebral ischemia, at the start of reperfusion, and 12 hours following reperfusion). Nimodipine was injected into the rats’ tail veins at the dose of 0.4 mg/kg at the start of reperfusion [21,24,25]. The same volume of normal saline was administered by intravenous injection into the rats’ tails in sham and I/R groups concurrently.
MCAO model
The rats were anesthetized using chloral hydrate (350 mg/kg, i.p.), and then, MCAO was performed as described previously, but with slight modification [26,27]. Briefly, the left common carotid artery, internal carotid artery (ICA) and external carotid artery (ECA) were exposed, and the latter was dissected distally. The ICA was isolated, and a monofilament nylon suture (diameter: 0.26 mm) with a rounded tip (diameter: 0.36 ± 0.02 mm) was inserted in the ECA lumen and then carefully introduced into the ICA lumen to occlude the MCA. To improve the adhesion force, the monofilament nylon suture was exposed to polylysine for 1 minute and dried at 60°C for 1 hour before the experiment. After 90 minutes of MCAO, the monofilament nylon suture was removed to restore blood flow (reperfusion). The incision was then sutured. The rats recovered under a heat lamp to maintain rectal temperature at 37 ± 0.5°C. The rats that did not lose the righting reflex or convulse during the ischemic episode were excluded from the experiment. The animals in the sham group underwent the same procedures except for the occlusion of the carotid arteries.
At 24 hours after reperfusion, neurological function was scored in the SD rats. Then, these rats were overdosed with 10% chloral hydrate and sacrificed by cervical dislocation. One part of the brain tissues was sectioned, stained, and fixed to calculate the infarct ratio; the other part of the brain tissues was homogenized for the detection of cytokines. The experiment was repeated 4 times with a total of 364 SD rats being used.
Assessment of neurological deficits
The neurological deficits of the animals were measured at 24 hours following reperfusion by one observer who had no knowledge of the identity of the groups. The neurological deficits were evaluated with Longa scoring method. This scoring method is based on a modified five-point scale: Grade 0 - No observable neurological deficit (normal); Grade 1 - Flexion of the contralateral torso and the forelimb on lifting the animal by its tail (mild); Grade 2 - Circling to the contralateral side but normal posture at rest (moderate); Grade 3 - Leaning to the contralateral side at rest (severe); and Grade 4 - No spontaneous motor activity (very severe) [28].
Measurement of infarct volume
Following 24 hours of reperfusion, the animals were anesthetized and then decapitated. The cerebral infarct volumes were measured using a method that has been described previously [29]. Briefly, the brains (Groups II-VI, n = 8; Group I, n = 3) were cut on a cutting block into 2-mm thick coronal sections and then immediately immersed in 2% 2,3,5-triphenyltetrazolium chloride for 20 minutes at 37°C. After that, the sections were immersed overnight in 4% paraformaldehyde. The infarct area was marked and then analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). Then the demarcation between the infarcted and noninfarcted tissue was outlined. The total infarct volume was determined by integrating the lesion areas from all sections. The infarct size was quantified by calculating the ratio of corrected infarct volume to the whole brain volume.
Brain homogenate preparation and measurement of TNF-α and IL-1β
Brain homogenate preparation
Eight animals per each group were deeply anesthetized and sacrificed. The brains were then quickly removed to obtain the ischemic cortex for the biochemical assays. The fresh ischemic cortical tissue was collected and washed with refrigerated saline and homogenized (1:10) immediately. The homogenate was centrifuged (3500 ×g, 4°C, 15 minutes), and the supernatant was frozen immediately in liquid nitrogen and then stored at −70°C until further processing.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-1β and TNF-α in the ischemic brain tissue homogenates were measured using specific ELISA kits following the instructions of the manufacturer (ShengGong Biological Technology, Shanghai, China).
Statistical analysis
Variables such as neurological deficit scores, infarct volumes, and IL-1β and TNF-α concentration were presented as means and standard deviations. One-way analysis of variance (ANOVA) with post hoc test was performed to compare the differences between the groups. The Bonferroni post hoc method was used for the multiple comparisons when equal variance was assumed, and the Dunnett’s T3 method was used when equal variance was not assumed. Statistical analyses were performed using IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY, USA). Two-tailed p < 0.05 indicated statistical significance.
RESULTS
Comparison of neurological deficit score between groups
Compared with the sham group, the mean neurological deficit score was significantly higher in I/R, nimodipine, Cys-Asn-Ser, D-Cys-Asn-Ser, and Arg-Cys-Asn-Ser groups (0 versus 1.9, 1.72, 1.64, 1.5, and 1.5, respectively; p ≤ 0.019 for all) (Figure 1).
FIGURE 1.
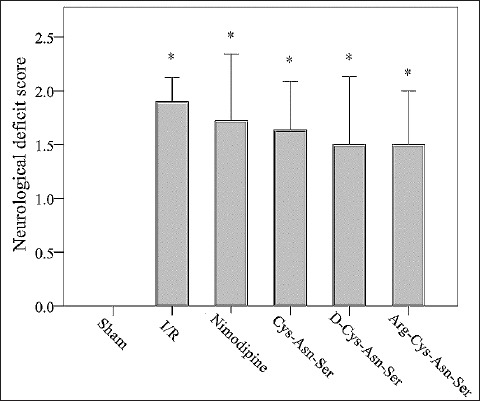
Comparison of neurological deficit score between groups. Effect of nimodipine and monocyte locomotion inhibitory factor (MLIF) analogs on the neurological deficit scores after ischemia-reperfusion (I/R) in the rats. The elevated neurological deficit scores after I/R were reduced by nimodipine and MLIF analogs. *p < 0.05 represents a statistically significant difference compared with the sham group.
Comparison of infarct volume between groups
Infarcts in the rat brains are illustrated in Figure 2A. The mean infarct volume was significantly higher in I/R and Cys-Asn-Ser groups compared with the sham group (313.6 and 203.24 mm3 versus 0 mm3 respectively, both p ≤ 0.046). There were no significant differences among the other groups [p > 0.05 for all] (Figure 2B).
FIGURE 2.
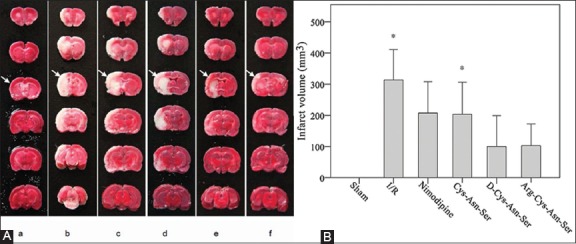
Comparison of infarct volumes between groups. The effect of nimodipine and monocyte locomotion inhibitory factor (MLIF) analogs on the cerebral infarct volume after ischemia-reperfusion (I/R) in the rats. (A) Representative 2,3,5-triphenyltetrazolium chloride -stained brain sections are shown where rats were subjected to 90 minutes of ischemia followed by 24 hours of reperfusion (I/R) with or without tail vein injections with nimodipine or MLIF analogs. The white is the infarct area and the red is the normal area. (a) Sham group; (b) Ischemia-reperfusion group; (c) Nimodipine treatment group; (d) Cys-Asn-Ser treatment group; (e) D-Cys-Asn-Ser treatment group; (f) Arg-Cys-Asn-Ser treatment group. (B) Quantification of the infarct volume at 24 hours. The ratio of corrected infarct volume to the whole brain volume was calculated for the cerebral infarct size. The infarct volume was decreased at 24 hours after the treatment with the MLIF analogs. The bars represent the mean ± standard error of the mean (n = 8). *p < 0.05 represents a statistically significant difference compared with the sham group.
Comparison of IL-1β concentration between groups
The mean IL-1β concentration was significantly lower in D-Cys-Asn-Ser and Arg-Cys-Asn-Ser groups compared with I/R group (44.24 and 39.71 pg versus 53.88 pg respectively, both p ≤ 0.046). It was also significantly lower in Arg-Cys-Asn-Ser group compared with the sham as well as nimodipine group [39.71 pg versus 52.42 and 53.6 pg, respectively, both p ≤ 0.05] (Figure 3).
FIGURE 3.
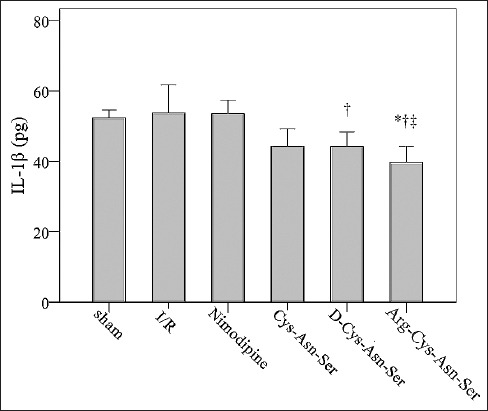
Comparison of interleukin-1β (IL-1β) levels between groups. Effect of the monocyte locomotion inhibitory factor analogs on the concentration of IL-1β after middle cerebral artery occlusion. *p < 0.05 represents a statistically significant difference compared with the sham group; †p < 0.05 represents a statistically significant difference compared with ischemia-reperfusion (I/R) group; ‡p < 0.05 represents a statistically significant difference compared with nimodipine group.
Comparison of TNF-α concentration between groups
The mean TNF-α concentration was 87.73 pg in the sham group, 138.14 pg in I/R, 109.71 pg in nimodipine, 116.92 pg in Cys-Asn-Ser, 104.33 pg in D-Cys-Asn-Ser, and 90.82 pg in Arg-Cys-Asn-Ser group. There were no significant differences in TNF-α concentration between the groups (p > 0.05) (Figure 4).
FIGURE 4.
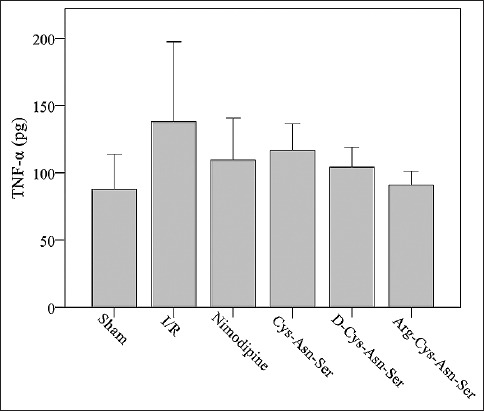
Comparison of tumor necrosis factor-alpha (TNF-α) levels between groups. The effect of the monocyte locomotion inhibitory factor analogs on the concentration of TNF-α after middle cerebral artery occlusion. I/R: ischemia-reperfusion.
DISCUSSION
In this study, two MLIF analogs, Arg-Cys-Asn-Ser and D-Cys-Asn-Ser, were synthesized with the aim of enhancing the penetrability and enzymatic stability of the anti-inflammatory MLIF pharmacophore group, tripeptide Cys-Asn-Ser. The results showed that in a MCAO rat model D-Cys-Asn-Ser and Arg-Cys-Asn-Ser provided greater neuroprotection than nimodipine or Cys-Asn-Ser. In our previous study, MLIF analogs Tyr-Cys-Asn-Ser and His-Cys-Asn-Ser appeared to attenuate focal ischemia injury resulting from the inflammatory response [21]. It seems that the introduction of a positively charged amino acid His or Arg at the N-terminal of Cys-Asn-Ser can significantly increase the protective effects of peptides in I/R injury. The enhancement of the anti-inflammatory activities of Arg-Cys-Asn-Ser and D-Cys-Asn-Ser may be due to the introduction of an Arg residue or the substitution of L-Cys by D-Cys, which could increase the BBB penetrability or the enzymatic stability of Cys-Asn-Ser. These MLIF analogs may have a therapeutic potential for patients with acute ischemic stroke.
Our results are consistent with several other studies that have used the MCAO rat or mouse model and found that certain substances can provide protection against cerebral I/R through attenuation of the pro-inflammatory response. Xin et al. [8] treated rats with apelin-13 following 24 hours of reperfusion and noted a marked reduction in neurological deficits and infarct volume and a decrease in several inflammatory cytokines. Xian et al. [9] injected macrophage-derived maresin-1 into the cerebral ventricles of mice and reported that maresin-1 significantly reduced the neurological deficits and infarct volume. Kraft et al. [10] assessed the effects of fingolimod (FTY720) on mice after reperfusion and found a reduction in infarct size and improved functional outcome. Ryu et al. [11] investigated the neuroprotective effects of the synthetic seco-nucleoside LMT497 in rats and found several benefits at the cellular level as well as a reduction in infarct volume and neurological deficits. In a study of Hwang et al. [12] rats treated with C. militaris extract WIB801C had smaller infarct volume and decreased cerebral edema, and showed a reduction in neurological deficits.
Excessive inflammation is becoming accepted as a critical factor in the pathogenesis of cerebral ischemia [2]. During ischemia, different types of cytokines such as IL-1, IL-6, TNF-α, transforming growth factor beta, and chemokines (e.g., cytokine-induced neutrophil chemoattractant and monocyte chemotactic protein-1) are produced by various activated cell types. These cell types include endothelial cells, microglia, neurons, platelets, leukocytes, and fibroblasts. The level of IL-1β is increased following either permanent or transient cerebral ischemia in microglia, astrocytes, and neurons [30]. TNF-α is a pleiotropic cytokine that has diverse pro-inflammatory actions. In both, the striatum and cortex, focal cerebral ischemia can induce a rapid and dramatic increase of the TNF-α level within and around the area of damage. The occurrence of TNF-α immunoreactivity in neurons and nerve fibers after MCAO suggests that TNF-α expressed in ischemic neurons might be delivered through axonal transport. However, the TNF-α immunoreactivity in the astrocyte end-feet and ependymal cells after MCAO suggests that TNF-α may play a role in the disruption of the BBB and initiation of inflammation in the brain [31]. Studies have shown that the injection of an antagonist of IL-1β and TNF-α can attenuate the ischemic injury [32]. Our previous results showed that after ischemia MLIF analogs markedly suppressed myeloperoxidase (MPO) activities and decreased the concentrations of pro-inflammatory cytokines IL-1β and TNF-α. Furthermore, after a modification, the effects of these peptides on the suppression of MPO activities and decrease of IL-1β concentration obviously increased, especially for the analog His-Cys-Asn-Ser [20]. In this study, we found that two additional, novel MLIF analogs, Arg-Cys-Asn-Ser and D-Cys-Asn-Ser, can clearly suppress the concentration of IL-1β after ischemia, and this was especially true of Arg-Cys-Asn-Ser. These findings indicate that the neuroprotective effects of these peptides are attributed mainly to their anti-inflammatory activities.
In this study, nimodipine was used as a positive control. Nimodipine can cross the BBB to dilate blood vessels and increase the blood flow in the brain, and has a high selectivity for blood vessels that lead to the brain. In addition, it also has neuroprotective effects independent of its vasodilator effects. Because the neuroprotective effects of nimodipine are widely applied in clinical practice, we speculate that the poor therapeutic effect of nimodipine in this study might be related to the quality and dilution of nimodipine as well as the route of nimodipine administration.
A limitation of this study was that we only tested two pro-inflammatory cytokines, IL-1β and TNF-α. Other cytokines will be tested in future studies as well as sequential signal transduction pathways.
CONCLUSION
Our results indicate that the anti-inflammatory activities of the newly synthesized MLIF analogs Arg-Cys-Asn-Ser and D-Cys-Asn-Ser are more potent than MLIF pharmacophore Cys-Asn-Ser, and this is especially true of Arg-Cys-Asn-Ser. Thus, with the increasing emergence of cerebral I/R injuries, Arg-Cys-Asn-Ser and D-Cys-Asn-Ser have features that make them desirable as novel chemotherapy agents for ischemic strokes. Additional studies should be performed to assess the therapeutic potential of these MLIF analogs.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81273440), and the Key National S&T Program “Major New Drug Development” of the Ministry of Science and Technology of China (2012ZX09504001-003).
REFERENCES
- [1].Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15(5):523–31. doi: 10.1586/14737175.2015.1035712. https://doi.org/10.1586/14737175.2015.1035712. [DOI] [PubMed] [Google Scholar]
- [2].Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy – from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754(1-2):253–62. doi: 10.1016/j.bbapap.2005.08.017. https://doi.org/10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- [3].Ormstad H, Verkerk R, Aass HC, Amthor KF, Sandvik L. Inflammation-induced catabolism of tryptophan and tyrosine in acute ischemic stroke. J Mol Neurosci. 2013;51(3):893–902. doi: 10.1007/s12031-013-0097-2. https://doi.org/10.1007/s12031-013-0097-2. [DOI] [PubMed] [Google Scholar]
- [4].Qiao M, Meng S, Foniok T, Tuor UI. Mild cerebral hypoxia-ischemia produces a sub-acute transient inflammatory response that is less selective and prolonged after a substantial insult. Int J Dev Neurosci. 2009;27(7):691–700. doi: 10.1016/j.ijdevneu.2009.07.004. https://doi.org/10.1016/j.ijdevneu.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [5].Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87(1):179–97. doi: 10.1016/j.pbb.2007.04.015. https://doi.org/10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- [6].Lin X, Yang DJ, Cai WQ, Zhao QY, Gao YF, Chen Q, et al. Endomorphins, endogenous opioid peptides, provide antioxidant defense in the brain against free radical-induced damage. Biochim Biophys Acta. 2003;1639(3):195–202. doi: 10.1016/j.bbadis.2003.09.007. https://doi.org/10.1016/j.bbadis.2003.09.007. [DOI] [PubMed] [Google Scholar]
- [7].Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, et al. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010;1323:174–83. doi: 10.1016/j.brainres.2010.01.079. https://doi.org/10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- [8].Xin Q, Cheng B, Pan Y, Liu H, Yang C, Chen J, et al. Neuroprotective effects of apelin-13 on experimental ischemic stroke through suppression of inflammation. Peptides. 2015;63:55–62. doi: 10.1016/j.peptides.2014.09.016. https://doi.org/10.1016/j.peptides.2014.09.016. [DOI] [PubMed] [Google Scholar]
- [9].Xian W, Wu Y, Xiong W, Li L, Li T, Pan S, et al. The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem Biophys Res Commun. 2016;472(1):175–81. doi: 10.1016/j.bbrc.2016.02.090. https://doi.org/10.1016/j.bbrc.2016.02.090. [DOI] [PubMed] [Google Scholar]
- [10].Kraft P, Göb E, Schuhmann MK, Göbel K, Deppermann C, Thielmann I, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44(11):3202–10. doi: 10.1161/STROKEAHA.113.002880. https://doi.org/10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- [11].Ryu S, Kwon J, Park H, Choi IY, Hwang S, Gajulapati V, et al. Amelioration of cerebral ischemic injury by a synthetic seco-nucleoside LMT497. Exp Neurobiol. 2015;24(1):31–40. doi: 10.5607/en.2015.24.1.31. https://doi.org/10.5607/en.2015.24.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hwang S, Cho GS, Ryu S, Kim HJ, Song HY, Yune TY, et al. Post-ischemic treatment of WIB801C, standardized cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J Ethnopharmacol. 2016;186(16):169–80. doi: 10.1016/j.jep.2016.03.052. https://doi.org/10.1016/j.jep.2016.03.052. [DOI] [PubMed] [Google Scholar]
- [13].Giménez-Scherer JA, Arenas E, Díaz L, Rico G, Fernández J, Kretschmer R. Effect of the monocyte locomotion inhibitory factor (MLIF) produced by Entamoeba histolytica on the expression of cell adhesion molecules (CAMs) in the skin of guinea pigs. Arch Med Res. 2000;31(4 Suppl):S92–3. doi: 10.1016/s0188-4409(00)00165-x. https://doi.org/10.1016/S0188-4409(00)00165-X. [DOI] [PubMed] [Google Scholar]
- [14].Utrera-Barillas D, Velazquez JR, Enciso A, Cruz SM, Rico G, Curiel-Quesada E, et al. An anti-inflammatory oligopeptide produced by Entamoeba histolytica down-regulates the expression of pro-inflammatory chemokines. Parasite Immunol. 2003;25(10):475–82. doi: 10.1111/j.1365-3024.2003.00657.x. https://doi.org/10.1111/j.1365-3024.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- [15].Walsh JA. Problems in recognition and diagnosis of amebiasis: Estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8(2):228–38. doi: 10.1093/clinids/8.2.228. https://doi.org/10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- [16].Silva-García R, Rico-Rosillo G. Anti-inflammatory defense mechanisms of Entamoeba histolytica. Inflamm Res. 2011;60(2):111–7. doi: 10.1007/s00011-010-0261-x. https://doi.org/10.1007/s00011-010-0261-x. [DOI] [PubMed] [Google Scholar]
- [17].Kretschmer RR, Rico G, Giménez JA. A novel anti-inflammatory oligopeptide produced by Entamoeba histolytica. Mol Biochem Parasitol. 2001;112(2):201–9. doi: 10.1016/s0166-6851(00)00367-4. https://doi.org/10.1016/S0166-6851(00)00367-4. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Chen J, Li F, Li D, Xiong Q, Lin Y, et al. Apentapeptide monocyte locomotion inhibitory factor protects brain ischemia injury by targeting the eEF1A1/endothelial nitric oxide synthase pathway. Stroke. 2012;43(10):2764–73. doi: 10.1161/STROKEAHA.112.657908. https://doi.org/10.1161/STROKEAHA.112.657908. [DOI] [PubMed] [Google Scholar]
- [19].Morales-Martínez ME, Silva-García R, Soriano-Correa C, Giménez-Scherer JA, Rojas-Dotor S, Blanco-Favela F, et al. The Cys-Asn-Ser carboxyl-terminal end group is the pharmacophore of the amebic anti-inflammatory monocyte locomotion inhibitory factor (MLIF) Mol Biochem Parasitol. 2008;158(1):46–51. doi: 10.1016/j.molbiopara.2007.11.010. https://doi.org/10.1016/j.molbiopara.2007.11.010. [DOI] [PubMed] [Google Scholar]
- [20].Barrientos-Salcedo C, Rico-Rosillo G, Giménez-Scherer JA, Soriano-Correa C. Computational study of the electronic structure characterization of a novel anti-inflammatory tripeptide derived from monocyte locomotion inhibitory factor (MLIF)-pentapeptide. Eur J Med Chem. 2009;44(8):3114–9. doi: 10.1016/j.ejmech.2009.03.003. https://doi.org/10.1016/j.ejmech.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [21].Yao J, Xu Y, Ji F, Wang C, Zhang Y, Ni J, et al. Protective effects of MLIF analogs on cerebral ischemia-reperfusion injury in rats. Peptides. 2011;32(5):1047–54. doi: 10.1016/j.peptides.2011.03.005. https://doi.org/10.1016/j.peptides.2011.03.005. [DOI] [PubMed] [Google Scholar]
- [22].Kretschmer R, Rico G, Giménez JA. Isolation and structural studies of the monocyte locomotion inhibitory factor (MLIF) produced by Entamoeba histolytica. Arch Med Res. 2000;31(4 Suppl):S76–7. doi: 10.1016/s0188-4409(00)00169-7. https://doi.org/10.1016/S0188-4409(00)00169-7. [DOI] [PubMed] [Google Scholar]
- [23].Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–8. doi: 10.1016/j.neuropharm.2008.01.005. https://doi.org/10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1-2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. https://doi.org/10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wei X, Liu H, Sun X, Fu F, Zhang X, Wang J, et al. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett. 2005;386(1):58–62. doi: 10.1016/j.neulet.2005.05.069. https://doi.org/10.1016/j.neulet.2005.05.069. [DOI] [PubMed] [Google Scholar]
- [26].Kim JY, Jeong HY, Lee HK, Kim S, Hwang BY, Bae K, et al. Neuroprotection of the leaf and stem of Vitis amurensis and their active compounds against ischemic brain damage in rats and excitotoxicity in cultured neurons. Phytomedicine. 2012;19(2):150–9. doi: 10.1016/j.phymed.2011.06.015. https://doi.org/10.1016/j.phymed.2011.06.015. [DOI] [PubMed] [Google Scholar]
- [27].Yin L, Ohtaki H, Nakamachi T, Kudo Y, Makino R, Shioda S. Delayed expressed TNFR1 co-localize with ICAM-1 in astrocyte in mice brain after transient focal ischemia. Neurosci Lett. 2004;370(1):30–5. doi: 10.1016/j.neulet.2004.07.083. https://doi.org/10.1016/j.neulet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- [28].Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. https://doi.org/10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [29].Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2009;276(1):13–26. doi: 10.1111/j.1742-4658.2008.06766.x. https://doi.org/10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- [30].Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66(3):232–45. doi: 10.1016/j.surneu.2005.12.028. https://doi.org/10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- [31].Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res. 1998;801(1-2):1–8. doi: 10.1016/s0006-8993(98)00489-2. https://doi.org/10.1016/S0006-8993(98)00489-2. [DOI] [PubMed] [Google Scholar]
- [32].Wang Q, van Hoecke M, Tang XN, Lee H, Zheng Z, Swanson RA, et al. Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis. 2009;36(1):223–31. doi: 10.1016/j.nbd.2009.07.018. https://doi.org/10.1016/j.nbd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


