Abstract
Purpose
This review will highlight the molecular and pathologic evidence that cervical cancer is driven by angiogenesis and present a summary of the recent clinical research in anti-angiogenesis therapy for advanced cervical cancer with a focus on the utilization of bevacizumab.
Methods
The articles chosen for this review reveal the rationale for anti-angiogenesis agents in cervical cancer from three perspectives: pathologic, molecular and clinical data.
Findings
Several translational investigations have shown that proangiogenic signaling cascades are active in cervical carcinogenesis which can be utilized to improve patient outcomes in advanced disease. For example, in a recently published study of patients with recurrent and metastatic cervical cancer, bevacizumab was the first targeted agent to improve overall survival in a gynecologic cancer when successfully combined with 2 different chemotherapy regimens.
Implications
Due to recent advances in screening, aggressive management of cervical intraepithelial neoplasia (CIN) and human papillomavirus (HPV) vaccination cervical cancer is preventable and curable with definitive surgery or radiation if detected early. Unfortunately, for patients with metastatic or recurrent disease there are limited effective therapeutic options for this aggressive life-threatening condition. However, molecularly targeted agents have provided a critical opportunity to improve patient outcomes beyond optimizing cytotoxic chemotherapy regimens so that they may benefit from other novel agents or emergent therapies in the future.
Keywords: Angiogenesis, recurrent cervical cancer, bevacizumab
INTRODUCTION
Since the 1950s widespread use of cervical cytology has been successful in reducing the incidence and mortality from cervical cancer dramatically in developed countries and is recognized as one of the greatest cancer prevention achievements to date. In patients with abnormal cervical cytology, one of the hallmarks of invasive disease is vascular aberrations. Mosaicism, punctuations and atypical vessels are all vascular markings that can be identified colposcopically and are indicative of angiogenesis. Angiogenesis is the process of formation of new blood vessels in the body, which is fundamental in the growth of new tissues, wound healing and embryogenesis; but is also essential for tumor proliferation, invasion and metastasis[1]. Neovascularization in cervical tumors is indicative of aggressive clinical behavior and poor prognosis [2].
PATHOLOGICAL EVIDENCE IN SUPPORT OF ANGIOGENESIS-DRIVEN CERVICAL CARCINOMA
Several key translational studies have demonstrated the association between markers of angiogenesis and prognosis in cervical cancer. Cooper et al. assessed intratumoral microvessel density (MVD) in 111 patients with locally advanced cervical cancer and found that higher tumor vascularity was associated with lower overall survival (OS) and locoregional control following treatment with pelvic irradiation [3]. Similarly, Obermair et al. demonstrated enhanced five-year survival with lower MVD (≤ 20 per high power field) of approximately 90% compared to 63% with higher MVD in 166 patients with Stage IB cervical cancer; MVD identified patients with early stage disease with negative nodes at high-risk for relapse [4]. Angiogenesis appears to be an early event in premalignant changes of the cervix from high-grade CIN and MVD increases significantly with malignant transformation suggesting it is a prerequisite for the development of invasive cancer [5–7]. Other authors have confirmed that cervical carcinomas characterized by strong staining for the endothelial marker CD31 (immunohistochemistry marker used to measure degree of tumor angiogenesis)and increased MVD are correlated with worse survival [8, 9].
In contrast to earlier studies, a prospective analysis performed 10 years later examined CD31-MVD in patients who received cisplatin-based chemotherapy along with adjuvant radiation after radical hysterectomy in high risk patients. Increased tumor angiogenesis as reflected by CD31-MVD was an independent prognostic factor for improved progression-free survival (PFS) and overall survival (OS). This observation was attributed to improved delivery of oxygen, nutrients and cytotoxic chemotherapy to well-vascularized and oxygenated tumors [10]. The vasculature associated with CD31+ endothelial cells tends to be more organized and may result in less tumor hypoxia, whereas endoglin, or CD105 (co-receptor for transforming growth factor, TGF-β) enriched endothelial cells are disorganized and CD105+ MVD is associated with an increased relative risk of treatment failure [11]. Observed differences in survival and pathologic tumor features may be related to the progression and stages of angiogenesis.
Investigation of several other pathologic features and immunohistochemictry (IHC) staining of cervical tumors have led to the description of other potentially clinically relevant biomarkers that may be correlated with prognosis and metastatic spread (listed in Table 1). For example, there is evidence that CD40 is overexpressed in HPV 16 and 18- positive cervical cancers and is associated with neovascularization via vascular endothelial growth factor (VEGF) induced angiogenesis. CD40 expression was also shown to be correlated with lymphatic metastasis [12]. Researchers have proposed that CD40 staining is a useful biomarker for evaluation the risk of developing cervical malignancies and better understanding the immune response against these tumors and may provide a potential target for future research in immunotherapy. Additionally, maspin is another example of a clinicopathologic biomarker that has been studied and is predictive with regards the correlation between tissue expression of maspin and prognosis in squamous cell cervical carcinomas. Maspin (a member of the serine protease inhibitors) has an inhibitory affect on angiogenesis and is thought to be potentially implicated in lymphagniogenesis in cervical cancer. Liu et al. demonstrated that both cytoplasmic and nuclear expression of maspin is significantly weaker in squamous cell carcinomas compared to high grade dysplasia and normal cervical specimens [13]. Sub-cellular expression of maspin was significantly decreased or absent in the presence of high lymphatic MVD, advanced clinical stage and lymph node metastases.
Table 1.
List of candidate genes and proteins of interest related to angiogenesis and endothelial-cell markers as prognostic indicators in cervical carcinoma
| Molecular target (gene/protein/biomarker) |
Pathologic and/or prognostic significance in Invasive Cervical Cancer (ICC) |
|---|---|
| Adrenomedullin (ADM) | Progangiogenic peptide upregulated in ICC, target of miR-126 |
| CD31 | Endothelial marker associated with MVD in ICC and prognostic for PFS/OS [10] |
| CD40 | Endothelial marker overexpressed with HPV 16/18+ ICC, associated with lymphatic metastasis and neovascularization [12] |
| Chintase 3 like 1(CHI3L1) | Overexpression of secreted glycoprotein correlates with prognosis and metastatsis in ICC [32] |
| Colony stimulating factor receptor (c-fms) | Proto-oncogene present in majority of ICC and absent in normal cervix, stimulates VEGF-mediated angiogenesis and involved in carcinogenesis [15] |
| Cyclooxygenase (COX- 2) | COX2 inhibition attenuated VEFG-C expression, potent mediator of lymphangiogenesis [33] |
| Endoglin (CD105) | Angiogenesis marker, highest in peritumoral areas and correlated with VEGF and EGFR overexpression [34] |
| Fibulin-4 | Glycoprotein upregulated in ICC, promotes angiogenesis and associated with poor clincopathologic characteristics [35] |
| Maspin | Tumor suppressor implicated in lymphangiogenesis and metastasis [13] |
| miR-126 | Micro RNA involved in angiogenesis and vascular integrity, down regulated [36] |
| Tc17 (cytotoxic T cells+IL17) | Infiltration by IL 17 producing T-cells in ICC correlated with lymph node metastasis and MVD [37] |
| Thrombospondin (TSP1) | Anti-angiogenesis factor that may regulate the angiogenic switch between CIN & ICC, mixed data on prognostic significance [10, 38, 39] |
| Tissue factor pathway inhibitor (TFPI2) | Serine protease inhibitor implicated in apoptosis, angiogenesis and progression of ICC [40] |
| Transforming growth factor β1 (TGFβ1) | Prognostic marker, strong expression associated with worse survival in CD105+ tumors [11] |
| Vasohibin | Angiogenesis inhibitor and potential marker, endothelial cell expression correlated with VEGFR-2 in ICC [41] |
Abbreviations: CIN, cervical intraepithelial neoplasia; COX, cyclooxygenase; HPV, human papillomavirus; ICC, invasive cervical cancer; IL, interleukin; MVD, microvessel density; OS, overall survival; PFS, progression free survival; TFPI, Tissue factor pathway inhibitor TSP, thrombospondin; VEGFR, vascular endothelial growth factor receptor.
Therefore, given the prominence of vascular aberrations and prognostic significance in cervical cancer, active agents that mediate angiogenesis were expected to aid in the development of more effective treatments. However, a more thorough molecular characterization of cervical cancer remains crucial to the development of safe and effective biologic therapies.
A MOLECULAR CASCADE LINKING VIRAL ONCOGENE EXPRESSION AND VEGF-DEPENDENT ANGIOGENESIS
One frequently studied candidate for novel biologic therapies involves the VEGF signaling pathway as it is one of the major drivers of angiogenesis in cervical cancer. Dobbs et al. established that VEGF receptor expression is correlated with MVD in cervical carcinomas [5]. Additionally, persistent infection with the oncogenic subtypes of HPV has been shown to increase angiogenic potential in tumors through up-regulation of VEGF. By all accounts this is an early event in the stages of carcinogenesis from chronic HPV infection or CIN, to invasive cancer [14, 15]. There is a wide range of cellular factors and pathways that have been linked to HPV genomic integration and downstream effects on targets that promote angiogenesis in cervical tumors; thus permitting neovascularization and enabling tumors to acquire the blood supply required for permissive growth and spread [16].
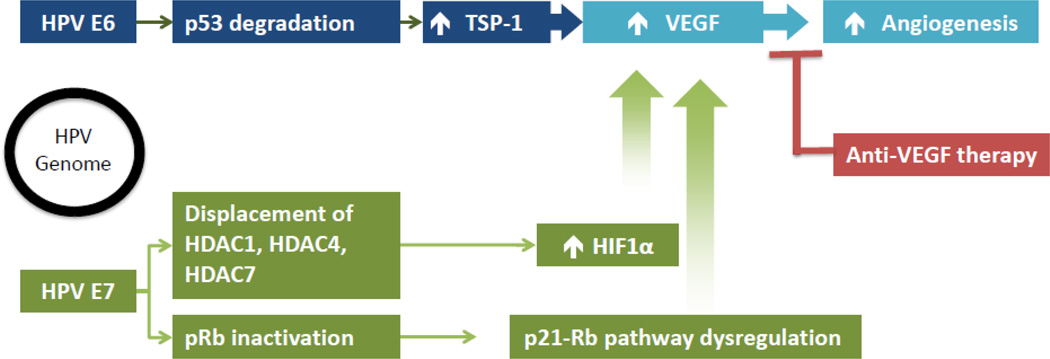
HPV subtypes 16 and 18 are responsible for the overwhelming majority of cervical cancer and one of the key steps in carcinogenesis involves integration of the HPV genome and host DNA. The responsible intermediaries, E6 and E7, are the only HPV gene products that are consistently retained in invasive cervical cancers and are responsible for the transformation and maintenance of the immortalized malignant phenotype caused by ongoing infection [17]. E6 and E7 code for proteins that knock out cellular (host) tumor suppressor gene products leading to several alterations in molecular signaling cascades that ultimately induce VEGF-dependent angiogenesis. The transcriptional repression of these viral oncogenes by E2, an HPV-related gene product) is disrupted during the process of viral integration. Consequently, E6 and E7 are expressed permitting transcription of certain oncoproteins that interact with other gene products to have two important effects: p53 degradation and retinoblastoma protein (pRb) inactivation, respectively. The pro-angiogenic signals that result from these molecular signaling changes following integration of high-risk HPV genomes into host cellular chromosomes are shown in Figure 1. Following DNA damage, HPV E6 proteins block the induction of p53 preventing the cell from going into cell cycle arrest to allow DNA repair and aborting programmed cell death or apoptosis thus allowing continued cellular proliferation. E6 expression can promote ubiquitination of p53 leading to rapid proteasomal protein degradation. In some cases, certain polymorphisms bind more ardently with oncogenic HPV E6 [2]. Individuals with HPV who carry the Arg variant (Arg 72), is one example where this particular polymorphism increases the likelihood of progression from cervical intraepithelial neoplasia to invasive cancer compared to the Pro variant (Pro72) [18]. E7-driven cell cycle progression resulting from abrogation of pRb function has the opposite affect from E6 by potentiating apoptosis through up regulation of the p53 pathway. The induced changes in several angiogenesis mediators that are regulated by p53 are differentially affected by the expression of E6 and E7. Thromobospondin-1 (TSP-1) and maspin are angiogenesis inhibitors that are normally positively regulated by p53 but are decreased in cells expressing E6 and E7. Conversely, VEGF (angiogenesis inducer), which is usually negatively regulated by p53 via the transcription factor hypoxia inducible factor 1 alpha (HIF1α), is up-regulated [16]. Additionally, that HPV E6 and E7 independently enhance the induction and stabilization of HIF1α in in vivo models [19]. The oncogenes again have different mechanisms of achieving similar effects. HPV E7 increases HIF1 transcriptional activity but E6 counteracts the repression of HIF1 through the p53 pathway [20]. Furthermore, E7 enhances HIF1-mediated transcription by inhibiting binding of histone deacetylases (HDAC) and promoting angiogenesis [20]. HIF1α is a transcription factor that controls the expression of over 40 different genes that encode various cytokines and growth factors involved in angiogenesis, including VEGF. Induction and stabilization of HIF1α can be accomplished by viral oncogenes, as described here, through dysregulation of tumor suppressor genes or invoked by other mechanism in response to environmental stressors like hypoxia. As tumors grow away from their existing blood supplies and decreased oxygen tension is encountered and triggers changes in gene expression that supports angiogenesis, which is independent from the effects of HPV E6 and E7.
Figure 1.
Proposed mechanism of HPV infection and the rationale behind angiogenesis inhibitor therapy.
Abbreviations: HDAC, histone deacetylase; HIF1α, hypoxia-inducible factor 1-alpha; HPV, human papilloma virus; pRb, retinoblastoma protein; TSP1, thrombospondin 1; VEGF, vascular endothelial growth factor.
Both oncogenes have a wide range of other targets that are not completely understood. However, the cumulative results of these modifications in gene transcription, protein function and the tumor microenvironment ultimately lead to increased VEGF and increased angiogenesis potential. Overexpression of VEGF has been associated with cancer progression and poor prognosis in many malignancies, including cervical carcinoma. Therefore, the rationale for anti-angiogenesis therapy in cervical cancer stems from the relationship between VEGF, pathologic neovascularization and the development of invasive disease.
However, there are other molecular alterations that contribute to the progression of cervical carcinogenesis beyond HPV infection alone because only a small proportion of these cases will progress to invasive cancer. Other genetic and environmental factors must play an important role in the balance between activating oncogenes and inhibiting the function of tumor suppressor genes. For example, researchers have been able to characterize several polymorphisms within the VEGF gene that affect not only angiogenesis but also influence susceptibility to cervical cancer and impact survival. This study compared four different VEGF genetic polymorphisms in the promoter region that could affect VEGF protein production or function ( −2578C>A, −460 T>C, +405 G>C, and +936 C>T) in 215 without cervical cancer and 199 patients with cervical cancer who underwent surgery [21]. The VEGF −2578 C>A genotype was associated with a decreased risk of cervical cancer (adjusted odds ratio =0.39, 95% CI 0.16–0.96). CD31 MVD was used as a marker for angiogenesis and was significantly decreased in patients with the VEGF +405C/C genotype. Additionally, decreased CD31 and MVD was an independent risk factor for disease recurrence and CD 31 MVD was significantly associated with disease-free survival (adjusted HR = 0.23, 95% CI 0.06–0.92). After controlling for other clinical prognostic factors, such as stage, tumor size, depth of invasion, lymphovascular space invasion, and adjuvant treatment VEGF +405 G>C played a detrimental role in cervical cancer patients based on Cox regression analysis. VEGF +405C/C and VEGF −2578C - −460 T - +405C haplotype were significantly related to shorter disease-free survival (adjusted HR=3.18, 95% CI 1.13–8.94, equally) and overall survival (adjusted HR=8.86, 95% CI 1.40–56.08). These findings suggest that genetic polymorphism are capable of modulating angiogenesis in tumors and thus may impact response to treatment and survival in early cervical cancers.
In summary, translational investigations have shown that proangiogenic signaling cascades are active in cervical carcinogenesis. Molecular alterations that upregulate pro-angiogenic factors such as VEGF, platelet derived growth factor (PDGF), and other small molecules have been correlated with advanced refractory disease and poor survival, but also present exciting opportunities for the development of novel therapeutic interventions.
CLINICAL RAMIFICATIONS OF ANGIOGENESIS INHIBITION IN ADVANCED DISEASE
This body of translational work has established biological plausibility to justify the investigation of angiogenesis inhibitors in cervical cancer, both on a molecular level and clinicopathologic basis. VEGF-mediated angiogenesis is so critical to carcinogenesis in cervical cancer it follows that disruption of this pathway with molecularly targeted agents may be useful in retarding tumor growth, progression, metastasis or perhaps even eliminating small volume residual disease. Furthermore, anti-angiogenesis agents have demonstrated efficacy in other solid malignancies with similar tumor biology, such as NSCLC. Bevacizumab is the single most studied agent in gynecologic neoplasms and other solid tumors.
Bevacizumab is an anti-VEGF monoclonal antibody that blocks tumor angiogenesis by binding and inactivating VEGF thereby inhibiting endothelial cell activation and proliferation, thus denying tumors the ability to recruit new vessel development, counteracts the survival (anti-apoptotic) signaling that supports the immature vasculature usually associated with neoplastic growth, and prevents constant endothelial remodeling required for local tumor spread and thus restoring normal structure and function to disorganized highly-permeable vessels typically seen in malignant tumors [22]. It is currently approved by the United States Food and Drug Administration (FDA) for use in the management of NSCLC, renal cell carcinoma, colorectal cancer and most recently—recurrent cervical cancer.
Based on the available literature, the use of cisplatin-based combination chemotherapy has been widely adopted as the standard treatment backbone in cervical cancer. Unfortunately, responses to chemotherapy are usually temporary with median durations typically lasting between 3–6 months [23]. Over 90% of the deaths attributed to cervical carcinoma occur within 5 years after diagnosis. Chemotherapy for recurrence is essentially palliative in nature because effective salvage therapies are lacking. This underlines the need for more effective treatment strategies for this clinical scenario. In order to improve the current management paradigm, researchers have developed therapeutic strategies that incorporate biologic agents with standard treatments. Bevacizumab was first shown in a small case series to exhibit activity in patients with recurrent cervical carcinoma when combined with cytotoxic chemotherapy [24].
The first prospective phase II trial of bevacizumab in cervical cancer was conducted through the cooperative research network led by the Gynecologic Oncology Group. GOG 227C was a multicenter phase II study of bevacizumab monotherapy that demonstrated the safety and efficacy of the drug in heavily pretreated patients with recurrent cervical cancer—bevacizumab performed even better than expected particularly compared to other historical control groups in this setting [25]. Subsequently, other agents with anti-angiogenic activity have also been studied in advanced and recurrent cervical cancer including oral tyrosine kinase inhibitors. Pazopanib (targets VEGFR, platelet derived growth factor receptor, and c-kit) and lapatinib (dual anti-epidermal growth factor receptor (EGFR) and anti-HER2/neu) were studied in a phase II trial comparing pazopanib (800 mg/day) or lapatinib (1,500 mg /day) monotherapy versus combination therapy with both drugs, however, the combination therapy treatment arm was closed for futility and imbalanced toxicity following the first interim-analysis [26]. This head to head comparison demonstrated the superiority of anti-angiogenesis over anti-EGF therapy. Pazopanib improved PFS compared to lapatinib (4.5 vs. 4.3 months; HR 0.66: 90% CI [0.48, 0.91]; p < 0.013), but did not result in an OS benefit (12.4 vs. 11 months; HR 0.67: 90% CI [0.46, 0.99]; p 0.045). The study provides additional support for pursuing further investigations of anti-VEGF treatments in cervical cancer, but unfortunately EGFR-based therapies, such as cetuximab and erlotinib have resulted in several negative clinical trials (data not shown).
The newest phase II study of another angiogenesis inhibitor was recently presented at the annual meeting of the European Society for Medical Oncology (ESMO) in September, 2014. Cediranib is a once daily oral tyrosine kinase inhibitor that was used in combination with a conventional chemotherapy in patients with metastatic or recurrent cervical cancer. Sixty-nine patients were randomized to carboplatin (AUC 5) and paclitaxel (175 mg/m2) every 21 days plus either cediranib 20 mg or a placebo daily [27]. The results demonstrated a statistically significant improvement in median PFS with chemotherapy plus cediranib compared to placebo (35 vs 30 weeks; HR 0.61; 80% CI [0.41, =0.89]: p=0.046). The median change in serum VEGF inhibition was also significantly improved with cediranib and toxicities appeared on par with other similar biologic agents. Overall, based on results reported cediranib appears safe and active in advanced cervical cancer. The study was not intended or powered to assess overall survival but further investigation with a larger phase III trial is warranted. Table 2 provides a summary of three prospective phase 2 clinical trials featuring VEGF-based therapies in advanced or recurrent cervical cancer.
Table 2.
Phase II studies of VEGF-based therapies in metastatic or recurrent cervical malignancies
| Study | Design | Response Rates | Median PFS | Median OS | Toxicity |
|---|---|---|---|---|---|
| Monk et al. [25] GOG 227C |
Bevacizumab monotherapy 15 mg/kg q 21 d (n=46) |
PR =10.9% PFS ≥ 6 mo = 23.9% |
3.4 mo (2.5–4.5) |
7.3 mo (6.1–10.4) |
Common G3/4 AEs: HTN (7), VTE (5), and GI (4); Grade 5 infection (1) |
| Monk et al. [26] VEG105281 |
Pazopanib monotherapy 800 mg once daily (arm P; n=74) Lapatinib monotherapy 1,500mg once daily (arm L; n=78) Combination therapy (discontinued for futility and unacceptable toxicity) |
arm P = 9 % arm L = 5% |
18.1 w (4.5 mo) 17.1 w (4.3 mo) HR = 0.66 (0.48–0.91) |
50.7 w (12.7 mo) 39.1 w (9.8 mo) HR =0.67 (0.46–0.99) |
Common AEs (%): Diarrhea (54 v 58; G3 =11 v 13); Nausea (36 v 33), HTN (30 v 3), Anorexia (28 v 32), any G4 (12 v 9) Arm P versus arm L , respectively |
| Symonds et al. CIRCCa [27] |
Carboplatin/Paclitaxel +Cediranib 20 mg daily (arm C; n =34) Carboplatin/Paclitaxel + placebo (arm Z; n =35) |
arm C = 66% arm Z= 42% |
35 wk (8.8 mo) 30 wk (7.5 mo) HR = 0.61 (0.41–0.89) |
59 wk (14.8 mo) 63 wk (15.8 mo) HR= 0.93 (0.64–1.36) |
G2–4 AEs (%): Diarrhea (50 v 18) HTN (34 v 12) Any grade (19 v 9) Arm C versus arm Z , respectively |
Abbreviations: AE, adverse event; d, days; CIRRCa, cediranib for advanced cervical cancer; G, grade; GI, gastrointestinal; GOG, Gynecologic Oncology Group; HR, hazard ratio; HTN, hypertension; mo, months; n, number of subjects; OS, overall survival; PFS, progression free survival; PR, partial response; v, versus; VEGF, vascular endothelial growth factor; VTE, venous thromboembolism.
The first phase III randomized controlled trial of anti-angiogenesis therapy in cervical cancer was initiated by the GOG and co-sponsored by the National Cancer Institute (NCI) to study the combination therapy of bevacizumab and standard cytotoxic chemotherapy in patients with recurrent, persistent or metastatic cervical cancer. GOG 240 was designed to address two critical issues in this setting—the effectiveness of anti-angiogenesis therapy and nonplatinum based chemotherapy doublets. A 2 × 2 factorial design was used to answer the biologic/antivascular hypothesis and the chemotherapy question of whether or not a nonplatinum doublet would have greater activity in the recurrent disease population. This was an important question due to the concern for possible platinum drug resistance given the widespread adoption of platinum-based chemoradiation in the primary treatment of locally advanced cancer with more than 70% of the patients in each group having had prior exposure to cisplatin.
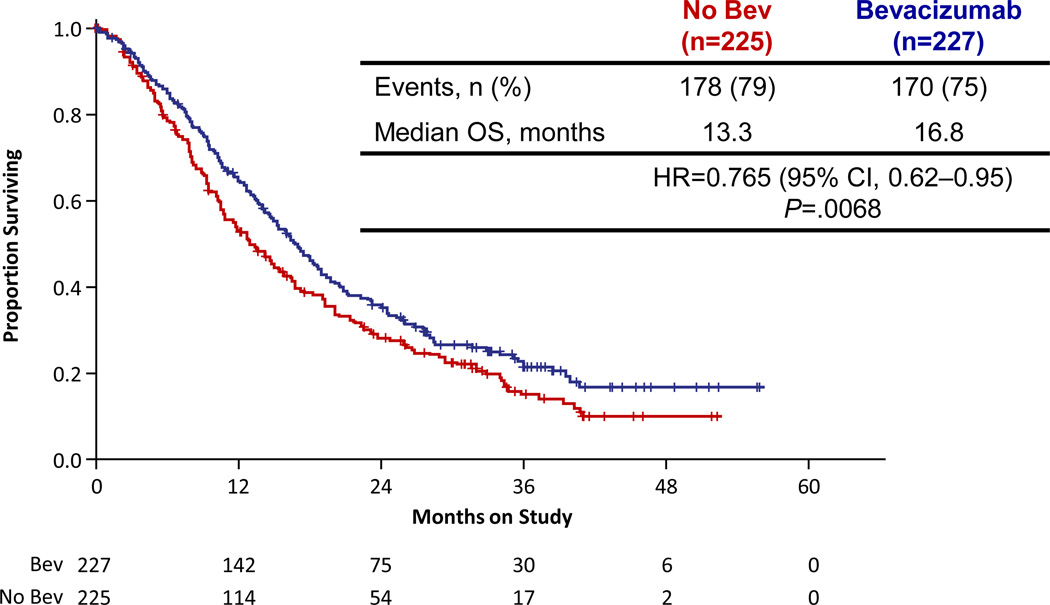
Consequently, patients were randomized to one of four regimens: cisplatin (50 mg/m2) plus paclitaxel (135or 175 mg/m2) with or without bevacizumab (15 mg/kg) or topotecan (0.75 mg/m2 on days 1–3) plus paclitaxel (135or 175 mg/m2) with or without bevacizumab (15 mg/kg) with cycles repeated every 21 days until disease progression or unacceptable toxicity. The results confirmed that the topotecan/paclitaxel chemotherapy doublet was not inferior to cisplatin/paclitaxel (Median OS 12.5 vs. 15 months; HR 1.20; 99% CI [0.82, 1.76]; p = 0.88) [28]. The final analysis did reveal superior outcomes when bevacizumab was added to either chemotherapy regimen leading to a hazard ratio (HR) of death of 0.71 (97.6%, CI [0.54, 0.94]; p = 0.0035). The survival curves are shown in Figure 2. The median OS for patients who received cisplatin plus paclitaxel was 14.3 months, significantly less than the 17.5 months for those who received cisplatin, paclitaxel, and bevacizumab (p = 0.0348). In parallel, the median OS for those who received topotecan plus paclitaxel was lower compared to when bevacizumab was added to that regimen, 12.7 months compared to 16.2 months respectively (p = 0.09. GOG 240 is a landmark trial because it is the first time that a targeted agent has reached its primary endpoint of improving OS in a gynecologic malignancy.
Figure 2.
GOG 240 Results: Overall Survival Curve
Adopted with permission from Tewari et al. [28] New England Journal of Medicine.
Copyright © 2014 Massachusetts Medical Society
Abbreviations: bev, bevacizumab; chemo, chemotherapy; CI, confidence interval; HR, hazard ratio; n, number; OS, overall survival.
Bevacizumab was also established as a “triple-threat” following publication of GOG 240. In addition to achieving the gold standard for the demonstration of clinical benefit in oncology trials—prolonged overall survival, patients who received bevacizumab, also showed improved PFS (HR 0.67; 95% CI [0.54, 0.82]; p = 0.0002) and higher response rates than controls 48% vs.36% (p = 0.008). Twenty eight patients who received bevacizumab had complete responses compared to 14 in the control groups. Even patients with disease contained within a previously irradiated pelvis experienced sustained clinical benefit from bevacizumab [28].
As with any other experimental regimens the benefits must outweigh the risks of treatment. Bevacizumab was associated with a reasonable toxicity profile that is similar to previous studies with this drug. The overall rate of serious adverse effects associated with bevacizumab-containing regimens was less than 10% in GOG 240. As expected from previous reports, treatment-related toxicities observed with the incorporation of bevacizumab included mainly: thromboembolism (8%) gastrointestinal or genitourinary fistulas (6%), and hypertension (25%). No new toxicities of bevacizumab were identified and no deterioration in health related quality of life was reported by patients receiving bevacizumab [28]. Following GOG 240 it is critical that all health care providers and patients are educated about these findings to ensure that all women with persistent, recurrent or metastatic cervical cancer who may benefit from bevacizumab are appropriately counseled about the risks and benefits of adding bevacizumab to standard chemotherapy. Individual treatment decisions should be made on a case-to-case basis and take into account prior treatment history, medical comorbidity, functional status, predisposition to certain toxicities and quality of life. Appropriate patient selection, close clinical monitoring and cautious attention to the management of treatment-related adverse effects will optimize efficacy as well as patient safety.
The National Cancer Institute (NCI) issued a practice changing press release after the initial results from GOG 240 were presented at the American Society of Clinical Oncology (ASCO) annual meeting in June 2013 in support of bevacizumab for late-stage cervical cancer [29]. In July 2013, the National Comprehensive Cancer Network (NCCN) updated their practice guidelines for Cervical Cancer Treatment to include the both cisplatin-paclitaxel-bevacizumab and topotecan-paclitaxel-bevacizumab triplets as first-line recommended therapy in recurrent and metastatic disease (category 1; updated from category 2 08/2014) [30]. Attaining expeditious FDA approval was a crucial step in enhancing the care of women with invasive cervical cancer because this regulatory milestone is required for coverage under Medicare and Medicaid. In August 2014, under the FDA’s priority review program bevacizumab became the first biologic agent approved for use in patient with late-stage cervical in less than four months and was the first drug approved in this patient population since 2006 [31]. This overwhelming and rapid response is a reflection of the importance of successfully addressing a historically unfulfilled clinical need.
FUTURE DIRECTIONS
The excitement created with publication of GOG 240 will promote the continued study of other classes of anti-angiogenesis inhibitors in recurrent or even frontline therapy for cervical cancer. The observed benefits associated with several anti-angiogenesis agents in the aforementioned phase 2 and phase 3 trials merit further investigation in order to further refine the most appropriate regimen for this population with tolerable toxicity. Moving forward, confirmatory phase 3 clinical trial proposals should include multifactorial study designs combining conventional chemotherapy backbones with known active biologic agents, including carboplatin or cisplatin/paclitaxel/bevacizumab with or without cediranib or pazopanib.
Unfortunately, despite the proven efficacy of anti-angiogenesis therapy in cervical cancer, disease recurrence is still problematic for these women. The majority of patients diagnosed with locally advanced or metastatic cervical cancer will experience disease recurrence. Historically, this disease is often refractory to chemotherapy resulting in disappointing responses to salvage therapies. However, if patients are living longer with anti-angiogenesis therapy there will be an increasing demand for second and third line therapies moving forward; resulting in an unmet clinical need for alternative agents and novel treatment paradigms for this disease in the future. Molecularly targeted drugs are needed to exploit other relevant signal transductions pathways, to disrupt the highly integrated tumor microenvironment, and immune system modulation will be critical to achieving improved oncologic outcomes for women affected by invasive cervical cancer.
Footnotes
Conflicts of Interest Statement:
The authors have declared relationships to this review as listed below:
LK has no conflicts of interest to disclose.
KT
Consultant: ROCHE/GENENTECH, CARIS, ADVAXIS
Advisory Board: ROCHE/GENENTECH, CARIS, ADVAXIS, VERMILLION
Speaker's Bureau: VERMILLION
Contracted Research: Genentech, Amgen, Endocyte, Astra-Zeneca
References
- 1.Kerbel RS. Tumor angiogenesis. The New England journal of medicine. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tewari KS, Monk BJ. New Strategies in Cervical Cancer: From Angiogenesis Blockade to Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-1099. [DOI] [PubMed] [Google Scholar]
- 3.Cooper RA, Wilks DP, Logue JP, Davidson SE, Hunter RD, Roberts SA, et al. High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 1998;4:2795–2800. [PubMed] [Google Scholar]
- 4.Obermair A, Wanner C, Bilgi S, Speiser P, Kaider A, Reinthaller A, et al. Tumor angiogenesis in stage IB cervical cancer: correlation of microvessel density with survival. American journal of obstetrics and gynecology. 1998;178:314–319. doi: 10.1016/s0002-9378(98)80018-5. [DOI] [PubMed] [Google Scholar]
- 5.Dobbs SP, Hewett PW, Johnson IR, Carmichael J, Murray JC. Angiogenesis is associated with vascular endothelial growth factor expression in cervical intraepithelial neoplasia. British journal of cancer. 1997;76:1410–1415. doi: 10.1038/bjc.1997.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellas A, Moch H, Schultheiss E, Feichter G, Almendral AC, Gudat F, et al. Angiogenesis in cervical neoplasia: microvessel quantitation in precancerous lesions and invasive carcinomas with clinicopathological correlations. Gynecologic oncology. 1997;67:27–33. doi: 10.1006/gyno.1997.4835. [DOI] [PubMed] [Google Scholar]
- 7.Tjalma W, Sonnemans H, Weyler J, Van Marck E, Van Daele A, van Dam P. Angiogenesis in cervical intraepithelial neoplasia and the risk of recurrence. American journal of obstetrics and gynecology. 1999;181:554–559. doi: 10.1016/s0002-9378(99)70492-8. [DOI] [PubMed] [Google Scholar]
- 8.Wiggins DL, Granai CO, Steinhoff MM, Calabresi P. Tumor angiogenesis as a prognostic factor in cervical carcinoma. Gynecologic oncology. 1995;56:353–356. doi: 10.1006/gyno.1995.1062. [DOI] [PubMed] [Google Scholar]
- 9.Sharma B, Singh N, Gupta N, Lal P, Pande S, Chauhan S. Diagnostic Modalities of Precancerous and Cancerous Cervical Lesions with Special Emphasis on CD31 Angiogenesis Factor as a Marker. Pathology research international. 2013;2013:243168. doi: 10.1155/2013/243168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randall LM, Monk BJ, Darcy KM, Tian C, Burger RA, Liao SY, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecologic oncology. 2009;112:583–589. doi: 10.1016/j.ygyno.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H, Huang CC, Ou YC, Huang EY, Changchien CC, Tseng CW, et al. High immunohistochemical expression of TGF-beta1 predicts a poor prognosis in cervical cancer patients who harbor enriched endoglin microvessel density. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2012;31:482–489. doi: 10.1097/PGP.0b013e31824c23a4. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Qu QX, Xie F, Zhang T, Hu JM, Chen YG, et al. CD40 is overexpressed by HPV16/18-E6 positive cervical carcinoma and correlated with clinical parameters and vascular density. Cancer epidemiology. 2011;35:388–392. doi: 10.1016/j.canep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Shi Y, Meng W, Liu Y, Yang K, Wu S, et al. Expression and localization of maspin in cervical cancer and its role in tumor progression and lymphangiogenesis. Archives of gynecology and obstetrics. 2014;289:373–382. doi: 10.1007/s00404-013-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Saia PJ, Di Saia PJ, Creasman WT, Di Saia PJ. Clinical gynecologic oncology. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2012. [Google Scholar]
- 15.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, et al. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecologic oncology. 2008;110:445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23:2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- 17.Baker CC, Phelps WC, Lindgren V, Braun MJ, Gonda MA, Howley PM. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. Journal of virology. 1987;61:962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habbous S, Pang V, Eng L, Xu W, Kurtz G, Liu FF, et al. p53 Arg72Pro polymorphism, HPV status and initiation, progression, and development of cervical cancer: a systematic review and meta-analysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6407–6415. doi: 10.1158/1078-0432.CCR-12-1983. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Bodily JM, Beglin M, Kyo S, Inoue M, Laimins LA. Hypoxia-specific stabilization of HIF-1alpha by human papillomaviruses. Virology. 2009;387:442–448. doi: 10.1016/j.virol.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodily JM, Mehta KP, Laimins LA. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer research. 2011;71:1187–1195. doi: 10.1158/0008-5472.CAN-10-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YH, Kim MA, Park IA, Park WY, Kim JW, Kim SC, et al. VEGF polymorphisms in early cervical cancer susceptibility, angiogenesis, and survival. Gynecologic oncology. 2010;119:232–236. doi: 10.1016/j.ygyno.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Monk BJ, Willmott LJ, Sumner DA. Anti-angiogenesis agents in metastatic or recurrent cervical cancer. Gynecologic oncology. 2010;116:181–186. doi: 10.1016/j.ygyno.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Current opinion in obstetrics & gynecology. 2014;26:314–321. doi: 10.1097/GCO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 24.Wright JD, Viviano D, Powell MA, Gibb RK, Mutch DG, Grigsby PW, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecologic oncology. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 27.Symonds P, Gourley C, Davidson S, et al. LBA25_PR-CIRCCa: A randomised double blind phase II trial of carboplatin-paclitaxel plus cediranib versus carboplatin-paclitaxel plus placebo in metastatic /recurrent cervical cancer. ESMO Abstract. 2014 doi: 10.1016/S1470-2045(15)00220-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. The New England journal of medicine. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NCI Press Release. Bevacizumab significantly improves survival for patients with recurrent and metastatic cervical cancer. www.cancer.gov/newscenter/newsfromnci/2013/GOG240.
- 30.NCCN Clinical Practice Guidelines in Oncology. Cervical Cancer Version 2. 2015. [Google Scholar]
- 31.FDA approves Avastin to treat patients with aggressive and late-stage cervical cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm410121.htm.
- 32.Ngernyuang N, Francescone RA, Jearanaikoon P, Daduang J, Supoken A, Yan W, et al. Chitinase 3 like 1 is associated with tumor angiogenesis in cervical cancer. The international journal of biochemistry & cell biology. 2014;51:45–52. doi: 10.1016/j.biocel.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Xiao J, Yang Y, Liu Y, Ma R, Li Y, et al. COX-2 expression is correlated with VEGF-C, lymphangiogenesis and lymph node metastasis in human cervical cancer. Microvascular research. 2011;82:131–140. doi: 10.1016/j.mvr.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Barbu I, Craitoiu S, Simionescu CE, Dragnei AM, Margaritescu C. CD105 microvessels density, VEGF, EGFR-1 and c-erbB-2 and their prognostic correlation in different subtypes of cervical adenocarcinoma. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2013;54:519–530. [PubMed] [Google Scholar]
- 35.Chen J, Zhang J, Liu X, Fang R, Zhao Y, Ma D. Overexpression of fibulin-4 is associated with tumor progression and poor prognosis in patients with cervical carcinoma. Oncology reports. 2014;31:2601–2610. doi: 10.3892/or.2014.3139. [DOI] [PubMed] [Google Scholar]
- 36.Huang TH, Chu TY. Repression of miR-126 and upregulation of adrenomedullin in the stromal endothelium by cancer-stromal cross talks confers angiogenesis of cervical cancer. Oncogene. 2014;33:3636–3647. doi: 10.1038/onc.2013.335. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Hou F, Liu X, Ma D, Zhang Y, Kong B, et al. Tc17 cells in patients with uterine cervical cancer. PloS one. 2014;9:e86812. doi: 10.1371/journal.pone.0086812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MP, Tzeng CC, Wu LW, Huang KF, Chou CY. Thrombospondin-1 acts as a fence to inhibit angiogenesis that occurs during cervical carcinogenesis. Cancer journal. 2004;10:27–32. doi: 10.1097/00130404-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Kodama J, Hashimoto I, Seki N, Hongo A, Yoshinouchi M, Okuda H, et al. Thrombospondin-1 and-2 messenger RNA expression in invasive cervical cancer: correlation with angiogenesis and prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:2826–2831. [PubMed] [Google Scholar]
- 40.Zhang Q, Zhang Y, Wang SZ, Wang N, Jiang WG, Ji YH, et al. Reduced expression of tissue factor pathway inhibitor-2 contributes to apoptosis and angiogenesis in cervical cancer. Journal of experimental & clinical cancer research : CR. 2012;31:1. doi: 10.1186/1756-9966-31-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshinaga K, Ito K, Moriya T, Nagase S, Takano T, Niikura H, et al. Roles of intrinsic angiogenesis inhibitor, vasohibin, in cervical carcinomas. Cancer science. 2011;102:446–451. doi: 10.1111/j.1349-7006.2010.01812.x. [DOI] [PubMed] [Google Scholar]