Abstract
Bevacizumab (BEV) is a common treatment for recurrent glioblastoma (GBM). After progression on BEV, there is no consensus on subsequent therapy, as multiple chemotherapy trials have failed to demonstrate discernible activity for salvage. A previous review (995 patients) estimated a progression free survival (PFS) on BEV of 4.2 months (SD±2.1) with an overall survival (OS) after progression on BEV at 3.8 months (SD +/− 1). We endeavored to establish a more rigorous historical control, both as a benchmark for efficacy, and a prognostic tool for clinical practice. A comprehensive literature review was performed utilizing PubMed and societal presentation abstracts. A total 2388 patients from 53 arms of 42 studies were analyzed in three groups: 1) thirty-two studies in which survival post-BEV was determined by subtracting PFS from OS (2045 patients): PFS on BEV =4.38 months (95% CI 4.09–4.68); OS post-BEV =3.36 months (95% CI 3.12–3.66); 2) two studies (94 patients) in which OS post-BEV is reported: OS= 3.26 (95% CI 2.39–4.42); 3) eight studies of salvage therapy after progression on BEV (249 patients): of OS post-BEV =4.46 months (95% CI 3.68–5.54). These estimates provide a firm historical control for PFS on BEV, as well as OS after disease progression on BEV therapy.
Keywords: bevacizumab, recurrent glioblastoma, overall survival
Introduction
Among primary brain cancers, glioblastoma (GBM) is both the most common and the most aggressive. Average survival from diagnosis is dismal at approximately 15 months [1]. Standard treatment consists of maximal surgical resection followed by concurrent radiotherapy with temozolomide and subsequent maintenance temozolomide. Unfortunately, glioblastomata uniformly recurs and effective salvage therapies at the time of recurrence are frustratingly limited.
One of the most frequently used salvage treatments is BEV, a humanized monoclonal antibody targeting vascular endothelial growth factor (VEGF). BEV received conditional accelerated American Food and Drug Administration (FDA) approval for treatment of recurrent GBM in 2009 based on promising non-controlled phase 2 trials. [2, 3]. (Two large phase 3 trials of BEV in the newly diagnosed setting failed to demonstrate improvement in overall survival over standard radiotherapy/temozolomide treatment [4, 5]). Even so, BEV remains a common choice for GBM therapy in the recurrent setting. Unfortunately, despite multiple trials of various salvage chemotherapy regimens, no chemotherapeutic agent has been demonstrated to significantly alter survival after tumor progression on BEV [6–12].
To date, we are aware of only one previous review exploring survival after progression on BEV. To establish a historical control for a phase 2 study of retreatment radiation, Magnuson et al performed a review of the literature including 922 patients, reporting a median overall survival (OS) after progression on BEV of 3.8 months (SD +/− 1.0 months) and a progression free survival (PFS) on BEV of 4.2 months (SD±2.1) [13]. This analysis, however, did not statistically incorporate confidence intervals of the included trials.
The study reported below was initiated in order to update this work and attempt a more rigorous statistical analysis of trials reporting outcomes in recurrent GBM therapy with BEV. The goal of this review is to provide a strong historical control/database, which can serve as a prognostic guide clinically for physicians, as well a target for success in future Phase II clinical trials of salvage therapies after progression on BEV.
Methods
Sources of data
We performed a comprehensive literature search via PubMed (updated through July 1, 2016) using the search words “bevacizumab”, “avastin” and “glioblastoma.” No language or date limitations were imposed. Abstracts and virtual meeting presentations from the American Society of Clinical Oncology conferences held between January 2010 and August 2015 and Society for Neuro-Oncology between November 2013 and November 2014 were also searched to identify relevant information. The reference lists of identified articles were examined for additional publications.
Study selection
The following selection criteria were applied: (i) the study population included only patients with histologically proven GBM (World Health Organization grade IV), all of whom had experienced tumor progression measurable on MRI and who received BEV as salvage chemotherapy; (ii) the study reported information on the diagnosis of recurrent GBM, treatment protocol, and reported data for the estimation of overall survival after progression on BEV (either directly, or as median PFS and OS after receiving BEV); and (iii) if there had been duplicate publication of the same patient cohort, the most recent or complete report was used for further analyses. Two authors (HIR, MDT) extracted the data.
Extraction of data
We extracted details regarding the number of patients and treatment information for all studies. For studies of BEV as therapy for recurrence median overall survival after BEV failure was used if available. If this value was not reported, median PFS was subtracted from median OS to estimate median overall survival post-progression on BEV. For studies of salvage therapies attempted after BEV failure median overall survival was extracted. Data reported in days were converted to months using 28 days per 1 month.
Statistical analyses
The analysis was conducted using a fixed effects parametric survival analysis model, assuming that survival (PFS and OS) follows an exponential distribution. The reported median and 95% confidence intervals (if reported) were used to estimate the parameters of the survival distribution for each study, utilizing the methods of moments estimator. The parametric bootstrap technique was utilized to estimate the survival parameters of the combined studies, which was then used to construct the pooled median survival times and corresponding 95% confidence intervals.
Results
A total of 2388 patients from 53 arms of 42 studies meeting the above selection criteria were identified (see Table 1 & 2). These data were analyzed in three separate groups:
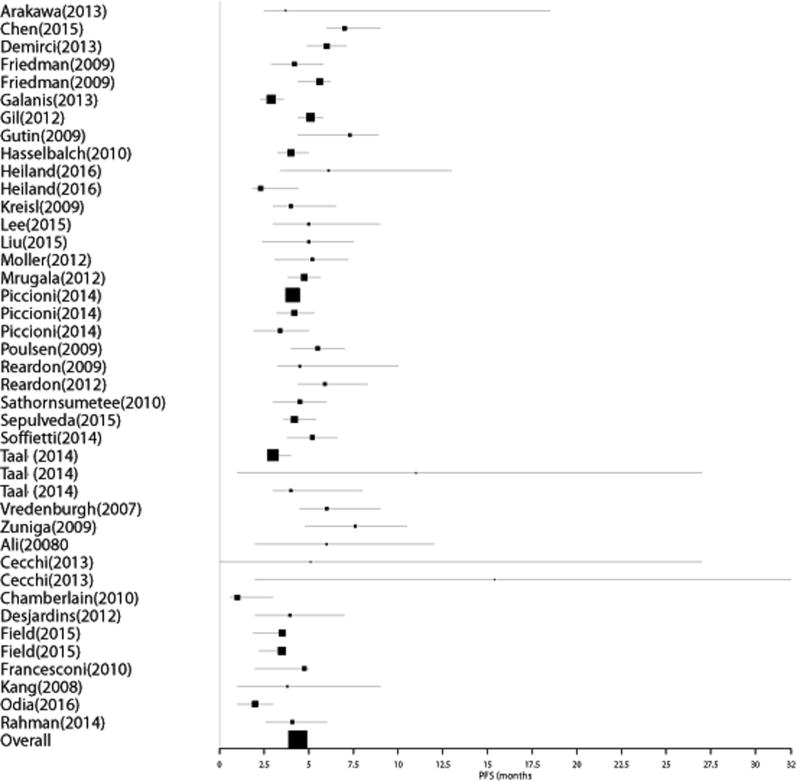
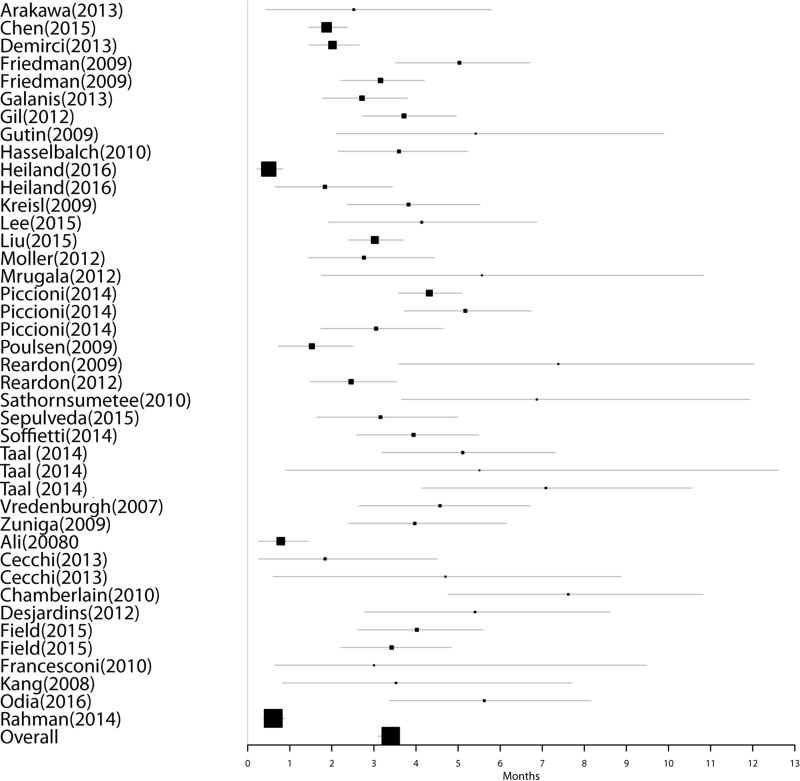
Group 1: Thirty-two studies reported data for 2045 BEV naïve patients undergoing therapy with BEV for recurrent GBM without directly reporting median overall survival post-progression on BEV. For these studies, the median overall survival post-progression on BEV was estimated from the difference between the reported median PFS and median OS. Nine of the 32 studies did not report confidence intervals for these data. Pooled median estimate of OS post-BEV failure for these patients was 3.36 months (95% CI 3.12 – 3.66); PFS was determined as 4.38 months (95% CI 4.09–4.68). Figures 1 & 2 represent Forest plots of PFS on BEV and OS post-BEV respectively in Group 1.
Group 2: Two additional studies reported data for 94 BEV-naïve patients undergoing therapy for recurrent GBM, but directly reported median OS after progression on BEV. Neither study reported confidence intervals for these values. Pooled median estimate of OS for these patients was 3.26 months (95% CI 2.39 – 4.42).
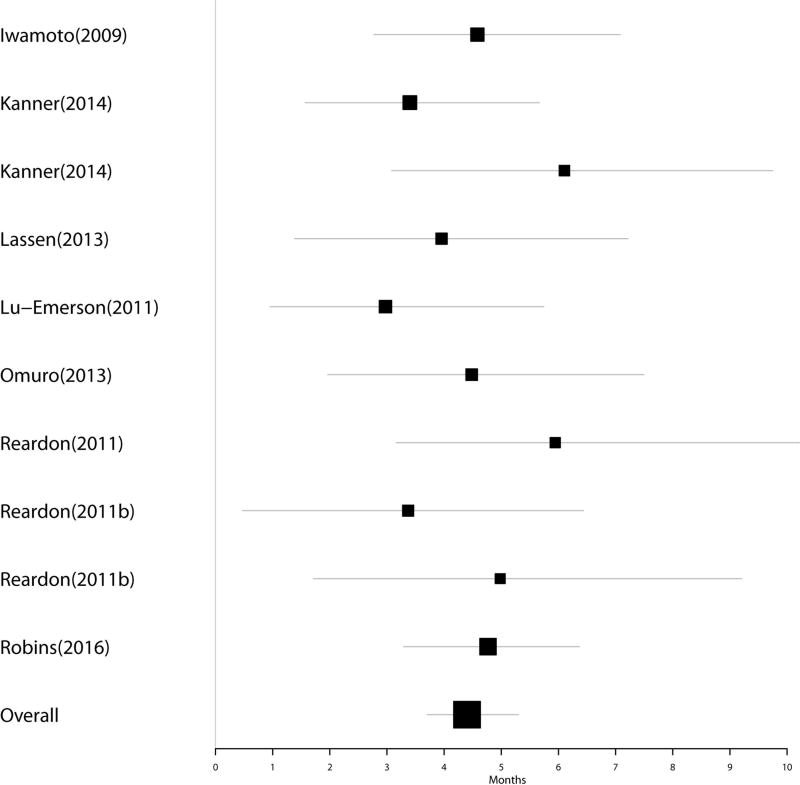
Group 3: Eight studies comprising 249 patients investigated salvage regimens for patients that had progressed on BEV. Five of these studies did not provide confidence intervals for the extracted data. Pooled median estimate of OS for these patients was 4.46 months (95% CI 3.68–5.54). Figure 3 represents a Forest plot of OS post-BEV in Group 3.
Table 1.
Reviewed studies of BEV in recurrent glioblastoma
| Study | Year | N | Agents | Median PFS on BEV |
95% CI | Median OS |
95% CI | Post-BEV Median OS |
|
|---|---|---|---|---|---|---|---|---|---|
| Group 1 BEV Studies | |||||||||
| Vredenburgh [15] | 2007 | 35 | BEV + CPT-11 | 6 | (4.5 – 9) | 10.5 | (8.75 – 15) | 4.5 | |
| Ali [16] | 2008 | 13 | BEV + CPT-11 | 6 | NR | 6.75 | NR | 0.75 | |
| Kang [17] | 2008 | 12 | BEV + CPT-11 | 3.8 | NR | 7.1 | NR | 3.3 | |
| Friedman [2] | 2009 | 85 | BEV | 4.2 | (2.9 – 5.8) | 9.2 | (8.2 – 10.7) | 5 | |
| Friedman [2] | 2009 | 82 | BEV + CPT-11 | 5.6 | (4.4 – 6.2) | 8.7 | (7.8 – 10.9) | 3.1 | |
| Gutin [18] | 2009 | 20 | BEV + RT | 7.3 | (4.4 – 8.9) | 12.5 | (6.9 – 22.8) | 5.2 | |
| Kreisl [3] | 2009 | 48 | BEV | 4 | (3 – 6.5) | 7.75 | (5.25 – 13.5) | 3.75 | |
| Poulsen [19] | 2009 | 27 | BEV + CPT-11 | 5.5 | (4 – 7) | 7 | (3.25 – 10.75) | 1.5 | |
| Reardon [20] | 2009 | 27 | BEV + VP-16 | 4.5 | (3.25 – 10) | 11.6 | (6.25 – 17.5) | 7.1 | |
| Zuniga [21] | 2009 | 37 | BEV + CPT-11 | 7.6 | (4.8 – 10.5) | 11.5 | (8.3 – 15.6) | 3.9 | |
| Chamberlain [22] | 2010 | 50 | BEV | 1 | NR | 8.5 | (3 – 174) | 7.5 | |
| Francesconi [23] | 2010 | 6 | BEV + CBDCA + VP-16 | 4.75 | NR | 7.475 | NR | 2.725 | |
| Hasselbalch [24] | 2010 | 43 | BEV + cetuximab + CPT-11 | 4 | (3.25 – 5) | 7.5 | (5.75 – 9.25) | 3.5 | |
| Sathornsumetee [25] | 2010 | 25 | BEV + erlotnib | 4.5 | (3 – 5.975) | 11.15 | (7.1 – 17.175) | 6.65 | |
| Desjardins [26] | 2012 | 32 | BEV + TMZ | 3.95 | NR | 9.275 | NR | 5.325 | |
| Gil [27] | 2012 | 92 | BEV + CPT-11 | 5.1 | (4.4 – 5.8) | 8.8 | (6.9 – 10.6) | 3.7 | |
| Møller [28] | 2012 | 32 | BEV + CPT-11 | 5.2 | (3.1 – 7.2) | 7.9 | (6.3 – 9.6) | 2.7 | |
| Mrugala [29] | 2012 | 14 | BEV + CBDCA | 4.75 | (3.825 – 5.65) | 10 | (7.675 – 15.325) | 5.25 | |
| Reardon [30] | 2012 | 40 | BEV + CBDCA + CPT-11 | 5.9 | (4.4 – 8.3) | 8.3 | (5.9 – 10.7) | 2.4 | |
| Arakawa [31] | 2013 | 8 | BEV + IFO, CBDCA, VP-16 | 3.7 | (2.5 – 18.5) | 6 | (3.2 –19.7) | 2.3 | |
| Cecchi [32] | 2013 | 9 | BEV | 5.1 | NR | 6.8 | NR | 1.7 | |
| Cecchi [32] | 2013 | 10 | BEV + CPT-11 | 15.4 | NR | 11.1 | NR | NA | |
| Demirci [33] | 2013 | 93 | BEV + CPT-11 | 6 | (4.9 – 7.1) | 8 | (6.6 – 9.4) | 2 | |
| Galanis [34] | 2013 | 54 | BEV + sorafenib | 2.9 | (2.3 – 3.6) | 5.6 | (4.7 – 8.2) | 2.7 | |
| Piccioni [35] | 2014 | 88 | BEV 2nd recurrence | 4.2 | (3.2 – 5.3) | 9.3 | (7.2 – 10.7) | 5.1 | |
| Piccioni [35] | 2014 | 264 | BEV 1st recurrence | 4.1 | (3.7 – 4.5) | 8.4 | (8 – 9.8) | 4.3 | |
| Piccioni [35] | 2014 | 36 | BEV 3rd+ recurrence | 3.4 | (1.9 – 5) | 6.4 | (5.1 – 9.4) | 3 | |
| Rahman [36] | 2014 | 42 | BEV + carmustine or CCNU | 4.075 | NR | 4.675 | NR | 0.6 | |
| Soffietti [37] | 2014 | 54 | BEV + fotemustine | 5.2 | (3.8 – 6.6) | 9.1 | (7.3 – 10.3) | 3.9 | |
| Taal [38] | 2014 | 44 | BEV + CCNU 90mg/m2 | 4 | (3 – 8) | 11 | (8 – 12) | 7 | |
| Taal [38] | 2014 | 50 | BEV | 3 | (3 – 4) | 8 | (6 – 9) | 5 | |
| Taal [38] | 2014 | 8 | BEV + CCNU 110mg/m2 | 11 | (1 – 27) | 16 | (2 – 34) | 5 | |
| Chen [39] | 2015 | 136 | BEV vs BEV + various chemo | 7 | (6 – 9) | 8.86 | (7.06 – 10.44) | 1.86 | |
| Field [40] | 2015 | 62 | BEV | 3.5 | (1.9 – 3.7) | 7.5 | NR | 4 | |
| Field [40] | 2015 | 60 | BEV + CBDCA | 3.5 | (2.2 – 3.7) | 6.9 | NR | 3.4 | |
| Lee [41] | 2015 | 24 | BEV + panobinostat | 5 | (3 – 9) | 9 | (6 – 19) | 4 | |
| Liu [42] | 2015 | 176 | BEV + fotemustine | 5 | (2.4 – 7.5) | 8 | (6.7 – 9.2) | 3 | |
| Sepúlveda [43] | 2015 | 32 | BEV + TMZ | 4.2 | (3.6 – 5.4) | 7.3 | (5.8 – 8.8) | 3.1 | |
| Heiland [44] | 2016 | 17 | BEV | 2.3 | (1.87 – 4.39) | 4.07 | (3.02 – 12.98) | 1.77 | |
| Heiland [44] | 2016 | 18 | BEV + CCNU | 6.11 | (3.41 – 12.98) | 6.59 | (5.51 – 16.3) | 0.48 | |
| Odia [45] | 2016 | 40 | BEV + enzastaurin | 2 | NR | 7.5 | NR | 5.5 | |
| Total N | 2045 | ||||||||
| Group 2 Directly Reported BEV Studies | |||||||||
| Nghiemphu [46] | 2009 | 44 | BEV + various chemo | 4.32 | |||||
| Raizer [47] | 2010 | 50 | BEV | 2.5 | |||||
| Total N | 94 | ||||||||
| Group 3 Post-BEV Salvage Studies | |||||||||
| Iwamoto [6] | 2009 | 37 | BEV + CPT-11 | 4.5 | NR | 4.5 | |||
| Lu-Emerson [12] | 2011 | 14 | BEV + dasatinib | 2.8 | (1.5 – 4.9) | 2.8 | |||
| Reardon [7] | 2011 | 25 | BEV + CPT-11 or CBDCA | 5.8 | (4 – 7) | 5.8 | |||
| Reardon [8] | 2011 | 13 | BEV + VP-16 | 4.8 | (2.8 – 6.4) | 4.8 | |||
| Reardon [8] | 2011 | 10 | BEV + TMZ | 3.2 | (1.2 – 5.8) | 3.2 | |||
| Lassen [48] | 2013 | 13 | BEV + temsirolimus | 3.8 | NR | 3.8 | |||
| Omuro [9] | 2013 | 18 | TMZ | 4.3 | NR | 4.3 | |||
| Kanner [10] | 2014 | 23 | Novo-TTF 100A | 6 | NR | 6 | |||
| Kanner [10] | 2014 | 21 | Various chemotherapies | 3.3 | NR | 3.3 | |||
| Robins [11] | 2016 | 75 | ABT888 + TMZ | 4.7 | NR | 4.7 | |||
| Total N | 249 | ||||||||
Abbreviations: NR = Not reported; BEV= bevacizumab; TMZ = temazolomide; CPT-11 = irinotecan; CBDCA = carboplatin; VP-16 = etoposide; IFO = ifosfamide; CCNU = lomustine; RT = radiotherapy
Table 2.
Pooled estimates across studies
| Pooled Median Estimate |
Lower 95% CI | Upper 95% | ||
|---|---|---|---|---|
| Group 1 OS post-BEV estimated from OS on BEV – PFS on BEV (n=2045) | PFS on BEV | 4.38 months | 4.09 months | 4.68 months |
| OS on BEV | 8.18 months | 7.67 months | 8.70 months | |
| OS post-BEV | 3.36 months | 3.12 months | 3.66 months | |
| Group 2 Directly reported OS after progression on BEV (n=94) | OS post-BEV | 3.26 months | 2.39 months | 4.42 months |
| Group 3 Studies of salvage after progression on BEV (n=249) | OS post-BEV | 4.46 months | 3.68 months | 5.44 months |
Figure 1.
Forest plot of PFS on BEV in Group 1
Figure 2.
Forest plot of OS post-progression on BEV in Group 1
Figure 3.
Forest plot of OS post-progression on BEV in Group 3
Discussion
Our pooled median estimate of post-BEV OS in BEV studies confirm a short median survival of 3.36 months, while our pooled median estimate of OS in post-BEV salvage studies is slightly longer at 4.46 months. This is consistent with the findings of Magnuson et al (mean OS of 3.8 months (SD ± 1.0), an estimate that combined both BEV studies and post-BEV salvage studies [13]. Our data also include more than twice as many patients and a more sophisticated statistical model than this previous estimate. The similarity of results from the two directly reported studies of BEV salvage studies (3.23 months vs 3.36 months) also help confirm the validity of our method of estimating survival after BEV failure using PFS and OS data.
As previously noted, our estimate of median survival after progression on BEV is slightly longer amongst the post-BEV salvage studies. Relative to this, it should be noted, that any gap in time between identification of progression on BEV and initiation of a subsequent salvage regimen would potentially reduce estimated overall survival contingent on the definition of OS. Even so, many of these studies reported slightly longer median overall survival than was typical amongst the BEV salvage studies. One likely explanation is that patients selected for participation in trials of further salvage therapy were generally healthier than the overall population of patients experiencing progression on BEV. By way of illustration, in one retrospective study of 37 patients having progressed on BEV, nearly half of patients had a Karnofsky Performance Status (KPS) of < 70 at the time of progression [6]. This would have excluded these patients from most of post-BEV salvage studies included in this review. Another consideration is that, as a whole, the post-BEV salvage studies tended to have smaller numbers of patients than the BEV salvage studies. Additionally, publication bias may have been stronger among post-BEV salvage studies than BEV salvage studies, as criteria for success would not generally include post-BEV survival in BEV salvage studies.
Taking the results of this review collectively, it is apparent that, to date, drug therapy has demonstrated minimal potential for salvaging patients who have progressed on BEV. It is of interest to note there are limited data suggesting there are at least two non-pharmacological options for prolonging survival after failure on BEV, i.e., Radiotherapy [14] and Tumor Treating Fields Therapy [10].
In summary, it is obvious that a concerted preclinical and clinical research effort is required to address the dismal prognosis of BEV refractory patients. It was the goal of this review to foster that effort, and provide a historical benchmark for efficacy to be used in establishing a “signal” in the context of future phase 2 clinical trials, as well as providing a prognostic tool for clinical practice.
Highlights.
Bevacizumab (BEV) is a common treatment for recurrent glioblastoma (r-GBM).
Post BEV survival (OS) & progression free survival (PFS) are not well defined.
Post BEV-OS & BEV-PFS were analyzed in r-GBM from 53 arms of 42 clinical trials.
PFS =4.38 M (95% CI 4.09–4.68); post-BEV-OS =3.36 M (95% CI 3.12–3.66) [n=2045]
These estimates provide a historical control for BEV-PFS & post BEV-OS.
Acknowledgments
Funded by P30 CA014520 Core Grant, University of Wisconsin Carbone Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Compliance with Ethical Standards
The authors have no conflicts of interests.
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–6. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, et al. Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer. 2011;117:5351–8. doi: 10.1002/cncr.26188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reardon DA, Desjardins A, Peters K, Gururangan S, Sampson J, Rich JN, et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2011;103:371–9. doi: 10.1007/s11060-010-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15:242–50. doi: 10.1093/neuonc/nos295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanner AA, Wong ET, Villano JL, Ram Z, Investigators E Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A™ system versus best physician's choice chemotherapy. Semin Oncol. 2014;41(Suppl 6):S25–34. doi: 10.1053/j.seminoncol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Robins HI, Zhang P, Gilbert MR, Chakravarti A, de Groot JF, Grimm SA, et al. A randomized phase I/II study of ABT-888 in combination with temozolomide in recurrent temozolomide resistant glioblastoma: an NRG oncology RTOG group study. J Neurooncol. 2016;126:309–16. doi: 10.1007/s11060-015-1966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu-Emerson C, Norden AD, Drappatz J, Quant EC, Beroukhim R, Ciampa AS, et al. Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. J Neurooncol. 2011;104:287–91. doi: 10.1007/s11060-010-0489-x. [DOI] [PubMed] [Google Scholar]
- 13.Magnuson W, Ian Robins H, Mohindra P, Howard S. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol. 2014;117:133–9. doi: 10.1007/s11060-014-1363-z. [DOI] [PubMed] [Google Scholar]
- 14.Torcuator RG, Thind R, Patel M, Mohan YS, Anderson J, Doyle T, et al. The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2010;97:401–7. doi: 10.1007/s11060-009-0034-y. [DOI] [PubMed] [Google Scholar]
- 15.Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 16.Ali SA, McHayleh WM, Ahmad A, Sehgal R, Braffet M, Rahman M, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 cases. J Neurosurg. 2008;109:268–72. doi: 10.3171/JNS/2008/109/8/0268. [DOI] [PubMed] [Google Scholar]
- 17.Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008;89:113–8. doi: 10.1007/s11060-008-9599-0. [DOI] [PubMed] [Google Scholar]
- 18.Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–63. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulsen HS, Grunnet K, Sorensen M, Olsen P, Hasselbalch B, Nelausen K, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009;48:52–8. doi: 10.1080/02841860802537924. [DOI] [PubMed] [Google Scholar]
- 20.Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, Sathornsumetee S, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101:1986–94. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Ellika S, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–36. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96:259–69. doi: 10.1007/s11060-009-9957-6. [DOI] [PubMed] [Google Scholar]
- 23.Francesconi AB, Dupre S, Matos M, Martin D, Hughes BG, Wyld DK, et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17:970–4. doi: 10.1016/j.jocn.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sørensen M, Kosteljanetz M, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12:508–16. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sathornsumetee S, Desjardins A, Vredenburgh JJ, McLendon RE, Marcello J, Herndon JE, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12:1300–10. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE, Bailey L, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118:1302–12. doi: 10.1002/cncr.26381. [DOI] [PubMed] [Google Scholar]
- 27.Gil MJ, de Las Peñas R, Reynés G, Balañá C, Peréz-Segura P, García-Velasco A, et al. Bevacizumab plus irinotecan in recurrent malignant glioma shows high overall survival in a multicenter retrospective pooled series of the Spanish Neuro-Oncology Research Group (GEINO) Anticancer Drugs. 2012;23:659–65. doi: 10.1097/CAD.0b013e3283534d3e. [DOI] [PubMed] [Google Scholar]
- 28.Møller S, Grunnet K, Hansen S, Schultz H, Holmberg M, Sorensen M, et al. A phase II trial with bevacizumab and irinotecan for patients with primary brain tumors and progression after standard therapy. Acta Oncol. 2012;51:797–804. doi: 10.3109/0284186X.2012.681063. [DOI] [PubMed] [Google Scholar]
- 29.Mrugala MM, Crew LK, Fink JR, Spence AM. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett. 2012;4:1082–6. doi: 10.3892/ol.2012.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J Neurooncol. 2012;107:155–64. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arakawa Y, Mizowaki T, Murata D, Fujimoto K, Kikuchi T, Kunieda T, et al. Retrospective analysis of bevacizumab in combination with ifosfamide, carboplatin, and etoposide in patients with second recurrence of glioblastoma. Neurol Med Chir (Tokyo) 2013;53:779–85. doi: 10.2176/nmc.oa2013-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cecchi M, Vaiani M, Ceroti M, Banfi R. A retrospective observational analysis to evaluate the off-label use of bevacizumab alone or with irinotecan in recurrent glioblastoma. Int J Clin Pharm. 2013;35:483–7. doi: 10.1007/s11096-013-9765-0. [DOI] [PubMed] [Google Scholar]
- 33.Demirci U, Tufan G, Aktas B, Balakan O, Alacacioglu A, Dane F, et al. Bevacizumab plus irinotecan in recurrent or progressive malign glioma: a multicenter study of the Anatolian Society of Medical Oncology (ASMO) J Cancer Res Clin Oncol. 2013;139:829–35. doi: 10.1007/s00432-013-1390-8. [DOI] [PubMed] [Google Scholar]
- 34.Galanis E, Anderson SK, Lafky JM, Uhm JH, Giannini C, Kumar SK, et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res. 2013;19:4816–23. doi: 10.1158/1078-0432.CCR-13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccioni DE, Selfridge J, Mody RR, Chowdhury R, Li S, Lalezari S, et al. Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol. 2014;16:815–22. doi: 10.1093/neuonc/nou028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman R, Hempfling K, Norden AD, Reardon DA, Nayak L, Rinne ML, et al. Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab. Neuro Oncol. 2014;16:1523–9. doi: 10.1093/neuonc/nou118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soffietti R, Trevisan E, Bertero L, Cassoni P, Morra I, Fabrini MG, et al. Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology) J Neurooncol. 2014;116:533–41. doi: 10.1007/s11060-013-1317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–53. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Ravelo A, Yu E, Dhanda R, Schnadig I. Clinical outcomes with bevacizumab-containing and non-bevacizumab-containing regimens in patients with recurrent glioblastoma from US community practices. J Neurooncol. 2015;122:595–605. doi: 10.1007/s11060-015-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field KM, Simes J, Nowak AK, Cher L, Wheeler H, Hovey EJ, et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EQ, Reardon DA, Schiff D, Drappatz J, Muzikansky A, Grimm SA, et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015;17:862–7. doi: 10.1093/neuonc/nou350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Zhang G, Zhu L, Wang J, Liu D, Lian L, et al. Retrospective analysis of bevacizumab in combination with fotemustine in chinese patients with recurrent glioblastoma multiforme. Biomed Res Int. 2015;2015:723612. doi: 10.1155/2015/723612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sepúlveda JM, Belda-Iniesta C, Gil-Gil M, Pérez-Segura P, Berrocal A, Reynés G, et al. A phase II study of feasibility and toxicity of bevacizumab in combination with temozolomide in patients with recurrent glioblastoma. Clin Transl Oncol. 2015;17:743–50. doi: 10.1007/s12094-015-1304-0. [DOI] [PubMed] [Google Scholar]
- 44.Heiland DH, Masalha W, Franco P, Machein MR, Weyerbrock A. Progression-free and overall survival in patients with recurrent Glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustine. J Neurooncol. 2016;126:567–75. doi: 10.1007/s11060-015-2002-z. [DOI] [PubMed] [Google Scholar]
- 45.Odia Y, Iwamoto FM, Moustakas A, Fraum TJ, Salgado CA, Li A, et al. A phase II trial of enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas. J Neurooncol. 2016;127:127–35. doi: 10.1007/s11060-015-2020-x. [DOI] [PubMed] [Google Scholar]
- 46.Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72:1217–22. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116:5297–305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 48.Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33:1657–60. [PubMed] [Google Scholar]