Abstract
Despite the advent of immune checkpoint blockade for effective treatment of advanced malignancies, only a minority of patients respond to therapy and significant immune-related adverse events remain to be minimized. Innovations in engineered drug delivery systems and controlled release strategies can improve drug accumulation at and retention within target cells and tissues in order to enhance therapeutic efficacy while simultaneously reducing drug exposure in off target tissues to minimize the potential for treatment-associated toxicities. This review will outline basic principles of the immune physiology of checkpoint signaling, the existing knowledge of dose-efficacy relationships in checkpoint inhibition, the influence of administration route on treatment efficacy, as well as the resulting checkpoint inhibitor antibody biodistribution profiles amongst target versus systemic tissues. It will also highlight recent successes in the application of drug delivery principles and technologies towards augmenting checkpoint blockade therapy in cancer. Delivery strategies that have been developed for other therapeutic and immunotherapy applications with as-of-yet underexplored potential in checkpoint inhibition therapy will also be discussed.
Keywords: cytotoxic T lymphocyte antigen-4, programmed cell death-1, cancer immunotherapy, tumor immunology, lymph node, drug delivery systems, controlled release, therapeutic antibody, immune-related associated toxicity
1. Introduction
Immune checkpoint blockade with anti-cytotoxic T-lymphocyte-associated protein (CTLA)-4, anti-programmed cell death (PD)-1, and anti-PD-ligand (PD-L) monoclonal antibody (mAb) drugs have emerged as a successful treatment approach that induces durable objective responses in patients with advanced melanoma, squamous cell lung cancer, renal cell carcinoma, and classical Hodgkin lymphoma due to role of these molecules in costimulatory signaling to T cells [1] that suppresses anti-tumor immunity in human cancers. However, despite clinical successes, objective tumor responses are achieved in only a minority of patients. Several complementary/overlapping tiers of immune regulation can contribute to anti-tumor immune suppression [2] that may limit treatment efficacy. Accordingly, biomarkers are in development to identify individuals most likely to benefit from checkpoint blockade [3,4]. Furthermore, considerable preclinical and clinical research focuses on how the efficacy of checkpoint inhibition may be improved when used in combination with agents with orthogonal but synergistic signaling activity, for example targeted therapies [5,6] and cancer vaccines [7], which expand the population of tumor antigen-specific lymphocytes. Significant immune-related adverse events (iRAE) and toxicities associated with treatment with checkpoint inhibitors when used alone or in combination (e.g. vemurafenib and ipimumab [8]) also remain to be minimized [9–11].
To this end, an emerging area of investigation aiming to augment checkpoint blockade therapy is the development of engineered delivery systems and controlled release innovations to improve mAb accumulation and retention within target cells and tissues in order to enhance immunotherapeutic efficacy and reduce off-target effects. This review will highlight such methods and their successes and, within the context of the basic principles of the immune physiology of checkpoint signaling, the known effects of delivered mAb dose and route of administration on treatment efficacy, as well as checkpoint inhibitor mAb biodistribution amongst target versus systemic tissues, delivery strategies that have been developed for other therapeutic applications with underexplored potential in checkpoint inhibition therapy.
2. Checkpoints and their tissues of action
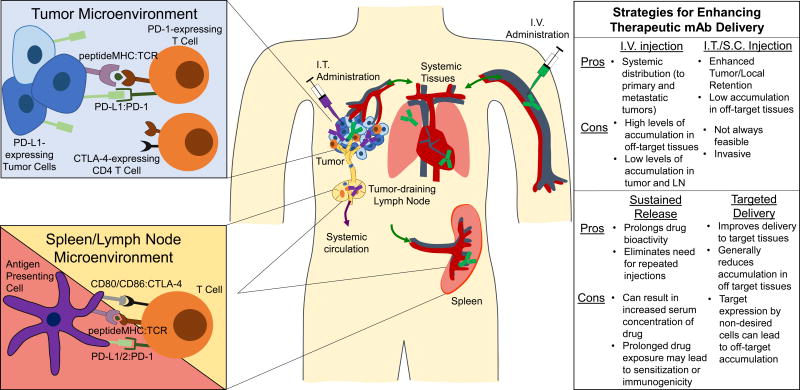
CTLA-4 and PD-1 as well as their ligands exhibit discrete expression profiles, signaling pathways, and molecular mechanisms that underlie their physiological and pathophysiological roles [12,13] (Figure 1). CTLA-4 attenuates T cell responses largely by inhibiting co-stimulatory signaling through CD28. This is facilitated in part by its out-competing CD28 binding to CD80 and CD86 [14], molecule’s whose expression is restricted to antigen presenting cells. Accordingly, CTLA-4’s suppression of anti-tumor immunity is considered to largely occur within secondary lymphoid organs (i.e. lymph nodes and spleen), tissues where T cell activation occurs [15–18] rather than within the tumor microenvironment. Since CTLA-4 is not expressed on the surface of naive and resting memory T cells [19], costimulation can occur upon antigen recognition. Augmented CD8 T cell responses resulting from anti-CTLA-4 treatment likely occurs through increased activation of CD4+ cells since CTLA-4 is also predominantly expressed on CD4 helper rather than CD8 T cells [20]. CTLA-4 also drives the suppressive function of regulatory T cells [21,22], which can locally inhibit anti-tumor immunity through their accumulation within the tumor microenvironment. Supporting the concept that CTLA-4’s effects are not solely localized to secondary lymphoid tissues and can also function within the tumor itself, CTLA-4 blockade exhibits anti-tumor effects even when lymphocyte egress from lymph nodes is inhibited [23].
Figure 1.
In the context of cancer, checkpoints are active within tumors and secondary lymphoid tissues. Left, Canonical and non-canonical checkpoint signaling in tumors and secondary lymphoid tissues. Right, Routes of drug administration and drug delivery systems that improve mAb delivery to target tissues with their relative advantages and limitations in conferring enhanced drug bioactivity and potential for toxicity. I.V., intravenous; I.T., intratumoral; S.C., subcutaneous; LN, lymph node. Green and purple syringe, mAb and lines roughly indicate distribution profiles resulting from i.v. and i.t. administration of mAb, respectively.
Like CTLA-4, resting naive and memory T cells lack PD-1 expression, which increases upon T cell receptor engagement and results in inhibition of T cell receptor-mediated effector functions. During T cell response to infection, PD-1 functions to restrain collateral tissue damage [24]. Accordingly, therapeutic PD-1 blockade in the context of tumor immunotherapy is thought to work predominantly within the tumor microenvironment, where PD-1 is highly expressed on tumor-infiltrating lymphocytes [25,26] and both tumor cells and tumor infiltrating leukocytes often over express its ligands [27]. Many human cancer types have been shown to overexpress PD-1 ligands [28] via mechanisms of either intrinsic resistance (resulting from genetic alterations or signaling pathway activation) or adaptive resistance (an adaptation of tumor cells to an inflammatory immune microenvironment) [12]. On the other hand, PD-1 also plays a role in early fate decisions of T cells recognizing antigens presented in the lymph node, affecting both the size of the proliferative T cell population upon antigen recognition and partially converting T cell tolerance to effector differentiation [29]. The proliferative burst of virus-specific CD8 T cells after PD-1 blockade in mice chronically infected with lymphocytic choriomeningitis virus also only occurs within lymphoid tissues [30].
3. Dosing Effects on Checkpoint Blockade Efficacy and Toxicity
The dosage of mAb administered is an important criterion that can greatly affect therapeutic response. Accordingly, clinical studies have established a dose-toxicity relationship for anti-CTLA-4 therapy indicating that higher doses lead to better response rates but with concurrent increases in iRAE. In a study with patients with advanced melanoma, anti-CTLA-4 mAb ipilimumab was administered at doses of 0.3, 3, or 10 mg/kg with the highest tested dose resulting in better overall response rates as well as higher total lymphocyte counts, a measurement used as a biomarker for anti-CTLA-4 therapy efficacy/pharmacodynamics [31]. In that same study, as the dose was increased, blood serum concentration of ipilimuamab and iRAE also increased in a linear fashion, however at all three doses, manageable safety profiles were achieved [31]. From a mechanistic standpoint, it has been shown in both preclinical and clinical models that CTLA-4 blockade leads to proliferation and activation of both regulatory and effector T cells, however, at lower doses in patients, regulatory T cells appear to be more sensitive to anti-CTLA4 treatment and therefore higher doses may be needed to affect effector T cells to result in anti-tumor immunity [32,33].
In contrast to CTLA-4, there are conflicting reports on the dose effects on the clinical efficacy and toxicity of PD-1 blockade. In patients with prostate, lung, and advanced melanoma, doses of anti-PD-1 nivolumab up to 10 mg/kg were well tolerated with no signs or indications of a dose-efficacy or dose-toxicity relationship [10,34]. However, in another trial in patients with advanced melanoma, a dose of 10 mg/kg anti-PD-1 mAb lambrolizumab every two weeks resulted in a superior rate of response (52%) compared to those achieved by lower doses (2 mg/kg every three weeks) or the same dose, just on a less frequent schedule (10 mg/kg every three weeks) (25% and 27% respectively) [35]. However, these dose-related improvements in response rates were accompanied by higher frequencies of iRAE, 23% for the group receiving 10 mg/kg every two weeks compared to 4% and 9% for the groups receiving 10 mg/kg every 3 weeks and 2 mg/kg every three weeks, respectively [35]. Similarly, in a preclinical study using the B16F10 melanoma model, improvements in tumor reduction were seen with increasing dose of mAb blocking PD-1 [36].
In contrast to checkpoint blockade with anti-CTLA-4 mAb and the conflicting reports on checkpoint blockade with anti-PD-1 mAb, the rate of patient response and frequency of iRAE with anti-PD-L1 treatment appears to be relatively dose independent. In a study involving patients with various advanced cancer types, clinical activity was seen with a dose as low as 1 mg/kg, although there appeared to be an improvement in response at higher doses, albeit not to a statistically significant level [37]. However, like anti-PD-1 mAb, preclinical studies have indicated a slight improvement in survival and tumor suppression with increasing anti-PD-L1 mAb dose [36,38], although toxicity effects have not to our knowledge been well studied in rodent models.
Other consistent themes seen in clinical testing are the dose-dependent blood pharmacokinetics and dose-independent blood pharmacodynamics or blood lymphocyte PD-1 or PD-L1 occupancy rates [34,35,37]. Specifically, as the dose of administered mAb increases, serum mAb concentration also increases in a direct manner. However, PD-1/PD-L1 expressing blood lymphocytes appear to be saturated at the lowest of tested dose (0.3 mgs/kg) suggesting that as the dose of mAb is increased and the serum concentration increases, unwanted accumulation in off-target tissues may result, leading to higher likelihoods of iRAE.
With these monotherapy studies in mind, the potential for combination therapy with anti-CTLA-4 and anti-PD-1 mAb drugs to achieve improved effects using lower doses compared to monotherapy was evaluated. In a study in patients with advanced melanoma, escalating doses of both nivolumab and ipilimumab were concurrently administered [39]. Doses at or above 3 mg/kg of nivolumab and 3 mg/kg of ipilimumab surpassed the maximum tolerated dose [39]. However, when reduced to 1 mg/kg nivolumab and 3 mg/kg ipilimumab, iRAE were reduced to acceptable levels while still achieving substantial rates of favorable responses (53%) [39]. In fact, all nine of the patients who responded to combination therapy exhibited tumor regression of 80% or more compared to less than 3% of patients who received nivolumamb or ipilimumab monotherapy at a dose of 3 mg/kg [39]. These phase I results suggest that combination therapy with nivolumab and ipilimumab can achieve high rates of patient response while maintaining manageable safety profiles depending on dose.
4. Biodistribution of Non-specific and Checkpoint Inhibitor mAb
Given the established dose-response relationships for some checkpoint inhibitor mAb with respect to both therapeutic and side effects, mAb biodistribution profiles within target versus off-target tissues may critically influence their effects both locally in addition to distant tissues resulting from the abscopal effects intrinsic to immunotherapy (Figure 1). When intravenously (i.v.) administered, IgG rapidly distributes throughout the body leading to accumulation primarily within blood-rich systemic organs including the liver, heart, kidneys, lungs, and spleen [40,41]. It also exhibits a long in vivo half-life, approximately 21 days which is due to recycling by the neonatal Fc receptor [42]. This long half-life can be advantageous in sustaining the effects of therapeutic mAb, but can also lead to significant exposure in non-target cells and tissues. As an alternative to i.v. infusion, Epenetos et al. investigated the effects of intratumoral (i.t.) injection on the accumulation and retention of mAb within tumors. Not surprisingly, i.t. administration led to tumor concentrations of mAb approximately 10 times greater than those achieved by i.v. injection up to 18 days post injection [43]. This may be favorable for tumor mAb retention, however depending on the tumor location, it may be infeasible, therefore requiring different administration routes. As with i.t. injection, IgG is largely retained at the administration site when administered subcutaneously (s.c.) resulting in slow and very low accumulation levels in systemic organs compared to i.v. injections [44]. Filipe et al. found s.c. injection required approximately 24 hours to achieve appreciable levels of IgG accumulation in systemic tissues, as opposed to several minutes with an i.v. infusion [44]. Moreover, no accumulation was observed or reported in the lymph nodes irrespective of administration route [44]. In addition to these studies, it is important to consider the therapeutic mAb closely when evaluating the half-life and distribution as these parameters can vary with the IgG isotype and host in part through their effects on neonatal Fc receptor affinity [45].
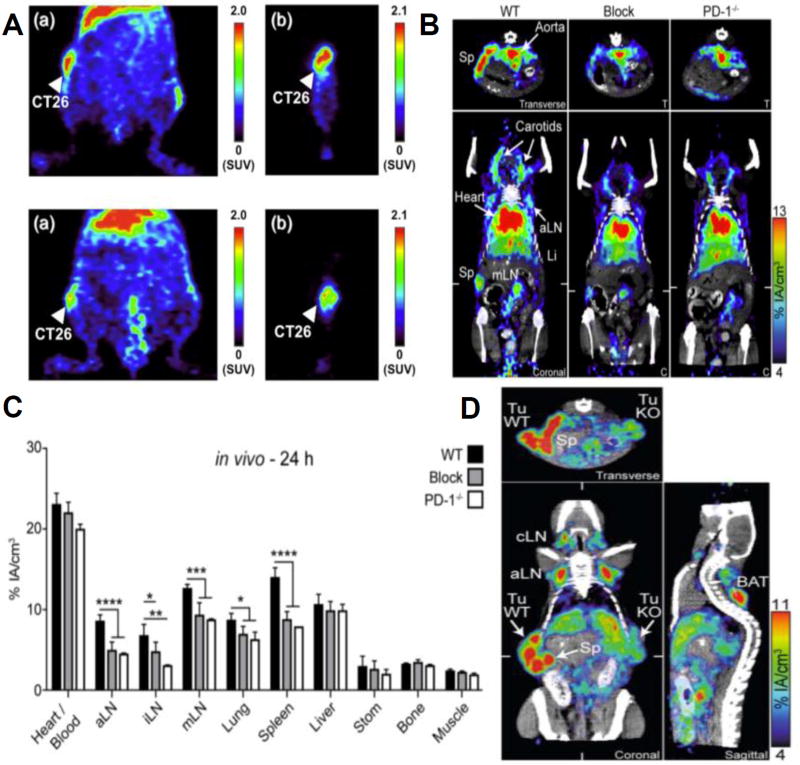
In addition to route of administration and IgG isotype, mAb distributions are drastically affected by target specificity. As a result, in addition to accumulating within systemic organs, checkpoint blockade mAb have been shown to distribute appreciably to secondary lymphoid organs, specifically lymph nodes and the spleen in addition to the tumor itself when administered i.v. and at levels dramatically higher than that seen with non-specific mAb (Figure 2). For example, Higashikawa et al. demonstrated that anti-CTLA-4 mAb exhibits enhanced accumulation in CT26 tumors compared to a control non-specific IgG antibody as a result of its binding to CTLA-4 expressing T cells [46] (Figure 2). Natarajan et al. also demonstrated highest accumulation levels of anti-PD-1 in the spleen, liver, blood, and tumor 24 hours post injection with this same trend continuing 48 hours post injection using a melanoma mouse xenograft and radiolabeling anti-PD-1 [47]. Moreover, when unlabeled anti-PD-1 was administered just prior to infusion of labeled anti-PD-1, significantly less labeled anti-PD-1 mAb was found to accumulate in the spleen and tumor, indicating specificity towards PD-1-expressing lymphocytes [47]. Interestingly, anti-PD-L1 shows similar biodistribution profiles to that of anti-PD-1 mAb when injected i.v., with high levels of accumulation within the liver, lungs, and kidneys [48,49]. Anti-PD-L1 mAb tissue distribution appears concentration dependent, an effect primarily attributed to the large abundance of PD-L1-expressing splenocytes. Thus the spleen acts as a sink for anti-PD-L1 mAb and as the dose increases, splenocytes become saturated, allowing anti-PD-L1 mAb to instead accumulate in other PD-L1-expressing tissues such as tumors [48–50]. Using a B16F10 mouse melanoma model, Hettich et al. evaluated the biodistribution of anti-PD-1 and anti-PD-L1 mAb using PD-1 or PD-L1-deficient mice as well as PD-L1-deficient B16F10 melanoma cells [51] (Figure 2). In both naïve and tumor bearing mice, anti-PD-1 accumulated significantly more in draining lymph nodes and the spleen compared to experiments where PD-1 was blocked by treatment with unlabeled anti-PD-1 mAb or when PD-1-deficient mice were used, indicating specificity towards PD-1 and confirming expression in these tissues [51] (Figure 2). Similar trends were also observed for anti-PD-L1 mAb distributions presumably due to PD-L1 expressing tumor cells and lymphocytes in the tumor, lymph nodes, and spleen, again confirming specificity towards PD-L1 [51] (Figure 2). However, in addition to the appreciable accumulation in secondary lymphoid tissues, anti-PD-L1 was also found to accumulate in the lungs and brown adipose tissue due to the expression of PD-L1 in these tissues [51]. Overall, mAb tend to accumulate in blood rich organs including the liver, lungs, and kidneys, however checkpoint blockade inhibitors alter the distribution to tissues with high expression of checkpoint receptors, mainly the spleen, tumor, and lymph nodes.
Figure 2.
Checkpoint expression and checkpoint blockade mAb biodistribution in naïve and tumor bearing mice. (A) PET images of BALB/c mice implanted with CT26 tumors 48 hours post administration of radiolabeled anti-CTLA-4 mAb (top row) or radiolabeled control IgG (bottom row). Coronal view (a) and sagittal view (b) are shown. (B) ImmunoPET/CT sections using radiolabeled anti-PD-1 mAb 24 hours post injection in naïve wildtype mice (C57BL/6) (WT), PD-1 blocked (Blocked) and PD deficient mice (PD-1−/−). White ticks in the coronal sections indicate the position of the Transverse section. (C) Quantification of B in various organs. * indicates significance relative to the Block and/or PD-1−/− groups using one-way ANOVA with Tukey’s multiple comparison test. P-values < 0.05 considered significant. (D) 10 days following implantation of C57BL/6 mice with wildtype CD133-expressing B16F10 melanoma cells on the left and CD133-expressing, PD-L1 knockout B16F10 melanoma cells on the right, radiolabeled anti-PD-L1 mAb was administered. ImmunoPET/CT images 24 hours post injection are shown here (coronal, transverse, and sagittal). (D) Reproduced from REF 46 (A) and REF 51 (B–D) with permission.
5. Route of Administration Effects on the Efficacy of Checkpoint Inhibition Cancer Therapy
The route of administration is another important parameter with potential to influence the effects of mAb therapy. Therapeutic mAb are administered i.v. clinically, however i.t., peri-tumoral (p.t.), and s.c. injection routes have been shown to improve mAb immunotherapy efficacy both by enhancing mAb delivery locally to the tumor as well as reducing systemic accumulation in preclinical models. For example, Fransen et al. showed that s.c. injection of anti-CTLA-4 mAb led to an effective anti-tumor response using a slow-release Montanide formulation [52]. Specifically, this administration route led to stimulation of T cells localized to the site of injection while using a mAb concentration four times lower than the intraperitoneal (i.p.) administration dose of 200 ug used in the majority of preclinical mouse studies [52]. This route of administration also greatly reduced mAb serum concentrations thereby preventing systemic toxicities associated with anti-CTLA-4 therapy [52]. In a similar study investigating the effect of checkpoint inhibitor route of administration on therapeutic efficacy, van Hooren et al. found i.t. injection to result in lower serum mAb concentrations while still slowing tumor growth compared to i.v. or s.c. (non-tumor bearing flank) injection [53]. When combining anti-PD-1 with anti-CTLA-4 mAb treatment, improvements in survival were achieved using p.t. injections of 30 ug per mouse at comparable rates to that induced by 100 ug i.v. injection of anti-PD-1 mAb [53]. Sandin et al. showed in a pancreatic adenocarcinoma model that p.t. injection of 30 ug anti-CTLA-4 mAb was just as effective as a 200 ug i.p. treatment in reducing tumor size while simultaneously resulting in a drastically lower serum concentrations of anti-CTLA-4 mAb [54]. Interestingly, this 30 ug peritumoral injection did not increase the regulatory T cell frequency in tumor-draining lymph nodes or spleen while the 200 ug i.p. injection significantly increased regulatory T cell frequencies in these tissues. It is also interesting to note that as the dose administered p.t. was increased to 90 ug, serum concentrations rose, leading to an increase in the frequency of regulatory T cells in the spleen, indicating that the 90 ug p.t. injection of anti-CTLA-4 had saturated available binding sites within the tumor [54]. Overall, these results suggest a local s.c., i.t., or p.t. injection route can be used to reduce serum levels of therapeutic mAb while still achieving an effective anti-tumor response, albeit in a dose dependent fashion, though to our knowledge this concept in the context of anti-PD-1 monotherapy has yet to be established.
6. Drug Delivery Systems Improving Checkpoint Blockade mAb Delivery to Target Tissues
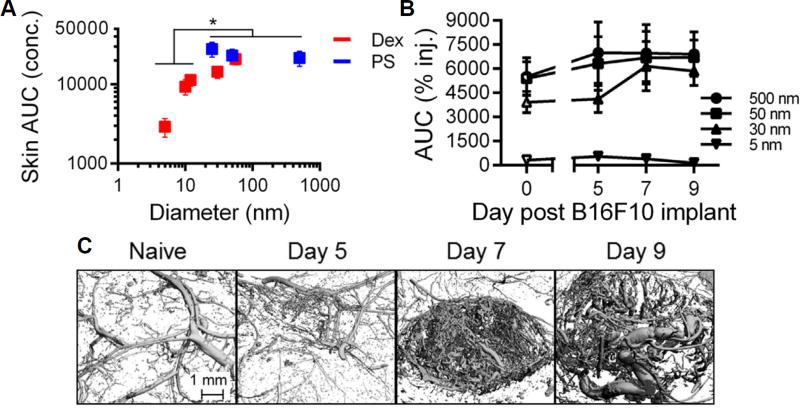
Due to iRAE and the requirement for repeated dosing in clinical checkpoint blockade therapeutic protocols, drug delivery platforms that improve mAb delivery to the tumor and achieve sustained release have garnered recent interest (Table 1). To this end, microparticle-based formulations aiming to prolong the retention of therapeutic agent at the site of injection have emerged as an attractive strategy since increasing carrier size enhances and prolongs retention at the site of injection [55,56] (Figure 3). We also recently demonstrated for the first time that this principle is conserved in malignant tissues, resulting in sustained retention within the tumor after i.t injection, despite the remodeled, irregular, and leaky tumor vasculature (Figure 3) [18]. Material systems can additionally be engineered to control the release of agent from its carrier in order to prolong its therapeutic effects. For example, Rahimian et al. engineered microparticles ~10–25 µm in diameter composed of a biodegradable poly(lactic-co-hydroxymethyl-glycolic-acid) polymer that could be modified to vary mAb release kinetics and loading efficiency [57]. The formulation the authors deemed optimal allowed for a burst release of about 20% of loaded mAb followed by a sustained release of the remaining 80% of mAb over approximately 30 days [57]. When compared to the same dose of mAb formulated in an incomplete Freund’s adjuvant formulation, the mAb-loaded microparticle system resulted in serum mAb levels that were significantly lower (5–10 fold) than the mAb administered in incomplete Freund’s adjuvant, although tumor-bearing animal survival was comparable for formulations [57]. Lei et al. engineered functionalized mesoporous silica based microparticles (12–15 um) for controlled mAb release [58]. By tuning particle functional groups (−COOH, −SO3H, −SH, etc.) and pore sizes up to ~30 nanometers, the entrapment and release of mAb was controlled [58]. This microparticle-based delivery platform resulted in improved mAb tumor retention and sustained release in a mouse melanoma model that slowed tumor growth and prolonged animal survival [58].
Table 1.
Drug delivery systems improving checkpoint blockade mAb delivery and therapy.
| Approach | Article | Drug Delivery System | Results |
|---|---|---|---|
| Microparticles for sustained mAb release | Lei et al. [58] | Silica microparticles | Funtionalizing mesoporous silica and modjtywig the pore sizes within the micropartides resulted in prolonged release mAb that improved antitumor immunity resulting from immunotherapy. |
| Rahimtan et al.[57] | Polymeric microparticles | Varying the polymer composition of micropartides influenced mAb release kinetics to enable tumor- localized sustained release over 30 days but resulted in comparable animal survival relative to a control formulation. | |
|
| |||
| Hyorogel for sustained mAb release | Li et al [36] | Alginate Hyorogel | Hyotogel-mediated sustained release of anti-PD-1 mAb into serum and retention in the tumor resulted in increased levels of CD4 and CD8 T cells while redudng regulatory Tcells in the spleen tumor draining lymph node, and tumor. |
|
| |||
| Microneedle (MN)- mediated sustained and local mAb release | Wang et al. 2016 [60] | Glucose triggered pH- reductions release mAb from MN-delivered nanopartides | MN administration of nanopartides encapsulating anti-PD-1 mAb improved anti-tumor immuraty via enhanced tumor retention of mAb causing local inflfration of CD8+ T cells as well as improved survival and tumor reduction |
| Ye et al. [59] | Hyaiuronidase triggered degradation of MN- delivered nanopartides | By improving the retention and sustained release of mAb within the tumor, a single administration of mAb loaded nanopartides via a MN patch improved anti-PD-1 mAb therapy, induoing enhanced tumor infiltration of CD8+ Tcells and prolonged animal survival | |
|
| |||
| Therapeutic mAb “hitehhidng” for tumor- targeted delivery | Wang et al. 2017 [64] | Platelet based Carrier | Decorating platelets with anti-PD-L1 mAb enabled its “hitchhikkig” to the tumor and triggered release following platelet actiesion with in tumors, resulting in enhanced accumulation of mAb with in the tumor and an increased CD8+to Treg ratio compared to i.v. administration which prolonged animal survival |
Figure 3.
Retention in skin is size dependent and remains relatively unchanged by tumor growth and progression. (A) Total exposure (AUC calculated from measured tissue concentrations) of tracers injected into naïve skin of C57Bl6 mice increases with increasing hydrodynamic size. * indicates significant for 5–12 vs 25, 50, and 500 nm tracers by one-way ANOVA and posthoc Fisher’s LSD tests. (B) Tracer retention and resulting exposure within the skin or melanoma site of injection is relatively unchanged by tumor growth. (C) Micro-computed tomography 3D reconstructions of the remodeling tumor blood vasculature in naïve skin and B16F10 melanomas. Day refers to day post B16F10 implant. Reproduced from REF 55 (B,C) and REF 56 (A) with permission.
In addition to the use of s.c. administered mAb-loaded microparticles, microneedle (MN)-based platforms may be an appealing administration strategy to improve mAb retention due to its ease of administration on superficial tumors such as those of the skin, and potential to more homogenously distribute therapeutic agent throughout the target tissue. Wang et al. and Ye et al. utilized MN to deliver nanoparticles composed of hyaluronic acid or dextran-alginate that encapsulate anti-PD-1 mAb and other small molecule therapeutics [59,60]. In this engineered MN system, the release of anti-PD-1 mAb from the nanoparticles was stimuli responsive to either hyaluronidase, an enzyme overexpressed in the tumor microenvironment [61] or glucose-triggered reductions in local pH. These MN-based approaches resulted in sustained anti-PD-1 mAb retention within the tumor, leading to a more robust immunotherapeutic response indicated by enhanced T cell infiltration into the tumor, reductions in tumor growth, and prolonged animal survival [59,60].
Another approach is the use of hydrogel-based platforms to improve release kinetics of delivered mAb. Wang et al. recently demonstrated that a s.c. injected alginate hydrogel improved the anti-tumor activity of both celecoxib and anti-PD-1 mAb when used individually or combined [36]. It is interesting to note that this system did not lower the serum concentration of anti-PD-1 mAb compared to an i.p. injection of free mAb [36]. However, the accumulation within the B16F10 melanoma was much higher compared to i.p. infusion [36].
Another approach developed to improve immune checkpoint mAb accumulation within tumors and resulting therapeutic effects is piggybacking on the intrinsic capacity of platelets to accumulate at wound sites, such as those created with tumor resection [62,63]. Wang et al. decorated platelets via a bifunctional maleimide linker and demonstrated the release of anti-PD-L1 mAb via platelet-derived microparticles following platelet activation or adhesion [64]. This approach improved survival and tumor suppression compared to i.v. injection of free anti-PD-L1 mAb in both melanoma and breast cancer models [64].
Lastly, combining checkpoint blockade inhibitors with a photothermal therapy through the use of nanoparticles or nanotubes has also led to improved checkpoint blockade therapy including enhanced immunological memory and systemic immunity towards metastatic cancer cells [65–67]. This is an attractive approach as the nanoparticles or nanotubes ablate the primary tumor resulting in released tumor-associated antigens that stimulate an anti-tumor immune response [66]. Ablation of the tumor also leads to infiltration of lymphocytes to the primary tumor site and when combined with an i.v. or i.p. injection of anti-CTLA-4, a substantial increase in CD8+ effector T cells infiltrating the tumor is achieved [65–67]. Taken together, these drug delivery approaches show great promise in improving checkpoint blockade by enhancing payload delivery without the need for repeated injections and potential for reducing systemic toxicity.
7. Opportunities and Potential Strategies for Improving Checkpoint Blockade Cancer Immunotherapy
Despite recent successes, enhancing checkpoint inhibitor mAb delivery to target tissues remains challenging. There are several excellent review papers that outline the challenges in mAb delivery to tumors that the reader is referred to [68,69] with two prevailing schools of thought that will be highlighted. First, tumors undergo significant remodeling that results in high levels of variation in the composition of the tumor vasculature and interstitium. Specifically, the tumor is comprised of a heterogeneous population of cells with a leaky, irregular vasculature, resulting in heterogeneous oxygenation of the tumor interstitium as well as an increase interstitial fluid pressure that can limit extravasation into the tumor from the blood [70]. Moreover, due to high cell densities and a highly cross-linked and dense extracellular matrix, the overall porosity of the tumor interstitium can be very low and result in the constriction of both tumor lymphatics and tumor blood vessels. Pending on the target antigen abundance, this can also greatly affect mAb distribution profiles within the tumor since mAb bind their targets strongly and therefore the total delivered dose may saturate peripheral cells prior to penetrating deeper within the tumor, an effect referred to as the “binding site barrier” [71]. Second, parameters of the mAb itself may influence their capacity to accumulate, penetrate, and evenly distribute within tumors. For example, the relatively large size of mAb can restrict diffusive tumor transport [72]. Antigen affinity and neonatal Fc receptor recycling also play key roles in altering tumor distribution via restraining mAb to the point of tumor entry and altering circulation time leading to clearance prior to tumor entry, respectively [73,74].
With the aforementioned challenges in mind, rational strategies and drug delivery systems have been engineered to improve mAb delivery and distribution. The reader is referred to reference [75] for an excellent review paper regarding drug delivery to tumors, of which we will only highlight a few examples. First, in a manner conceptually similar to the use of photothermal tumor therapy to enhance checkpoint inhibition, direct modulation of the tumor microenvironment has been explored to improve drug delivery to tumors, specifically by normalizing the tumor matrix using extracellular matrix-degrading enzymes such as collagenase or extracellular matrix antagonists to improve the diffusivity and penetration of agents within the tumor [76–78]. Normalization of the tumor microvasculature using anti-angiogenic therapies can also reduce tumor interstitial hypertension and matrix remodeling, leading to improved drug entry and penetration [79]. Nanoparticles have also emerged as an attractive tumor mAb delivery approach as they can either encapsulate or be decorated with mAb. And by virtue of their propensity for prolonged circulation times relative to microparticles as well as propensity to accumulate within tumors and for cellular uptake, nanoparticles can improve mAb delivery to tumors as well as be used to control mAb release [80–82]. However, nanoparticles may be stymied from penetration into the tumor interstitium due to their size, accumulate in non-target cells, and may negatively affect the mAb structure/binding.
In addition to solid tumors as tissue targets, checkpoint inhibitor mAb delivery to secondary lymphoid tissues is also attractive in the context of tumor therapy for several reasons. First, many cancers metastasize via the lymphatic system, resulting in metastatic tumors within tumor-draining lymph nodes. Therefore, targeting anti-cancer therapeutics to lymph nodes may be of great interest to eradicate lymph node metastases. Lymph nodes in addition to the spleen harbor liquid tumor cells, providing further rationale for targeting. Second, one of the major tissues responsible for the priming of anti-tumor immune responses are tumor-draining lymph nodes [17]. This is because tumor antigens drain from the primary tumor microenvironment to the tumor-draining lymph node via the lymphatic vasculature [17,18,55]. Furthermore, tumor-draining lymph nodes often display altered immunological microenvironments relative to non-tumor associated lymph nodes, suggesting active regulation of the tumor-draining lymph node microenvironment by the tumor to suppress anti-tumor immunity. We and others have noted lymph node remodeling associated with tumor lymphatic drainage [17,18,83–85], effects that may alter antigen, cytokine, growth factor, or even therapeutic agent accumulation within and distribution throughout tumor-draining lymph nodes to alter their signaling activity [18]. Furthermore, increased frequencies of regulatory T cells [86] and low densities of CD169+ subcapsular sinus macrophages [87] within tumor-draining lymph nodes predicts poor patient outcome in melanoma [86]. Importantly, CTLA-4 and PD-1 play roles in directing early fate decisions of T cells recognizing antigens presented within lymph nodes [29].
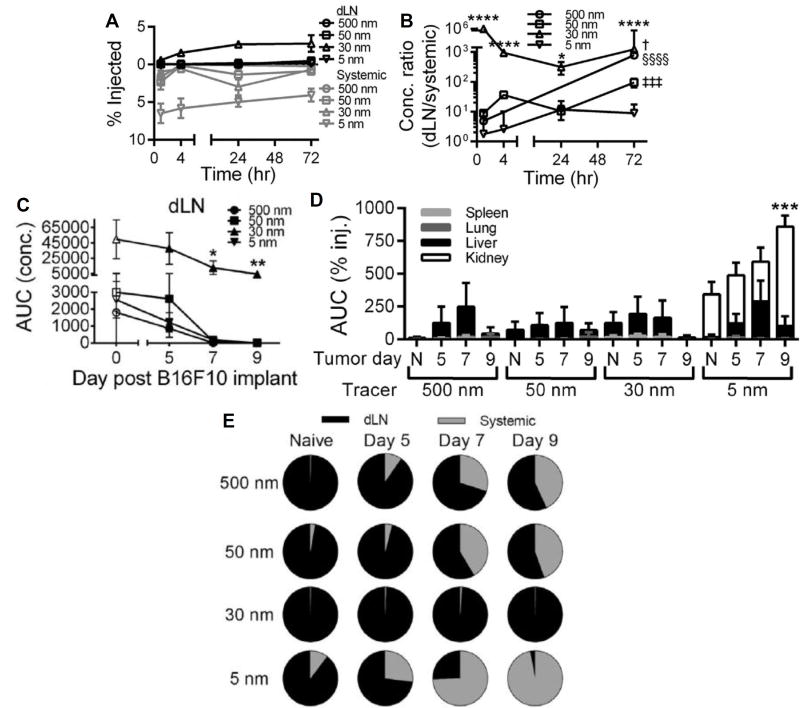
Accordingly, technologies and/or methodologies targeting checkpoint inhibitor mAb to tumor-draining lymph nodes, such as those that have been used for delivery of other classes of immunotherapeutic agents and demonstrated the ability to enhance their immunotherapeutic effects while simultaneously reducing accumulation in off-target tissues [88,89], have potential to enhance checkpoint inhibitor therapeutic effects. Due to the nature and anatomy of the lymphatic system [18,90], localization of high concentrations of immunomodulatory agents to lymph nodes is difficult to obtain through the administration of free drug or by conventional microparticle-based formulations (Figure 4). However, lymphatic uptake and accumulation within draining lymph nodes after intradermal, s.c., and intramuscular injection is optimum for formulations roughly tens of nanometers in hydrodynamic diameter [91,92] (Figure 4), which may facilitate the use of a significantly lower total administered dose to reduce treatment-associated toxicities [93] by reducing levels of accumulation in off target tissues [83,93,56] (Figure 4). This principle has been explored for lymph node targeting by our group and others using nanoformulations comprised of synthetic materials including Pluronic-stablized poly(propylene sulfide) [94,95], polymer dendrimers [96,97] and poly(γ-glutamic acid)-l-phenylalanine ethylester [98] as well as biologically derived biopolymers such as hyaluronic acid [99] and albumin [93]. These formulations have been used to enhance the delivery and bioactivity of small molecules [94,97], oligonucleotides [93,94,100], as well as proteins and peptides [93,101,102] within lymph nodes. However, as the result of extracellular matrix and vascular remodeling within the tumor microenvironment, lymphatic transport in solid tumors is significantly attenuated [103,104], which has the potential to undermine a lymphatic mediated drug delivery strategy to tumor-draining lymph nodes via i.t. administration. This may undermine the feasibility of lymphatic-targeting delivery systems to enhance lymph node delivery for melanoma immunotherapy. However, size-based principles of lymph node-targeting [90] were recently shown by our group to be conserved in malignant skin (Figure 4) [55]. In particular, although the extent of accumulation of nanoscale dextran tracers administered i.t. within tumor-draining lymph nodes substantially diminished as melanomas advanced, significant levels of accumulation of 30 nm, but not 5 nm or 50–500 nm tracers, within tumor-draining lymph nodes was still appreciable (Figure 4) [55]. Furthermore, when analyzed for their relative distribution amongst local tumor-draining lymph node versus systemic tissues, only tracers 30 nm in hydrodynamic diameter exhibited tumor-draining lymph node-selective accumulation profiles whereas all other tested sizes resulted instead in significant levels of systemic accumulation that increased with tumor stage (Figure 4) [55]. These results suggest that delivery systems ~30 nm in hydrodynamic size are ideal for lymphatic exposure even in cancers that are known to induce significant remodeling of the lymphatic vasculature. Therefore, 30 nm based formulations for immunotherapeutic delivered have the potential to simultaneously localize high levels of payload to both the tumor (Figure 3) as well as its draining lymph nodes (Figure 4) while minimizing exposure in off target tissues to minimize the risk of treatment-associated toxicities when administered i.t.. In addition to size, we also recently demonstrated carrier flexibility versus rigidity to have effects on lymph node accumulation after intradermal administration [56]. Notably, flexible dextrans accumulated at levels roughly an order of magnitude higher than size-matched rigid polystyrene spheres [56]. Alternatively direct injection into lymph nodes has also been explored as a means to localize payload activity to lymph nodes, an approach amenable to controlled release strategies to tune therapeutic activity [105].
Figure 4.
Size-based principles of lymph node drug targeting are conserved in tumors. Time-resolved accumulation of tracers in draining lymph nodes (dLN) (A) and ratio of accumulating tracer concentrations within dLN to systemic tissues (B). * indicates significance relative to all other tracers at the same time point by two-way ANOVA and post-hoc Tukey’s tests. § indicates significance for 500 nm tracer relative to all other time points, ‡ for 50 nm tracer relative to all other time points, † for 30 nm tracer vs all other time points by one-way ANOVA and post-hoc Fisher’s LSD tests. One, two, three, and four symbols denoting statistical significance represent p < 0.05, 0.01, 0.001, and 0.0001, respectively. Tumor growth reduces tracer exposure within dLN (C) and increases systemic tracer accumulation in the spleen, lungs, and liver, and kidneys (D). C, *p<0.05 and **p<0.01 relative to all other tracers within same tumor day group by one-way ANOVA with post-hoc Fisher’s LSD test. D, *** indicates significance for 5 nm tracer at day 9 in kidney vs all other groups by two-way ANOVA and post-hoc Tukey’s tests. (E) Despite these distribution and transport changes resulting from tumor growth, the relative enrichment of 30 nm but not 5, 50, or 500 nm exposure within dLN relative to systemic tissues (AUC calculated from measured levels of concentrations of injected tracers in individual tissues 1–72 h p.i) seen in naïve skin is conserved. Reproduced from REF 55 with permission.
Lastly, the route of administration may be one of the most important parameters to consider when trying to optimize mAb therapy [90]. I.v. injection leads to significant accumulation within the primary tumor and spleen and may be particularly attractive in treating metastatic cancers but will also lead to accumulation within other systemic tissues, potentially resulting in more iRAE. I.t. injection on the other hand may improve tumor retention and enhance drainage to tumor-draining lymph nodes, but it may not be feasible pending the location of the tumor. Finally, in addition to different profiles of accumulation within various tissues, the distribution within the tumor may vary significantly pending the administration route. Whereas an i.t. injection would more likely result in mAb localized primarily to the core of the tumor, an i.v. injection would instead most likely result in levels of mAb accumulation being the highest immediately surrounding the tumor blood vasculature, potentially resulting in mAb interactions with different tumor-resident cell populations.
8. Conclusions
Engineered drug delivery systems offer the significant advantages of enabling more finely tuned control of tissue and cell targeting as well as rate of therapeutic agent release within target tissues to improve the immunotherapeutic effects of checkpoint inhibitor mAb drugs. The success of such systems will likely be defined as either increasing the proportion of patients who respond to treatment or enhancing drug safety profiles, though ideally both. With checkpoint inhibition likely to be approved for an even broader array of malignancies pending the success of innumerable ongoing clinical trials, the number of translational targets and opportunities for engineers as well as materials and formulation scientists to innovative solutions within this nascent but growing field is sure to continue to grow.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health (NIH) Grant R01CA207619, CCR15330478 grant from Susan G. Komen®, and Department of Defense Grant CA150523.
References
- 1.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 2.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesterhuis WJ, Bosco A, Millward MJ, Small M, Nowak AK, Lake RA. Dynamic versus static biomarkers in cancer immune checkpoint blockade: unravelling complexity. Nat Rev Drug Discov. 2017 doi: 10.1038/nrd.2016.233. [DOI] [PubMed] [Google Scholar]
- 5.Robert L, Ribas A, Hu-Lieskovan S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2? Semin Immunol. 2016;28:73–80. doi: 10.1016/j.smim.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermel DJ, Ott P. Combining forces: the promise and peril of synergistic immune checkpoint blockade and targeted therapy in metastatic melanoma. Cancer Metastasis Rev. 2017 doi: 10.1007/s10555-017-9656-2. [DOI] [PubMed] [Google Scholar]
- 7.Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine. 2015;33:7377–7385. doi: 10.1016/j.vaccine.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 8.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 9.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:451–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, Ribas A. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra41. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. http://www.ncbi.nlm.nih.gov/pubmed/7534620. [DOI] [PubMed] [Google Scholar]
- 15.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189:2181–2190. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund AW, Duraes FV, Hirosue S, Raghaven V, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. Tumor VEGF-C promotes immune tolerance and tumor antigen cross-presentation by lymphatics. Cell Rep. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SN, Rohner NA, Edwards EE. Implications of Lymphatic Transport to Lymph Nodes in Immunity and Immunotherapy. Annu Rev Biomed Eng. 2016;18:207–233. doi: 10.1146/annurev-bioeng-101515-014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jago CB, Yates J, Camara NO, Lechler RI, Lombardi G. Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol. 2004;136:463–471. doi: 10.1111/j.1365-2249.2004.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan DV, Gibson HM, Aufiero BM, Wilson AJ, Hafner MS, Mi QS, Wong HK. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 2014;15:25–32. doi: 10.1038/gene.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science (80) 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 22.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thangavelu G, Gill RG, Boon L, Ellestad KK, Anderson CC. Control of in vivo collateral damage generated by T cell immunity. J Immunol. 2013;191:1686–1691. doi: 10.4049/jimmunol.1203240. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadzadeh M, Johnson La, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg Sa, Dc W. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Rubin M, Grosso JF, Harris TJ, Getnet D, Whartenby Ka, Dirk G, Dubensky TW, Chen L, Pardoll DM, Drake CG, Tuder RM, Joseph F, Brockstedt DGTWD., Jr Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2014;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbé C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 32.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavanagh B, Brien SO, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3 + regulatory and activated effector CD4 + T cells in a dose-dependant fashion CTLA4 blockade expands FoxP3 + regulatory and activated effector CD4 + T cells in a dose-dependant fashion. Online. 2008;112:1175–1184. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Supplementary - Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, Li W, Wu G, Ren J, Wang Z, Zou W, Wang L. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology. 2016;5:e1074374. doi: 10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, Watkins A, Mullins S, Chodorge M, Andrews J, Bannister D, Dick E, Crawford N, Parmentier J, Alimzhanov M, Babcook JS, Foltz IN, Buchanan A, Bedian V, Wilkinson RW, McCourt M. Identification and Pre-clinical Characterization of MEDI4736, an Antagonistic anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res. 2015;3:1052–1063. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 39.Wolchok JD, Kluger H, Callahan MK, Postow Ma, Rizvi Na, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K, Burke MM, Caldwell A, Kronenberg Sa, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yip V, Palma E, Tesar DB, Mundo EE, Bumbaca D, Torres EK, Reyes NA, Shen BQ, Fielder PJ, Prabhu S, Khawli LA, Boswell CA. Quantitative cumulative biodistribution of antibodies in mice: Effect of modulating binding affinity to the neonatal Fc receptor. MAbs. 2014;6:689–696. doi: 10.4161/mabs.28254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kijanka G, Bee JS, Bishop SM, Que I, Löwik C, Jiskoot W. Fate of Multimeric Oligomers, Submicron, and Micron Size Aggregates of Monoclonal Antibodies Upon Subcutaneous Injection in Mice. J. Pharm. Sci. 2016;105:1693–1704. doi: 10.1016/j.xphs.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J. Clin. Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bamias A, Krausz T. Uptake and Distribution of Specific and Control Monoclonal Antibodies in Subcutaneous Xenografts following Intratumor Injection. Cancer Res. 1991;51:3251–3256. [PubMed] [Google Scholar]
- 44.Filipe V, Que I, Carpenter JF, Löwik C, Jiskoot W. In vivo fluorescence imaging of IgG1 aggregates after subcutaneous and intravenous injection in mice. Pharm. Res. 2014;31:216–227. doi: 10.1007/s11095-013-1154-9. [DOI] [PubMed] [Google Scholar]
- 45.Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs. 2011;3:422–430. doi: 10.4161/mabs.3.5.16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higashikawa K, Yagi K, Watanabe K, Kamino S, Ueda M, Hiromura M, Enomoto S. 64Cu-DOTA-anti-CTLA-4 mAb enabled PET visualization of CTLA-4 on the T-cell infiltrating tumor tissues. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjug. Chem. 2015;26:2062–2069. doi: 10.1021/acs.bioconjchem.5b00318. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee S, Lesniak WG, Gabrielson M, Lisok A, Wharram B, Sysa-Shah P, Azad BB, Pomper MG, Nimmagadda S. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget. 2016;7:10215–10227. doi: 10.18632/oncotarget.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josefsson A, Nedrow JR, Park S, Banerjee SR, Rittenbach A, Jammes F, Tsui B, Sgouros G. Imaging, biodistribution, and dosimetry of radionuclide-labeled PD-L1 antibody in an immunocompetent mouse model of breast cancer. Cancer Res. 2016;76:472–479. doi: 10.1158/0008-5472.CAN-15-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, Chiu H, McBride J, Johnson C, Xin Y, Koeppen H, Leabman M, Iyer S. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. MAbs. 2016;8:593–603. doi: 10.1080/19420862.2015.1136043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hettich M, Braun F, Bartholom?? MD, Schirmbeck R, Niedermann G. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics. 2016;6:1629–1640. doi: 10.7150/thno.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fransen MF, Van Der Sluis TC, Ossendorp F, Arens R, Melief CJM. Controlled local delivery of CTLA-4 blocking antibody induces CD8 + T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin. Cancer Res. 2013;19:5381–5389. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 53.van Hooren L, Sandin LC, Moskalev I, Ellmark P, Dimberg A, Black P, Tötterman TH, Mangsbo SM. Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. Eur. J. Immunol. 2016;46:1–9. doi: 10.1002/eji.201646583. [DOI] [PubMed] [Google Scholar]
- 54.Sandin LC, Eriksson F, Ellmark P, Loskog AS, Tötterman TH, Mangsbo SM. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology. 2014;3:e27614. doi: 10.4161/onci.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohner NA, Thomas SN. Melanoma growth effects on molecular clearance from tumors and biodistribution into systemic tissues versus draining lymph nodes. J Control Release. 2016;223:99–108. doi: 10.1016/j.jconrel.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohner NA, Thomas SN. Flexible Macromolecule versus Rigid Particle Retention in the Injected Skin and Accumulation in Draining Lymph Nodes Are Differentially Influenced by Hydrodynamic Size. ACS Biomater. Sci. Eng. 2017;3:153–159. doi: 10.1021/acsbiomaterials.6b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahimian S, Fransen MF, Kleinovink JW, Amidi M, Ossendorp F, Hennink WE. Polymeric microparticles for sustained and local delivery of antiCD40 and antiCTLA-4 in immunotherapy of cancer. Biomaterials. 2015;61:33–40. doi: 10.1016/j.biomaterials.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 58.Lei C, Liu P, Chen B, Mao Y, Engelmann H, Shin Y, Jaffar J, Hellstrom I, Liu J, Hellstrom KE. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J. Am. Chem. Soc. 2010;132:6906–6907. doi: 10.1021/ja102414t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang C, Gu Z. Synergistic Transcutaneous Immunotherapy Enhances Antitumor Immune Responses through Delivery of Checkpoint Inhibitors. ACS Nano. 2016;10:8956–8963. doi: 10.1021/acsnano.6b04989. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 61.Manuscript A. NIH Public Access. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009;18:281–287. HYALURONDIASE. [Google Scholar]
- 62.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 63.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front. Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 64.Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z, Baker D, Masterson T, Pace R, Constable W, Wanebo H, Lukianova-Hleb EY, Stephan SB, Demicheli R, Retsky M, Hrushesky W, Baum M, Gukas I, Ceelen W, Pattyn P, Mareel M, Klevorn LE, Teague RM, O’Sullivan D, Pearce EL, Robert C, Postow MA, Sharma P, Allison JP, Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z, Zou W, Wolchok JD, Chen L, Buchbinder EI, Hodi FS, Smyth EC, Cunningham D, Rosenberg JE, Naidoo J, Mellati M, Boutros C, Larkin J, Chen L, Han X, Weber JS, Kahler KC, Hauschild A, Woo SR, Corrales L, Gajewski TF, Hegde PS, Karanikas V, Evers S, Spranger S, Gajewski TF, Schreiber H, Fu YX, Fesnak AD, June CH, Levine BL, Yoo JW, Irvine DJ, Discher DE, Mitragotri S, Tamagawa-Mineoka R, Franco AT, Corken A, Ware J, Hu CM, Harker LA, Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E, Gay LJ, Felding-Habermann B, Nash GF, Turner LF, Scully MF, Kakkar AK, Hu Q, Garraud O, Morrell CN, Aggrey AA, Chapman LM, Modjeski KL, Semple JW, Italiano JE, Freedman J, Elzey BD, Seifert L, Topalian SL, Drake CG, Pardoll DM, Siljander PRM, Li J, Sharkey CC, Wun B, Liesveld JL, King MR, Ruggeri ZM, Mendolicchio GL, Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C, Tripathi S, Guleria I, Headley MB, Flaumenhaft R, Rand ML, Wang H, Bang KW, Packham MA, Freedman J, Lu Y, Aimetti AA, Langer R, Gu Z, Janowska-Wieczorek A, Cheville NF, Stasko J, Zimmerman M, Hu X, Liu K, Fischer AH, Wang C. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 2017;1:11. [Google Scholar]
- 65.Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014;26:8154–8162. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 66.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. for Effective Cancer Immunotherapy. Nat. Commun. 2016;7:1–13. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD, Cruz CRY, Fernandes R. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma, Nanomedicine Nanotechnology. Biol. Med. 2016;13:771–781. doi: 10.1016/j.nano.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12:33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jang SH, Wientjes MG, Lu D, Au JLS. Drug delivery and transport to solid tumors. Pharm. Res. 2003;20:1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 71.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J. Nucl. Med. 1990;31:1191–1198. [PubMed] [Google Scholar]
- 72.de Davies CL, Berk DA, Pluen A, Jain RK. Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br. J. Cancer. 2002;86:1639–1644. doi: 10.1038/sj.bjc.6600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High affinity restricts the localization and tumor penetration of single-chain Fv antibody molecules. Cancer Res. 2001;61:4750–4755. [PubMed] [Google Scholar]
- 74.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR Expression in Bone Marrow-Derived Cells Functions to Protect Serum IgG from Catabolism. J. Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 75.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int. J. Nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- 77.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22:276–284. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 78.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Pnas. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 80.Arruebo M, Valladares M, González-Fernández Á. Antibody-conjugated nanoparticles for biomedical applications. J. Nanomater. 2009. 2009 [Google Scholar]
- 81.Sousa F, Castro P, Fonte P, Kennedy PJ, Neves-Petersen MT, Sarmento B. Nanoparticles for the delivery of therapeutic antibodies: Dogma or promising strategy? Expert Opin. Drug Deliv. 2016;0:1–14. doi: 10.1080/17425247.2017.1273345. [DOI] [PubMed] [Google Scholar]
- 82.Turner CT, Mcinnes SJP, Voelcker NH, Cowin AJ. Therapeutic Potential of Inorganic Nanoparticles for the Delivery of Monoclonal Antibodies, 2015. 2015 [Google Scholar]
- 83.Rohner NA, McClain J, Tuell SL, Warner A, Smith B, Yun Y, Mohan A, Sushnitha M, Thomas SN. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 2015;29:4512–4522. doi: 10.1096/fj.15-274761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riedel A, Shorthouse D, Haas L, Hall BA, Shields J. Tumor-induced stromal reprogramming drives lymph node transformation. Nat Immunol. 2016;17:1118–1127. doi: 10.1038/ni.3492. http://www.nature.com/ni/journal/v17/n9/abs/ni.3492.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira ER, Jones D, Jung K, Padera TP. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin Cell Dev Biol. 2015;38:98–105. doi: 10.1016/j.semcdb.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohos A, Sebestyen T, Liszkay G, Plotar V, Horvath S, Gaudi I, Ladanyi A. Immune cell profile of sentinel lymph nodes in patients with malignant melanoma - FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome. J Transl Med. 2013;11:43. doi: 10.1186/1479-5876-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saito Y, Ohnishi K, Miyashita A, Nakahara S, Fujiwara Y, Horlad H, Motoshima T, Fukushima S, Jinnin M, Ihn H, Takeya M, Komohara Y. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol Res. 2015;3:1356–1363. doi: 10.1158/2326-6066.CIR-14-0180. [DOI] [PubMed] [Google Scholar]
- 88.Chandrasekaran S, King M. Microenvironment of Tumor-Draining Lymph Nodes: Opportunities for Liposome-Based Targeted Therapy. Int. J. Mol. Sci. 2014;15:20209–20239. doi: 10.3390/ijms151120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andorko JI, Hess KL, Jewell CM. Harnessing Biomaterials to Engineer the Lymph Node Microenvironment for Immunity or Tolerance. AAPS J. 2014;17:323–338. doi: 10.1208/s12248-014-9708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas SN, Schudel A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 2015;7:65–74. doi: 10.1016/j.coche.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oussoren C, Zuidema J, Crommelin DJA, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta-Biomembr. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 92.Swartz MA. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 93.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, Swartz MA. Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaminskas LM, Kota J, McLeod VM, Kelly BD, Karellas P, Porter CJ. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J. Control. Release. 2009;140:108–116. doi: 10.1016/j.jconrel.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 97.Ryan GM, Kaminskas LM, Bulitta JB, McIntosh MP, Owen DJ, Porter CJH. PEGylated polylysine dendrimers increase lymphatic exposure to doxorubicin when compared to PEGylated liposomal and solution formulations of doxorubicin. J. Control. Release. 2013;172:128–136. doi: 10.1016/j.jconrel.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Shima F, Uto T, Akagi T, Baba M, Akashi M. Size effect of amphiphilic poly(??-glutamic acid) nanoparticles on cellular uptake and maturation of dendritic cells in vivo. Acta Biomater. 2013;9:8894–8901. doi: 10.1016/j.actbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 99.Bagby TR, Cai S, Duan S, Thati S, Aires DJ, Forrest L. Impact of molecular weight on lymphatic drainage of a biopolymer-based imaging agent. Pharmaceutics. 2012;4:276–295. doi: 10.3390/pharmaceutics4020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, Van Der Vlies AJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. 2013;110:19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci U S A. 2011;108:E989–E997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas SN, van der Vlies AJ, O’Neil CP, Reddy ST, Yu SS, Giorgio TD, Swartz MA, Hubbell JA. Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials. 2011;32:2194–2203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 103.Hagendoorn J, Tong R, Fukumura D, Lin Q, Lobo J, Padera TP, Xu L, Kucherlapati R, Jain RK. Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 2006;66:3360–3364. doi: 10.1158/0008-5472.CAN-05-2655. [DOI] [PubMed] [Google Scholar]
- 104.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 105.Jewell CM, Lopez SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci U S A. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]