Abstract
The main objective of this novel study was to develop chlorpheniramine maleate orally disintegrating films (ODF) using hot-melt extrusion technology and evaluate the characteristics of the formulation using in vitro and in vivo methods. Modified starch with glycerol was used as a polymer matrix for melt extrusion. Sweetening and saliva-simulating agents were incorporated to improve palatability and lower the disintegration time of film formulations. A standard screw configuration was applied, and the last zone of the barrel was opened to discharge water vapors, which helped to manufacture non-sticky, clear, and uniform films. The film formulations demonstrated rapid disintegration times (6–11 s) and more than 95% dissolution in 5 min. In addition, the films had characteristic mechanical properties that were helpful in handling and storage. An animal model was employed to determine the taste masking of melt-extruded films. The lead film formulation was subjected to a human panel for evaluation of extent of taste masking and disintegration.
Keywords: Hot-melt extrusion, Orally disintegrating film, Taste-masking, Pediatric and geriatric, Modified starch, Human panel taste evaluation, Continuous manufacturing, Chlorpheniramine maleate
Graphical abstract
Development of orally disintegrating film utilizing hot melt extruder
1. Introduction
It is estimated that 26–50% of the patient population find difficulty in swallowing tablets and hard gelatin capsules [1]. These patients mainly include the elderly who have difficulty taking conventional oral dosage forms because of hand tremors and dysphagia, and pediatric patients who are often fearful of taking solid oral dosage forms owing to their underdeveloped muscular and nervous systems [2]. In addition, patients who are mentally ill, developmentally disabled, uncooperative, on reduced liquid-intake plans or nauseated, and travelers who may not have access to clean water also are candidates for ODFs [3, 4].
The traditional alternative to swallowing difficulties is formulating a drug substance in liquid dosage form. However, liquid dosage forms have several limitations, such as the need for measuring, bulkiness, physical, chemical, and microbial stability issues, spoilage, inaccurate dosing, and organoleptic properties of drug and drug formulations [5].
Conventional solid oral formulations contributed significantly to minimizing the shortcomings of liquid dosage forms. The crushing of tablets or opening of capsules is a straightforward way for patients or caregivers to lessen the swallowing difficulties. However, serious consequences may be associated with modified-release, enteric-coated, and cytotoxic or hormonal medicines, as these formulations are designed for special cases [6]. Moreover, European Medical Agency does not recommend the splitting or crushing of tablets because the active pharmaceutical ingredient (API) is not evenly distributed in the tablet [7, 8]. Thus, it is very convenient to develop a formulation that disintegrates in the oral cavity and eases the swallowing process.
In recent years, fast disintegrating oral formulations established their importance in patient population suffering from dysphagia, stroke, thyroid disorder, Parkinson’s disease, multiple sclerosis, and cerebral palsy [9]. Commercially available orodispersible tablets (ODT) and orally disintegrating films or orodispersible films (ODF) are the most successful platforms for pharmaceutical product development. ODTs are solid oral dosage forms that disintegrate rapidly, typically within 30 s, with or without the administration of additional water [10]. They provided great comfort to patients with swallowing difficulties [11]. Despite the benefits of ODTs, there are some challenges in their processing and handling owing to their fragility and brittleness, which warrant special package for protection during storage and transportation [12]. The films are flexible and not as fragile as most ODTs. Hence, there is ease in transportation, consumer handling, and storage of ODFs.
ODF can be defined as a dosage form that employs a water-soluble polymer (generally a hydrocolloid, which may be a bioadhesive polymer), which allows the dosage form to quickly wet, adhere, and dissolve to release the drug when placed on the tongue or in the oral cavity [5]. ODF alleviated patient discomforts associated with swallowing disabilities without compromising the therapeutic effect. In addition, it could ease the administration of drugs to pediatric patient population [13]. Moreover, ODF can be helpful in curtailing dose variations in younger patients, in whom liquid formulations are the most accepted way of drug delivery.
Currently, solvent casting methods are commonly employed to produce ODFs, owing to its ease of production and low set up costs [14, 15]. Despite its wide application, products with batch-to-batch variation may be produced because of multiple steps involved in the production. In addition, air entrapment in the films is commonly observed in solvent casting methods, which leads to dose variations and inappropriate esthetic appearance of the product [15]. The use of large amounts of solvent is one of the biggest shortcomings of this method as solvent removal and disposal is a long and tedious process. Thus, it is very beneficial to develop a solvent-free, quick, and continuous process that could diminish the shortcomings of the current manufacturing method.
Hot melt extrusion (HME) is a one-step, solvent-free continuous manufacturing process, which established itself in the pharmaceutical arena for the development of various solid oral formulations [16–25]. This technology involves the use of temperature and shear to process polymer blends and extrude them through a die of the desired design [26]. HME could be an effective alternative to the solvent casting method as it diminishes the inherent shortcomings, such as the use of solvents and problems involved in the mixing and drying steps. This ultimately makes HME process efficient and cost effective for patients [27, 28].
This study has three main objectives: to 1) develop a robust patient-friendly orally fast disintegrating film of chlorpheniramine maleate (CPM); 2) evaluate these formulations with different in vitro and in vivo techniques, and 3) demonstrate the feasibility of HME techniques for continuous manufacturing of ODF without the use of solvents. To the best of our knowledge, there is no published literature on the manufacturing of orally fast disintegrating formulations using HME technology and evaluation of films using in vitro and in vivo techniques.
2. Materials and Methods
2.1. Materials
CPM was purchased from MP Biomedicals, LLC (Solon, OH, USA). Lycoat® RS 780 (modified starch) was supplied by Roquette America Inc. (Keokuk, IA, USA). Citric acid and glycerol were ordered from Fisher Scientific (Pittsburgh PA, USA). Magnasweet® sample was gifted by Mafco worldwide LLC (Camden, NJ, USA). Sucralose was supplied by JK Sucralose Inc. (Edison, New Jersey, USA).
2.2. Thermal analysis
Thermogravimetric analysis (TGA) studies (Perkin Elmer Pyris 1, Shelton, CT, USA) were performed to estimate the thermal stability of the API and excipients during HME processing. Data were analyzed using Pyris software. The API excipients were heated from 30–160°C at 20°C /min.
2.3. Material preparation and blending
CPM, citric acid, and Lycoat® RS 780 were dry mixed at amounts outlined in Table 1 using a V-shell blender (GlobePharma, Maxiblend, New Brunswick, NJ, USA) after passing through an ASTM #30 mesh. The plasticizer (glycerol with dissolved sucralose and Magnasweet®) was incorporated slowly into a high-shear mixer (Model RSI 3VG, Robot Coupe Industrial Division, Ridgeland, MS, USA) containing the previously mixed blend with all excipients and allowed to blend for 10 min.
Table 1.
HME formulations
| Experiment Number | Formulation (%w/w) | Temperature (°C) | Sucralose | Magnasweet | Citric acid | CPM | GLY | Lycoat 780 | Screw speed (rpm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | N 1 | 100 | 0.5 | 0.5 | 4 | 5 | 20 | 70 | 50 |
| 2 | N 2 | 110 | 0.5 | 0.5 | 4 | 5 | 17 | 73 | 30 |
| 3 | N 3 | 100 | 0.5 | 0.5 | 6 | 5 | 17 | 71 | 50 |
| 4 | N 4 | 110 | 0.5 | 0.5 | 6 | 5 | 20 | 68 | 30 |
| 5 | N 5 | 100 | 0.5 | 0.5 | 4 | 10 | 20 | 65 | 30 |
| 6 | N 6 | 110 | 0.5 | 0.5 | 4 | 10 | 17 | 68 | 50 |
| 7 | N 7 | 100 | 0.5 | 0.5 | 6 | 10 | 17 | 66 | 30 |
| 8 | N 8 | 110 | 0.5 | 0.5 | 6 | 10 | 20 | 63 | 50 |
| 9 | N 9 | 105 | 0.5 | 0.5 | 5 | 7.5 | 18.5 | 68 | 40 |
2.4. Hot melt extrusion
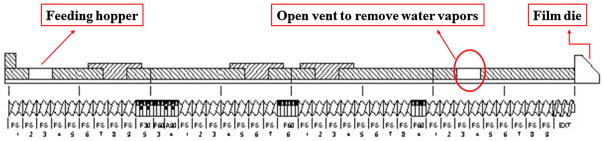
The blends were melt-extruded using a co-rotating twin-screw extruder (16 mm Prism EuroLab, ThermoFisher Scientific, Pittsburgh, PA, USA) at 30–50 rpm over a temperature range of 100– 110°C. A degassing port was introduced in the last zone of the barrel to release excess water vapor, which would otherwise produce unwanted bubbles in the films. Additionally, the film die was installed with preset thickness. The physical blend of the formulation was manually fed into the hopper, and the films were collected, wrapped in wax paper, sealed, and stored in polyethylene bags at 25°C with 40% relative humidity.
2.5. Film characterizations
2.5.1 Film thickness and mechanical properties
The mechanical properties of the films were evaluated using the TA.XTPlus texture analyzer equipped with 5 kg load cell (Texture Technologies, Scarsdale, NY, USA). The films were cut into dumbbell shaped specimens with a width and length of 1.55 and 15.5 mm, respectively, and placed longitudinally in tensile grip probe on the texture analyzer. The films were tested at a crosshead speed of 2 mm/min and held between two clamps positioned at 5 mm. The results of film samples that broke at and not between the clamps were not included in the calculations. Each film formulation was measured with ten replicates [29]. The tensile strength (Ts) and percent elongation (%E) were calculated using the results from texture analyzer. Film thickness was measured using an electronic caliper (Fisher Scientific, Pittsburgh, PA, USA) at different positions.
2.5.2 Disintegration test
The film was cut into an appropriate size as per the dose (4 mg) and placed in a petri dish. Then, 100 μL artificial salivary media was added, and the time for complete disintegration of the film was recorded (n =10).
2.5.3 Surface pH of film
The film was moistened using 5 μL water and a contact electrode touched the surface of the film (Oakton™ pH meter, Fisher Scientific, Pittsburgh, PA, USA), followed by pH measurement (n=6)
2.6. Analytical method
A Waters® high performance liquid chromatography (HPLC) system equipped with a Water® 600 binary pump, Waters® 2489 UV/detector, and Waters® 717 plus autosampler (Waters Technologies Corporation, Milford, MA, USA), and a Phenomenex Luna® 5 μm C18 (2) 250 × 4.6 mm column (Torrance, CA, USA) were used at a detection wavelength of 254 nm. The mobile phase consisted of 7.5 mM monobasic potassium phosphate in methanol and water at a ratio of 62.5:37.5 (v/v). The mobile phase flow rate was maintained at 1.0 mL/min, and an injection volume of 10 μL was used [30]. HPLC data were analyzed using Empower 2 software (Milford, MA, USA).
2.7. In vitro dissolution studies
The films for dissolution studies were cut into sizes relative to the dose of CPM (4 mg). The drug profile was evaluated using a USP dissolution apparatus-I (Hanson SR8, Chatsworth, CA) maintained at 37 ± 0.5°C and having a shaft rotation speed of 100 rpm. The dissolution test was performed using 900 mL phosphate buffer (pH 6.8). The samples were withdrawn at 5, 10, and 30 min and analyzed using the HPLC- UV system.
2.8. X-ray diffraction studies (XRD)
X-Ray diffraction (Bruker D8 Advance, Madison, MI, USA) was used to determine the physical state of the drug, excipients, and film formulations. The X-ray diffraction apparatus used CuK radiation at 40 mA, 40 kV, a scanning speed of 2°/min, and diffraction angle (2θ) range of 5– 55.
2.9. Scanning electron Microscope (SEM)
The surface morphology of the films was evaluated using SEM analysis. The samples were mounted on adhesive carbon pads placed on aluminum and sputter coated with gold using a Hummer sputtering system (Anatech Ltd, Springfield, VA, USA) in a high vacuum evaporator. A JEOL JSM-5600 SEM operating at an accelerating voltage of 10 kV was used for imaging.
2.10. In vivo taste evaluation
Twenty-one naïve adult male Sprague-Dawley rats (175–200 g) were ordered from Harlan Laboratories (Houston, TX, USA) for the study. The rats were housed in Plexiglass cages with Corncob bedding in a vivarium that maintained a 12 h light/dark cycle and an ambient temperature of ~22°C. Food and water were available without any restriction, except during the training and taste evaluation experiments as mentioned below (2.10.1). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Mississippi, University, USA (protocol no. 15-026). This study was performed as per the procedure in our previous publication on taste assessment method for bitter drugs[31].
2.10.1. Training paradigm
The rats were trained for licking behavior (response to thirst) by depriving them of water for 22 h, but they had ad libitum access to food. After the water deprivation period, the Plexiglass cage was divided using plastic transparent dividers to provide an individual water bottle to each animal. Eventually, the rats were provided with graduated water bottle for 30 min, and the amount consumed at 15 and 30 min were recorded. This training paradigm was performed for 2 days before the taste evaluation experiment.
2.10.2. Evaluation of bitterness sensitivity of rats
To determine the concentration of CPM for this study, a sensitivity test for bitterness was performed in rats. After depriving the rats of water for 22 h, sensitivity toward 0.5 mg/mL CPM solution was evaluated on the first day, followed by a washout period of 24 h. Subsequently, the effect of 1 mg/mL CPM solution was examined, and the results were recorded.
2.10.3. Experiment
The experiment was performed for 30 min with 30 mL test formulation following the 22 h water deprivation period. After each experiment, the rats had a washout period of 24 h to avoid any memory of the taste of the previous formulation. The rats had ad libitum access to food during the experiment and washout period. The amount of solution remaining at 15 and 30 min was noted and subtracted from the original test volume. Varying results caused by spilling of the test solution while measuring or leaking of bottle knob were omitted from the study. Notably, animal behavior responses such as jaw smacking, oral grooming, and retreating were observed, which was not the focus of this study. Formulations N2, N7, and N9 at 0.5 mg/mL CPM were used for bitterness evaluation study in rat model. The average amount of solution consumed by all animals was calculated and expressed as the mean standard deviation. The mean scores between the physical mixture and formulation were compared using a student t-test at 95% confidence level and P<0.05 was considered statistically significant.
2.11. Film evaluation by human panel
The evaluation of film for palatability, disintegration time (DT), and organoleptic characteristics was performed at the Institute for Drug Delivery and Biomedical Research, Bangalore India (Protocol number VIPS/2013/12). The subjects were recruited after obtaining informed consent. This study is also in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The experimental procedure for this study was as per our previously published work [10, 32].
2.11.1. Human subject selection criteria
Six human subjects belonging to either sex were recruited. They were asked to abstain from coffee/tea and other beverages for 12 h. The subjects were allowed to drink only water for 12 h. Moreover, they were asked not to eat chocolates or other candies for over 6 h. The inclusion criterion was healthy human subjects aged 18–42 years, and the exclusion criteria were subjects suffering from fever, mouth ulcers, dry mouth, cold, nose block, and wounds as well as smokers.
2.11.2. Data collection
Before data collection, the subjects were asked to wash their mouth with water at ambient temperature. The surface temperature of the tongue was recorded using an infrared (IR) thermometer, and a difference of ±5°C relative to the body temperature was considered an exclusion criteria.
2.11.2.1. Bitterness perception
The subjects were asked to taste aqueous solutions of CPM, beginning with very dilute solutions and progressing to higher concentrations, by placing 2 mL solution for 30 s on the tongue/buccal cavity. The concentrations screened were 0, 0.5, 1, 2.5, and 4 mg. The volunteers were asked to report the perception each time: 1- I feel bitter taste, 2- I feel something but cannot identify the taste, and 3-I do not feel the taste. The subjects who reported 2 or 3 were asked to taste higher concentrations of the solution until they expressed perception 1. This was recorded as the threshold for an individual. For individuals who reported a score of 1, at least 1/5th the drug concentration of the actual dose was only allowed for testing the products. A few high concentration API solutions above the individual s perception threshold were made for tasting, and the subjects were subsequently asked to provide a score for each solution (Table 3). The highest concentration of the solution contained CPM equivalent to the dose present in the products tested. The scoring pattern followed was according to modified hedonic scale: 0-no taste, 1- taste something (threshold), 2-slightly bitter, 3-moderately bitter, 4-bitter, and 5-strongly bitter.
Table 3.
Human taste perception response
| Amount of CPM/2ml | Volunteer 1 | Volunteer 2 | Volunteer 3 | Volunteer 4 | Volunteer 5 | Volunteer 6 |
|---|---|---|---|---|---|---|
| 0 | ||||||
| 0.5 | 1 | 1 | 3 | 3 | 3 | 1 |
| 1 | 3 | 2 | 4 | 5 | 4 | 2 |
| 2.5 | 4 | 3 | 5 | 5 | 3 | |
| 4 | 5 | 5 | 5 |
(0- no taste, 1- taste something (threshold), 2-slightly bitter, 3-moderately bitter, 4-bitter, and 5-strongly bitter)
2.11.2.2. Formulation evaluation and data analysis
A washout interval of 12–24 h was allowed after screening the standard solution. The individuals were asked to taste the products (physical mixture or ODF) randomly (blinded) and score the product. The products were placed on the tongue/buccal cavity for 30–40 s, and the subjects were asked to score the bitterness on a scale of 0–5 for each product. Moreover, volunteers were asked to report the time for complete disintegration of the film. Sufficient washout time was allowed between the products, and the volunteers were allowed to drink copious amounts of water after tasting each product. The average of the scores given by all individuals were taken and expressed as the mean standard deviation. The mean scores between the physical mixture and formulation were compared using a student t-test at 95% confidence level, and P<0.05 was considered statistically significant.
3. Results and Discussion
3.1. Preparation of hot-melt extruded film
Modified starch is very difficult to extrude because of its high glass transition temperature. Thus, there is a need to introduce a plasticizer during extrusion, which could reduce the melt viscosity and increase the free volume of starch chains. For this study, glycerin was used as a plasticizer in different proportions, and it exhibited excellent extrudability with significantly lower torque (4.8–7.2 Nm) values than typically encountered. The barrel design was modified with a degassing port to remove excess amount of water vapor from the molten mass. Initial studies without a degassing port demonstrated the presence of bubbles as well as unequal distribution of drug in the film samples.
Standard screw configuration (Figure 1) with three mixing zones was utilized for this study. It provided enough shear for dispersive and distributive mixing of the drug and helped get excellent content uniformity in all the extruded film formulations.
Figure 1.
Film extrusion screw design
The extruded films were stretched using the roll connected to the extruder assembly. This aided in making thin films with uniform thickness, and the roll speed was optimized for steady collection of the film. The combination of processing and formulation parameters helped to manufacture uniform, clear, and very thin films (60–110 μm) using melt extruder.
3.2. Physiochemical evaluation of films
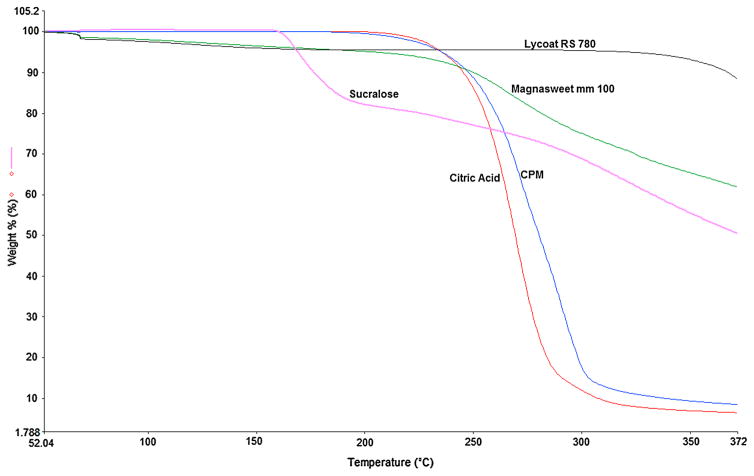
TGA is very critical before performing HME because the drug and excipients are exposed to high temperature during the extrusion process, and there are possibilities of drug degradation or thermally-induced chemical reactions or both [16]. The TGA results (Figure 2) specified that API, polymer, and excipients were chemically stable in the HME processing temperature range. Lycoat® RS 780 demonstrated a loss of weight (<3%), which was attributed to the moisture present in the polymer. These results confirmed that all materials had excellent thermal stability and fit for the melt extrusion process [33].
Figure 2.
TGA thermograms of chlorpheniramine maleate, polymer, and excipients
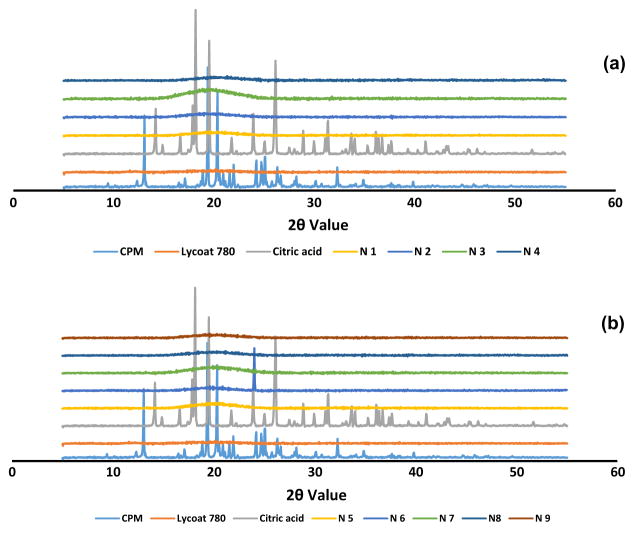
XRD was used to investigate the physical state of the drug after HME process. The XRD results (Figure 3a & 3b) of CPM illustrated prominent peaks at 2θ angles of approximately 13, 19, and 20 degrees, while citric acid showed peaks at 2θ angles of 18, 19, and 26. The melt-extruded formulation did not show any peak that confirmed the presence of drug in an amorphous form. The reasons behind the complete conversion of drug to an amorphous form were the high shear during extrusion, low drug load, and relatively high amounts of glycerin. The presence of CPM and excipients in an amorphous form aided the flexibility and clear appearance of the film.
Figure 3.
(a) (b). X-ray diffraction profiles of CPM, polymer, and melt-extruded film formulations
3.3. Dissolution studies
Lycoat® RS 780 is a comparatively new modified starch-based polymer, which demonstrated its significance in film coating for tablets and oral film development using solvent casting method [34, 35]. Being a non-gelling and highly water-soluble polymer, it provides rapid disintegration and dissolution to formulations. Visual inspection during dissolution demonstrated rapid disintegration of the film when it touched the dissolution media. This characteristic helps in the rapid onset of action of the formulation, because the drug can diffuse from the oral mucosa and reach the systemic circulation [36].
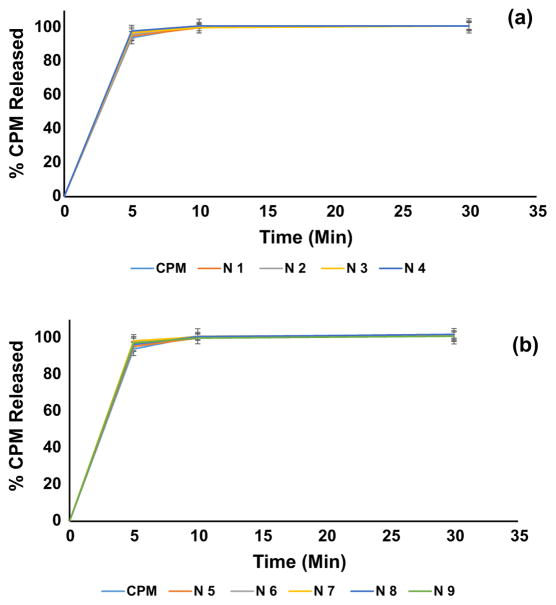
During dissolution studies, the formulations rapidly release CPM, and it was attributed to hydrophilic excipients and Biopharmaceutics Classification System (BCS) class I drug. These films had very low thickness (60–110 μm) and higher surface area, which enabled interaction with dissolution media and rapid disintegration following complete dissolution. Dissolution results (Figure 4a & 4b) showed ~95% drug release in the first 5 min of the dissolution experiment, and at 10-min time points, there was complete release of the drug.
Figure 4.
(a) (b). Dissolution release profiles of CPM and melt-extruded film formulations
3.4. SEM evaluation
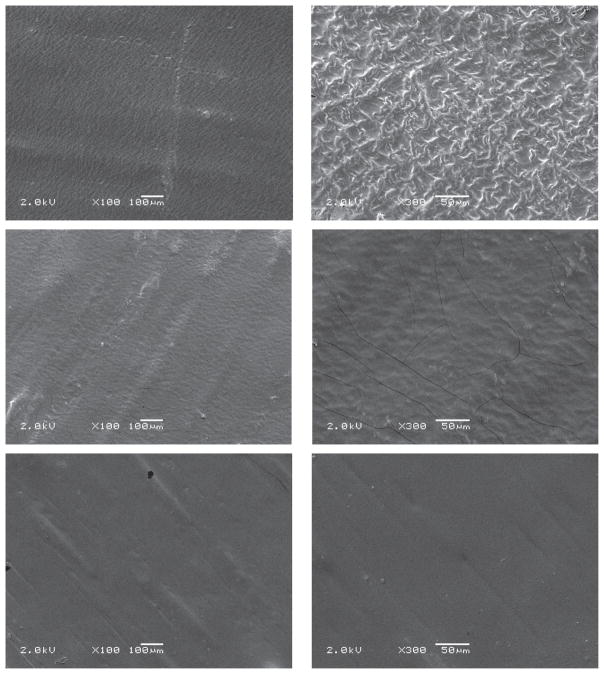
The surface morphology was examined by SEM for all film formulations. SEM images did not exhibit CPM crystals, indicating an amorphous nature of drug in formulations (Figure 5). The formulations showed very smooth surface at low magnification. This affirmed the smooth texture of film surface, which is one of the esthetic attributes of films. However, at microscopic level, there was high surface area, which helped in the rapid disintegration of the film.
Figure 5.
Scanning electron microscopy (SEM) images of films: formulation N2 (a, b), N7 (a, b), and N9 (a, b)
3.5. Film characterizations
As illustrated in Table 2, the film formulations demonstrated excellent D.T of 6–11 s, which was attributed to the thickness of the film and presence of water-soluble materials in the film. The formulations contained water-soluble excipients and APIs such as CPM, citric acid, glycerin, and modified starch. The most crucial parameter for disintegration is the low thickness of the film. As the films had a thickness range of 60–110 μm, they aided in the faster disintegration of all film formulations. In addition to the low thickness of the film, the amount of saliva in the oral cavity is very critical for rapid disintegration. The normal flow of saliva in a healthy person is 0.34 mL/min, and it can be increased by the addition of agents that simulate salivary production, including citric, malic, lactic, ascorbic, and tartaric acids [14]. Citric acid is the most preferred saliva-stimulating agent, and it was estimated that citric acid could increase salivary flow approximately 5-fold in 2– 6% proportion in the formulation [14]. With the addition of citric acid, the pH of the films was found to be in the range of 2.9–3.4 and it could contribute in improving rate of salivary flow after administration of formulation which will aid in rapid disintegration of film product.
Table 2.
Film Evaluation
| Formulation | Strength (Mpa) | Elongation (%) | Thickness (μm) | pH | D.T (Seconds) |
|---|---|---|---|---|---|
| N 1 | 10.036 (±1.017) | 9.930 (±0.702) | 70 (±10) | 3.130 (± 0.006) | 6 (±1) |
| N 2 | 20.879 (±1.014) | 7.918 (±0.367) | 70 (±10) | 3.000 (± 0.050) | 7 (±1) |
| N 3 | 5.795 (±0.487) | 14.000 (±0.958) | 70 (±15) | 2.880 (± 0.025) | 6 (±2) |
| N 4 | 7.803 (±1.014) | 12.040 (±0.467) | 100 (±20) | 2.900 (± 0.035) | 11 (±1) |
| N 5 | 3.556 (±0.941) | 127.120 (±10.400) | 110(±13) | 3.390 (± 0.076) | 9 (±2) |
| N 6 | 10.826 (±0.146) | 9.880 (±0.163) | 100 (±10) | 3.370 (± 0.023) | 7 (±1) |
| N 7 | 6.840 (± 0.440) | 9.770 (± 0.061) | 60 (±18) | 3.216 (± 0.032) | 6 (±1) |
| N 8 | 11.040 (± 0.920) | 11.300 (± 0.390) | 110 (±21) | 3.180 (± 0.006) | 6 (±1) |
| N 9 | 5.390 (± 0.590) | 16.340 (± 6.720) | 100 (±14) | 3.110 (± 0.006) | 7 (±1) |
All the film formulations were tested for their Ts and %E (Table 2). Ideally, the film should have desirable mechanical properties so that it can remain intact during handling and transport. ODFs showed appropriate strength and %E. These excellent mechanical properties were attributed to the presence of glycerol, citric acid, and CPM, which reduced film stiffness via disruption of intermolecular forces of the polymer owing to the accommodation of these compounds between the strands, thereby providing elasticity to the films [37, 38].
3.6. In vivo taste evolution
Animal preference tests demonstrated a great potential for taste evaluation of bitter APIs and the outcome from these studies exhibited the comparison to results obtained from the human panel [39, 40]. The rodent brief-access taste aversion (BATA) model is one of the popular approaches used for taste evaluation [39–45]. In this model, rodents such as mice or rats, are mildly water-deprived and then put into as “lickometer” which records the number of “licks” that the rodents make to different concentrations of the compound under test samples presented in several sipper tubes [46]. Typically, a low number of licks compared to water will indicate an aversive taste [46].
The taste discrimination rat model employed in this study was based on drinking pattern of test solution by animals and compared the outcome to pure drug and water consumption. Higher the consumption of test solution compared to pure drug solution will suggest the reduction in bitterness.
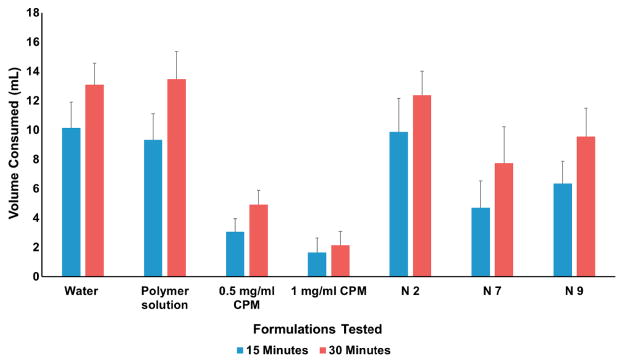
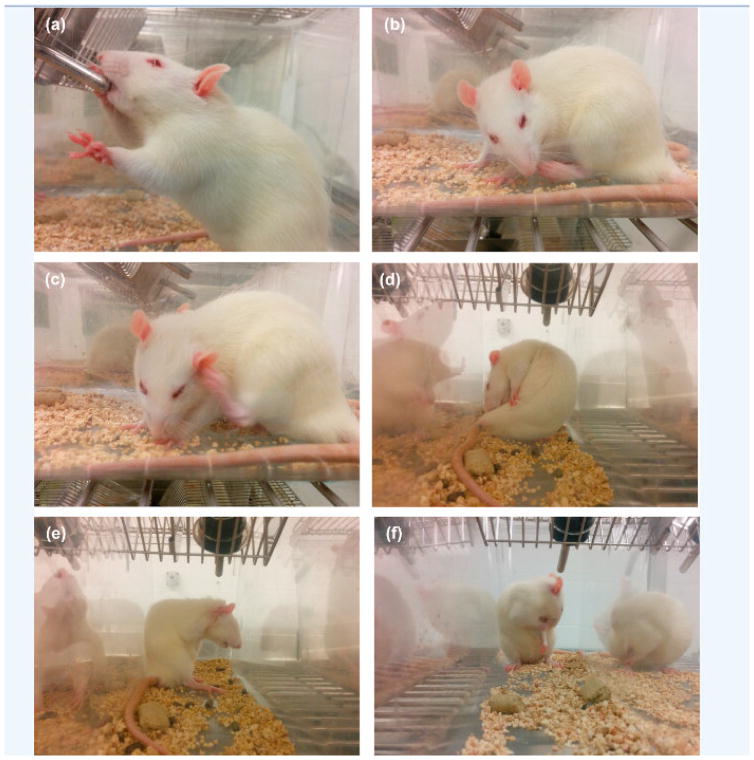
Firstly, the taste perception of rats was evaluated by administering 0.5 and 1 mg/mL CPM dissolved in distilled water. These results were important to avoid taste variability between animals (Figure 6), and showed that the rats consumed ~10 and ~14 mL of water in 15 and 30 min, respectively. The results of this study were comparable with those of the study published by Tiwari et.al. Thus, the rate and extent of consumption of water were reduced significantly to ~3 and ~5 mL in 15 and 30 min with the administration of 0.5 mg/mL CPM solution. At a higher concentration of 1 mg/mL, there was notable reduction in consumption of water to ~1.6 and ~2 mL at 15 and 30 min. Notably, this reduction in consumption of CPM solution despite deprivation of water for 22 h affirmed an aversion toward CPM. Moreover, aversion behaviors (Figure 7), such as jaw smacking, oral grooming, nose wrinkle, paw wipe, forelimb flail, head shake, paw shakes, and retreating confirmed the dislike of rats toward the drug solution[31].
Figure 6.
Taste evaluation in rat model
Figure 7.
Behavioral response of rats after administration of CPM and formulation solutions, a) Normal Drinking b) Paw licking c) Scratching by paw d) Biting e) Scratching by both paws f) Oral grooming
As illustrated in Figure 6, the rats consumed ~10 and 12.5 mL of N 2 solution (5% CPM in the film) in the first 15 and 30 min, and the amount was comparable with the consumption of water. In addition, N7 (10% CPM) exhibited consumption of ~ 4.7 and ~8 mL at 15 and 30 min. Furthermore, N9 (7.5% CPM) showed consumption of ~6.3 and 10 mL at 15- and 30-min time point. These results indicated that with increasing concentrations of CPM, there was noticeable reduction in the consumption of formulation. The rats did not show aversion behavior such as forelimb flail with N2 formulation. However, there was a surge in the aversion behavior response upon increasing the drug concentrations in N7 and N9,.
The results of this study were very helpful to understand about the taste of pure drug and formulation. It provided an insight into the taste of products, which helped to screen this formulation for human studies.
3.7. Film evaluation by human panel
Before evaluation of taste of the formulation, it is very important to understand the taste perception of human volunteers to minimize intra-subject variability. Taste perception study was performed on six healthy human volunteers. Initially, different concentrations of CPM in 2 mL of water were administered to the human subjects. Three subjects had threshold at 0.5 mg and the remaining three subjects reported moderate bitterness at the same concentration. A dose of 2.5 mg demonstrated bitterness in all subjects, and only three volunteers could taste higher concentration of CPM (4 mg, Table 3). This initial evaluation confirmed the appropriateness of the subjects for taste masking study.
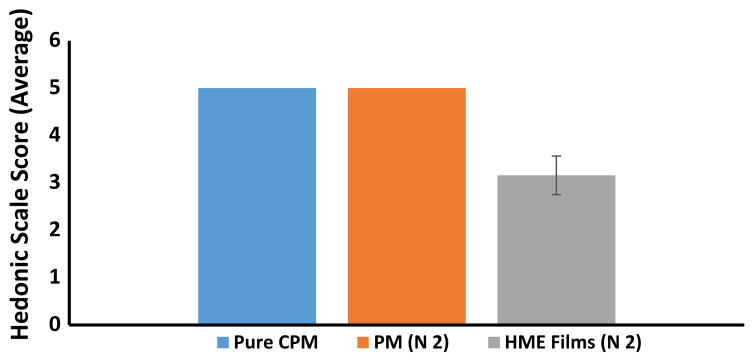
The results of taste masking evaluation in the animal model suggested that formulation N2 with 5% CPM had significant taste masking. Moreover, this formulation had an excellent D.T (7 s) in in vitro studies. Based on these results, N2 formulation (4 mg CPM in each film) was considered for human panel taste and disintegration studies to ascertain the product attributes in human subjects. The physical mixture of N2 exhibited disagreeable and unendurable taste in human volunteers (an average score of 5 on a scale of 1–5; Figure 8). These findings asserted that the administration of CPM with modified starch and other excipients (physical mixture) did not assist in diminishing the bitterness of the drug, and it required a pertinent formulation approach. When the film formulation was administered, there was notable reduction in bitterness to ~3 on the scale of 1–5. Statistical analysis of the human panel data suggested a significant difference in bitterness (p<0.0001) between the physical mixture and melt-extruded ODF samples.
Figure 8.
Human taste panel evaluations: pure CPM, physical mixture (N2), and melt-extruded film (N2)
The volunteers were asked to report the time for complete disintegration of films in the oral cavity, and the average D.T was 16±4.5 s for N2 formulation. The rapid D.T was attributed to the thickness of film (~70 μm) and the use of hydrophilic polymer in the formulation. Notably, the thickness of N2 formulation was comparable with commercially available mouth-refreshing films (~60 μm) [47]. Furthermore, human subjects did not report any stickiness or difficulties in handling and no particulate matter after disintegration of films. Currently, there are no regulatory guidelines available for the thickness, D.T, and other quality attributes of the film; however, there are guidelines for ODT suggesting a D.T of 30 s. We are considering 30 s as a reference D.T for the film product.
4. Conclusion
This innovative study demonstrated the utilization of HME technology for continuous manufacturing of orally disintegrating films. A standard screw configuration with a degassing port was used to manufacture thin, bubble-free, uniform, and esthetic film formulations. The films showed immediate disintegration and dissolution, which were attributed to the thinness and presence of hydrophilic excipients in the formulations. The rat model exhibited excellent bitterness discrimination in different drug-loaded formulations. The human panel study confirmed the reduction of bitterness in the films compared to the pure drug and physical mixture. Moreover, the disintegration results were in accordance with those of the in vitro method. This formulation could be used as a platform for the development of solvent-free thin film formulations focusing on pediatric and geriatric patients.
Acknowledgments
This work was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH. The authors also thank the Pii Center for Pharmaceutical Technology for contributions in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen O, Zweidorff OK, Hjelde T, Rodland EA. Problems when swallowing tablets. A questionnaire study from general practice. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke. 1995;115:947–949. [PubMed] [Google Scholar]
- 2.Slowson M, Slowson S. What to do when patients cannot swallow their medications. Pharma Times. 1985;51:90–96. [Google Scholar]
- 3.Chang RK, Xiaodi G, Burnside BA, Couch RA. Fast-dissolving tablets. Pharmaceutical technology. 2000;24:52–58. [Google Scholar]
- 4.Lindgreen S, Janzon L. Dysphagia: Prevalence of swallowing complaints and clinical findings. Med Clin North Am. 1993;77:3–5. doi: 10.1007/BF02493524. [DOI] [PubMed] [Google Scholar]
- 5.Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert opinion on drug delivery. 2011;8:1521–1529. doi: 10.1517/17425247.2011.628311. [DOI] [PubMed] [Google Scholar]
- 6.Wright D. Swallowing difficulties protocol: medication administration. Nursing Standard. 2002;17:43–45. doi: 10.7748/ns2002.12.17.14.43.c3319. [DOI] [PubMed] [Google Scholar]
- 7.Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, Khan MA. Tablet splitting of a narrow therapeutic index drug: a case with levothyroxine sodium. Aaps Pharmscitech. 2010;11:1359–1367. doi: 10.1208/s12249-010-9515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao N, Zidan A, Tawakkul M, Sayeed VA, Khan M. Tablet splitting: product quality assessment of metoprolol succinate extended release tablets. International journal of pharmaceutics. 2010;401:25–31. doi: 10.1016/j.ijpharm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: a review. Acta pharmaceutica. 2011;61:117–139. doi: 10.2478/v10007-011-0020-8. [DOI] [PubMed] [Google Scholar]
- 10.Pimparade MB, Morott JT, Park JB, Kulkarni VI, Majumdar S, Murthy S, Lian Z, Pinto E, Bi V, Durig T. Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro–in vivo evaluations. International journal of pharmaceutics. 2015;487:167–176. doi: 10.1016/j.ijpharm.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slavkova M, Breitkreutz J. Orodispersible drug formulations for children and elderly. European Journal of Pharmaceutical Sciences. 2015;75:2–9. doi: 10.1016/j.ejps.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Dahiya M, Saha S, Shahiwala AF. A review on mouth dissolving films. Current drug delivery. 2009;6:469–476. doi: 10.2174/156720109789941713. [DOI] [PubMed] [Google Scholar]
- 13.Reiner V, Giarratana N, Monti NC, Breitenbach A, Klaffenbach P. Rapidfilm: an innovative pharmaceutical form designed to improve patient compliance. Int J Pharm. 2010;393:55–60. doi: 10.1016/j.ijpharm.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 14.Dixit RP, Puthli SP. Oral strip technology: overview and future potential. Journal of controlled release: official journal of the Controlled Release Society. 2009;139:94–107. doi: 10.1016/j.jconrel.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Low AQ, Parmentier J, Khong YM, Chai CC, Tun TY, Berania JE, Liu X, Gokhale R, Chan SY. Effect of type and ratio of solubilising polymer on characteristics of hot-melt extruded orodispersible films. Int J Pharm. 2013;455:138–147. doi: 10.1016/j.ijpharm.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Morott JT, Pimparade M, Park JB, Worley CP, Majumdar S, Lian Z, Pinto E, Bi Y, Durig T, Repka MA. The effects of screw configuration and polymeric carriers on hot-melt extruded taste-masked formulations incorporated into orally disintegrating tablets. Journal of pharmaceutical sciences. 2015;104:124–134. doi: 10.1002/jps.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vo AQ, Feng X, Morott JT, Pimparade MB, Tiwari RV, Zhang F, Repka MA. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e V. 2016;98:108–121. doi: 10.1016/j.ejpb.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng X, Vo A, Patil H, Tiwari RV, Alshetaili AS, Pimparade MB, Repka MA. The effects of polymer carrier, hot melt extrusion process and downstream processing parameters on the moisture sorption properties of amorphous solid dispersions. The Journal of pharmacy and pharmacology. 2016;68:692–704. doi: 10.1111/jphp.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albers J, Alles R, Matthée K, Knop K, Nahrup JS, Kleinebudde P. Mechanism of drug release from polymethacrylate-based extrudates and milled strands prepared by hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71:387–394. doi: 10.1016/j.ejpb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Almeida A, Possemiers S, Boone MN, De Beer T, Quinten T, Van Hoorebeke L, Remon JP, Vervaet C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e V. 2011;77:297–305. doi: 10.1016/j.ejpb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Forster A, Hempenstall J, Rades T. Characterization of glass solutions of poorly water-soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. Journal of Pharmacy and Pharmacology. 2001;53:303–315. doi: 10.1211/0022357011775532. [DOI] [PubMed] [Google Scholar]
- 22.Desai D, Wang J, Wen H, Li X, Timmins P. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharmaceutical development and technology. 2013;18:1265–1276. doi: 10.3109/10837450.2012.660699. [DOI] [PubMed] [Google Scholar]
- 23.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, Martin C, McGinity JW. Pharmaceutical applications of hot-melt extrusion: Part II. Drug development and industrial pharmacy. 2007;33:1043–1057. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 24.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, McGinity JW, Martin C. Pharmaceutical applications of hot-melt extrusion: part I. Drug development and industrial pharmacy. 2007;33:909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 25.Vo AQ, Feng X, Pimparade M, Ye X, Kim DW, Martin ST, Repka MA. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. European Journal of Pharmaceutical Sciences. 2017 doi: 10.1016/j.ejps.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Just S, Sievert F, Thommes M, Breitkreutz J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85:1191–1199. doi: 10.1016/j.ejpb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Preis M, Woertz C, Kleinebudde P, Breitkreutz J. Oromucosal film preparations: classification and characterization methods. Expert opinion on drug delivery. 2013;10:1303–1317. doi: 10.1517/17425247.2013.804058. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann EM, Breitenbach A, Breitkreutz J. Advances in orodispersible films for drug delivery. Expert opinion on drug delivery. 2011;8:299–316. doi: 10.1517/17425247.2011.553217. [DOI] [PubMed] [Google Scholar]
- 29.Low AQJ, Parmentier J, Khong YM, Chai CCE, Tun TY, Berania JE, Liu X, Gokhale R, Chan SY. Effect of type and ratio of solubilising polymer on characteristics of hot-melt extruded orodispersible films. International journal of pharmaceutics. 2013;455:138–147. doi: 10.1016/j.ijpharm.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 30.Moyano MA, Rosasco MA, Pizzorno MT, Segall AI. Simultaneous determination of chlorpheniramine maleate and dexamethasone in a tablet dosage form by liquid chromatography. Journal of AOAC International. 2005;88:1677–1683. [PubMed] [Google Scholar]
- 31.Tiwari RV, Polk AN, Patil H, Ye X, Pimparade MB, Repka MA. Rat Palatability Study for Taste Assessment of Caffeine Citrate Formulation Prepared via Hot-Melt Extrusion Technology. AAPS PharmSciTech. 2015:1–8. doi: 10.1208/s12249-015-0447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juluri A, Popescu C, Zhou L, Murthy RN, Gowda VK, Kumar C, Pimparade MB, Repka MA, Murthy SN. Taste masking of griseofulvin and caffeine anhydrous using Kleptose Linecaps DE17 by hot melt extrusion. Aaps Pharmscitech. 2016;17:99–105. doi: 10.1208/s12249-015-0374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pimparade CPMB, Juluri A, Kulkarni V, Zhou L, Murthy SN, Repka MA. Improving Clotrimazole Release from a Hydrophobic Matrix Formulated by Hot Melt Extrusion: Evaluation of a New Dissolution Initiator. 2013 AAPS Annual Meeting and Exposition; San Antonio, TX. 2013. [Google Scholar]
- 34.Parissaux X, Joshi AA, Francois A, Lefevre P. Evaluation of a novel modified starch polymer in an easy to formulate thin-film drug delivery system and comparison with some marketed formulations. Young. 2007;1070:323. [Google Scholar]
- 35.El-Setouhy DA, El-Malak NSA. Formulation of a novel tianeptine sodium orodispersible film. Aaps Pharmscitech. 2010;11:1018–1025. doi: 10.1208/s12249-010-9464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garsuch V, Breitkreutz J. Novel analytical methods for the characterization of oral wafers. European Journal of Pharmaceutics and Biopharmaceutics. 2009;73:195–201. doi: 10.1016/j.ejpb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Entwistle C, Rowe R. Plasticization of cellulose ethers used in the film coating of tablets. Journal of Pharmacy and Pharmacology. 1979;31:269–272. doi: 10.1111/j.2042-7158.1979.tb13499.x. [DOI] [PubMed] [Google Scholar]
- 38.Stubberud L, Arwidsson HG, Hjortsberg V, Graffner C. Water-solid interactions. III. Effect of glass transition temperature, Tg, and processing on tensile strength of compacts of lactose and lactose/polyvinyl pyrrolidone. Pharmaceutical development and technology. 1996;1:195–204. doi: 10.3109/10837459609029894. [DOI] [PubMed] [Google Scholar]
- 39.Devantier HR, Long DJ, Brennan FX, Carlucci SA, Hendrix C, Bryant RW, Salemme FR, Palmer RK. Quantitative assessment of TRPM5-dependent oral aversiveness of pharmaceuticals using a mouse brief-access taste aversion assay. Behavioural pharmacology. 2008;19:673–682. doi: 10.1097/FBP.0b013e3283123cd6. [DOI] [PubMed] [Google Scholar]
- 40.Clapham D, Kirsanov D, Legin A, Rudnitskaya A, Saunders K. Assessing taste without using humans: rat brief access aversion model and electronic tongue. International journal of pharmaceutics. 2012;435:137–139. doi: 10.1016/j.ijpharm.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 41.Contreras RJ, Carson CA, Pierce CE. A novel psychophysical procedure for bitter taste assessment in rats. Chemical senses. 1995;20:305–312. doi: 10.1093/chemse/20.3.305. [DOI] [PubMed] [Google Scholar]
- 42.Clarke SN, Bernstein IL. NaCl preference increases during pregnancy and lactation: assessment using brief access tests. Pharmacology Biochemistry and Behavior. 2001;68:555–563. doi: 10.1016/s0091-3057(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 43.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of α-gustducin to taste-guided licking responses of mice. Chemical senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 44.Bhat MG, Jordt RM, Khan MA, Foley CE, Gilbertson TA. Validation of a rat behavioral avoidance model from a drug delivery perspective. International journal of pharmaceutics. 2005;303:31–36. doi: 10.1016/j.ijpharm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Guenthner C, McCaughey S, Tordoff M, Baird J. Licking for taste solutions by potassium-deprived rats: specificity and mechanisms. Physiology & behavior. 2008;93:937–946. doi: 10.1016/j.physbeh.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soto J, Sheng Y, Standing JF, Gul MO, Tuleu C. Development of a model for robust and exploratory analysis of the rodent brief-access taste aversion data. European Journal of Pharmaceutics and Biopharmaceutics. 2015;91:47–51. doi: 10.1016/j.ejpb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Dave RH, Shah DA, Patel PG. Development and evaluation of high loading oral dissolving film of aspirin and acetaminophen. Journal of Pharmaceutical Sciences and Pharmacology. 2014;1:112–122. [Google Scholar]