Abstract
Currently, the most efficient and promising approach for generating large numbers of engraftable human skeletal myogenic progenitors from pluripotent stem cells requires the conditional in vitro overexpression of PAX7 using lentiviral vectors. Because a non-integrating approach would be preferable to eliminate or minimize the risk associated with random genomic integration, here we investigate whether transient expression of PAX7 using minicircle DNA would enable the generation of functional pluripotent stem cell-derived myogenic progenitors, equivalent to those generated by lentivirus. Our results demonstrate that upon multiple transfections, the minicircle approach allows for the scalable generation of myogenic progenitors and these undergo efficient terminal differentiation in vitro. However, transplantation of minicircle-generated myogenic progenitors resulted in limited engraftment. This is probably due to less efficient delivery and more transient PAX7 expression in these cultures since PAX7 downregulation is accompanied by high level of spontaneous differentiation. Thus, although the in vitro data shows that the minicircle approach could potentially replace the use of lentivirus, improvements in the transfection/expression system will be necessary before it will be a feasible strategy for the generation of myogenic progenitors for cell replacement therapy.
Keywords: Lentivirus, non-viral minicircle, skeletal myogenic progenitors, engraftment
1. INTRODUCTION
Over the past few years, several studies using hPS cells focused on the use of transgene-free strategies to recapitulate embryonic skeletal myogenesis in vitro (Barberi et al., 2007; Chal et al., 2016; Hwang et al., 2013; Shelton et al., 2014; Xi et al., 2017; Zheng et al., 2006). However, most of the current transgene-free myogenic differentiation protocols have limitations for potential clinical translation. The primary problems are the heterogeneity of cell preparations and the lack of evidence for the in vivo regenerative potential of generated myogenic cells (Kim et al., 2017). To date, the most homogeneous approaches for the generation of a myogenic-restricted population from PS cells relies on the introduction of exogenous DNA for the myogenic regulatory factor MYOD (Albini et al., 2013; Goudenege et al., 2012; Tedesco et al., 2012; Young et al., 2016) or PAX7 (Darabi et al., 2012; Kim et al., 2017), a paired box transcription factor essential for the commitment and maintenance of muscle stem cells (Gros et al., 2005; Lagha et al., 2008; Seale et al., 2000),
Few of these studies have documented transplantation of PS-cell derived progenitors, but of those that have, most have shown minimal engraftment (Barberi et al., 2007; Hwang et al., 2013; Kim et al., 2017; Zheng et al., 2006). In contrast to these studies, the approach of temporally overexpressing PAX7 enables the generation of large numbers of proliferating PAX7+ myogenic progenitors and when these are transplanted, they contribute to significant skeletal muscle regeneration (Darabi et al., 2012; Kim et al., 2017; Magli et al., 2017). To date, the PAX7 approach has used lentiviral (LV) delivery of PAX7, which introduces the possibility of insertional mutagenesis.
Alternative approaches to LV are non-viral vectors such as RNA or DNA, which are considered safer due to their integration-free nature (Hardee et al., 2017). However, manufacturing RNA is expensive and difficult because RNA has a propensity to degrade (Yin et al., 2014). On the other hand, DNA plasmids are relatively economical and stable but are inefficient in terms of cellular uptake and intracellular transport into the host (Nehlsen et al., 2006). A miniaturized plasmid lacking bacterial DNA sequences, known as a minicircle (MC) shows several benefits over these vectors, including: i) much smaller size of DNA for higher diffusion rate and increased biological activity both in vitro and in vivo (Chabot et al., 2013; Chen et al., 2003); ii) lack of bacterial-related genes and CpG motifs (Ismail et al., 2012; Mayrhofer and Iro, 2012); and iii) reduced risk of possible transgene integration into host genome. It has been previously shown that a MC encoding the pluripotent transcription factors Oct4, Nanog, Lin28 and Sox2, enables the reprogramming of human adipose cells into iPS cells (Jia et al., 2010; Narsinh et al., 2010). However, the application of MC for lineage-specific differentiation from PS cells has not yet been reported.
In the present study, we assess the feasibility of delivering PAX7 through MC to generate scalable and engraftable PAX7+ myogenic progenitors from PS cells, equivalent to those generated using LV.
2. MATERIAL AND METHODS
2.1. Production of MC expressing hPAX7
To generate the hPAX7 expressing MC construct (Figure 1A), a segment of hPAX7-T2A-GFP from its carrying vector (hPAX7-T2A-GFP-pRRL) and EF1α-RFP fragment from the MC parental plasmid (PP, CMV-MCS-EF1α-RFP-SVPolyA, MN512A-1, System Biosciences) were isolated. The two linearized fragments, hPAX7-T2A-eGFP and CMV-MCS-SVPolyA were then ligated to generate hPAX7 expressing PP construct (Figure 1A). The MC was produced and isolated according to the manufacturer’s instructions using E. coli ZYCY10P10P3S2T cells (System Biosciences). MC was isolated using a NucleoBond midi kit (Clontech) and treated with Minicircle-safe-DNase to remove bacterial genomic and PP DNA contaminants.
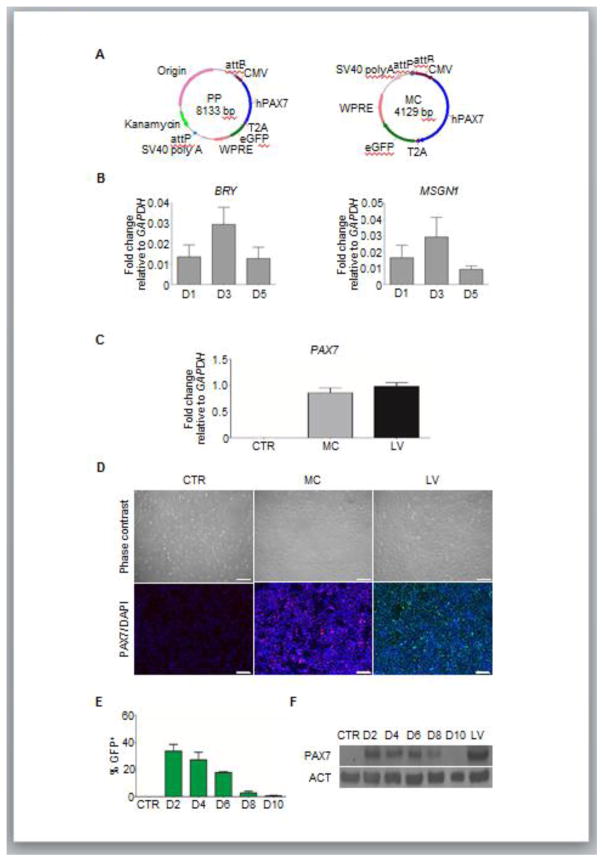
Figure 1. Characterization of PAX7 MC delivery in mesodermal cells.
(A) Schematic diagram of MC and its PP constructs.
(B) Quantitative RT-PCR analysis for BRY and MSGN1 expression in differentiating EB cultures at different time points after GSK3β inhibition. Values represent mean ± SEM (n=3 biological replicates).
(C–D) Quantitative RT-PCR analysis for PAX7 expression in transfected cells 2 days after MC delivery (C). PAX7-induced myogenic progenitors were used as positive control, whereas non-transfected cells served as negative control. Values represent mean ± SEM (n=3 biological replicates). Immunofluorescence analysis of respective samples (D). PAX7 staining in red for (MC) or green (LV), and DAPI staining in blue for nuclei. Scale bar = 200 μm (n=3 biological replicates).
(E–F) Persistence of transgene expression analyses. Graph shows GFP expression in transfected cells, measured by FACS. Cells were analyzed every other day, beginning 2 days after transfection (D2) until day 10 (D10) (E). Non-transfected cells served as negative control (values represent mean ± SEM; n=3). Western blot analysis for PAX7 expression in respective samples (F). PAX7-induced myogenic progenitors were used as positive control. ACT was used as housekeeping protein. Lane 1 = Non-transfected cells; Lane 2 = D2 MC-transfected cells; Lane 3 = D4 MC-transfected cells; Lane 4= D6 MC-transfected cells; Lane 5 = D8 MC-transfected cells; Lane 6 = D10 MC-transfected cells; Lane 7 = PAX7-induced myogenic progenitors. (n=2 biological replicates).
2.2. Propagation of hPS cells
Unmodified human H9 ES and PLZ iPS cells, as well as their respective Dox-inducible iPAX7 LV-transduced counterparts (iPAX7 H9 and iPAX7 PLZ cells) (Darabi et al., 2012) were cultured on matrigel (BD Biosciences)-coated dishes in mTeSR1 medium (Stem Cell Technologies). Cells were passaged as aggregates.
2.3. Generation of myogenic progenitors using the MC approach
As previously described (Kim et al., 2016), undifferentiated PS cell colonies were harvested using Accumax, and cultured in a non-adherent 60 mm Petri Dish (1 x 106 cells/well) with EB medium (IMDM medium (Life Technologies) containing 15% fetal bovine serum (Gibco), 10% horse serum (Gibco), 1% penicillin/streptomycin (Gibco), 1% glutamax (Gibco), 0.45 μM monothioglycerol (MP Biomedicals), 0.5 mM ascorbic acid (Sigma), and supplemented with 10 μM GSK3β inhibitor (CHIRON99021, Tocris) for 3 days. Next, the dissociated EBs were transfected with MC using GeneIn transfection kit (MTI-Global Stem) and seeded onto gelatin (Sigma)-coated 6-well plates (4 x 105 cells/well) in EB medium, supplemented with 10 μM Y-27632 and 10 ng/ml FGF-2 (R&D Systems). Cells were passaged by trypsinization every 3 days along with transfections (total of 3). At day 11, GFP+ (PAX7+) cells were isolated by FACS using a FACS Aria (BD Biosciences). Cells were cultured on a gelatin-coated 24-well dish for additional 6 days in the EB medium supplemented with 10 ng/ml FGF-2 or until they were confluent (>95%) prior to terminal differentiation by switching the culture medium as described (Darabi et al., 2012; Kim et al., 2016).
2.4. Generation of myogenic progenitors using the LV system
The initial procedure, from day 0 to 3, was carried out as described above. At day 3, dissociated EBs were seeded onto gelatin-coated 6-well plates (4 x 105 cells/well) in EB medium supplemented with 10 μM Y-27632, 1 ng/ml Dox and 10 ng/ml FGF-2. At day 7, the red fluorescent protein positive (RFP+) or GFP+ (PAX7+) cells were sorted and maintained on gelatin-coated plates for additional 6–10 days in EB medium supplemented with 1 ng/ml Dox and 10 ng/ml FGF-2. The terminal differentiation procedure was carried out as described above.
2.5. Quantitative RT-PCR
The procedures were conducted as described (Kim et al., 2017). The gene-specific Taqman probes used were BRY (Hs00610080_m1), MSGN1 (s03405514_m1), PAX7 (Hs00242962_m1), MYOG (Hs01072232_m1), MHC (Hs01074230_m1) and GAPDH (GAPDH, Hs02758991_g1) from Thermo Fisher Scientific.
2.6. Western blot
The procedures were carried out as described (Kim et al., 2017). The primary antibodies used were PAX7 (PAX7, DSHB), MYOG (F5D, DSHB), MHC (MF-20, DSHB) and ACT (JLA20, DSHB). The proteins were visualized using ECL Western blotting detection reagents (Thermo Fisher Scientific).
2.7. Immunofluorescence staining
The procedures for staining cell cultures and muscle cryosections were carried out as described (Kim et al., 2017). The primary antibodies used for cell culture were PAX7 (PAX7, DSHB), MYOG (F5D, DSHB) and MHC (MF-20, DSHB). For muscle cryosections, slides were stained with human DYS (mouse; clone 2C6; Millipore) primary antibody in blocking solution, overnight at 4°C. On the next day, slides were stained with human LMNA-C primary antibody (rabbit; clone ERP4100; Abcam) for 1 hr at room temperature. Alexa Fluor® conjugated secondary antibodies (anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 555; Thermo Fisher Scientific) were applied for 45 min at room temperature, followed by subsequently counterstaining with DAPI. The slides were washed 3 times with PBS between steps. Fluorescence images were captured using the Zeiss upright fluorescence microscope Axio Imager M2 using Zen Application Suite software.
2.8. Transplantation studies
Animal experiments were carried out according to protocols approved by the University of Minnesota Institutional Animal Care and Use Committee. Cell preparations (pre-treated with 10 μM Y-27632 for 24 hours) were injected into TA muscles of 6–7 weeks-old NSG male mice (Jackson Laboratories) that had been pre-injured with cardiotoxin (Sigma), at 5 x 105 cells/10 μl of PBS (Darabi et al., 2012). Engraftment was assessed 6 weeks later.
2.9. Statistics
Differences between samples were assessed by using the Student’s two-tailed t-test for independent samples.
3. RESULTS
3.1. PAX7 MC delivery in mesodermal cells
To determine the ability of MC delivery to induce PAX7-mediated myogenesis in differentiating PS cells, we transfected H9 ES cells at day 3 of EB cultures, a time point that coincides with the peak of mesoderm formation, as shown by expression of BRY and MSGN1 (Figure 1B). Two days after transfection, we observed up-regulation of PAX7 by gene expression (Figure 1C) and immunofluorescence staining (Figure 1D), which was equivalent to those observed in LV-generated iPAX7 H9 ES cell-derived myogenic progenitors (referred as iPAX7 hereafter) (Figure 1C–D). Next, we investigated the persistence of transgene expression by FACS analysis. As expected, transfected cells showed transient expression of GFP (PAX7+, Figure 1A), which peaked after 2 days of transfection and decreased gradually, eventually becoming undetectable by 10 days (Figure 1E). The temporary transgene expression of transfected cells was confirmed by western blot analysis for PAX7 (Figure 1F), which correlated with the GFP expression pattern (Figure 1E).
3.2. PAX7 expression following multiple MC transfections
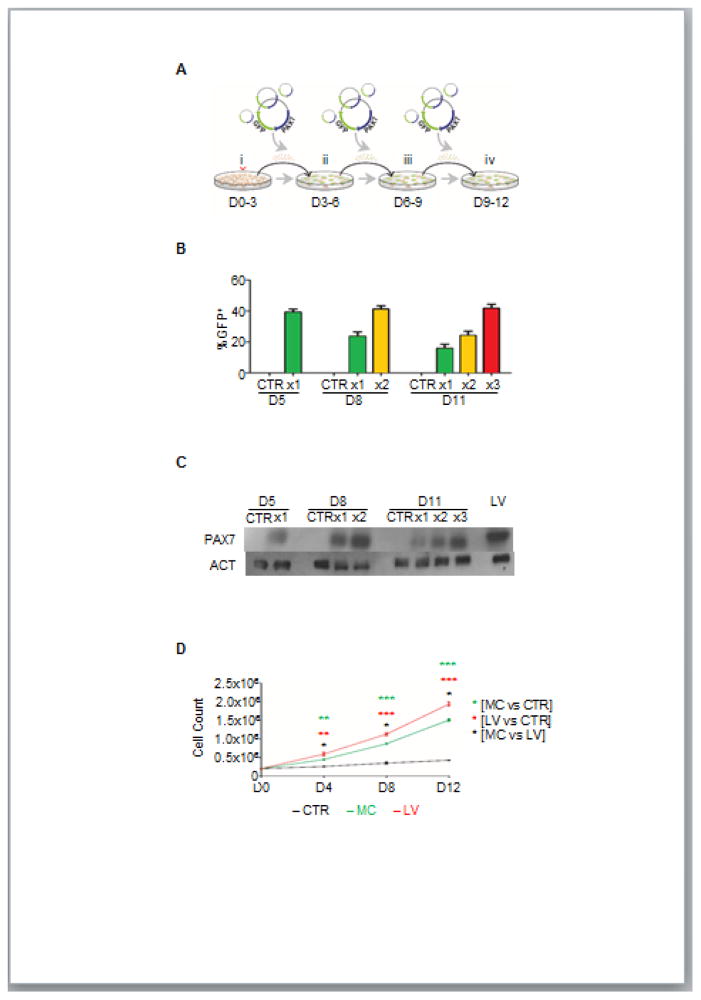
Our initial results indicate that transient PAX7 expression using MC peaks 2 days after transfection but it is not sustained at this level, which is necessary for generating myogenic progenitors. Therefore, we assessed whether multiple transfections would yield sustained high expression of PAX7 (Figure 2A). FACS analysis for GFP expression (Figure 2B), followed by western blot for PAX7 (Figure 2C) confirmed that multiple transfections (n=3) resulted in sustained high levels of PAX7 and therefore, myogenic progenitor identity. Next, we assessed the in vitro scalability of transfected cells. As observed in Figure 2D, MC-generated myogenic progenitors demonstrated exponential growth but at a significantly lower rate than the iPAX7 counterparts (LV). As expected, non-transfected cells showed almost no cell proliferation (Figure 2D). This further confirms the necessity of maintaining high levels of PAX7, equivalent to those in iPAX7 cells, for the expansion of mesoderm-derived myogenic progenitors.
Figure 2. PAX7 expression and expansion following multiple MC transfections.
(A) Schematic diagram of differentiation protocol outlining multiple MC transfections (i = CHIR99021; ii–iv = single cell seeding, MC transfections, medium containing FGF2).
(B–C) Graph shows expression levels of GFP measured by FACS in cells that had been subjected to one (x1) or multiple MC transfections, x2, or x3. Cells were analyzed 2 days after each transfection. Non-transfected cells were used as a negative control. Values represent mean ± SEM (n=3 biological replicates) (B). Western blot analysis for PAX7 expression in respective samples (C). ACT was used as housekeeping protein. Lane 1 = D5 non-transfected cells; Lane 2 = D5 MC-transfected cells (x1); Lane 3 = D8 non-transfected cells; Lane 4 = D8 MC-transfected cells (x1); Lane 5 = D8 MC-transfected cells (x2); Lane 6 = D11 non-transfected cells; Lane 7 = D11 MC-transfected cells (x1); Lane 8 = D11 MC-transfected cells (x2); Lane 9 = D11 MC-transfected cells (x3) and Lane 10 = PAX7-induced myogenic progenitors. (n=2 biological replicates).
(D) Growth curve of cells that had been transfected with MC every 3 days, side-by-side with non-transfected cells (negative control) and PAX7-induced myogenic progenitors (positive control). Cell counts were recorded every 4 days, beginning at day 4 (D4). Values represent mean ± SEM. (n=3 biological replicates). *p < 0.05; **p < 0.01; ****p < 0.0001.
3.3. Terminal differentiation of MC-generated PAX7+ myogenic progenitors
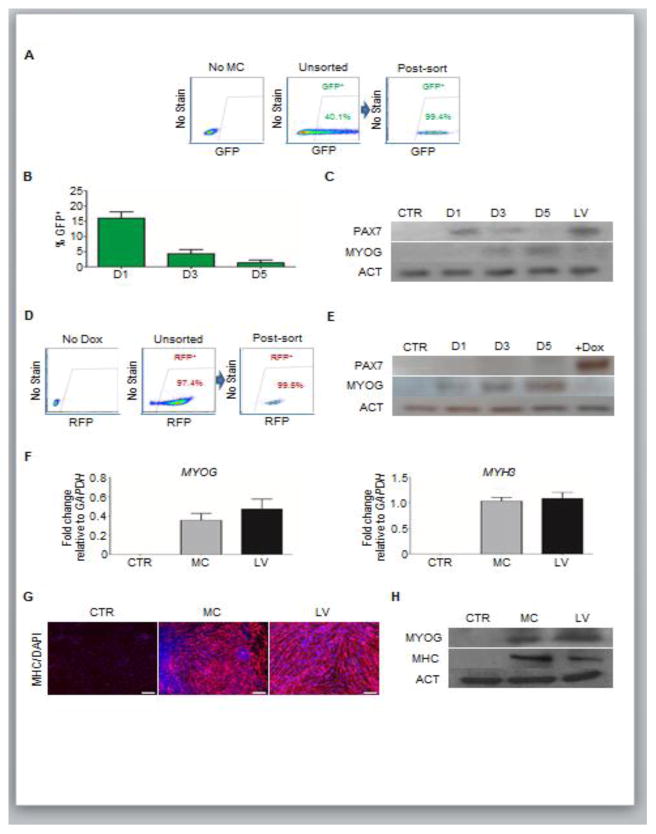
Following the expansion of myogenic progenitors (Figure 2D), GFP+ (PAX7+) cells were FACS purified to exclude any PAX7neg cells that could reduce the efficiency of myogenic differentiation. MC cultures contained approximately 40–50% of transgene expressing cells (Figure 3A, middle panel), which was much lower compared to iPAX7 myogenic progenitors (>90% RFP+ cells; Figure 3D, middle panel). Following purification, we observed an abrupt decline in the GFP expression of PAX7+ myogenic progenitors, which was almost absent by 5 days (Figure 3B). Accordingly, western blot analysis also showed a decline of PAX7 expression (Figure 3C). Interestingly, this coincided with a steady increase of MYOG expression (Figure 3C), indicating that PAX7+ cells readily lose their progenitor identity and differentiate towards myoblasts upon PAX7 down-regulation, as expected (Singh and Dilworth, 2013). Similarly, iPAX7 myogenic progenitors showed downregulation of PAX7 expression, however only upon Dox withdrawal, which is accompanied by steady increase of MYOG expression, suggesting more efficient control of PAX7 expression than in the MC approach (Figure 3E).
Figure 3. Terminal muscle differentiation of MC-generated PAX7+ myogenic progenitors.
(A) Representative FACS plots show GFP expression, and gates for the purification of MC-generated PAX7+ (GFP+) myogenic progenitors. Non-transfected cells (left), MC-transfected unsorted (middle) and MC-transfected FACS-purified cells (post-sort analysis; right) (n=3 biological replicates).
(B–C) Persistence of transgene expression analyses using MC. (B) Graph shows decay of GFP expression in FACS-purified GFP+ cells. Cells were analyzed every other day, beginning one day post-sorting seeding (D1). Values represent mean ± SEM (n=3). (C) Western blot analysis for PAX7 and MYOG expression in respective samples. ACT was used as a housekeeping protein. Lane 1 = Non-transfected cells; Lane 2 = GFP+ cells one day post-sorting (D1); Lane 3 = GFP+ cells three days post-sorting (D3); Lane 4 = GFP+ cells five days post-sorting (D5); Lane 5 = PAX7-induced myogenic progenitors. (n=2 biological replicates).
(D–E) Regulated PAX7 expression using LV. (D) Representative FACS plots show RFP (PAX7) expression in PAX7-induced myogenic progenitors, and gates for the purification of PAX7+ (RFP+) myogenic progenitors (D). Control non-induced iPAX7 cells (left), unsorted (middle) and post-sort (right) PAX7-induced myogenic progenitors (n=3 biological replicates). (E) Representative western blot analysis for PAX7 and MYOG expression in RFP+ cells with no Dox induction following sorting. ACT was used as a housekeeping protein. Lane 1 = Control non-induced iPAX7 cells (no Dox); Lane 2 = RFP+ cells, 1 day of no Dox induction after sorting of PAX7+ myogenic progenitors (D1); Lane 3 = RFP+ cells, 3 days of no Dox induction after sorting (D3); Lane 4 = RFP+ cells, 5 days of no Dox induction after sorting (D5); Lane 5 = PAX7-induced myogenic progenitors. (n=2 biological replicates).
(F–H) Quantitative RT-PCR analysis for MYOG and MHC in myotubes derived from MC- and LV-generated PAX7+ myogenic progenitors (F). Non-transfected cells served as negative control. Values represent mean ± SEM (n=3 biological replicates). (G–H) Immunofluorescence (G) and western blot (H) analyses of respective samples. (G) Staining for MHC in red and DAPI in blue. Scale bar = 200 μm (n=3 biological replicates). (H) Western blot for MYOG and MHC. ACT was used as housekeeping protein. Lane 1 = Non-transfected cells; Lane 2 = Myotubes from MC-generated PAX7 progenitor cells; Lane 3 = Myotubes from PAX7-induced progenitor cells (LV). (n=2 biological replicates).
To assess the ability of MC-derived PAX7+ myogenic progenitors to differentiate into MHC+ myotubes, cultures were allowed to reach confluency of >95% and induced to differentiate by serum withdrawal for up to one week. MC-derived myotubes showed similar levels of MYOG and MHC to LV-derived counterparts (Figure 3F–H). These results were further confirmed using chemically-defined monolayer (CDM) cultures (Kim et al., 2017) of H9 and PLZ PS cell lines, which showed similar results to EB cultures: i) PAX7 expression 2 days after transfection (Figure S1A–B); and ii) purified GFP+ (PAX7+) cells show robust terminal differentiation into MHC+ myocytes (Figure S1C–F). Thus, these data suggest that multiple MC transfections generate PAX7+ myogenic progenitors that can terminally differentiate into MHC+ myotubes in vitro.
3.4. Marginal engraftment potential of MC-generated PAX7+ myogenic progenitors
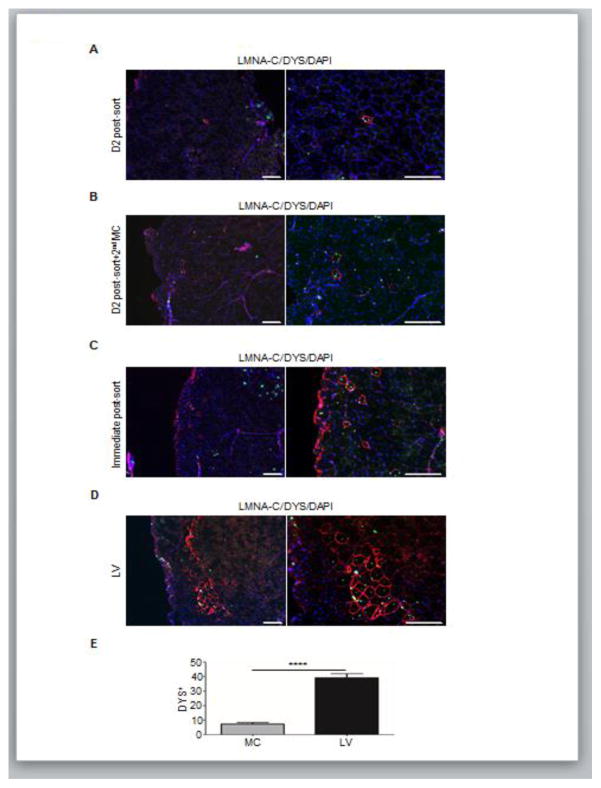
To assess the in vivo regenerative potential of MC-generated PAX7+ myogenic progenitors, we transplanted cells, at different time points of the differentiation protocol, into TA muscles of NSG mice. First, we injected GFP+ cells after 2 days of FACS isolation, which resulted in minimal engraftment (Figure 4A). Alternatively, we transplanted GFP+ sorted cells that had been re-transfected. However, no significant changes were observed (Figure 4B). Based on our observation regarding the rapid loss of PAX7 that is accompanied by differentiation towards myoblasts after sorting for GFP+ cells, we reasoned that it might be more effective to inject GFP+ cells soon after FACS purification. As observed in Figure 4C, this strategy improved engraftment. However, still a minority of transplanted cells survived and contributed to LMNA-C+/DYS+ myofibers. On the other hand, transplantation of iPAX7 myogenic progenitors showed a significantly higher number of donor-derived myofibers (Figure 4D). A similar outcome was obtained using CDM culture conditions for H9 (Figure S2A–B) and PLZ (Figure S2C) PS cells. Thus, myogenic progenitors generated using the MC approach possesses limited ability to contribute to myofiber formation in vivo.
Figure 4. Engraftment potential of MC-generated PAX7+ myogenic progenitors.
(A–D) Representative immunofluorescence analysis of MC-generated (A–C) PAX7+ myogenic progenitors and LV-counterparts (D) for LMNA-C and DYS in transplanted NSG mice. For the MC approach, muscles were injected with (A) PAX7+ cells 2 days following FACS purification for GFP, (B) Day 2 cultures of PAX7+ cells that had been re-transfected with MC one day after FACS purification for GFP, and (C) PAX7+ cells directly after FACS sorting for GFP. Transplantation with LV-generated PAX7-induced myogenic progenitors served as reference (D). Staining for LMNA-C is shown in green, DYS in red and DAPI in blue. Scale bar = 200 μm (n=4 biological replicates).
(E) Respective quantifications were recorded from muscle sections (C–D). Values represent mean ± SEM (n=4 biological replicates). ****p < 0.0001.
4. DISCUSSION
Although numerous protocols are available for the in vitro differentiation of hPS cells into myogenic cells, the method that generates progenitors with functional force-generating potential, suitable for translation into a potential cell replacement therapy, is the one involving conditional lentiviral expression of PAX7 or PAX3 (Darabi et al., 2012; Darabi et al., 2008; Darabi et al., 2011; Filareto et al., 2012; Filareto et al., 2013). The LV system allows for the generation of large quantities of PAX7+ myogenic progenitors that upon transplantation, contribute to significant muscle regeneration in vivo. Here we tested an alternative non-viral delivery approach to induce PAX7 expression, and generate PS cell-derived myogenic progenitors. The in vitro results were comparable between MC- (after 3 transductions) and LV-generated myogenic progenitors. However, because the transgene expression mechanism of MC is non-integrative and PAX7+ myogenic progenitors rapidly divide when they are induced with PAX7 expression, we identified several disadvantages for the use of this approach for the generation of PS cell-derived myogenic progenitors: i) requirement of multiple transfections to sustain high levels of PAX7 expression, necessary to enable expansion and maintenance of PAX7+ myogenic progenitor identity, which is labor intensive and costly; ii) multiple transfections introduce toxicity, reducing the rate of cell growth even when the cell death inhibitor Y-27632 was used; and iii) inability to expand and maintain a homogeneous population of PAX7+ myogenic progenitors after FACS purification. On the other hand, the LV system only requires a single transduction, which results in stable and efficient transgene expression (>90%). This enables the expansion of a homogeneous population of PAX7+ myogenic progenitors with minimal cell death upon Dox induction.
However, efficient in vitro differentiation does not necessarily predict efficient in vivo function (Kim et al., 2017). The major caveat observed for the MC approach relates to the very limited in vivo regenerative potential of generated PAX7+ myogenic progenitors. This is likely due to the dilution of the MC, resulting in diminished PAX7 expression and subsequent rapid differentiation towards myoblast. It is shown here and previously that the transplantation of heterogeneous population of cells greatly reduces the efficacy of myogenic contribution in vivo (Kim et al., 2017). It might be possible to transplant a homogeneous population of MC-induced PAX7+ myogenic progenitors by directly sorting GFP+ (PAX7+) progenitors. However, FACS is highly stressful and transplanted cells are prone to apoptosis, limiting likelihood of successful engraftment. In the LV approach, in contrast, a homogeneous population of iPAX7 myogenic progenitors can be harvested with minimal stress in a significantly shorter time frame, and these have more efficient muscle regeneration contribution.
In conclusion, the MC approach described in this study provides a platform to generate a population of human PAX7+ myogenic progenitors from PS cells that can be terminally differentiated into MHC+ myocytes in vitro that is equivalent to iPAX7 cells. However, further development of the methodology will be necessary to significantly increase the in vivo regenerative potential before it could be considered as an alternative to the LV expression system for translational therapeutic applications.
Supplementary Material
HIGHLIGHTS.
PAX7 delivery using DNA minicircle (MC) induces myogenesis
Multiple rounds of PAX7 minicircle delivery produces proliferating PAX7+ cells
MC-generated myogenic progenitors differentiate in vitro into MHC+ myotubes
MC-generated myogenic progenitors have minimal in vivo regenerative potential
Delivery optimization will be necessary for cell replacement therapy applications
Acknowledgments
This project was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR055299, the Parent Project Muscular Dystrophy (PPMD), ADVault Inc., and MyDirectives.com (R.C.R.P.). The monoclonal antibody to MHC was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa. We thank Cynthia Dekay for assistance in graphic design.
Abbreviations
- hPS
human pluripotent stem
- MYOD
Myogenic differentiation
- PAX7
Paired box 7
- Dox
doxycycline
- LV
lentivirus
- NSG
NOD-scid gamma
- TA
tibialis anterior
- MC
minicircle
- PP
parental plasmid
- iPS
induced pluripotent stem
- EB
embryoid body
- CTR
control
- FACS
fluorescence-activated cell sorting
- BRY
Brachyury
- MSGN1
Mesogenin 1
- CDM
chemically-defined monolayer
- MYOG
Myogenin
- MHC
Myosin-Heavy-Chain
- LMNA-C
Lamin A/C
- DYS
Dystrophin
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- iPAX7
inducible PAX7
- GAPDH
Glyceraldehyde phosphate dehydrogenase
- ACT
Actin
- FGF-2
Fibroblast growth factor-2
- Origin
bacterial origin of replication
- CMV
Cytomegalovirus promoter
- WPRE
Woodchuck hepatitis virus post-transcriptional regulatory element
- SV40 poly A
Simian virus 40 poly A tail
- Kanamycin
Kanamycin resistance gene
Footnotes
AUTHORSHIP CONTRIBUTIONS
J.K. designed and conducted experiments, analyzed the data, and wrote the manuscript. V.K.P.O. and A.Y. performed experiments. R.C.R.P. supervised the study, analyzed the data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albini S, Coutinho P, Malecova B, Giordani L, Savchenko A, Forcales S, Puri PL. Epigenetic reprogramming of human ES cells into skeletal muscle cells and generation of contractile myospheres. Cell reports. 2013;3:661–670. doi: 10.1016/j.celrep.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- Chabot S, Orio J, Schmeer M, Schleef M, Golzio M, Teissie J. Minicircle DNA electrotransfer for efficient tissue-targeted gene delivery. Gene Ther. 2013;20:62–68. doi: 10.1038/gt.2011.215. [DOI] [PubMed] [Google Scholar]
- Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, Cherrier T, Nesmith AP, Parker KK, Pourquie O. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protocols. 2016;11:1833–1850. doi: 10.1038/nprot.2016.110. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Molecular Therapy. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Darabi R, Arpke RW, Irion S, Dimos John T, Grskovic M, Kyba M, Perlingeiro RR. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RCR. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- Darabi R, Pan W, Bosnakovski D, Baik J, Kyba M, Perlingeiro RCR. Functional Myogenic Engraftment from Mouse iPS Cells. Stem Cell Rev and Rep. 2011;7:948–957. doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filareto A, Darabi R, Perlingeiro RCR. Engraftment of ES-Derived Myogenic Progenitors in a Severe Mouse Model of Muscular Dystrophy. Journal of stem cell research & therapy. 2012;10:S10–001. doi: 10.4172/2157-7633.S10-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filareto A, Parker S, Darabi R, Borges L, Iacovino M, Schaaf T, Mayerhofer T, Chamberlain JS, Ervasti JM, McIvor RS, et al. An ex vivo gene therapy approach to treat muscular dystrophy using inducible pluripotent stem cells. 2013;4:1549. doi: 10.1038/ncomms2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, Gekas J, Rousseau J, Tremblay JP. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Molecular Therapy. 2012;20:2153–2167. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Hardee CL, Arévalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in Non-Viral DNA Vectors for Gene Therapy. Genes. 2017;8:65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y, Suk S, Lin S, Tierney M, Du B, Seo T, Mitchell A, Sacco A, Varghese S. Directed In Vitro Myogenesis of Human Embryonic Stem Cells and Their In Vivo Engraftment. PLoS ONE. 2013;8:e72023. doi: 10.1371/journal.pone.0072023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail R, Allaudin ZN, Lila MAM. Scaling-up recombinant plasmid DNA for clinical trial: Current concern, solution and status. Vaccine. 2012;30:5914–5920. doi: 10.1016/j.vaccine.2012.02.061. [DOI] [PubMed] [Google Scholar]
- Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Meth. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Magli A, Chan S, Oliveira V, Wu J, Darabi R, Kyba M, Perlingeiro R. Expansion and purification are required for the therapeutic application of pluripotent stem cell-derived myogenic progenitors. Stem Cell Reports. 2017 doi: 10.1016/j.stemcr.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Magli A, Perlingeiro RCR. Working with Stem Cells. Cham: Springer International Publishing; 2016. Efficient Generation of Skeletal Myogenic Progenitors from Human Pluripotent Stem Cells; pp. 277–285. [Google Scholar]
- Lagha M, Sato T, Bajard L, Daubas P, Esner M, Montarras D, Relaix F, Buckingham M. Regulation of skeletal muscle stem cell behavior by Pax3 and Pax7. Cold Spring Harb Symp Quant Biol. 2008;73:307–315. doi: 10.1101/sqb.2008.73.006. [DOI] [PubMed] [Google Scholar]
- Magli A, Incitti T, Kiley J, Swanson SA, Darabi R, Rinaldi F, Selvaraj S, Yamamoto A, Tolar J, Yuan C, et al. PAX7 Targets, CD54, Integrin α9β1 and SDC2, Allow Isolation of Human ES/iPS Cell-Derived Myogenic Progenitors Cell Reports. 2017 doi: 10.1016/j.celrep.2017.06.005. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer P, Iro M. Minicircle-DNA. In: Thalhamer J, Weiss R, Scheiblhofer S, editors. Gene Vaccines. Vienna: Springer Vienna; 2012. pp. 297–310. [Google Scholar]
- Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, Wu JC. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protocols. 2010;6:78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlsen K, Broll S, Bode J. Replicating minicircles: Generation of nonviral episomes for the efficient modification of dividing cells. Gene Ther Mol Biol. 2006;10:233–244. [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cellls. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shelton M, Metz J, Liu J, Carpenedo RL, Demers SP, Stanford WL, Skerjanc IS. Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Reports. 2014;3:516–529. doi: 10.1016/j.stemcr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Dilworth FJ. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS Journal. 2013;280:3991–4003. doi: 10.1111/febs.12188. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Gerli MFM, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Science Translational Medicine. 2012;4:140ra189–140ra189. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- Xi H, Fujiwara W, Gonzalez K, Jan M, Liebscher S, Van Handel B, Schenke-Layland K, Pyle AD. In Vivo Human Somitogenesis Guides Somite Development from hPSCs. Cell reports. 2017;18:1573–1585. doi: 10.1016/j.celrep.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- Young Courtney S, Hicks Michael R, Ermolova Natalia V, Nakano H, Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack Jerome A, et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell. 2016;18:533–540. doi: 10.1016/j.stem.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JK, Wang Y, Karandikar A, Wang Q, Gai H, Liu AL, Peng C, Sheng HZ. Skeletal myogenesis by human embryonic stem cells. Cell Res. 2006;16:713–722. doi: 10.1038/sj.cr.7310080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.