Abstract
Background
The anatomy and physiology of the pig nervous system is more similar to humans compared to traditional rodent models. This makes the pig an attractive model to answer questions relating to human health and disease. Yet the technical and molecular tools available to pig researchers are limited compared to rodent researchers
New Method
We developed simple and rapid methods to isolate the trigeminal, nodose (distal vagal), and dorsal root ganglia from neonatal pigs. We selected these ganglia due to their broad applicability to basic science researchers and clinicians
Results
Use of these methods resulted in reproducible isolation of all three types of ganglia as validated by histological examination.
Comparison with Existing Method(s)
There are currently no methods that describe a step-by-step protocol to isolate these porcine ganglia.
Conclusions
In conclusion, these methods for ganglia collection will facilitate and accelerate future neuroscience investigations in pig models of human disease.
Keywords: porcine, trigeminal, vagal, dorsal root ganglia, nodose
INTRODUCTION
Large animal models are increasingly utilized in biomedical research as a useful and alternative model for the study of human diseases1–3. Large animals models offer an advantage in that the anatomy and physiology of many organ systems more closely parallel human organs compared to traditional rodent models4–8. For example, the growth and development of the porcine brain is more similar to humans when compared to rodents, carnivores, and other ungulates8. Yet rodent animals dominate many areas of research, including neuroscience9. This is due in part to the greater number of resources and tools available to rodent researchers. Therefore, developing and making available tools and resources for large animal models10 might expand research programs utilizing large animal models and accelerate new research discoveries. This might be particularly relevant given the recent surge in neuroscience funding initiatives11.
Here, we describe user-friendly methods for dissection and collection of the trigeminal, nodose (distal vagal), and dorsal root ganglia. These major ganglia provide sensory feedback from multiple organ systems and are involved in autonomic control. Their association with nociception and pain12, which is a common manifestation of various neurological diseases13, is also of broad interest. Following the methods herein allows for rapid and reproducible isolation of ganglia that may be used for numerous downstream processes, such as histology, RNA isolation, and cell culturing.
METHODS
Animals: Newborn pigs (~1 week of age, n=2, male, n = 2 female) were used for this study. Pigs were sedated and anesthetized by masked inhalation of 8% Sevothesia (Henry Schein Animal Health) and euthanized by intravenous Euthasol (Virbac). Tissues (n=8 ganglia, right and left ganglia from each pig) were collected following protocols that were developed from combined experiences (LR and DM) to result in simple and repeatable approach for each ganglia (trigeminal, nodose, dorsal root) in pigs of both sexes. The University of Florida Animal Care and Use Committee approved all procedures.
In young pigs, basic dissecting tools that are required include a scalpel, thumb forceps, mayo scissors (or chicken shears) and occasionally a bone rongeur. In older animals with additional calcification of bones, additional tools (e.g. oscillating saw, hand saws, etc.) may be necessary.
Histology: After collection, tissues were fixed in 10% neutral buffered formalin (~7–10 days) then routinely processed, paraffin-embedded, sectioned (~4 μm) and stained with Masson’s trichrome stain. Tissues were examined by a veterinary pathologist (DKM) for confirmation of ganglia and digital images were collected with specialized equipment (BX51 microscope and DP73 digital camera, Olympus) and software (CellSens Pathology Edition, Olympus).
RESULTS
Nodose Ganglion
The pig is placed in dorsal recumbency. A midline incision is made at the neck and the skin is reflected back to form a window viewing the larynx and soft tissues surrounding the trachea (Figure 1A, B right side). The soft tissues (muscles, adipose tissue) ventral and lateral to the trachea are removed so as to see the neurovascular tracts containing nerve, artery and vein (Figure 1B). From this point, the nerve is carefully dissected and followed cranially (1–3+ cm) to find the bulge associated with the nerve – which is the nodose ganglion (Figure 1C). This approach helps to avoid accidental collection of other ganglia that are in the region such as the cranial cervical ganglion14. Moreover, this approach provides a very simple strategy to identify the nodose ganglion (via tracing of the nerve towards the skull); such descriptions are lacking in other resources15,16.
Figure 1.
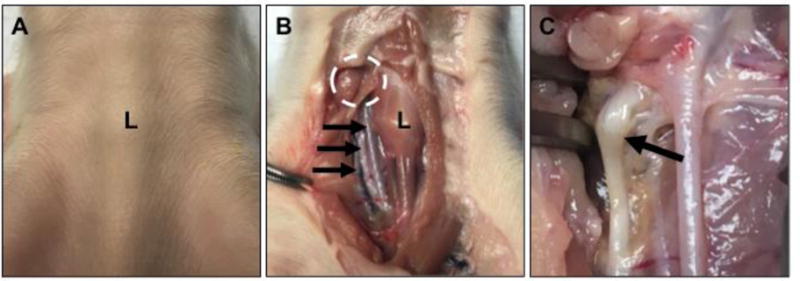
Cervical neck, ventral midline view with the cranial aspect being in the upper portion of image. A) The larynx (L) can be seen as a landmark in the newborn pig. B) The overlying skin is removed to form a window over the larynx (L) and adjacent soft tissues (see example of soft tissues on right side of larynx in image). These soft tissues are carefully dissected away from the larynx/trachea (see dissected example on left side of larynx in image) to reveal the nearby tracts of artery (top arrow), vagus nerve (middle arrow) and vein (bottom arrow). Once the vagus nerve is identified, it is traced cranially toward the expected locale of the nodose ganglion (dashed circle). C) The nodose ganglion (arrow) is morphologically defined as a “bulge” along the vagus nerve.
Trigeminal Ganglion
The cervical spine (near C1) is sectioned so as to disarticulate and decapitate the head from the spine (Figure 2A). The skin over the head is removed. The skull is cut in the horizontal plane so as to remove the calvarium and the brain. The bone tissue overlying each globe and optic nerve is cut as shown in Figure 2A (dashed lines) and detached to expose the eyes and orbital sockets (Figure 2B). The globe, optic nerve and adjacent soft tissues are removed to reveal the trigeminal nerve (Figure 2C, arrows). A larger window is cut through the ventral temporal bone to further uncover the trigeminal nerve (Figure 2D, blue arrows). The trigeminal nerve is traced caudally towards its exit point from the skull, where the trigeminal ganglia are located (Figure 2D, white arrow). Cutting the nerve at the exit point frees it from the base of the skull and allows for easy manipulation and removal, while keeping the ganglia intact (Figure 2E, white dashed circle). The intact ganglia appear as bulbous tissue (Figure 2F, white arrows) adjacent to the trigeminal nerve near its origin. Collection in this manner allows for functional and morphological characterization of the trigeminal ganglia17.
Figure 2.
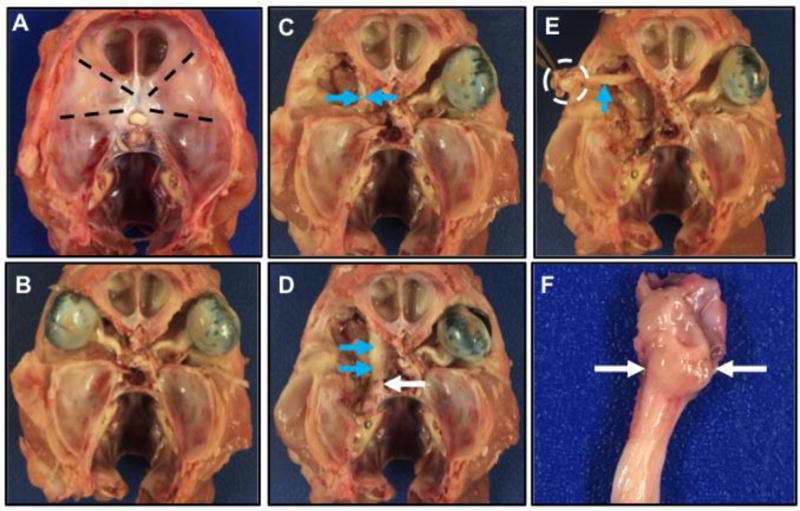
Cranial view of the skull with the calvarium and brain removed. A, B) The bones overlying the eyes (bulbus oculi) are removed in a roughly triangular pattern (A, dashed lines) to expose the eyes and the optic nerve (B). C) The eye, optic nerve and soft tissues are carefully removed and the large trigeminal nerve (blue arrows) can start to be seen tracking along the medial aspects. D) The trigeminal nerve (blue arrows) is carefully dissected caudally towards its origin (white arrow) near its attachment to the brain. E) The trigeminal nerve (blue arrow) can be dissected free from the adjacent tissue and ganglia will generally be located near its origin (dashed circle). F) The trigeminal ganglia (arrows, slight bulge that is less white than adjacent nerve) and nerve near its origin (top of image).
Dorsal Root Ganglia (DRG)
For the purposes of this methods paper, we will focus on the cervical DRG, but DRG collection in the thoracic or lumbar regions can be similarly made using slight modifications in approach. For cervical DRG, the cervical spine (near C1) is sectioned as done for the trigeminal collection (see above) and the spine is also sectioned in the thoracic spine. The epaxial muscles and ribs are removed for easier handling (Figure 3A). After initial sectioning, the cervical spine can be examined at the sectioned interface for the spinal nerves (Figure 3A, arrow) that can be often seen exiting the vertebral canal. Dissection in this region can further expose the spinal nerves (Figure 3B, small arrows) traversing the neuroforamina into the tissues and DRGs (Figure 3B–C, large arrows) are seen as prominent bulges along the spinal nerves. This technique is useful for all applications, but is optimal for collection of intact DRG to be used for ganglia morphology and morphometric applications.
Figure 3.
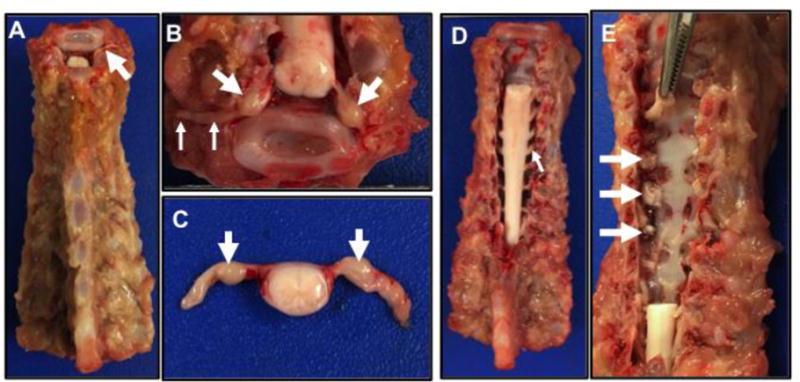
Cervical to thoracic spine, dorsal view. A) The spine is sectioned from the head (at top of image) with the epaxial muscles and ribs removed. Note the small spinal nerve root (arrow) from the sectioning of the cervical spine. B) Cervical section through the spine (see top of A) that has been further dissected to reveal the dorsal root ganglia (large arrows) and spinal nerves (small arrows) extending from the spinal cord. C. Section of spinal cord and nerves with dorsal root ganglia (arrows). D) Dorsal view of cervical spine following laminectomy to reveal the spinal cord and nerve roots (arrow). E) The cervical spinal cord (see D) with spinal nerves was removed. Remnant spinal nerves can be seen exiting into the neuroforamina (arrows). Forceps (top of image) can be inserted into the neuroformina to grasp and remove the spinal nerve with dorsal root ganglia.
DRGs are also collected for molecular studies (e.g. RNA transcription) and another modification to the technique can provide larger quantity of DRG for multiple molecular analyses. In this approach, the spine is prepared as in Figure 3A. Then using rongeurs or scissors, the vertebral lamina are bilaterally sectioned over a defined region (e.g. cervical spine) to remove the posterior covering of the spinal canal. Here, the spinal cord and spinal nerve roots can be clearly seen (Figure 3D). The spinal nerves are cut with scissors near the spinal cord and the spinal cord is removed leaving visible the remnant spinal nerves in their respective neuroforamina (Figure 3D, arrows). Forceps can be used to reach into the neuroforamina and remove the spinal nerve and DRG (Figure 3E). The tissues can sometimes acquire crush artifacts during this process that compromise the DRG morphology, so this technique may be best suited for molecular studies. Histological examination is useful to confirm proper collection of the ganglia, especially when the techniques are first being learned. Also, histological images of ganglia can be useful for data publication to further corroborate the proper collection of ganglia for molecular studies. The respective ganglia in this study were readily identified histologically by aggregates of neurons adjacent to the respective nerves (Figure 4 A–D) and histological evidence of ganglia were found in all samples (100%).
Figure 4.
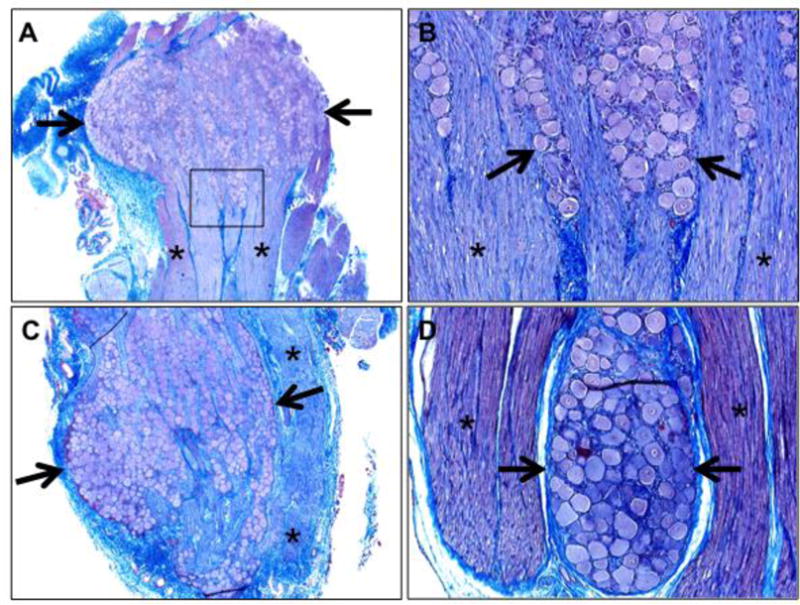
Histological confirmation of ganglia collection. A) Trigeminal ganglia, 20×. B) Trigeminal ganglia (box in figure 4A), 100×. C) Nodose ganglia, 40×. D) Dorsal root ganglia, 100×. Note the ganglia that are observed by aggregates of neurons (A-D, arrows) and are adjacent to nerves (A-D, highlighted by asterisks), Masson’s trichrome stains.
DISCUSSION
Pigs are studied as models of human disease because of their similar anatomy, physiology, metabolism, and size5,18. In recent years, their use in translational research has significantly expanded due to the development of genetically modified and experimentally-induced pig models of human disease. These include cystic fibrosis19–21, muscular dystrophy22, diabetes23, cardiovascular disease24,25,26, diabetes27, cancer28,29, environmental toxicology30, cutaneous wound healing31, metabolic syndrome32, among others. Porcine models have also been used to investigate traumatic brain injury3, neurodegenerative diseases33,34, brain development35, seizures36, cognition37, and neurogenesis38.
In the current study, we demonstrated practical methods for the collection of three key ganglia (the trigeminal, nodose, and dorsal root) from juvenile pigs. The trigeminal ganglia provide sensory innervation to the face, oral and nasal cavities and dura mater of the CNS39. These neurons relay mechanosensitive, thermosensitive, and nociceptive information to the brainstem and upper spinal cord. Thus, the trigeminal ganglia are often implicated in pain associated with toothaches, headaches, migraines, rhinosinusitis, temporomandibular joint (TMJ) disorder and trigeminal neuralgia. The vagal ganglia, consisting of the jugular and nodose ganglion, send projections to multiple visceral organs, including the respiratory40, gastrointestinal41, and cardiovascular42 systems. The vagal ganglia have been implicated in several diseases, including airway hyperreactivity43,44, chronic pulmonary disease45, inflammatory bowel disease46, esophageal motility disorders47, and obesity48. The DRG are a collection of sensory neurons that convey information from the periphery to the CNS49. They have been associated with neuropathic pain following nerve injury50. Thus, the trigeminal, nodose, and dorsal root ganglia are of broad interest to basic scientists and clinicians.
The bilateral nature of ganglia can be a strategic advantage for investigators. First, both ganglia can be collected as samples of treatment effects on each animal, to help mitigate intragroup variances and outliers. Second, investigators can utilize each ganglia for different approaches such as one for those requiring intact morphology (histopathology, immunohistochemistry, immunofluorescence, in situ hybridization, etc.) and the other ganglia for molecular techniques where the morphology is not essential (e.g. proteomics, RNA, and DNA analyses). This approach of parallel usage of ganglia from the same animal/experiment can increase the redundancy and rigor of data for a given project51.
While pigs are increasingly studied as preclinical models of disease, compared to rodent species, there is a paucity of published resources, expertise, reagents and other tools available. The implementation of the National Swine Resource and Research Center10 and the increased recognition of the importance of porcine models is helping to close this gap. As more investigators embrace large animal models, this technical resource, along with additional emerging resources, will continue to enhance the research landscape.
Highlights.
Reproducible and rapid techniques for isolating three common porcine ganglia
Image-guided methods to accommodate novice to experienced audiences
Methods emphasize preservation of ganglia structure for morphometric examination
Dual approaches for DRG collection depending on anticipated downstream uses
Acknowledgments
The authors thank Yan-Shin Liao, Katelyn Davis, Joshua Dadural, and Emily Collins for helpful technical assistance. This work was supported by the National Institutes of Health (R00 HL119560-03, P01 HL051670, P01 HL091842, P30 DK054759) and the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ziegler A, Gonzalez L, Blikslager A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell Mol Gastroenterol Hepatol. 2016;2:716–724. doi: 10.1016/j.jcmgh.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoegger MJ, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cullen DK, et al. A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods Mol Biol. 2016;1462:289–324. doi: 10.1007/978-1-4939-3816-2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi E, Hishikawa S, Teratani T, Lefor AT. The pig as a model for translational research: overview of porcine animal models at Jichi Medical University. Transplant Res. 2012;1:8. doi: 10.1186/2047-1440-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers CS, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan N, Lai L. Genetically modified pig models for human diseases. J Genet Genomics. 2013;40:67–73. doi: 10.1016/j.jgg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Reznikov LR. Cystic Fibrosis and the Nervous System. Chest. 2017;151:1147–1155. doi: 10.1016/j.chest.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lind NM, et al. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council. Policy and Global Affairs. Division on Earth and Life Studies. Institute of Medicine. Board on Health Sciences Policy. Committee on Science, T., and Law. Institute for Laboratory Animal Research. Forum on Neuroscience and Nervous System Disorders. Pankevich Diana E, Wizemann Theresa M, Mazza Anne-Marie, Altevogt Bruce M. Rapporteurs. Animals in Neuroscience Research. 2012:29–42. [Google Scholar]
- 10.Walters EM, Wells KD, Bryda EC, Schommer S, Prather RS. Swine models, genomic tools and services to enhance our understanding of human health and diseases. Lab Anim (NY) 2017;46:167–172. doi: 10.1038/laban.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funding big neuroscience. Nat Neurosci. 2014;17:1. doi: 10.1038/nn.3621. [DOI] [PubMed] [Google Scholar]
- 12.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- 13.Borsook D. Neurological diseases and pain. Brain. 2012;135:320–344. doi: 10.1093/brain/awr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabak M, Orhan IO, Haziroglu RM. Macro anatomical investigations of the cranial cervical ganglion in domestic pig (Sus scrofa domesticus) Anat Histol Embryol. 2005;34:199–202. doi: 10.1111/j.1439-0264.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 15.Hayes D, Jr, et al. Identification of the nodose ganglia and TRPV1 in swine. Lung. 2013;191:445–447. doi: 10.1007/s00408-013-9496-y. [DOI] [PubMed] [Google Scholar]
- 16.Ding P, Tufano RP, German RZ. Anatomical anomalies of the laryngeal branches of the vagus nerve in pigs (Sus scrofa) Lab Anim. 2012;46:338–340. doi: 10.1258/la.2012.012091. [DOI] [PubMed] [Google Scholar]
- 17.Reznikov LR, et al. CFTR-deficient pigs display peripheral nervous system defects at birth. Proc Natl Acad Sci U S A. 2013;110:3083–3088. doi: 10.1073/pnas.1222729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aigner B, et al. Transgenic pigs as models for translational biomedical research. J Mol Med (Berl) 2010;88:653–664. doi: 10.1007/s00109-010-0610-9. [DOI] [PubMed] [Google Scholar]
- 19.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostedgaard LS, et al. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoltz DA, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–2693. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klymiuk N, et al. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum Mol Genet. 2013;22:4368–4382. doi: 10.1093/hmg/ddt287. [DOI] [PubMed] [Google Scholar]
- 23.Wolf E, Braun-Reichhart C, Streckel E, Renner S. Genetically engineered pig models for diabetes research. Transgenic Res. 2014;23:27–38. doi: 10.1007/s11248-013-9755-y. [DOI] [PubMed] [Google Scholar]
- 24.McKenney-Drake ML, et al. Epicardial Adipose Tissue Removal Potentiates Outward Remodeling and Arrests Coronary Atherogenesis. Ann Thorac Surg. 2017;103:1622–1630. doi: 10.1016/j.athoracsur.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tung R, et al. Scar voltage threshold determination using ex vivo magnetic resonance imaging integration in a porcine infarct model: Influence of interelectrode distances and three-dimensional spatial effects of scar. Heart Rhythm. 2016;13:1993–2002. doi: 10.1016/j.hrthm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Davis BT, et al. Targeted disruption of LDLR causes hypercholesterolemia and atherosclerosis in Yucatan miniature pigs. PLoS One. 2014;9:e93457. doi: 10.1371/journal.pone.0093457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renner S, et al. Comparative aspects of rodent and nonrodent animal models for mechanistic and translational diabetes research. Theriogenology. 2016;86:406–421. doi: 10.1016/j.theriogenology.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Sieren JC, et al. Development and translational imaging of a TP53 porcine tumorigenesis model. J Clin Invest. 2014;124:4052–4066. doi: 10.1172/JCI75447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schook LB, et al. A Genetic Porcine Model of Cancer. PLoS One. 2015;10:e0128864. doi: 10.1371/journal.pone.0128864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond E, et al. Computed Tomography and Magnetic Resonance Imaging for Longitudinal Characterization of Lung Structure Changes in a Yucatan Miniature Pig Silicosis Model. Toxicol Pathol. 2016;44:373–381. doi: 10.1177/0192623315622303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56:127–138. doi: 10.1093/ilar/ilv016. [DOI] [PubMed] [Google Scholar]
- 32.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 33.Holm IE, Alstrup AK, Luo Y. Genetically modified pig models for neurodegenerative disorders. J Pathol. 2016;238:267–287. doi: 10.1002/path.4654. [DOI] [PubMed] [Google Scholar]
- 34.Beraldi R, et al. A novel porcine model of ataxia telangiectasia reproduces neurological features and motor deficits of human disease. Hum Mol Genet. 2015;24:6473–6484. doi: 10.1093/hmg/ddv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad MS, Dilger RN, Johnson RW. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev Neurosci. 2012;34:291–298. doi: 10.1159/000339311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller SM, et al. Neonatal seizures are associated with redistribution and loss of GABAA alpha-subunits in the hypoxic-ischaemic pig. J Neurochem. 2016;139:471–484. doi: 10.1111/jnc.13746. [DOI] [PubMed] [Google Scholar]
- 37.Radlowski EC, et al. A neonatal piglet model for investigating brain and cognitive development in small for gestational age human infants. PLoS One. 2014;9:e91951. doi: 10.1371/journal.pone.0091951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrad MS, Harasim S, Rhodes JS, Van Alstine WG, Johnson RW. Early postnatal respiratory viral infection alters hippocampal neurogenesis, cell fate, and neuron morphology in the neonatal piglet. Brain Behav Immun. 2015;44:82–90. doi: 10.1016/j.bbi.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durham PL, Garrett FG. Development of functional units within trigeminal ganglia correlates with increased expression of proteins involved in neuron-glia interactions. Neuron Glia Biol. 2010;6:171–181. doi: 10.1017/S1740925X10000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kollarik M, Ru F, Brozmanova M. Vagal afferent nerves with the properties of nociceptors. Auton Neurosci. 2010;153:12–20. doi: 10.1016/j.autneu.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams EK, et al. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hattori T, et al. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ Res. 2009;105:279–286. doi: 10.1161/CIRCRESAHA.109.202036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reznikov LR, et al. Acid-Sensing Ion Channel 1a Contributes to Airway Hyperreactivity in Mice. PLoS One. 2016;11:e0166089. doi: 10.1371/journal.pone.0166089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Undem BJ, Kollarik M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:355–360. doi: 10.1513/pats.200504-033SR. discussion 371–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. 2010;7:37. doi: 10.1186/1742-2094-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland CT, Satchell PM, Farrow BR. Vagal afferent dysfunction in naturally occurring canine esophageal motility disorder. Dig Dis Sci. 1994;39:2090–2098. doi: 10.1007/BF02090355. [DOI] [PubMed] [Google Scholar]
- 48.Kentish SJ, Page AJ. The role of gastrointestinal vagal afferent fibres in obesity. J Physiol. 2015;593:775–786. doi: 10.1113/jphysiol.2014.278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion - a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38. doi: 10.2147/JPR.S26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou XF, Deng YS, Xian CJ, Zhong JH. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci. 2000;12:100–105. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 51.Casadevall A, Fang FC. Rigorous Science: a How-To Guide. MBio. 2016;7 doi: 10.1128/mBio.01902-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


